Abstract
Toxoplasma gondii is a widely distributed protozoan pathogen that causes devastating ocular and central nervous system disease. We show that the endochin-like quinolone (ELQ) class of compounds contains extremely potent inhibitors of T. gondii growth in vitro and is effective against acute and latent toxoplasmosis in mice. We screened 50 ELQs against T. gondii and selected two lead compounds, ELQ-271 and ELQ-316, for evaluation. ELQ-271 and ELQ-316, have in vitro IC50 values of 0.1 nM and 0.007 nM, respectively. ELQ-271 and ELQ-316 have ED50 values of 0.14 mg/kg and 0.08 mg/kg when administered orally to mice with acute toxoplasmosis. Moreover, ELQ-271 and ELQ-316 are highly active against the cyst form of T. gondii in mice at low doses, reducing cyst burden by 76–88% after 16 d of treatment. To investigate the ELQ mechanism of action against T. gondii, we demonstrate that endochin and ELQ-271 inhibit cytochrome c reduction by the T. gondii cytochrome bc1 complex at 8 nM and 31 nM, respectively. We also show that ELQ-271 inhibits the Saccharomyces cerevisiae cytochrome bc1 complex, and an M221Q amino acid substitution in the Qi site of the protein leads to >100-fold resistance. We conclude that ELQ-271 and ELQ-316 are orally bioavailable drugs that are effective against acute and latent toxoplasmosis, likely acting as inhibitors of the Qi site of the T. gondii cytochrome bc1 complex.
Keywords: antiparasitic agents, electron transport, antimalarial, quinoline, opportunistic infection
The widely distributed protozoan parasite Toxoplasma gondii causes devastating ocular and central nervous system disease. Approximately 1 billion people worldwide are seropositive for T. gondii, including more than 10.8% of the United States population (1). In developing countries, seroprevalence can be as high as 80% (2). T. gondii infection is acquired by ingesting T. gondii oocysts or undercooked, infected meat. Frequently, T. gondii infection is asymptomatic or causes an influenza-like illness and lymphadenopathy. However, 2–25% of people infected with T. gondii develop ocular lesions, making T. gondii the leading cause of blindness in South America and the leading cause of posterior uveitis worldwide (2). When T. gondii infects pregnant women, it can cross the placenta and infect the developing fetus. Fetal exposure results in up to 4,000 congenital infections per year in the United States, causing neurocognitive deficits, chorioretinitis, and abortion (1).
After initial infection, T. gondii establishes latent infection. Reactivation of latent infection in immunocompromised persons causes encephalitis, myocarditis, and pneumonitis. Most immunocompromised individuals with AIDS live in the developing world, and do not have access to first-line anti-Toxoplasma therapy. Moreover, the impact of toxoplasmosis is expected to increase as immunosuppression for solid-organ and stem-cell transplant patients becomes more frequent in the developing world, where latent T. gondii infection is common (3).
Because of the large global burden of disease and the shortcomings of current therapeutic options, there is an urgent need for better anti-Toxoplasma drugs. Current therapy for toxoplasmosis suppresses active infection but does not cure latent infection and is poorly tolerated. In AIDS patients, therapy is continued until 6 mo after immune reconstitution with antiretroviral therapy. For some patients, immune suppression is life-long, requiring indefinite drug suppression. Without prolonged suppressive treatment up to 80% of cases relapse, and 20–30% of patients on suppressive therapy relapse. Drug side effects have led to discontinuation of therapy in up to 40% of patients (4, 5). Moreover, current drugs do not prevent relapsing ocular disease that causes cumulative scarring and leads to blindness. An ideal anti-Toxoplasma drug would be potent, nontoxic, and eliminate latent infection.
The drug endochin provides a scaffold for promising anti-Toxoplasma drugs. Endochin is a 4-(1H)-quinolone initially investigated as an antimalarial drug in an avian model of malaria (Fig. 1) by Salzer et al. (6) in 1948. Gingrich and Darrow (7) subsequently found endochin to be active against avian and murine toxoplasmosis in 1951. Recent 4-(1H)-quinolone derivatives, endochin-like quinolones (ELQ), exhibit an in vitro IC50 against Plasmodium falciparum as low as 0.1 nM (8). Although highly active in vitro, the initial series of ELQs exhibited poor aqueous solubility and were unstable in the presence of rat and human microsomes (8). A library of 4(1H)-quinolone-3-diarylethers was made to improve these properties. Of the 4(1H)-quinolone-3-diarylethers synthesized in our laboratory, we found that ELQ-271 and ELQ-316 have the greatest efficacy against T. gondii. Herein, we describe the in vitro and in vivo efficacy of ELQ-271 and ELQ-316 against T. gondii and provide evidence that the mechanism of action of ELQ-271 is inhibition of the T. gondii cytochrome bc1 complex at the Qi site.
Fig. 1.
Chemical structures of endochin, ELQ-271, and ELQ-316.
Results
In Vitro Inhibition of T. gondii and Host-Cell Toxicity.
The growth inhibition of T. gondii by ELQ-271 and ELQ-316 was tested against the 2F strain of T. gondii expressing β-galactosidase, allowing colorimetric quantitation of T. gondii. The results are shown in Table 1. ELQ-271 and ELQ-316 inhibit T. gondii at an IC50 of 0.1 nM and 0.007 nM, respectively. By comparison, under these experimental conditions, atovaquone inhibits T. gondii at an IC50 of 138 nM.
