Abstract
Purse-string healing is driven by contraction of actin/myosin cables that span cells at wound edges, and it is the predominant mode of closing small round wounds in embryonic and some adult epithelia. Wounds can also heal by cell crawling, and my colleagues and I have shown previously that the presence of unconstrained, straight edges in sheets of epithelial cells is a sufficient signal to induce healing by crawling. Here, it is reported that the presence of highly concave edges, which are free or physically constrained by an inert material (agarose), is sufficient to induce formation of purse strings. It was determined that neither of the two types of healing required cell damage or other potential stimuli by using the particularly gentle procedure of introducing gaps by digesting agarose blocks imbedded in the cell sheets. Movement by crawling depends on signaling by the EGF receptor (EGFR); however, this was not required for purse-string contraction. A migrating epithelial cell sheet usually produces finger-like projections of crawling cells. The cells between fingers contain continuous actin cables, which were also determined to contain myosin IIA and exhibit additional characteristics of purse strings. When crawling was blocked by inhibition of EGFR signaling, the concave regions continued to move, suggesting that both mechanisms contribute to propel the sheets forward. Wounding epithelial cell sheets causes activation of the EGFR, which triggers movement by crawling. The EGFR was found to be activated only at straight and convex edges, which explains how both types of movement can coexist at leading epithelial edges.
Keywords: wound healing, cell migration, cell motility, corneal epithelium, cell extrusion
Epithelia have the ability to cover wounds rapidly, which is clearly important for maintaining the integrity of organisms (1, 2). In most cases, epithelia respond initially to injury by moving to cover denuded areas and, only later, are lost cells replenished by proliferation. Movement can occur by extension of lamellipodia from cells at or near wound edges in a mechanism that is similar to the crawling movement of single cells (3–5). In a different mode of motility, purse-string healing, movement is mediated through contraction of an actin/myosin cable that spans cells at the wound edge, and additional forces may be provided by other cytoskeletal elements or adjacent tissues (6–8). Purse-string healing is prominent in embryos and in certain adult tissues, including the gut and corneal epithelium, and is important in extrusion of apoptotic cells from epithelia (9–14). Purse strings form preferentially in small circular wounds, which suggests that the shape of wound edges is important in determining which type of healing is induced (6, 7, 15, 16).
Signaling elicited by wounding has usually been studied by creating linear wounds by scraping cell layers with a needle or a pipette tip (17–22). Although simple in execution, these procedures actually provide a complex set of signals: cells are broken and release intracellular components, some cells become transiently permeable to extracellular molecules, and extracellular matrix covered previously by cells becomes abruptly accessible. Also, loss of cells results in disruption of cell–cell interactions, changes the mechanical forces in the cell layer, and allows receptors present at lateral edges of cells to interact with ligands present in apical regions. These stimuli contribute variously to EGF receptor (EGFR) activation, which is required for healing of large wounds in many types of epithelia (17–22). Of this great number of stimuli, my colleagues and I determined previously that the presence of physically unconstrained edges in the cell sheet is sufficient to induce activation of the EGFR and increase cell motility and, thus, constitutes a minimal trigger that can induce the epithelium to move (23).
Signaling has been studied extensively in cell crawling, but signaling in purse-string healing is less well-characterized. One goal of the present studies was to identify the minimal trigger for induction of purse strings by creating a procedure to produce concave edges that generates the least possible number of other potential signals. Because the EGFR is known to be central for induction of motility by crawling, the relationship of signaling by this receptor and purse-string healing was analyzed. Finally, moving edges of sheets of epithelial cells usually contain protruding cell clusters headed by very active “leader cells,” which have prominent lamellipodia and produce forces to move the epithelium forward by cell crawling (24, 25). The cells at the edges of the intervening concave regions have few lamellipodia and are known to contain actin cables that are continuous from cell to cell. Therefore, whether such regions contribute to movement by a purse-string–like contraction was analyzed.
Results
A Small Round Discontinuity in an Epithelial Cell Sheet Is a Sufficient Signal to Induce Purse Strings.
