Abstract
P-glycoprotein, an ATP-driven drug efflux pump, is a major obstacle to the delivery of small-molecule drugs across the blood-brain barrier and into the CNS. Here we test a unique signaling-based strategy to overcome this obstacle. We used a confocal microscopy-based assay with isolated rat brain capillaries to map a signaling pathway that within minutes abolishes P-glycoprotein transport activity without altering transporter protein expression or tight junction permeability. This pathway encompasses elements of proinflammatory- (TNF-α) and sphingolipid-based signaling. Critical to this pathway was signaling through sphingosine-1-phosphate receptor 1 (S1PR1). In brain capillaries, S1P acted through S1PR1 to rapidly and reversibly reduce P-glycoprotein transport activity. Sphingosine reduced transport by a sphingosine kinase-dependent mechanism. Importantly, fingolimod (FTY720), a S1P analog recently approved for treatment of multiple sclerosis, also rapidly reduced P-glycoprotein activity; similar effects were found with the active, phosphorylated metabolite (FTY720P). We validated these findings in vivo using in situ brain perfusion in rats. Administration of S1P, FTY720, or FTY729P increased brain uptake of three radiolabeled P-glycoprotein substrates, 3H-verapamil (threefold increase), 3H-loperamide (fivefold increase), and 3H-paclitaxel (fivefold increase); blocking S1PR1 abolished this effect. Tight junctional permeability, measured as brain 14C-sucrose accumulation, was not altered. Therefore, targeting signaling through S1PR1 at the blood-brain barrier with the sphingolipid-based drugs, FTY720 or FTY720P, can rapidly and reversibly reduce basal P-glycoprotein activity and thus improve delivery of small-molecule therapeutics to the brain.
Keywords: ABC transporters, chemotherapy, brain endothelium, ABCB1, breast cancer related protein, multidrug resistance-associated protein
Delivering drugs across the blood-brain barrier to treat CNS diseases (e.g., brain cancer, neuroAIDS, epilepsy, and brain injury) is one “final frontier” of pharmacotherapy (1). The brain capillary endothelium that comprises this barrier possesses a unique phenotype. Passive permeability across the brain capillary endothelium is limited by tight junctional complexes between cells and by a low rate of transcytosis. Endothelial cells also express ATP-driven drug efflux pumps [e.g., P-glycoprotein and breast cancer related protein (Bcrp), on the luminal, blood-facing, plasma membrane). For small-molecule drugs, especially lipophillic drugs, P-glycoprotein presents a formidable obstacle (2). Knocking out P-glycoprotein or administering potent competitors increases brain uptake of therapeutics in animal models, with greatly improved efficacy of chemotherapeutics against implanted brain tumors (3). However, translation of this strategy to the clinic is limited by systemic toxicity of the inhibitors themselves (4–6). One potentially powerful but untested alternative to the use of transport inhibitors would be to specifically target within brain capillary endothelial cells a signaling pathway that normally maintains basal transporter activity, the goal being to rapidly but transiently reduce efflux transport activity and thus increase drug delivery. Achieving this targeting requires the identification of a suitable signaling pathway, a “drugable” target within that pathway, and a clinically practical means of hitting that target.
Here we identify a signaling pathway that rapidly and reversibly reduces basal P-glycoprotein transport activity in rat brain capillaries in vitro and at the blood-brain barrier in vivo. We show that sphingolipid signaling through sphingosine-1-phosphate (S1P) and S1P receptor 1 (S1PR1) provides a suitable pharmacological target and demonstrate that the target can be hit using fingolimod (FTY720), a prodrug that is phosphorylated to form a nonselective S1PR agonist. FTY720 is a clinically feasible therapeutic because it is currently used to treat patients with relapsing multiple sclerosis (7). These findings disclose a promising signaling-based strategy to improve drug delivery to the CNS in patients where blood-brain barrier P-glycoprotein limits pharmacotherapy.
Results
S1P Signals Downstream of TNF-α to Rapidly Reduce P-Glycoprotein Transport Activity.
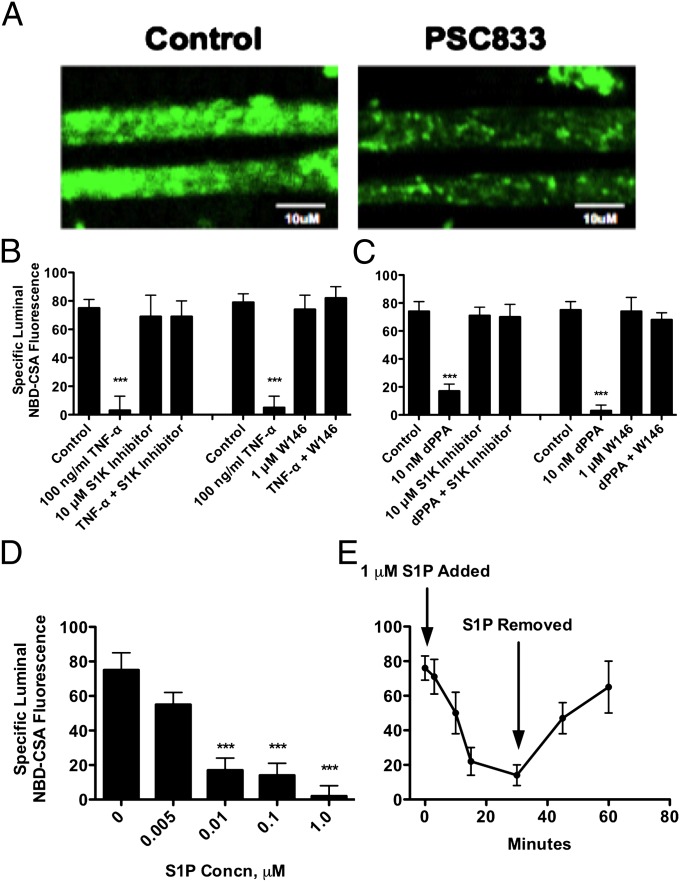
We previously established a confocal imaging-based assay to assess P-glycoprotein transport activity in freshly isolated brain capillaries from rats and mice (8, 9). This assay measures within capillary lumens accumulation of [N-ε(4-nitrobenzofurazan-7-yl)-d-Lys(8)]-cyclosporin A (NBD-CSA), a fluorescent P-glycoprotein substrate. Fig. 1A shows representative confocal images of capillaries incubated to steady state in medium containing 2 μM NBD-CSA (control) or NBD-CSA plus 5 μM PSC833, a specific inhibitor of P-glycoprotein. Note the loss of luminal fluorescence in the image from the capillary exposed to PSC833; this concentration of inhibitor maximally and specifically reduces luminal NBD-CSA accumulation. We previously used the PSC833-sensitive component of luminal NBD-CSA accumulation as a measure of specific P-glycoprotein transport activity in rat brain capillaries. As previously discussed (10, 11), we use here PSC833-sensitive, luminal NBD-CSA accumulation as a measure of specific P-glycoprotein transport activity.