Table 1.
In vitro and in vivo drug efficacy against T. gondii and host cell toxicity
| Drug | In vitro IC50 nM (95% CI) | In vitro HFF TD50 nM (95% CI) | In vivo ED50 mg/kg (95% CI) | In vivo ED90 mg/kg (95% CI) |
| Endochin | 0.003 (0.0000003–43) | >50,000 | ND | ND |
| ELQ-271 | 0.1 (0.004–2.4) | 9,349 (2,473–35,346) | 0.14 (0.07–0.31) | 1.06 (0.24–4.63) |
| ELQ-316 | 0.007 (0.00006–0.086) | >50,000 | 0.08 (0.04–0.15) | 0.33 (0.09–1.14) |
| Atovaquone | 138 (96–201) | 37,811 (20,762–68,862) | 0.85 (0.45–1.61) | 3.51 (0.52–23.78) |
T. gondii growth inhibition and host-cell toxicity of selected drugs were tested in vitro. Efficacy against experimental toxoplasmosis was tested in a murine acute infection model (Fig. 2). HFF, human foreskin fibroblasts; ND, not done.
Host-cell viability was measured colorimetrically using CellTiter 96Aqueous One Solution Reagent to evaluate host-cell toxicity that may influence antiparasite activity. This measurement also provides an initial indication of potential human toxicity. The TD50 dose of ELQ-271 against human foreskin fibroblast (HFF) cell culture was 9.3 μM, whereas toxicity was not observed with ELQ-316 or endochin at >50 μM. Based on these results, the calculated in vitro therapeutic index (TI) is 93,490 for ELQ-271 and >7.1 × 106 for ELQ-316. The TI calculated for atovaquone is 274, which is 341-fold lower than ELQ-271 and at least 25,912-fold lower than ELQ-316.
Human and Rat Microsome Metabolism of ELQ-271 and ELQ-316.
No measurable degradation of ELQ-271 and ELQ-316 was seen in the presence of human or rat liver microsomes, with or without cofactors for chromosome P450- and glucuronosyltransferase-mediated metabolism, suggesting that both compounds would be subject to low hepatic metabolic clearance in vivo.
Efficacy of ELQ-271 and ELQ-316 Against Acute Murine Toxoplasmosis.
We tested ELQ-271 and ELQ-316 in comparison with atovaquone against acute murine toxoplasmosis in two separate experiments (Fig. 2). The initial experiment included drug concentrations of 50, 20, 5, and 1 mg/kg or the vehicle, polyethylene glycol (PEG) 400, only. The subsequent experiment included drug concentrations of 5, 1, 0.2, and 0.04 mg/kg and the vehicle-only control. In both experiments, mice were divided into groups of four and inoculated i.p. with 20,000 RH strain T. gondii tachyzoites that express YFP. Beginning 48 h after inoculation, drugs were administered daily for 5 d. Twenty-four hours after the final drug administration, mice were killed and underwent peritoneal lavage with PBS. The lavage fluid was stained with allophycocyanin (APC)-conjugated anti-mouse CD45 antibody and analyzed by flow cytometry. The anti-mouse CD45 antibody stains bone marrow-derived cells other than red blood cells and platelets. Cells infected with T. gondii and cell-free tachyzoites are counted by flow cytometry as YFP-positive events. We calculated the T. gondii burden of infection as the percentage of YFP-positive events out of the number of all CD45-positive events and YFP-positive events. The use of anti-CD45 antibody staining excludes from analysis red blood cells introduced at the time of peritoneal puncture. One of the eight mice in the control group that exhibited a very low level of infection and one of the four mice in the 0.2-mg/kg atovaquone group that exhibited a very high burden of disease were excluded as outliers.
Fig. 2.
The efficacy of ELQ-271, ELQ-316, and atovaquone against acute T. gondii infection in mice. The T. gondii burden reflects the percentage of T. gondii infection. Treatment groups consisted of the control (n = 7), 0.04 mg/kg (n = 4), 0.2 mg/kg (n = 4; one value from the atovaquone 0.2 mg/kg group was excluded from analysis as an outlier because it was excessively high), 1 mg/kg (n = 8), 5 mg/kg (n = 8), 20 mg/kg (n = 4), and 50 mg/kg (n = 4) of each drug. ED50 and ED90 values for each compound are listed in Table 1. Error bars represent SEM. ATQ, atovaquone.
ELQ-271 and ELQ-316 had an ED50 of 0.14 and 0.08 mg/kg and an ED90 of 1.06 and 0.33 mg/kg, respectively. By comparison, atovaquone was given as a positive control and had an ED50 of 0.85 mg/kg and an ED90 of 3.51 mg/kg. Mice in the high-dose groups of 50 mg/kg showed no signs of overt toxicity (i.e., weight loss, inactivity, or ruffled fur).
Efficacy of ELQ-271 and ELQ-316 Against Latent Murine Toxoplasmosis.