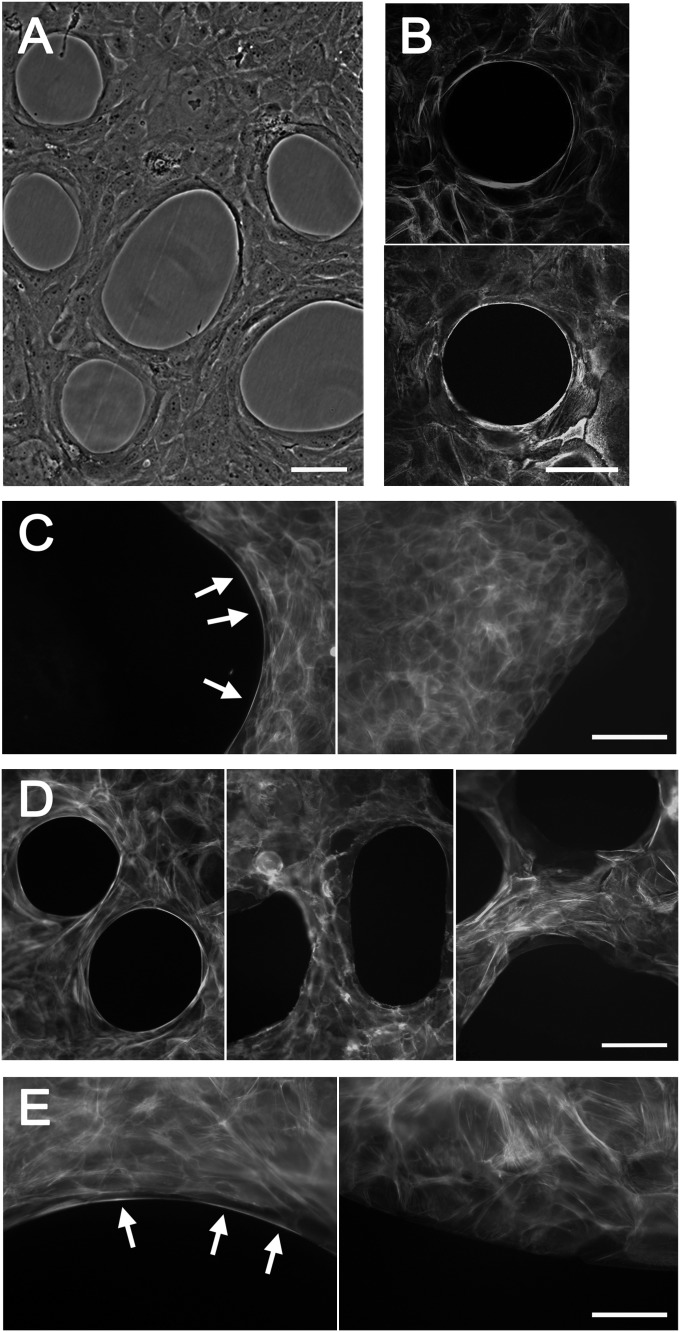
An agarose solution was sprayed on tissue culture dishes, and immortalized human corneal limbal epithelial (HCLE) cells (26) were grown to form a continuous sheet around the agarose droplets. The cells at the edges of the droplets were highly refractile in phase-contrast microscopy and contained prominent actin cables that were continuous from cell to cell (Fig. 1 A and B). To examine the effects of the shapes of edges, the agarose was also applied as strips in zigzag patterns on the dishes. The cables formed at concave edges of the cell sheets but not at straight or convex edges (Fig. 1C). They contained myosin IIA and were disrupted by treatment with a cell-permeable derivative of exoenzyme C3 transferase, which inhibits the small GTP-binding protein Rho (27), and by blebbistatin, which inhibits the myosin II ATPase (28) (Fig. 1D). The cables, therefore, exhibit the characteristics of purse strings described in other systems (15, 16, 29). Interestingly, the holes in the cell layer around the agarose droplets increased noticeably in size after the drug treatments, which is consistent with the presence of contractile forces in the actin–myosin cables that are relaxed after the inhibitors are added.
Fig. 1.