Fig. 1.
TNF-α and dPPA act through sphingolipids to reduce luminal P-glycoprotein transport activity in isolated rat brain capillaries. (A) Representative confocal images of brain capillaries after a 60-min incubation with 2 μM NBD-CSA; note the high luminal fluorescence in the control capillary and decreased luminal fluorescence in capillaries exposed to 5 μM PSC833. (Scale bars, 10 μm.) (B) TNF-α acts through SKI, S1PR1, to reduce P-glycoprotein activity. (C) dPPA, a PKCβ1 agonist, acts through SK and S1PR1 to reduce P-glycoprotein transport activity. (D) S1P concentration-dependence of P-glycoprotein–mediated transport. (E) Rapid time course of S1P action. Capillaries were incubated to steady-state (60 min) in medium with 2 μM NBD-CSA. Then 1 μM S1P was added to the medium (time 0 on graph); 30 min later, capillaries were washed and S1P-free medium was added. Each bar represents the mean value for 12–16 capillaries from a single preparation (pooled tissue from five to seven rats); variability is shown as SE bars. Units are arbitrary fluorescence. Statistical comparisons: ***significantly lower than control, P < 0.001.
In rat brain capillaries, TNF-α signals through TNFR1, endothelin (ET)B, inducible nitric oxide synthase (iNOS), and PKCβ1 to rapidly reduce basal P-glycoprotein activity (9, 12, 13). In endothelial cells, TNF-α signaling stimulates S1P production from sphingosine through activation of sphingosine kinase 1 (SK) (14, 15). Two findings indicate that the TNF-α/ ETB/iNOS/PKCβ1 pathway is coupled to sphingolipid signaling in rat brain capillaries. First, we exposed capillaries to TNF-α in the absence or presence of an SK inhibitor or the S1PR1 antagonist, W146. As shown previously (12), TNF-α greatly reduced P-glycoprotein transport activity (Fig. 1B). Importantly, both the SK inhibitor and the S1PR1 antagonist blocked this TNF-α effect. Second, we repeated the experiment using the PKCβ1 activator, dPPA (12-deoxyphorbol-13-phenylacetate-20-acetate), to reduce P-glycoprotein activity. Again, both the SK inhibitor and the S1PR1 antagonist blocked the reduction in transporter activity (Fig. 1C). Taken together, these experiments indicate that SK and S1PR are downstream of the previously characterized TNF-α/ETBR/iNOS/PKCβ1 pathway.
The endogenous ligand for S1PR is S1P, a bioactive lipid metabolite generated from sphingosine by SK, which acts through a subfamily of G protein-coupled receptors expressed in nearly all cells, including endothelial cells (16). Exposing brain capillaries to 0.005–1 μM S1P for 1 h reduced P-glycoprotein transport activity in a concentration-dependent manner (Fig. 1D); using nonlinear regression we calculated the S1P concentration causing 50% loss of activity as 7 ± 2 nM. With 1 μM S1P, specific transport activity was essentially abolished. To determine the time course of S1P action, we first incubated capillaries to steady state (60 min) in medium with 2 μM NBD-CSA and then added 1 μM S1P. As shown in Fig. 1E, luminal NBD-CSA fell rapidly after addition of S1P. Within 10 min, luminal NBD-CSA was significantly lower than controls and within 30 min it was roughly the same as in capillaries exposed to 5 μM PSC833, indicating complete loss of transport activity. Removing S1P from the medium after 30 min caused a rapid return to control luminal fluorescence levels (Fig. 1E). This is the same rapid and reversible time course of action seen with TNF-α, ET-1, and the PKCβ1 activator, dPPA (9, 12). Other experiments demonstrated that: (i) P-glycoprotein protein expression (Western blots) did not change with several hours of S1P exposure (Fig. S1A); (ii) the TNF-α–, ET-1–. and PKCβ1-induced reduction in transport was not affected when transcription was inhibited with actinomycin D or when translation was inhibited with cyclohexamide (9, 12, 13); and (iii) transport activity was still significantly reduced with S1P exposures lasting 6 h (control, 40 ± 9 fluorescence units; 0.1 μM S1P, 24 ± 4 fluorescence units; n = 20 capillaries for each; P < 0.05).
We previously showed that concentrative bath to capillary lumen transport mediated by two other ATP-driven efflux transporters expressed at the luminal membrane of the brain capillary endothelium, multidrug resistance-associated protein 2 (Mrp2) and Bcrp (17, 18), was not reduced when exposure to TNF-α, ET-1, and dPPA reduced P-glycoprotein transport activity (9, 12, 13). This finding was taken to mean that signaling specifically affected P-glycoprotein activity and that neither tight junction permeability nor ATP availability was altered. Exposing capillaries to 1 μM S1P for 60 min had no effect on steady-state luminal accumulation of the Mrp2 substrate, Texas red (Fig. S1B), or the Bcrp substrate, bodipy-prazosin (Fig. S1C). However, as shown previously (9), increasing tight junctional permeability by adding 100 mM sucrose to the medium (hyperosmotic barrier opening) or poisoning metabolism with NaCN reduced Texas red accumulation in capillary lumens (Fig. S1B). Clearly, as with TNF-α, ET-1, and dPPA, S1P did not affect concentrative and ATP-dependent cell-to-lumen transport mediated by Mrp2 or Bcrp and, by inference, tight junction permeability.