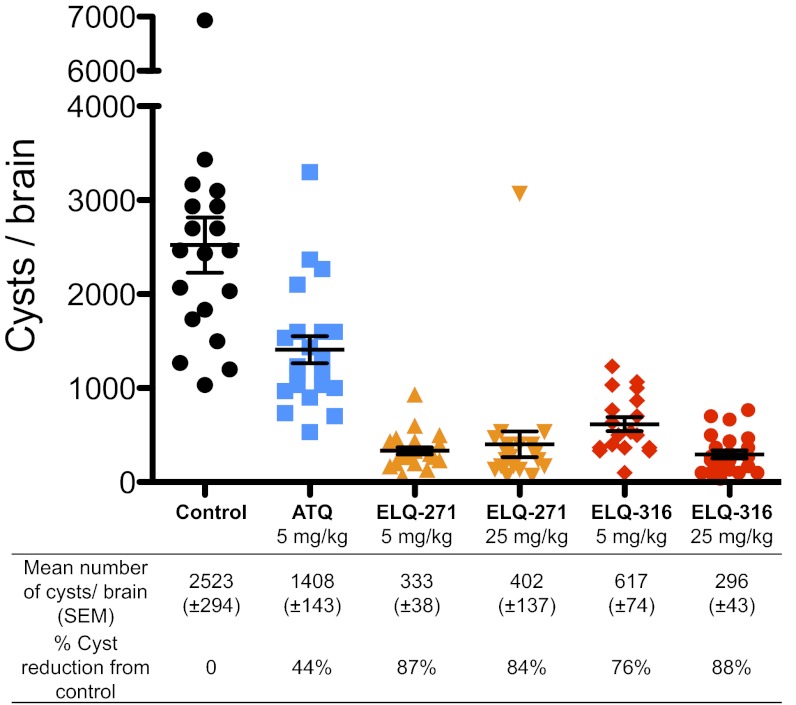
We tested ELQ-271, ELQ-316, and atovaquone against latent murine toxoplasmosis (Fig. 3). Mice were infected i.p. with the cyst-forming ME49 strain of T. gondii. Five weeks after infection, mice were treated once daily with 5 mg/kg of atovaquone or either 5 or 25 mg/kg of ELQ-271 or ELQ-316 for 16 d. Two weeks after the final drug dose, mice were killed, and slides were prepared from brain homogenate for cyst enumeration. The control group of mice that were given vehicle alone had a mean of 2,523 cysts per brain. Atovaquone at 5 mg/kg reduced the number of brain cysts by 44%. ELQ-271 at 5 mg/kg and 25 mg/kg reduced the number of brain cysts by 87% and 84%, respectively. ELQ-316 at 5 mg/kg and 25 mg/kg reduced the number of brain cysts by 76% and 88%. The number of cysts per brain was significantly fewer for each ELQ group than for the atovaquone group (P < 0.0001).
Fig. 3.
The efficacy of ELQ-271, ELQ-316, and atovaquone against latent T. gondii infection. The number of T. gondii cysts per brain was reduced by atovaquone, ELQ-271, and ELQ-316 compared with the control. Five weeks after inoculation with the T. gondii ME49 strain, mice were treated with each drug for 16 d. Brain cysts were counted 2 wk after the final day of drug administration. The number of cysts per brain was significantly lower for each ELQ group than for the atovaquone group (P < 0.0001). Error bars represent SEM. ATQ, atovaquone.
Inhibition of T. gondii Cytochrome c Reductase.
Based on our prior published work demonstrating that ELQ analogs inhibit Plasmodium yoelli oxygen consumption, we suspected that ELQs target the T. gondii bc1 complex (9). The cytochrome bc1 complex is a membrane-bound enzyme complex located in the inner mitochondrial membrane that contributes to pyrimidine biosynthesis and oxidative phosphorylation (10). Electron transfer through the cytochrome bc1 complex proceeds via the Q-cycle, resulting in ubiquinol oxidation at the Qo site, ubiquinone reduction at the Qi site, and cytochrome c reduction that shuttles electrons to cytochrome c oxidase. Atovaquone is known to bind to the Qo site, and point mutations in cytochrome b confer resistance to atovaquone (11, 12). We selected ELQ-271, atovaquone, and endochin to test against cytochrome bc1 function in an enriched T. gondii mitochondrial preparation. ELQ-271, atovaquone and endochin inhibition of the T. gondii bc1 was compared to the inhibition of the HFF cytochrome bc1 (Table 2).
Table 2.
Inhibition of T. gondii and host-cell cytochrome c reductase activity
| Drug | T. gondii IC50 nM | HFF IC50 nM |
| Atovaquone | 785 | >10,000 |
| Endochin | 8.2 | >10,000 |
| ELQ-271 | 30.6 | 818 |
The rate of cytochrome c reduction was measured spectrophotometrically in the presence and absence of inhibitors. HFF, human foreskin fibroblasts.
Cytochrome c reduction was measured spectrophotometrically at 550–542 nm. T. gondii cytochrome bc1 complex has an apparent Km of 7.4 μM for ubiquinol that is several-fold lower than the 50 μM decylubiquinol used in our experimental system. ELQ-271, endochin, and atovaquone inhibited T. gondii cytochrome c reduction at IC50s of 30.6 nM, 8.2 nM, and 785 nM, respectively. The IC50 against HFF cytochrome c reduction was 818 nM for ELQ-271 and >10 μM for endochin and atovaquone.
Inhibition of Saccharomyces cerevisiae Cytochrome c Reductase.