Characterization of purse strings. (A) Phase-contrast micrograph of cells cultured around agarose droplets. (B) Cells cultured around agarose droplets were fixed, permeabilized, and stained with Alexa Fluor 546–conjugated phalloidin (Upper) and an antibody against myosin IIA and Alexa Fluor 488–conjugated secondary antibody (Lower). Stacks of sequential confocal images are shown. (C) Cells were cultured around zigzag-shaped agarose strips and stained with labeled phalloidin. Arrows indicate purse strings at concave edges of the cell sheet. (D) Cells were grown around agarose droplets and stained with labeled phalloidin after no treatment (Left), 20 μM blebbistatin (2.5 h) (Center), or 5 μg/mL cell-permeable exoenzyme C3 transferase (6 h) (Right). Staining after the latter treatment was weak, indicating a general dissolution of f-actin in the cells, and the brightness was increased to visualize the signals. (E) Cells were grown on zigzag-shaped thin plastic strips and stained with labeled phalloidin. Arrows indicate purse strings at concave edges of the cell sheet. [Scale bars: 100 μm (A–D); 50 μm (E).]
Agarose droplets provide inert physical barriers to the epithelial cells (23), and to test whether edges of epithelial cells also form purse strings at concave edges in the absence of such barriers, the cells were cultured on thin plastic strips (23) formed in zigzag patterns. As is seen in Fig. 1E, prominent actin cables spanning several cells formed at concave but not at straight or convex edges. They contained myosin IIA and were sensitive to blebbistatin and exoenzyme C3 transferase (Fig. S1). Hence, purse strings form whether the edges are constrained or free, but the edges must be concave.
Purse String Healing Proceeds Independently of EGFR Activation in Epithelial Cell Sheets.
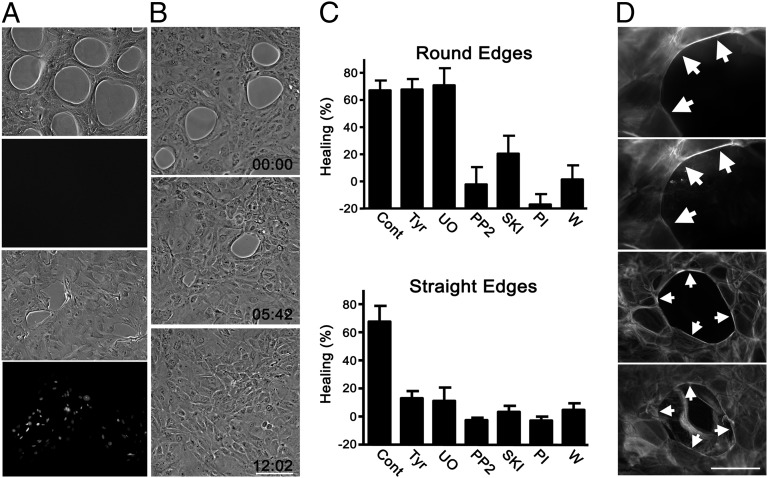
The agarose droplets were digested by treating with agarase. This was expected to be a very gentle way of removing the physical blocks provided by the droplets, and it did not result in detectable cell damage (Fig. 2A). After digestion, the holes in the cell layer closed within 10–12 h. Analysis by time-lapse microscopy revealed that contraction occurred initially with smooth edges, but after 2–4 h, ruffles appeared in some of the cells bordering the holes (Fig. 2B and Movie S1; see Fig. S6). To visualize the purse strings during healing, the cells were transfected with an expression vector coding for an enhanced green fluorescent protein/actin fusion protein. The actin cable was clearly visible in cells at the edges of transfected cells (Movie S2). The cells were very mobile, and individual cells moved away from the edges as the circumference of the wounds decreased during healing. The purse-string actin cables generally disappeared as the cells left the wound edges. This great mobility of cells at epithelial wound edges has been reported previously after mechanical wounding of corneas (30).
Fig. 2.