Signaling Through S1PR1.
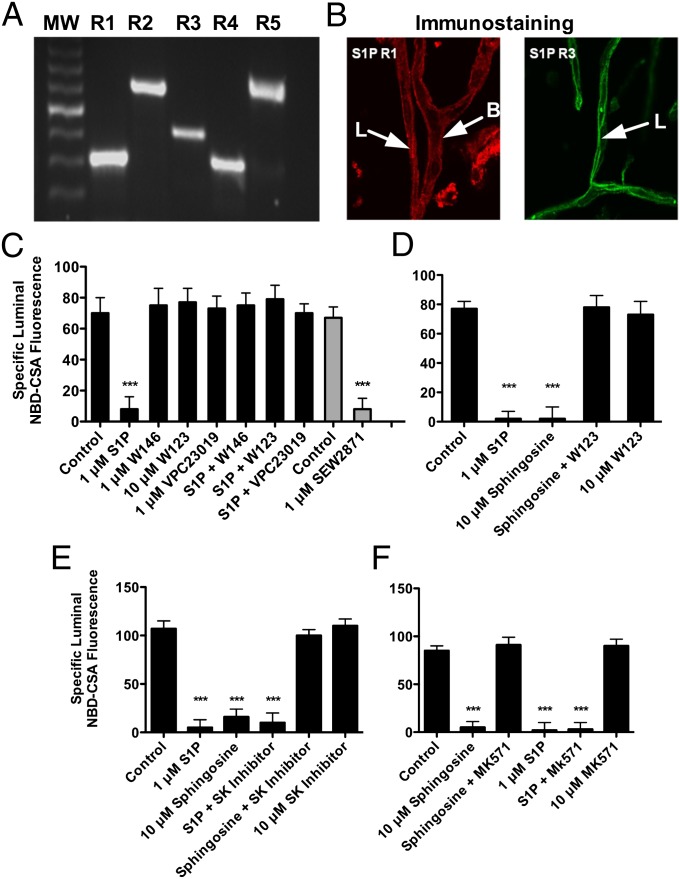
S1P signals through five extracellular receptors (16). Using sequence-targeted primers for each of the rat S1PR transcripts in a RT-PCR assay we detected transcripts for all five S1PRs in total RNA preparations isolated from rat brain capillaries (Fig. 2A). However, using receptor subtype-specific antibodies and immunofluorescence, we only detected protein expression of S1PR1 and S1PR3 (Fig. 2B). Using two different epitope-specific primary antibodies, we did not detect any signal above background for S1PR2, S1PR4, and S1PR5. Close inspection of immunostained capillaries revealed that S1PR1 was expressed on both sides of the endothelium, but S1PR3 was expressed only on the luminal side (Fig. 2B). Exposing rat brain capillaries to three S1PR antagonists blocked the reduction of basal transport activity signaled by S1P (Fig. 2C). Of these drugs, W123 and W146 are S1PR1-specific, but VPC23019 blocks S1PR1 and S1PR3. Consistent with those results, the S1PR1-selective agonist, SEW2871, significantly reduced basal P-glycoprotein transport activity (Fig. 2C). Finally, exposing capillaries to pertussis toxin, which blocks G-protein interaction with G protein-coupled receptors, abolished the effects of S1P on P-glycoprotein activity (Fig. S2). This finding indicates additional signaling downstream of S1PR1. Moreover, this result shows that the effects of S1P and other S1PR agonists do not involve direct interactions with P-glycoprotein.
Fig. 2.
S1PR expression and function in rat brain capillaries. (A) RT-PCR using mRNA isolated from brain capillaries. (Lane 1) 1 kb molecular weight marker; (lane 2) S1PR1; (lane 3) S1PR2; (lane 4) S1PR3; (lane 5) S1PR4; (lane 6) S1PR5. (B) Representative confocal images (magnification, 40×) of capillaries immunostained for S1PR1 and S1PR3. (C) S1PR1 antagonists block the action of S1P on P-glycoprotein transport activity (black bars); an S1PR1 agonist reduces transport activity (gray bars). (D) Sphingosine-induced reduction in P-glycoprotein activity is S1PR1-dependent. (E) Sphingosine reduction in P-glycoprotein activity is SK-dependent. (F) Sphingosine effects on transport depend on a Mrp. Each bar represents the mean value for 8–15 capillaries from a single preparation (pooled tissue from three to six rats); variability is shown as SE bars. Units are arbitrary fluorescence. Statistical comparisons: ***significantly lower than control, P < 0.001. B, basolateral membrane; L, luminal membrane.
The S1P precursor sphingosine reduced P-glycoprotein transport activity, an effect blocked by a S1PR1 antagonist (Fig. 2D) and a SK inhibitor (Fig. 2E). To access S1PR1, S1P produced intracellularly must be transported out of the cell. Previous experiments suggest that one or more Mrps can mediate S1P efflux from cells (19, 20). This result appears to be the case in brain capillaries, because MK571, a nonselective Mrp inhibitor, blocked the effects of sphingosine—but not S1P—on P-glycoprotein transport activity (Fig. 2F). Taken together, these data identify S1PR1 as the receptor through which S1P signals to reduce P-glycoprotein transport activity. These data also show that added sphingosine can be phosphorylated to S1P, which is transported out of the endothelial cells to target S1PR1, a pattern of inside-out signaling characteristic of sphingolipids (21). The signaling pathway from TNF-α through S1PR1 is shown in Fig. 3F.