To investigate further the mechanism of action of ELQ-271, we tested the activity of ELQ-271 against a panel of Saccharomyces cerevisiae strains that have deletions of ATP-binding cassette transporter genes and amino acid substitutions in the Qo and Qi site of the cytochrome bc1 complex. The S. cerevisiae cytochrome bc1 complex has been used as a model to understand the mechanism of atovaquone and other cytochrome bc1 complex inhibitors against T. gondii, P. falciparum, and Pneumocystis jirovecii (13, 14). This model is valuable, because neither crystallization of the T. gondii cytochrome bc1 complex nor direct manipulation of the T. gondii mitochondrial genome, which includes the cytochrome b gene, has been accomplished. Hill et al. (15) demonstrated that deleting efflux transporter genes from S. cerevisiae resulted in susceptibility to atovaquone. This susceptibility allows the testing of cytochrome b inhibitors against S. cerevisiae strains with point mutations introduced to the cytochrome b gene with a growth-inhibition assay.
We tested 14 S. cerevisiae strains with point mutations in the Qi or Qo site and the parental strain (AD1-9) for resistance to ELQ-271 on glycerol medium that forces S. cerevisiae to rely on respiration for ATP production (Table S1). Of these strains, the mutant strain with a point mutation that results in an amino acid substitution of methionine by glutamine at position 221 in the Qi site shows drug resistance compared with AD1-9 (Fig. 4). Growth of the M221Q strain was not inhibited by ELQ-271 when grown on fermentable medium.
Fig. 4.
Comparison of growth inhibition by disk diffusion of ELQ-271 against S. cerevisiae strain AD 1-9 with and without the M221Q point mutation. ELQ-271 concentrations are listed below the disks.
We then evaluated the inhibitory activity of ELQ-271 against cytochrome c reduction of the parental strain and the M221Q mutant. Atovaquone was included as a positive control. The IC50 of ELQ-271 against parental cytochrome c reduction and M221Q mutant cytochrome c reduction were 61 nM and >10 μM, respectively. The IC50 of atovaquone against parental cytochrome c reduction and M221Q mutant cytochrome c reduction was 45 nM and 78 nM, respectively. The >100-fold ELQ-271 resistance of the M221Q mutant, compared with the parental strain, strongly suggests that ELQ-271 inhibits the S. cerevisiae cytochrome bc1 complex by targeting the Qi site and that replacing methionine with glutamine at position 221 interferes with ELQ-271 binding.
Discussion
Current clinically used anti-Toxoplasma drugs have limited efficacy against ocular and congenital toxoplasmosis and leave immunocompromised patients susceptible to recurrent infection. Despite advances in understanding T. gondii biology and identification of multiple unique drug targets, current first-line anti-Toxoplasma therapy is based on the discovery of synergy between pyrimethamine and sulfadiazine by Eyles and Coleman in 1953, 14 y after T. gondii was proven to be a pathogen (16). The need for improved therapy that is safe, effective, and well tolerated should be a global priority.
We describe the efficacy of two orally active drugs, ELQ-271 and ELQ-316, in vitro and against acute and latent murine toxoplasmosis. It is difficult to compare the ED50 of the ELQs with the published efficacy of other anti-Toxoplasma drugs because of heterogeneous experimental designs and variations in T. gondii virulence. However, the atovaquone efficacy that we found is comparable to that described by Araujo et al. (17, 18), who showed that atovaquone doses <10 mg/kg partially suppressed T. gondii RH strain infection during the first 8 d of infection. The observation that ELQ-271 and ELQ-316 are threefold and 10-fold more effective than atovaquone is promising and indicates that these compounds and structurally similar 4-(1H)-quinolone-3-diphenylethers should be evaluated further for potential clinical use against acute toxoplasmosis. Moreover, the remarkable efficacy of ELQ-271 and ELQ-316 in decreasing T. gondii brain cysts by up to 88% after 16 d of treatment suggests that they have the potential to eradicate latent infection at clinically applicable doses. A drug capable of eliminating latent infection would revolutionize current T. gondii therapy by preventing T. gondii reactivation in patients who are immunosuppressed or who are anticipating immunosuppression for stem-cell or solid-organ transplantation. This treatment paradigm would prevent toxoplasmosis and supplant the need for prolonged suppressive anti-Toxoplasma therapy that often is toxic or poorly tolerated.
We chose atovaquone for comparison in these studies because ELQs have a similar mechanism of action. Atovaquone, a Qo-site inhibitor and the only clinically used cytochrome bc1 complex inhibitor, provides a basis for speculation regarding the clinical potential for bc1 inhibitors. Prospective clinical studies of atovaquone as monotherapy and in combination with sulfadiazine or pyrimethamine against Toxoplasma encephalitis in AIDS patients have established atovaquone as an alternative anti-Toxoplasma regimen (19, 20). As for latent infection, a retrospective study of patients with ocular toxoplasmosis suggests that atovaquone may decrease the frequency of disease recurrence more than other anti-Toxoplasma drugs (21). The efficacy against recurrent ocular toxoplasmosis is likely related to atovaquone’s activity against latent infection that has been demonstrated against T. gondii brain cysts in mice (22, 23).