Differential healing at concave and straight edges. (A) Wounding was performed either by digestion of agarose droplets (upper two images) or with a pipette tip (lower two images). Phase-contrast micrographs or incorporated propidium iodide are shown. (B) Phase-contrast micrographs at the times indicated after wounding (Movie S1). Numbers indicate times after initiation of the movie (h:min). (C) Healing of wounds 7 h after removal of agarose droplets (Upper) or 14 h after removal of straight agarose strips (Lower) with addition of vehicle (cont), 0.25 μM tyrphostin AG 1478 (Tyr), 10 μM UO126 (UO), 10 μM PP2, 10 μM Src kinase inhibitor I (SKI), 1 μM PI-103 (PI), or 0.25 μM wortmannin (W). Values are means of quadruplicates ± SD. (D) Formation of purse strings at holes acutely induced by mechanical wounding. Cells were stained 3.5 h after wounding with labeled phalloidin (first and third images from the top) or antibodies against myosin IIA (second and fourth images from the top). Cellular debris is very prominent after staining with myosin antibodies. Tyrphostin AG 1478 (0.25 μM) was added to the cells depicted in the two lower images before wounding. Arrows indicate purse strings. [Scale bars: 100 μm (B and D).]
To test the requirement of EGFR signaling on purse-string healing, tyrphostin AG 1478, which potently inhibits the EGFR kinase (31, 32), was added. Importantly, the drug did not inhibit healing and had no noticeable morphological effect on the cells during purse-string contraction (Movie S3). Also, UO126, which inhibits activation of the important down-stream targets of the EGFR, extracellular regulated kinase (ERK)1/2 (33), had no effect.
When HCLE cells were induced to migrate by digestion of agarose strips with straight edges, lamellipodia were apparent within 15–20 min in virtually all of the cells at the border, which is similar to observations with other epithelial cells at straight wound edges (24, 25) (Fig. S2). The early response at concave wound edges is, therefore, very different from that at straight edges. Importantly, and in sharp contrast to their effects after dissolution of agarose droplets, both UO126 and tyrphostin AG 1478 blocked healing in wounds with straight edges (Fig. 2B). This shows that the shapes of the edges determine whether EGFR-ERK1/2 signaling is required for motility. Inhibitors of Src family kinases [PP2 and Src kinase inhibitor I (SKI)] and phosphatidylinositol 3-kinase signaling (PI-103 and wortmannin) (34) blocked healing in wounds of either shape (Fig. 2C).