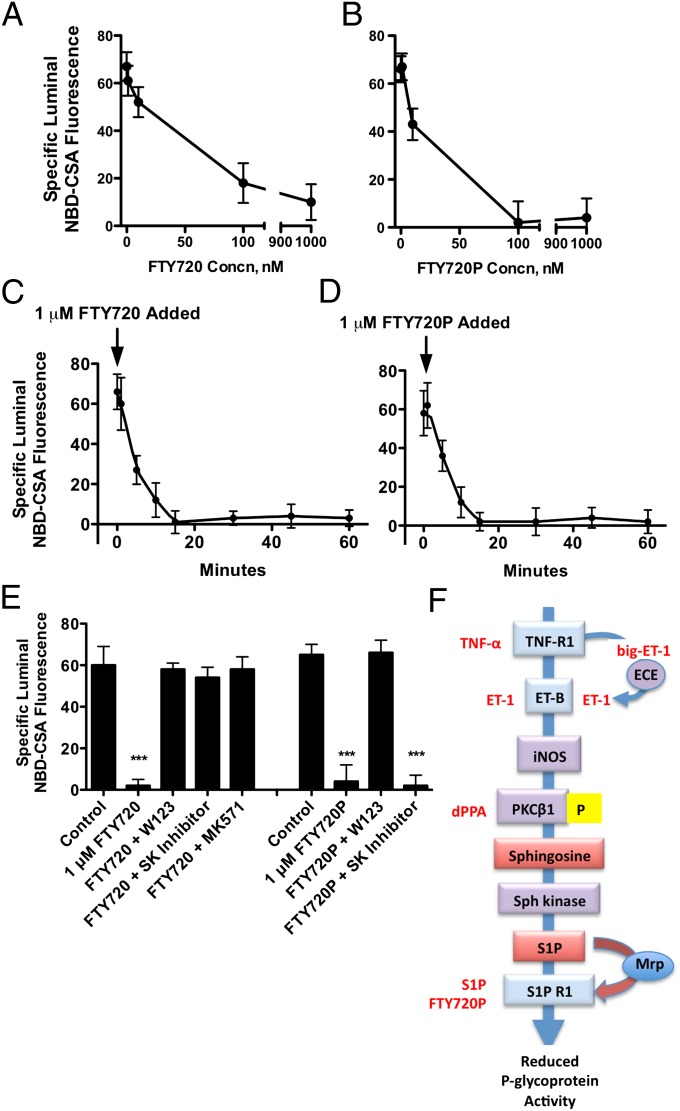
Fig. 3.
Fingolimod (FTY720) and its active metabolite, FTY720P, reduce P-glycoprotein transport activity. (A) FTY720 concentration dependence, (B) FTY720P concentration dependence, (C) FTY720 time course of action, (D) FTY720P time course of action. For the time courses, capillaries were loaded to steady state (60 min) in medium with 2 μM NBD-CSA. Then 1 μM FTY720 or FTY720P was added to the medium (time 0 on graph). (E) FTY720 and FTY720P require SK and S1PR1 to reduce P-glycoprotein activity. Each point represents the mean value for 10–15 capillaries from a single preparation (pooled tissue from 5 to 10 rats); variability is shown as SE bars. Units are arbitrary fluorescence. Extended signaling pathway through which TNF-α rapidly reduces basal P-glycoprotein transport activity. Statistical comparisons: ***significantly lower than control, P < 0.001. (F) Agents that activate signaling at various points in the pathway are shown in red. For details on events upstream of S1PR1, see refs. 9, 12, and 13.
Fingolimod (FTY720), a prodrug that is phosphorylated to form a nonselective S1PR agonist (FTY720P), is a highly effective oral treatment for relapsing-remitting multiple sclerosis (7). Preclinical studies suggest that FTY720 has promise for treatment of ischemic stroke (22). In multiple sclerosis, FTY720 appears to target both peripheral sites (lymphocytes) and sites within the CNS, including astrocytes (23, 24). Thus, the drug crosses the blood-brain barrier and in doing so has the potential to interact with S1PR within the brain capillary endothelium. Exposing brain capillaries to FTY720 or FTY720P for 1 h reduced P-glycoprotein transport activity in a concentration-dependent manner (Fig. 3 A and B). Like TNF-α (12), the PKCβ1 activator dPPA (13), and S1P (Fig. 1E), both FTY720 and FTY720P rapidly reduced P-glycoprotein transport activity (Fig. 3 C and D). Fifteen-minute exposure to either drug at 1 μM essentially abolished transport activity. The effects of both drugs were abolished when capillaries were exposed to an S1PR1 antagonist (Fig. 3E). Consistent with intracellular activation of the prodrug by phosphorylation, FTY720 effects on transport were abolished by an inhibitor of SK; this was not the case for FTY720P (Fig. 3E). Thus, in isolated rat brain capillaries, the prodrug FTY720 is converted to FTY720P by SK and FTY720P targets S1PR1 to rapidly reduce P-glycoprotein activity.
S1PR1 Ligands Reduce P-Glycoprotein-Mediated Transport in Vivo.
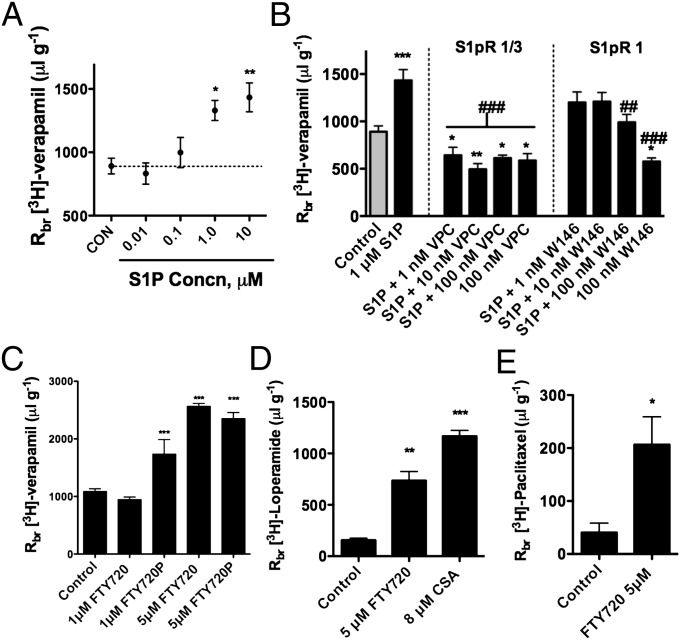
We used in situ brain perfusion to measure brain accumulation of the P-glycoprotein substrate, [3H]-verapamil (25). Adding 10 μM S1P to the perfusate roughly doubled brain accumulation of [3H]-verapamil (Fig. 4A). This effect was blocked by addition to the perfusate of VPC, an antagonist selective for S1PR1 and S1PR3, and by W146, an antagonist selective for S1PR1 (Fig. 4B). Note that, both VPC (all concentrations) and W146 (at 100 nM) by themselves caused small but significant reductions in brain accumulation of [3H]-verapamil. Nevertheless, neither S1PR antagonist affected brain sucrose accumulation (Fig. S3B). This finding suggests displacement of endogenous S1P from the receptors, an effect not detected in our in vitro experiments with isolated brain capillaries. If that were the underlying mechanism, it would imply some level of tonic inhibition by circulating, endogenous ligand.