Cytochrome bc1 complex inhibitors bind to the Qo site or the Qi site of the enzyme complex. The marked resistance against ELQ-271 inhibition caused by the M221Q substitution in the S. cerevisiae Qi site suggests that ELQ-271 targets the Qi site. The M221Q substitution is a reversion mutation from a respiratory-deficient M221K substitution, and its effects on enzyme kinetics are well characterized (24-26). The methionine at position 221 is thought to form a hydrophobic interaction with ubiquinone (26). The M221Q substitution causes resistance to multiple Qi site inhibitors (14, 25). The structure and function of the Qi and Qo sites are highly conserved across a wide range of species. Currently, there are no clinically used Qi site inhibitors. Although further investigation into the mechanism of action of ELQ-271 and ELQ-316 against T. gondii is needed, the present experiments suggest that ELQ-271 and potentially other ELQs act at the T. gondii Qi site. New ELQs may be designed to exploit the Qi site further.
Subsequent investigation of ELQ-271 and ELQ-316 will include more extensive studies of pharmacokinetics, toxicology, and optimization of the route of administration and dosing schedule for eradication of latent T. gondii infection. We suspect that either increased penetration of the blood–brain barrier or more favorable pharmacokinetics underlie ELQ-271’s excellent activity against T. gondii brain cysts at 5 mg/kg, despite its lower intrinsic potency and efficacy against acute infection as compared with ELQ-316. Future studies also will evaluate synergy with other clinically used anti-Toxoplasma agents.
Atovaquone typically is combined with other agents to prevent resistance and for synergy. Resistance mutations have developed in the cytochrome b gene when atovaquone is used alone to treat Plasmodium and Pneumocystis (27, 28). Atovaquone-resistance mutations have not been found in clinical T. gondii isolates but have been generated experimentally (11, 29). Although it is unclear if a drug that acts at the Qi site will have a similar threshold for the development of resistance, discovering synergistic combinations that enhance the antiparasitic effect and shorten the duration of therapy will diminish the risk of drug toxicity. Synergy between atovaquone and clindamycin against murine toxoplasmosis has been observed, and we expect that similar combinations may enhance efficacy against acute and latent toxoplasmosis (23).
The results presented demonstrate that ELQ-271 and ELQ-316 are orally available drugs that are remarkably effective against acute and latent murine toxoplasmosis at low doses. Further in vivo toxicology studies are warranted, but our current limited evaluation does not prompt concerns for toxicity. Mechanistic studies of ELQ-271 show inhibition of the T. gondii bc1 complex. Based on the high-level resistance caused by the M221Q amino acid substitution in the S. cerevisiae cytochrome b Qi site, it is likely that ELQ-271 and potentially other members of the ELQ series act at the T. gondii cytochrome b Qi site. Qi site inhibition offers a mechanism of action that differs from current clinically used anti-Toxoplasma therapy. ELQ-271 and ELQ-316 are promising candidates for the treatment and prevention of toxoplasmosis.
Methods
Chemicals and Biologic Strains.
The chemical structures of endochin, ELQ-271, and ELQ-316 are shown in Fig. 1. The methods for the synthesis of ELQ-271 and ELQ-316 are described in the supporting information (SI Methods). Atovaquone was obtained from Sigma-Aldrich or DeF PharmaChemical Co. HFF cells were obtained from American Type Culture Collection, and YFP2 RH strain T. gondii was obtained from Boris Striepen (University of Georgia, Athens GA). Mutations of the cytochrome b gene were introduced into the yeast mitochondrial genome by site-directed mutagenesis and the mitochondrial transformation procedure previously described (15). Alternatively, mutations resulted from the selection of respiratory-competent revertants from respiratory-deficient mutants with a point mutation in the cytochrome b gene (24). The mutated and wild-type mitochondrial genomes were transferred by cytoduction into the nuclear background AD1-9 (Mat α ura3 his1, yor1Δ::hisG, snq2Δ::hisG, pdr5Δ::hisG, pdr10Δ::hisG, pdr11Δ::hisG, ycf1Δ::hisG, pdr3Δ::hisG, pdr15Δ::hisG, pdr1Δ::hisG) (kindly given by M. Ghislain, Catholic University of Louvain, Louvain, Belgium).
In Vitro Inhibition of T. gondii And Host Cell Toxicity.
Drugs were evaluated for growth inhibition of T. gondii using a 96-well assay in which T. gondii expressing β-galactosidase were cultured in an HFF cell monolayer and were quantified colorimetrically. This assay was reported originally by Mcfadden et al. (30) and has been modified by Jones-Brando et al. (31). Compounds dissolved in DMSO were added to the first column of HFF cells (320 μM) and then were diluted serially across the plate by dilutions of 0.5 log10, leaving the final column drug free. Fifty T. gondii tachyzoites were added to each well in six of the eight rows. After 4 d of incubation at 37 °C/5% CO2, chlorophenol red-β-d-galactopyranoside (CPRG) was added, and on the fifth day absorbance was measured at 570–650 nm. To measure cell viability, CellTiter 96Aqueous One Solution Reagent (Promega Corp.) was added to the two rows of uninfected HFF cells, and absorbance at 490–650 nm was measured after 3 h. Color reactions were read in a Vmax microplate reader (Molecular Devices). The IC50 and TD50 were calculated using CalcuSyn software (Biosoft). A therapeutic index for each compound was calculated as TD50/IC50.