A limitation of the agarose-removal approach is that the effects of the inhibitors on formation of purse strings cannot be analyzed. Holes were, therefore, also induced by mechanical wounding, and although debris often obscured analysis, purse strings were clearly visible in about one-third of the holes, as detected by staining with phalloidin or antibodies to myosin IIA (Fig. 2D), and they formed even in the presence of tyrphostin AG 1478. Under these conditions, the small round wounds also closed independently of EGFR and ERK1/2 signaling (Fig. S3).
Role for Purse Strings in Forward Movement of Leading Edges of Epithelial Cell Sheets.
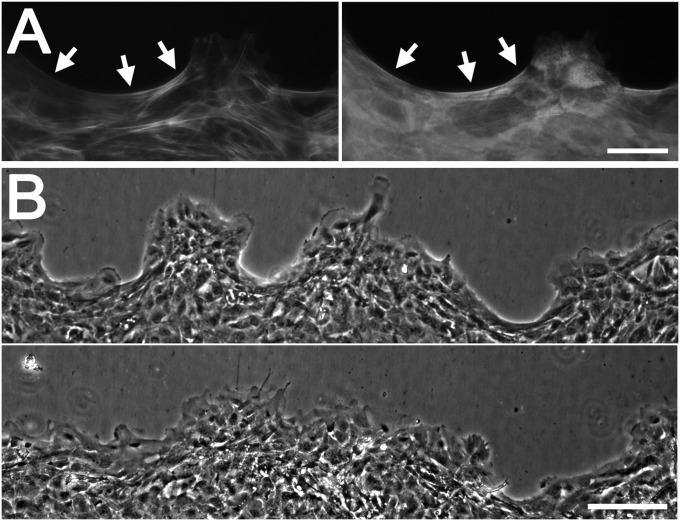
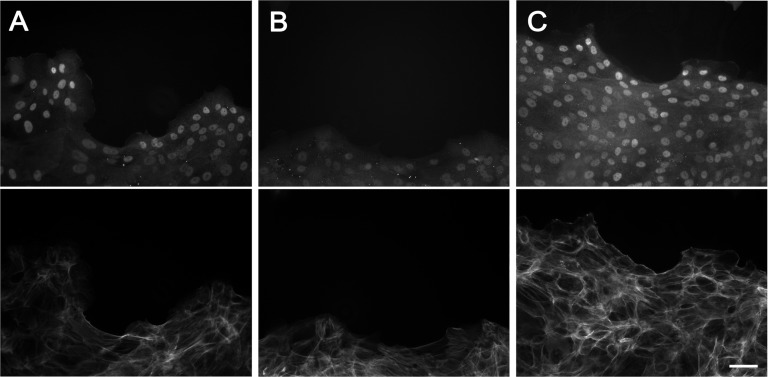
Moving edges of HCLE cells segregate into regions that contain protruding cell clusters and concave regions that have few lamellipodia (Fig. 3A). The latter regions contained actin cables that were continuous from cell to cell; they also contained myosin IIA and were sensitive to exoenzyme C3 transferase and blebbistatin, and were characterized as purse strings (Fig. 3A and Fig. S4). To examine the role of purse-string–type movement, tyrphostin AG 1478, which reversibly inhibits overall forward movement of HCLE cells (32), was added to moving cell sheets. The concave regions were seen to fill out whereas the advance of the protruding lamellipodia-rich regions was blocked (Fig. 3B and Movie S4). To support this conclusion, activation of the EGFR was also blocked with the LA1 antibody, which prevents EGFR activation by blocking its transactivation by ligands (32). The LA1 antibody blocked overall forward movement of the cell sheets (32) but did not prevent concave regions from filling out (Fig. S5). These observations suggest that both crawling and purse-string–type movement occurs at the leading edge and that both contribute to propel the cell sheet forward.
Fig. 3.
Role for purse strings in movement of epithelial sheets. (A) Cells at a moving epithelial edge were stained with labeled phalloidin (Left) or antibodies to myosin IIA (Right). Arrows indicate purse-string–like structures at concave regions. (B) Edge at the time of addition of 0.25 μM tyrphostin AG 1478 (Upper) and the same region 14 h later (Lower). (Scale bars: 100 μm.)
EGFR Activation Overrides Purse String Healing.
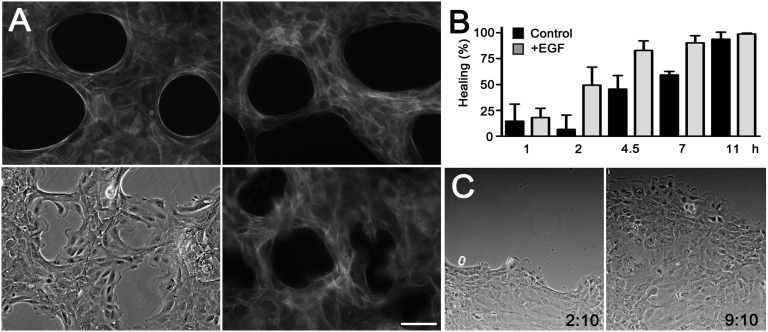
Addition of EGF caused dissolution of the purse strings around agarose droplets, which was apparent after ∼8 h (Fig. 4A). When the agarose droplets were digested in the presence of EGF, the purse strings disassembled quickly and the cells extended numerous fibrillar actin (f-actin)-rich lamellipodia containing prominent focal adhesions (Fig. 4A, Fig. S6, and Movie S5). This was associated with increased rates of healing (Fig. 4B). Blocking cell division did not affect acceleration of healing, demonstrating that enhanced proliferation induced by EGF does not contribute significantly to acceleration of healing (Fig. S7).
Fig. 4.