Fig. 4.
S1P, FTY720, and FTY720P reduce P-glycoprotein activity (increased brain accumulation of [3H]-verapamil, [3H]-loperamide, and [3H]-paclitaxel) in vivo. (A) Concentration-dependence for S1P-induced increase in brain [3H]-verapamil accumulation. Estimated EC50 from the curve fit is 230 nM. (B) S1P effects on [3H]-verapamil accumulation are blocked by S1PR1 receptor antagonists: VPC is selective for S1PR1 and S1PR3, W146 is selective for the S1PR1. (C) FTY720 and FTY720P reduce P-glycoprotein transport activity in vivo (increased [3H]-verapamil accumulation). (D) FTY720 increases brain accumulation of [3H]-loperamide. (E) FTY720 increases brain accumulation of [3H]-pacitaxel. Results are expressed as the ratio of disintegrations per minute in the brain to disintegrations per minute in the perfusate (Rbr μL/g). Each point or bar represents the mean value from four to eight rats; variability is shown as SE bars. Significant differences determined using one-way ANOVA with a Neuman-Keuls post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; ##P < 0.01, ###P < 0.001 vs. S1P (10 μM).
Adding 5 μM FTY720 or 1 or 5 μM FTY720P to the perfusate significantly increased verapamil accumulation in brain (Fig. 4C). Indeed, 5 μM FTY720 or FTY720P increased [3H]-verapamil accumulation nearly fourfold. In additional experiments with somewhat better P-glycoprotein substrates; the opioid receptor agonist loperamide and the chemotherapeutic paclitaxel, 5 μM FTY720 increased brain accumulation of both drugs fivefold (Fig. 4 D and E). In comparison, adding 8 μM CSA to the perfusate increased [3H]-verapamil accumulation sixfold (Fig. S3A) and [3H]-loperamide accumulation eightfold (Fig. 4D). This concentration of CSA causes near-complete inhibition of blood-brain barrier P-glycoprotein activity in brain perfusion experiments (25).
As in our in vitro experiments, increased accumulation of [3H]-verapamil, loperamide, and paclitaxel in brain tissue could have resulted from reduced P-glycoprotein activity or increased tight junctional permeability. To test for altered passive permeability, we measured the brain distribution of [14C]-sucrose, a vascular permeability marker that poorly penetrates an intact blood-brain barrier (25). We previously showed that 5 μM CSA increased brain accumulation of P-glycoprotein substrates, but did not alter [14C]-sucrose accumulation, whereas hyperosmotic mannitol increased [14C]-sucrose accumulation, likely through tight junction disruption (13, 25). As shown in Fig. S3B, neither S1P nor S1PR antagonists (VPC, W146) nor SIPR agonists (FTY720 and FTY720P) affected brain distribution of [14C]-sucrose, indicating that altered [3H]-verapamil uptake in response to these chemicals was not because of opening of tight junctions. Taken together, these in vivo data are consistent with our vitro findings showing that S1P, FTY720 and FTY720P reduce the basal transport activity of P-glycoprotein.
Discussion
The wide specificity limits of P-glycoprotein, its luminal location within brain capillary endothelial cells, and its ability to generate and maintain large blood-to-brain concentration gradients, make this efflux transporter a primary determinant of small drug entry into the CNS (2). Thus, an understanding of the mechanisms that regulate blood-brain barrier P-glycoprotein is a critical step toward improving CNS pharmacotherapy with small-molecule drugs. Recent studies show regulation occurs through signals that increase transporter protein expression and those that alter transport activity without affecting transporter expression (2). On the one hand, transporter expression at the blood-brain barrier increases in response to inflammatory (26), ischemic (27), and oxidative stress (28), epileptic seizures (29), and to exposure to certain therapeutic drugs and environmental pollutants (2, 10, 11). Such increases are mediated by specific transcription factors and by ligand-activated nuclear receptors. Increased transporter expression and the resulting increase in transport activity reduce the ability of drugs that are P-glycoprotein substrates to enter the CNS (10, 11, 27). On the other hand, two distinct signaling pathways rapidly and reversibly reduce basal transport activity without changing P-glycoprotein expression. Activating either pathway increases drug delivery to the CNS without affecting blood-brain barrier tight junctional permeability or transport activity of Mrp2 and Bcrp. In one pathway VEGF signals through a flk-1 receptor and Src kinase (25). In the other pathway, TNF-α signals through TNFR1, ETBR, iNOS, and PKCβ1 (9, 12, 13). We recently showed that activating blood-brain barrier PKCβ1 restores drug delivery to the brain in rats exhibiting up-regulated P-glycoprotein expression and reduced drug delivery (30).
Here we extend the TNF-α/PKCβ1 signaling pathway through sphingolipid-based steps (Fig. 3F). In contrast, two earlier reports suggest that exposure to sphingolipids increases P-glycoprotein activity. In one report, Honig et al. (31) found that exposing T cells to ∼15 μM FTY720 for 1 h decreased accumulation of a fluorescent P-glycoprotein substrate (increased transport activity). It is not clear from that study whether the increase in activity reflects increased transporter protein expression. In the other report, Pilorget et al. (32) found that overexpression of SK1 increased P-glycoprotein expression and activity in a cell line derived from rat brain endothelial cells. In those cells, added S1P signaled through S1PR1/3 to increase transporter activity but not expression. Clearly, the present results for rat brain capillaries and intact rat blood-brain barrier contradict Pilorget et al.’s (32) findings for a cell-culture model of the blood-brain barrier. We suspect that these differences reflect changes in cell physiology that occurred when the endothelial cells were isolated and developed into a continuous line (33).