Human and Rat Microsome Metabolism of ELQ-271.
Human and rat liver microsomes (BD Gentest, Discovery Labware, Inc.) were suspended in 0.1 M phosphate buffer (pH 7.4) at a final protein concentration of 0.4 mg/mL and incubated with compounds (0.5 or 1 μM) at 37 °C. An NADPH-regenerating system (1 mg/mL NADP, 1 mg/mL glucose-6-phosphate, 1 U/mL glucose-6-phosphate dehydrogenase) and MgCl2 (0.67 mg/mL) was added to initiate the metabolic reaction, and mixtures were quenched with ice-cold acetonitrile at time points ranging from 0–60 min. Samples also were incubated in the absence of the NADPH-regenerating system to monitor for non–cytochrome P450-mediated metabolism in the microsomal matrix. Samples were centrifuged, and the amount of parent compound remaining in the supernatant was assessed by LC-MS. The first-order rate constant for substrate depletion was determined by fitting the data to an exponential decay function. The rate constant was used to calculate the in vitro intrinsic clearance value (CLint) and the predicted in vivo intrinsic clearance value (CLint vivo) (32). The predicted in vivo hepatic extraction ratio (EH) was calculated using the following relationship: EH = CLint vivo/(Q + CLint vivo) where Q is liver blood flow (20.7 mL⋅min−1⋅kg−1, 55.2 mL⋅min−1⋅kg−1, and 90 mL⋅min−1⋅kg−1 in humans, rats, and mice, respectively) (33). Potential binding of compounds to microsomal protein was not taken into account in these calculations.
Efficacy Against Acute Murine Toxoplasmosis.
We used a model of acute murine toxoplasmosis that is adapted from a protocol described by Bajohr et al. (34). Twenty thousand T. gondii tachyzoites were inoculated i.p. into 4- to 5-wk-old female CF-1 mice with four mice in each experimental group. Drugs were dissolved in PEG 400 and administered by oral gavage 48 h after inoculation at concentrations of 0.04, 0.2, 1, 5, 25, and 50 mg/kg/d for 5 d. The control group received PEG 400 only. On the eighth day, the mice were killed, and 3 mL of PBS (pH 7.4) was injected into the peritoneum. Peritoneal fluid and PBS were withdrawn and examined by flow cytometry using a BD FACSCalibur. Then 200 μL of peritoneal fluid was stained with 2 μg of anti-mouse APC-conjugated CD45 antibody for 20 min at 4 °C, centrifuged at 1,000 × g, and resuspended in 500 μL of PBS. CD45 staining was performed to distinguish uninfected macrophages from red blood cells that might have been introduced at the time of peritoneal lavage. The T. gondii burden of disease was calculated as the percentage of FACS events that were positive for both YFP and CD45 plus YFP alone events, out of the events that were positive for both YFP and CD45 plus YFP alone events plus CD45 alone events. Analysis included 1 × 105 FACS events. Nonlinear regression analysis was performed with GraphPad Prism 5.0 software (GraphPad Software). This protocol was approved by the Institutional Animal Care and Use Committee of the Portland Veterans Administration Research Foundation.
Treatment of Latent Toxoplasma Infections in Mice.
Six groups of 7-wk-old CBA/J strain mice (Jackson Laboratory) (23 mice per group) were injected i.p. with 200 μL of sterile PBS containing 18 cysts of ME49 strain T. gondii (type II genotype) from infected CBA/J donor mice. Zero to four mice per group succumbed to the infection, leaving 19–23 mice per group available for treatment. Beginning 5 wk postinfection, mice were injected i.p. daily for 16 consecutive days with 50 μL solvent (DMSO) or drug dissolved in DMSO. Atovaquone was administered at 5 mg/kg; ELQ-271 and ELQ-316 were administered at 5 mg/kg and 25 mg/kg, respectively. Mice were euthanized humanely 2 wk after the final injection. The mouse brains were placed in 1 mL sterile PBS and individually minced with scissors, vortexed, and homogenized by three or four passages through a 22-G needle and syringe. Three 10-μL samples (30 μL total) of each brain homogenate were mixed on a glass microscope slide, smeared, air dried, fixed with ethanol for 15 min, dried further, and then mounted on a coverslip in Eukitt mounting medium (Sigma-Aldrich). Penicillin and streptomycin (Gibco) (50 U/mL and 50 μg/mL final concentrations, respectively) were added to remaining brain homogenate for storage at 4 °C and resampling as necessary. Cysts were enumerated by phase-constrast microscopy without knowledge of the sample's treatment group. This protocol was approved by the Committee on the Use and Care of Animals of the University of Michigan.
T. gondii Cytochrome c Reductase Inhibition.
T. gondii tachyzoites were harvested from lysed HFF cell culture, filtered through a 3-μm polycarbonate filter, and centrifuged at 8,000 × g for 5 min. The T. gondii pellet was washed in PBS containing 1 mM PMSF and centrifuged at 8,000 × g for 10 min. The T. gondii pellet then was resuspended in ice-cold lysis buffer (75 mM sucrose, 225 mM mannitol, 5 mM MgCl2, 5 mM KH2PO4, 1 mM EDTA, 1 mM PMSF, 5 mM Hepes, at pH 7.4). This suspension was homogenized in a cooled glass Dounce homogenizer. Cell debris was removed after centrifugation at 800 × g for 5 min, and then mitochondrial particles were centrifuged at 20,000 × g for 30 min. The pellet was suspended in lysis buffer and mixed to a final concentration of 30% (vol/vol) glycerol. Aliquots of mitochondrial fragments were frozen at −80 °C until needed.