Switch to the crawling mode of healing by EGF signaling. (A) Cells were grown around agarose droplets and were either untreated (Upper Left) or treated with 10 nM EGF for 10 h without removing the agarose droplets (Upper Right) and stained with Alexa Fluor 546–conjugated phalloidin. Cells were incubated with 10 nM EGF and photographed in phase contrast (Lower Left) or stained with phalloidin 3 h after removal of agarose (Lower Right). (Scale bar: 100 μm.) (B) Healing of holes after removal of agarose droplets. EGF (10 nM) was added where indicated. Means of triplicates ± SD. (C) Progression of moving epithelial sheets at the time of addition of 10 nM EGF and 7 h later (Movie S7).
In the leading edge of moving sheets of HCLE cells, purse strings are dynamic structures that form at seemingly random regions (Movie S6). After addition of EGF, the purse strings dissolved and large lamellipodia were uniformly present in the cells at the edge (Fig. 4C and Movie S7). As previously described, this is associated with an increased rate of healing of wounds with straight edges (23). Thus, in both of the examined systems, addition of EGF results in overriding the purse-string mechanism and in increased rates of wound closure.
EGFR Signaling Is Selectively Activated at Convex and Straight Wound Edges.
The EGFR is activated upon wounding epithelial cell sheets (17–23). The finding that EGFR signaling disrupts purse strings suggested that activation occurs only at straight and convex edges. Direct localization of activated EGFR after wounding is complicated because of limitation of antibodies and down-regulation of the receptor (32). Transcription of c-fos is triggered by EGFR activation (35, 36), and c-fos accumulation was used as a marker of EGFR activity. c-fos accumulated at protruding regions of the wound edges where lamellipodia were abundant but was not increased at concave regions (Fig. 5A). Also, no activation was seen in cells at edges after digestion of agarose droplets (Fig. S8). As controls, c-fos accumulation was inhibited when wounding was performed in the presence of tyrphostin AG 1478 (Fig. 5B) or the LA1 antibody (Fig. S9) (37). When EGF was added, c-fos accumulated in cells at edges of any shape, demonstrating that the EGFR signaling pathway was not blocked at concave edges (Fig. 5C).
Fig. 5.
EGFR signaling is selectively activated at convex and straight edges. Moving edges of sheets of HCLE cells after no treatment (A), 4 h with 0.25 μM tyrphostin AG 1478 (B), or 1 h with 10 nM EGF (C). Upper images show staining with an anti–c-fos antibody, and the exposures are directly comparable. Lower images show staining with labeled phalloidin. (Scale bar: 100 μm.)
Discussion
Impaired resolution of wounds is a very significant source of morbidity (38, 39), and it is, therefore, important to understand the healing process in depth. Epithelialization is an essential part of the proliferative phase of healing, and it is initially driven by cells moving to cover wounds. Here, it was found that the two known modes of epithelial movement, cell crawling and purse-string contraction, have different signaling requirements because the latter does not require signaling by the EGFR. Indeed, stimulation of the EGFR promotes transition of purse-string healing to cell crawling. It was found that the presence of a concave edge in an epithelial cell sheet is a sufficient signal for induction of purse strings, but that other stimuli, such as cell damage, are dispensable. The shape of a wound edge is, therefore, a critical signal that determines which type of healing is induced. In accordance with the differing signaling requirements of the two types of healing, closure of small round wounds does not require EGFR signaling, in contrast to healing of larger wounds. An implication of this is that developing procedures to enhance healing of large wounds may involve stimulating different signaling pathways from promoting epithelial resealing of small wounds. Small wounds can result, for instance, from extrusion of apoptotic cells from epithelia (14).
The cues that initiate the two types of epithelial healing are quite different. The presence of free straight edges in epithelial cell sheets is sufficient to induce EGFR activation and to promote cell crawling (23). The present data show that formation of purse strings does not depend on EGFR signaling but, rather, requires the presence of concave edges, in accordance with previous observations that purse strings form predominantly in small round wounds (6, 7, 15, 16). The edges can be free or constrained by an inert material such as agarose. This raises the issue of how curvatures of edges are detected: it is increasingly apparent that mechanical forces can have profound effects on almost every aspect of cell behavior and that cells communicate extensively by mechanical means (40–44). The intracellular distribution of forces within cells is complex and is likely to depend on the shapes of edges. Forces can be detected by a variety of different sensors, including components of the cytoskeleton, focal adhesions, and cell–cell junctions (45–48). Tension in the plasma membrane, which is detected by stress-activated ion channels (49), could also be different in cells at concave and convex edges. Our results suggest that apoptotic cells in epithelia could be detected as inert bodies similar to agarose droplets and their recognition and extrusion may not require any specific signals released from the cells (14, 50).