We believe that the extended signaling pathway depicted in Fig. 3F is incomplete. Certainly, we anticipate further signaling elements downstream of S1PR1 and these could provide additional targets of opportunity. In addition, the mechanism by which P-glycoprotein activity decreases is not clear. Because this loss of activity is rapid and fully reversible, it could occur through trafficking of the transport protein away from the exterior surface of the luminal plasma membrane or through reduced transporter turnover number with no change in location. Recent experiments with an in vivo protease K protection assay explored the former possibility for the two separate signaling pathways known to reduce basal P-glycoprotein activity at the blood-brain barrier: VEGF/src-kinase signaling (25) and PKCβ1 signaling (34). PKCβ1 signals downstream of TNF-α and directly upstream of SK and S1PR1 (Fig. 3F). The experiments showed that signaling through VEGF/src-kinase pathways provided protection from proteolysis, indicating that it removed P-glycoprotein from the surface of the luminal plasma membrane, likely through trafficking (34). In the same study, activation of PKCβ1 provided no detectable protection, indicating no change in the location of the transporter (34). Thus, for the TNF-α/PKCβ1/S1PR1 pathway, covalent modification of the transporter or changes in its lipid or protein microenvironment may underlie the rapid loss of transport activity.
At present, we do not fully understand the physiological significance of the rapid reduction in P-glycoprotein activity in response to TNF-α. This result may relate to reduced efflux into the capillary lumen of endogenous metabolites produced during the endothelium’s inflammatory response and transported by P-glycoprotein. One candidate is platelet activating factor (PAF), a lipid-signaling molecule that plays a role in thrombotic diseases and is an important mediator of immune responses (35). PAF is produced in several types of blood cells and in the endothelium. In messangial cells and in a renal epithelial cell line, PAF efflux is blocked specifically by P-glycoprotein inhibitors (36, 37). We speculate that reducing blood-brain barrier P-glycoprotein transport activity in the short-term reduces the PAF-mediated cascade of neuroinflammatory signals from the CNS into the vasculature, limiting a more widespread response. Note that PAF has been implicated in the pathogenesis of multiple sclerosis (38, 39), as has P-glycoprotein (40, 41). It remains to be seen whether this mechanism contributes to the therapeutic effects of FTY720.
The present study provides proof of principle that targeting a signaling pathway regulating basal efflux transporter activity at the blood-brain barrier can substantially increase delivery of P-glycoprotein substrates to the brain. Indeed, in our animal model, increased drug uptake can be accomplished in a rapid and transient manner using a drug that is already approved for use in the clinic. We found that carotid infusion of FTY720 or FTY720P increased brain verapamil uptake nearly fourfold and brain loperamide and paclitaxel uptake fivefold. The magnitude of FTY720/FTY720P-induced increase in verapamil and loperamide uptake was roughly 60% of what we saw when 8 μM CSA was added to the perfusate (Fig. 4D), which may have come close to inhibiting the transporter fully (25). In P-glycoprotein–null mice, brain accumulation of verapamil is increased about sevenfold (42), but for more lipophillic drugs (e.g., some chemotherapeutics) the increase can be an order of magnitude or more when measured over several hours (43). One would expect to see larger fold-increases with those drugs when P-glycoprotein activity is reduced by FTY720/FTY720P.
Note that although FTY720 and FTY720P were effective at 10–100 nM in our experiments with isolated brain capillaries (Fig. 3), 1–5 μM was needed in the short-term, carotid infusion studies (Fig. 4). The latter concentration range is substantially higher than peak plasma concentrations measured in multiple sclerosis patients taking FTY720 (44). However, we propose using the drug acutely and locally, through carotid artery perfusion, to transiently open a window that allows therapeutic drugs to enter the brain; doing this requires micromolar FTY720 or FTY720P concentrations in the perfusate. This use will provide a substantial first-pass effect, followed by rapid dilution in the systemic circulation. We show that these concentrations have no effect on tight junction permeability as measured by sucrose accumulation, a very sensitive, small-molecule probe of blood-brain barrier perturbation.
Thus, an improved understanding of the signals that affect basal efflux transporter activity at the blood-brain barrier has the potential to provide new ways to improve drug delivery to the brain without compromising neuroprotection.
Materials and Methods
Reagents.
NBD-CSA was custom synthesized by R. Wenger (Sandoz Pharma AG, Basel, Switzerland). PSC833 (valspodar) was a gift from Novartis (Basel, Switzerland). dPPA and B-0312 were from Biomol (Enzo Life Sciences). S1P receptor 1, 3, and 6 antibodies were: EDG-1 (targets histidine 60), EDG-1 (targets alanine 6), EDG-3 (targets histidine 15), EDG-3 (targets histidine 70), EDG-3 (targets methionine 17), EDG-6 (targets threonine 20), and EDG-6 (targets cysteine 20) from Santa Cruz Biotechnology. ABC transporter antibodies were C219 (targets P-glycoprotein) purchased from Abcam, and M2III-6 (targets multidrug resistance-associated protein 2) and BXP-53 (targets Bcrp), both purchased from Alexis (Enzo Life Sciences). Recombinant rat TNF-α and S1P were from. Other chemicals were obtained from Sigma and Cayman.
Brain Capillary Isolation.
Experiments were performed in compliance with National Institutes of Health animal care and use guidelines and approved by the National Institute on Environmental Health Sciences Animal Care and Use Committee. Brain capillaries were isolated from retired male breeder Sprague–Dawley rats, as described previously (9, 12). Rats were killed by CO2 inhalation, decapitated, and brains were removed to PBS buffer (KCl 2.7 mM, KH2PO4 1.5 mM, NaCl 136.9 mM, Na2HPO4 4.3 mM, CaCl2 0.7 mM, MgCl2 0.5 mM, d-glucose 5 mM, and pyruvate 1 mM, pH 7.4) at 4 °C. Cortical gray matter was removed and the remaining tissue was homogenized and centrifuged in 15% (wt/vol) Ficoll PM 400 for 20 min at 5,800 × g and 4 °C. The pellet was resuspended in PBS with 1% (wt/vol) BSA, filtered through a 300-μm nylon mesh, and passed through a glass bead column. Capillaries were collected from the column using mechanical agitation and aspiration, washed three times in PBS and used directly for transport studies, membrane isolation, or immunostaining.