Cytochrome c reduction was monitored at 550 nm using 542 nm as the baseline with an Agilent Diode Array 8453 spectrophotometer. Mitochondrial T. gondii protein (40 μg) solubilized in 2 mg/mL dodecyl maltoside was added to a cuvettete containing 50 μM oxidized cytochrome c (horse heart; Sigma-Aldrich), 50 μM decylubiquinol, 2 mM KCN, 100 mM KCl, 50 mM Tricine, pH 8.0. Drugs were dissolved in DMSO. The initial rate of cytochrome c reduction in the presence and absence of inhibitors was measured after the addition of mitochondrial protein and after sufficient time to measure the background reaction between decylubiquinone and cytochrome c.
S. cerevisiae Growth Inhibition.
S. cerevisiae strains were grown overnight in liquid YPG medium [1% yeast extract, 2% (wt/vol) peptone, and 3% (vol/vol) glycerol]. Overnight cultures were diluted to an OD600 of 0.05 and grown for 2 h. Cultures were combined with YPG containing 0.6% melted agar for a total volume of 10 mL and a final OD600 of 0.008 and were applied evenly to 2% (wt/vol) agar YPG plates. Drugs were dissolved in DMSO at various concentrations. Ten microliters of each concentration was applied to 7-mm diameter, 3-μm-thick filter paper. The disks were placed on the YPG agar plates. Plates were incubated at 30 °C. Images were obtained after 4 d.
S. cerevisiae Cytochrome c Reductase Inhibition.
Enriched mitochondrial preparations were obtained from S. cerevisiae strains grown overnight in 250 mL of YPD liquid medium [1% yeast extract, 2% (wt/vol) peptone, 2% (wt/vol) dextrose]. Cells were washed with distilled water and then were resuspended in buffer [100 mM Tris⋅H2SO (pH 9.4), 10 mM DTT] and incubated for 20 min at 30 °C. Cells were resuspended in buffer [2.4 M sorbitol, 1 M Hepes⋅KOH (pH 7), 1 M NaN3, 0.5 M EDTA] containing 5 mg Zymolase (Sigma-Aldrich) / g cells and incubated at 30°C for 30 min with shaking. Cells were resuspended in ice-cold lysis buffer and homogenized in a cooled glass Dounce homogenizer. The suspension was centrifuged at 1,500 × g for 5 min. The supernatant was centrifuged at 4,000 × g for 5 min, and the resulting supernatant was centrifuged at 12,000 × g for 15 min. The pellet was suspended in lysis buffer and mixed to a final concentration of 30% (vol/vol) glycerol. Aliquots of mitochondrial fragments were frozen at −80 °C until needed. Cytochrome c reductase activity was measured as described above for T. gondii.
Supplementary Material
Acknowledgments
This project was supported by funds from the Stanley Medical Research Institute and the Medicines for Malaria Venture, by National Institutes of Health National Institute of Allergy and Infectious Diseases Grant 1-R01-AI079182, and by the US Department of Veterans Affairs Merit Review Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208069109/-/DCSupplemental.
References
- 1.Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am J Trop Med Hyg. 2007;77:405–410. [PubMed] [Google Scholar]
- 2.Arevalo JF, Belfort R, Jr, Muccioli C, Espinoza JV. Ocular toxoplasmosis in the developing world. Int Ophthalmol Clin. 2010;50:57–69. doi: 10.1097/IIO.0b013e3181d26bf4. [DOI] [PubMed] [Google Scholar]
- 3.Mulanovich VE, et al. Toxoplasmosis in allo-SCT patients: Risk factors and outcomes at a transplantation center with a low incidence. Bone Marrow Transplant. 2011;46:273–7. doi: 10.1038/bmt.2010.102. [DOI] [PubMed] [Google Scholar]
- 4.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 5.Renold CAS, et al. Toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Medicine (Baltimore) 1992;71:224–239. doi: 10.1097/00005792-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Salzer W, Timmler H, Andersag H. A new type of compound active against avian malaria. Chem Ber. 1948;81:12–19. [Google Scholar]
- 7.Gingrich WD, Darrow EM. The effect of endochin on experimental toxoplasmosis. Am J Trop Med Hyg. 1951;31:12–17. doi: 10.4269/ajtmh.1951.s1-31.12. [DOI] [PubMed] [Google Scholar]
- 8.Winter R, et al. Optimization of endochin-like quinolones for antimalarial activity. Exp Parasitol. 2011;127:545–551. doi: 10.1016/j.exppara.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter RW, et al. Antimalarial quinolones: Synthesis, potency, and mechanistic studies. Exp Parasitol. 2008;118:487–497. doi: 10.1016/j.exppara.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vercesi AE, Rodrigues CO, Uyemura SA, Zhong L, Moreno SN. Respiration and oxidative phosphorylation in the apicomplexan parasite Toxoplasma gondii. J Biol Chem. 1998;273:31040–31047. doi: 10.1074/jbc.273.47.31040. [DOI] [PubMed] [Google Scholar]
- 11.McFadden DC, Tomavo S, Berry EA, Boothroyd JC. Characterization of cytochrome b from Toxoplasma gondii and Q(o) domain mutations as a mechanism of atovaquone-resistance. Mol Biochem Parasitol. 2000;108:1–12. doi: 10.1016/s0166-6851(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 12.Kessl JJ, Ha KH, Merritt AK, Meshnick SR, Trumpower BL. Molecular basis of Toxoplasma gondii atovaquone resistance modeled in Saccharomyces cerevisiae. Mol Biochem Parasitol. 2006;146:255–258. doi: 10.1016/j.molbiopara.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Kessl JJ, Meshnick SR, Trumpower BL. Modeling the molecular basis of atovaquone resistance in parasites and pathogenic fungi. Trends Parasitol. 2007;23:494–501. doi: 10.1016/j.pt.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Vallieres C, et al. 2012. HDQ, A potent inhibitor of Plasmodium falciparum proliferation binds to the Qi site of the bc1 complex. Antimicrob Agents Chemother 56:3739–47.