The forces that produce movement are also different in the two types of healing. Movement by crawling depends on formation of lamellipodia, whereas purse-string healing is thought to, at least in part, depend on contraction of an actin/myosin cable that encircles the wounds (8, 51, 52). During cell crawling, forces that propel the sheet forward are generated in cells at the leading edge and in many rows behind them (3, 53–55). It is possible that cells behind the edge also contribute some forces to close purse-string wounds, although it is notable that blocking EGFR signaling, which drives lamellipodial crawling, has no detectable effects on purse-string healing.
EGF was found to induce a switch to movement by crawling, so although EGFR signaling is not required for purse-string closure of wounds, it profoundly affects healing. Purse strings can nonetheless form at the leading edges of moving epithelial cell sheets because EGFR activation occurs only at straight and convex edges. Wounding sheets of corneal epithelial cells causes release of heparin-binding EGF-like growth factor and amphiregulin (19, 20, 22, 32). Both of these ligands bind strongly to negatively charged glycans in the extracellular matrix and the cell surface (56–58). This limits their diffusion and provides an explanation why the receptor is activated only very locally.
Edges of moving sheets of epithelial cells are typically segregated into protruding regions interspersed with concave regions that contain few lamellipodia (24, 25). The traditional view is that the leader cells at the tips of the protrusions pull neighboring cells forward and that the forces are propagated through actin cables that connect the cells in the concave regions (24, 25, 55). Here, it was found that the actin cables have the characteristics of purse strings and that the concave regions move forward when overall forward movement is blocked by inhibiting EGFR signaling. This suggests an active role of concave regions in the movement of the cell sheets and that both crawling and purse-string modes contribute to propel the sheets forward. Cells engaged in crawling protrude at the cell edge in accord with more rapid movement by this mechanism.
The presence of two different modes of generating force at the leading edge has important implications for understanding epithelial movement. Creation of mathematical models is complicated by the existence of the two modes of motility and the influence of the shapes of the edges. Models based on a single class of cells that obey a single set of rules presumably do not describe movement accurately (54, 55, 59–62). Also, our finding that stimulation of the EGFR causes a switch to cell crawling underscores that the many factors in the wound environment that can stimulate this receptor must be taken into account for a complete understanding of epithelial wound healing.
Methods
For a more detailed description, see SI Methods.
To create small circular holes in the cell layer, 3.5-cm Petri dishes were sprayed with a 0.125% low-melting point agarose (Promega) with 0.2% (wt/vol) glycerol and dried overnight at room temperature. Cells were then seeded using standard techniques. The agarose was removed by incubating the plates with 250 μL of Eagle’s MEM with 2% (vol/vol) newborn calf serum and 0.5 units of agarase for 10–15 min at 37 °C, and the cells were washed and incubated as required in the same medium with 10% (vol/vol) newborn calf serum. After appropriate treatments and incubations, the cells were fixed with 3.7% (wt/wt) formaldehyde and stained with 0.05% gentian violet. For quantitation, four areas of the dishes were photographed using a Nikon TS100 inverted microscope with a 2× objective and a Spot RT camera (Diagnostics Instruments). The areas of the holes were determined using MetaMorph software (Molecular Devices) using the thresholding function. To create straight edges, strips of agarose were made with plastic molds as described (20) and digested as above. To examine the effects of unconstrained edges, cells were grown on plastic strips cast on top of agarose layers as described (23). Moving edges of epithelial cell sheets were produced by culturing and differentiating cells around agarose strips (20), transferring to MEM with 10% (vol/vol) newborn calf serum, and removing the strips 16 h before use.