Transport Assay.
Assay procedures are described in refs. 9 and 12. Briefly, brain capillaries in PBS buffer were transferred to microscope chamber slides with cover-slip bottoms. In all but the time-course experiment, capillaries were pretreated with signaling or transport inhibitors for 30 min and then exposed for 1 h to S1P or TNF-α along with the fluorescent substrates [2 μM NBD-CDSA for P-glycoprotein, 2 μM Texas red (sulforhodamine 101) for MRP2, or 2 μM Bodipy-prazosin for Bcrp]. Confocal images of capillaries were obtained using a Zeiss 510 metalaser scanning confocal microscope fitted with a 40× water-immersion objective (N.A. 1.2). Luminal substrate fluorescence was measured as described previously using ImageJ software. To document the rapid time course of S1P, FTY720, and FTY720P action, capillaries were first incubated to steady state (60 min) in medium with 2 μM NBD-CDSA and multiple times after addition of drug (Fig. 1E).
Western Blotting.
Capillary membranes were isolated (29) following treatment with activators or inhibitors of signaling. Western blots were performed using the NuPage Bis-Tris electrophoresis system (Invitrogen). Blocking and antibody incubation were carried out using the Millipore Snap I.D. blotting system according to the manufacturer’s specifications. Briefly, PVDF transfer membranes were incubated in Odyssey (Li-Cor) blocking solution for 1 min (room temperature). This solution was then pulled through the membrane using the Snap I.D. system and the membrane was incubated with primary antibody for 10 min and secondary antibody (Odyssey Goat anti-mouse IR Dye 800CW or Goat anti-rat IR dye 680CW) for an additional 10 min. Western blot fluorescence was detected using the Li-Cor Odyssey infrared imaging system. For each sample, target band intensities were normalized to β-actin.
Immunohistochemistry.
Isolated rat brain capillaries on glass cover-slips were fixed in 3% (wt/vol) paraformaldehyde/0.2% (vol/vol) glutaraldehyde for 10 min, washed in PBS, permeabilized in 1% (vol/vol) triton X-100 in PBS, and blocked for 1 h with 1% (wt/vol) BSA in PBS. Capillaries were incubated with primary antibody S1P receptor 1 or 3 antibodies (Santa Cruz Biotechnology) overnight at 4 °C. Capillaries were washed in 1% (wt/vol) BSA, followed by exposure to 2 μg/mL of either Alexa Fluor-488–conjugated goat anti-mouse IgG (Invitrogen) or Alexa Fluor-563–conjugated goat anti-mouse IgG (Invitrogen) for 1 h at room temperature. Negative controls had primary antibody omitted in the first incubation step. Immunostained capillaries were imaged by confocal microscopy (40× water objective; N.A 1.2).
In Situ Brain Perfusion.
Brain perfusion was carried out as described previously (25). Rats were anesthetized by intraperitoneal injection with 1 mL/kg ketamine mixture (79 mg/mL ketamine, 3 mg/mL xylazine, 0.6 mg/mL acepromazine) and administered heparin (10 kU/kg). The common carotid arteries were exposed by midline incision at the neck, and perfused with oxygenated Ringer’s solution at 37 °C (NaCl 117 mM, KCl 4.7 mM, MgSO4 0.8 mM, NaHCO3 24.8 mM, KH2PO4 1.2 mM, CaCl2 2.5 mM, d-glucose 10 mM, plus 39 g/L, 170 kDa dextran, 1 g/L BSA, and 0.055 g/L Evans blue) at 3 mL/min. [14C]-sucrose (0.5 μCi/mL), [3H]-verapamil (0.1 μCi/mL), [3H]-loperamide (0.1 μCi/mL), or [3H]-paclitaxel (0.5 μCi/mL), were infused into the circuit via syringe pump at 0.5 mL/min for 20 min. Samples of perfusate were collected from the cannulae at the end of each experiment. Brains were removed, stripped of meninges, midbrain, and choroid plexuses, and minced. Tissue and 100 μL perfusate samples were solubilized and counted (25). Results were expressed as the ratio of disintegrations per minute in the brain to disintegrations per minute in the perfusate (Rbr μL/g).
Statistical Analyses.
Data are expressed as mean ± SE. Differences between means were deemed statistically significant when P < 0.05 using one-way ANOVA with a Tukey–Kramer post test (for multiple comparisons) or Student’s unpaired t test (GraphPad Prism 5.0c).
Supplementary Material
Acknowledgments
We thank Destiny Sykes for excellent technical support and Dr. Lindsay Smith for careful reading of the manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203534109/-/DCSupplemental.