- 15.Hill P, et al. Recapitulation in Saccharomyces cerevisiae of cytochrome b mutations conferring resistance to atovaquone in Pneumocystis jiroveci. Antimicrob Agents Chemother. 2003;47:2725–2731. doi: 10.1128/AAC.47.9.2725-2731.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubey JP. The history of Toxoplasma gondii—the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 17.Araujo FG, Huskinson J, Remington JS. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991;35:293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araujo FG, Suzuki Y, Remington JS. Use of rifabutin in combination with atovaquone, clindamycin, pyrimethamine, or sulfadiazine for treatment of toxoplasmic encephalitis in mice. Eur J Clin Microbiol Infect Dis. 1996;15:394–397. doi: 10.1007/BF01690096. [DOI] [PubMed] [Google Scholar]
- 19.Torres RA, et al. Atovaquone/Toxoplasmic Encephalitis Study Group Atovaquone for salvage treatment and suppression of toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis. 1997;24:422–429. doi: 10.1093/clinids/24.3.422. [DOI] [PubMed] [Google Scholar]
- 20.Chirgwin K, et al. Randomized phase II trial of atovaquone with pyrimethamine or sulfadiazine for treatment of toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome: ACTG 237/ANRS 039 Study. AIDS Clinical Trials Group 237/Agence Nationale de Recherche sur le SIDA, Essai 039. Clin Infect Dis. 2002;34:1243–1250. doi: 10.1086/339551. [DOI] [PubMed] [Google Scholar]
- 21.Winterhalter S, et al. Does atovaquone prolong the disease-free interval of toxoplasmic retinochoroiditis? Graefes Arch Clin Exp Ophthalmol. 2010;248:1187–1192. doi: 10.1007/s00417-010-1379-9. [DOI] [PubMed] [Google Scholar]
- 22.Araujo FG, Huskinson-Mark J, Gutteridge WE, Remington JS. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother. 1992;36:326–330. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djurković-Djaković O, Milenković V, Nikolić A, Bobić B, Grujić J. Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii. J Antimicrob Chemother. 2002;50:981–987. doi: 10.1093/jac/dkf251. [DOI] [PubMed] [Google Scholar]
- 24.Coppée JY, et al. Analysis of revertants from respiratory deficient mutants within the center N of cytochrome b in Saccharomyces cerevisiae. FEBS Lett. 1994;339:1–6. doi: 10.1016/0014-5793(94)80373-0. [DOI] [PubMed] [Google Scholar]
- 25.Brasseur G, Brivet-Chevillotte P. Characterization of mutations in the mitochondrial cytochrome b gene of Saccharomyces cerevisiae affecting the quinone reductase site (QN) Eur J Biochem. 1995;230:1118–1124. doi: 10.1111/j.1432-1033.1995.tb20663.x. [DOI] [PubMed] [Google Scholar]
- 26.Rotsaert FA, Covian R, Trumpower BL. Mutations in cytochrome b that affect kinetics of the electron transfer reactions at center N in the yeast cytochrome bc1 complex. Biochim Biophys Acta. 2008;1777:239–249. doi: 10.1016/j.bbabio.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Walker DJ, et al. Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J Infect Dis. 1998;178:1767–1775. doi: 10.1086/314509. [DOI] [PubMed] [Google Scholar]
- 28.Looareesuwan S, et al. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg. 1996;54:62–66. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- 29.Meneceur P, et al. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob Agents Chemother. 2008;52:1269–1277. doi: 10.1128/AAC.01203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFadden DC, Seeber F, Boothroyd JC. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob Agents Chemother. 1997;41:1849–1853. doi: 10.1128/aac.41.9.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62:237–244. doi: 10.1016/s0920-9964(02)00357-2. [DOI] [PubMed] [Google Scholar]
- 32.Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27:1350–1359. [PubMed] [Google Scholar]
- 33.Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 34.Bajohr LL, et al. In vitro and in vivo activities of 1-hydroxy-2-alkyl-4(1H)quinolone derivatives against Toxoplasma gondii. Antimicrob Agents Chemother. 2010;54:517–521. doi: 10.1128/AAC.01001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.