References
- 1.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fellner S, et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002;110:1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferry DR, Traunecker H, Kerr DJ. Clinical trials of P-glycoprotein reversal in solid tumours. Eur J Cancer. 1996;32A:1070–1081. doi: 10.1016/0959-8049(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 5.Krishna R, St-Louis M, Mayer LD. Increased intracellular drug accumulation and complete chemosensitization achieved in multidrug-resistant solid tumors by co-administering valspodar (PSC 833) with sterically stabilized liposomal doxorubicin. Int J Cancer. 2000;85:131–141. doi: 10.1002/(sici)1097-0215(20000101)85:1<131::aid-ijc23>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Liang XJ, Aszalos A. Multidrug transporters as drug targets. Curr Drug Targets. 2006;7:911–921. doi: 10.2174/138945006778019264. [DOI] [PubMed] [Google Scholar]
- 7.Killestein J, Rudick RA, Polman CH. Oral treatment for multiple sclerosis. Lancet Neurol. 2011;10:1026–1034. doi: 10.1016/S1474-4422(11)70228-9. [DOI] [PubMed] [Google Scholar]
- 8.Bauer B, et al. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–1219. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 9.Hartz AM, Bauer B, Fricker G, Miller DS. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol. 2004;66:387–394. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Hawkins BT, Miller DS. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J. 2011;25:644–652. doi: 10.1096/fj.10-169227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Sykes DB, Miller DS. Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol Pharmacol. 2010;78:376–383. doi: 10.1124/mol.110.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartz AM, Bauer B, Fricker G, Miller DS. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–470. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- 13.Rigor RR, Hawkins BT, Miller DS. Activation of PKC isoform beta(I) at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J Cereb Blood Flow Metab. 2010;30:1373–1383. doi: 10.1038/jcbfm.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia P, Wang L, Gamble JR, Vadas MA. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem. 1999;274:34499–34505. doi: 10.1074/jbc.274.48.34499. [DOI] [PubMed] [Google Scholar]
- 15.De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: A novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 16.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer B, et al. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab. 2008;28:1222–1234. doi: 10.1038/jcbfm.2008.16. [DOI] [PubMed] [Google Scholar]
- 18.Hartz AM, Mahringer A, Miller DS, Bauer B. 17-β-Estradiol: A powerful modulator of blood-brain barrier BCRP activity. J Cereb Blood Flow Metab. 2010;30:1742–1755. doi: 10.1038/jcbfm.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitra P, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanfin Z, Serrano-Sanchez M, Leiber D. ATP-binding cassette ABCC1 is involved in the release of sphingosine 1-phosphate from rat uterine leiomyoma ELT3 cells and late pregnant rat myometrium. Cell Signal. 2011;23:1997–2004. doi: 10.1016/j.cellsig.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeilschifter W, Czech B, Neumann-Haefelin T. Targeting the sphingolipid signaling pathway in stroke. Stroke. 2010;41:e193. doi: 10.1161/STROKEAHA.110.578278. author reply e194. [DOI] [PubMed] [Google Scholar]
- 23.Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–777. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- 24.Choi JW, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkins BT, Sykes DB, Miller DS. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30:1417–1425. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 27.ElAli A, Hermann DM. Apolipoprotein E controls ATP-binding cassette transporters in the ischemic brain. Sci Signal. 2010;3:ra72. doi: 10.1126/scisignal.2001213. [DOI] [PubMed] [Google Scholar]
- 28.Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008;22:2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer B, et al. Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol. 2008;73:1444–1453. doi: 10.1124/mol.107.041210. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Hawkins BT, Miller DS. Activating PKC-β1 at the blood-brain barrier reverses induction of P-glycoprotein activity by dioxin and restores drug delivery to the CNS. J Cereb Blood Flow Metab. 2011;31:1371–1375. doi: 10.1038/jcbfm.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honig SM, et al. FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J Clin Invest. 2003;111:627–637. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilorget A, et al. Modulation of P-glycoprotein function by sphingosine kinase-1 in brain endothelial cells. J Neurochem. 2007;100:1203–1210. doi: 10.1111/j.1471-4159.2006.04295.x. [DOI] [PubMed] [Google Scholar]
- 33.Calabria AR, Shusta EV. A genomic comparison of in vivo and in vitro brain microvascular endothelial cells. J Cereb Blood Flow Metab. 2008;28:135–148. doi: 10.1038/sj.jcbfm.9600518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkins BT, Rigor RR, Miller DS. Rapid loss of blood-brain barrier P-glycoprotein activity through transporter internalization demonstrated using a novel in situ proteolysis protection assay. J Cereb Blood Flow Metab. 2010;30:1593–1597. doi: 10.1038/jcbfm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards LJ, Constantinescu CS. Platelet activating factor/platelet activating factor receptor pathway as a potential therapeutic target in autoimmune diseases. Inflamm Allergy Drug Targets. 2009;8:182–190. doi: 10.2174/187152809788681010. [DOI] [PubMed] [Google Scholar]
- 36.Raggers RJ, Vogels I, van Meer G. Multidrug-resistance P-glycoprotein (MDR1) secretes platelet-activating factor. Biochem J. 2001;357:859–865. doi: 10.1042/0264-6021:3570859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ernest S, Bello-Reuss E. Secretion of platelet-activating factor is mediated by MDR1 P-glycoprotein in cultured human mesangial cells. J Am Soc Nephrol. 1999;10:2306–2313. doi: 10.1681/ASN.V10112306. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues DH, et al. Absence of PAF receptor alters cellular infiltrate but not rolling and adhesion of leukocytes in experimental autoimmune encephalomyelitis. Brain Res. 2011;1385:298–306. doi: 10.1016/j.brainres.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 39.Callea L, et al. Platelet activating factor is elevated in cerebral spinal fluid and plasma of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 1999;94:212–221. doi: 10.1016/s0165-5728(98)00246-x. [DOI] [PubMed] [Google Scholar]
- 40.Kooij G, et al. P-glycoprotein acts as an immunomodulator during neuroinflammation. PLoS ONE. 2009;4:e8212. doi: 10.1371/journal.pone.0008212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kooij G, et al. T lymphocytes impair P-glycoprotein function during neuroinflammation. J Autoimmun. 2010;34:416–425. doi: 10.1016/j.jaut.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Dagenais C, Zong J, Ducharme J, Pollack GM. Effect of mdr1a P-glycoprotein gene disruption, gender, and substrate concentration on brain uptake of selected compounds. Pharm Res. 2001;18:957–963. doi: 10.1023/a:1010984110732. [DOI] [PubMed] [Google Scholar]
- 43.Mizuno N, Niwa T, Yotsumoto Y, Sugiyama Y. Impact of drug transporter studies on drug discovery and development. Pharmacol Rev. 2003;55:425–461. doi: 10.1124/pr.55.3.1. [DOI] [PubMed] [Google Scholar]
- 44.David OJ, Kovarik JM, Schmouder RL. Clinical pharmacokinetics of fingolimod. Clin Pharmacokinet. 2012;51:15–28. doi: 10.2165/11596550-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.