Supplementary Material
Acknowledgments
We thank Kira Lathrop for superb assistance with live cell imaging and preparation of the figures and Ethan Block for useful comments on the manuscript. This work was supported by the National Institutes of Health National Eye Institute Grant EY-008098, Research to Prevent Blindness, and the Eye and Ear Foundation (Pittsburgh, PA).
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210992109/-/DCSupplemental.
References
- 1.Fini ME, Stramer BM. How the cornea heals: Cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24(8 Suppl):S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- 2.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 3.Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- 4.Bugyi B, Carlier MF. Control of actin filament treadmilling in cell motility. Annu Rev Biophys. 2010;39:449–470. doi: 10.1146/annurev-biophys-051309-103849. [DOI] [PubMed] [Google Scholar]
- 5.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 7.Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121:565–578. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Fernandez B, Campos I, Geiger J, Santos AC, Jacinto A. Epithelial resealing. Int J Dev Biol. 2009;53:1549–1556. doi: 10.1387/ijdb.072308bg. [DOI] [PubMed] [Google Scholar]
- 9.Danjo Y, Gipson IK. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci. 1998;111:3323–3332. doi: 10.1242/jcs.111.22.3323. [DOI] [PubMed] [Google Scholar]
- 10.Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: Embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359:777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bullen TF, et al. Characterization of epithelial cell shedding from human small intestine. Lab Invest. 2006;86:1052–1063. doi: 10.1038/labinvest.3700464. [DOI] [PubMed] [Google Scholar]
- 12.Hantash BM, Zhao L, Knowles JA, Lorenz HP. Adult and fetal wound healing. Front Biosci. 2008;13:51–61. doi: 10.2741/2559. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem. 2009;48:137–161. doi: 10.1016/s0065-2423(09)48006-5. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 15.Russo JM, et al. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology. 2005;128:987–1001. doi: 10.1053/j.gastro.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamada M, Perez TD, Nelson WJ, Sheetz MP. Two distinct modes of myosin assembly and dynamics during epithelial wound closure. J Cell Biol. 2007;176:27–33. doi: 10.1083/jcb.200609116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puddicombe SM, et al. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 18.Tokumaru S, et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:813–820. doi: 10.1167/iovs.03-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: Role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- 21.Myhre GM, Toruner M, Abraham S, Egan LJ. Metalloprotease disintegrin-mediated ectodomain shedding of EGFR ligands promotes intestinal epithelial restitution. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1213–G1219. doi: 10.1152/ajpgi.00149.2004. [DOI] [PubMed] [Google Scholar]
- 22.Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp Eye Res. 2007;85:130–141. doi: 10.1016/j.exer.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Block ER, et al. Free edges in epithelial cell sheets stimulate epidermal growth factor receptor signaling. Mol Biol Cell. 2010;21:2172–2181. doi: 10.1091/mbc.E09-12-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci USA. 2003;100:10788–10793. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poujade M, et al. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci USA. 2007;104:15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gipson IK, et al. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 27.Aktories K, Wilde C, Vogelsgesang M. Rho-modifying C3-like ADP-ribosyltransferases. Rev Physiol Biochem Pharmacol. 2004;152:1–22. doi: 10.1007/s10254-004-0034-4. [DOI] [PubMed] [Google Scholar]
- 28.Kovács M, Tóth J, Hetényi C, Málnási-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 29.Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: Characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol. 1996;135:1097–1107. doi: 10.1083/jcb.135.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danjo Y, Gipson IK. Specific transduction of the leading edge cells of migrating epithelia demonstrates that they are replaced during healing. Exp Eye Res. 2002;74:199–204. doi: 10.1006/exer.2001.1115. [DOI] [PubMed] [Google Scholar]
- 31.Ellis AG, et al. Preclinical analysis of the analinoquinazoline AG1478, a specific small molecule inhibitor of EGF receptor tyrosine kinase. Biochem Pharmacol. 2006;71:1422–1434. doi: 10.1016/j.bcp.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Block ER, Klarlund JK. Wounding sheets of epithelial cells activates the epidermal growth factor receptor through distinct short- and long-range mechanisms. Mol Biol Cell. 2008;19:4909–4917. doi: 10.1091/mbc.E08-01-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 34.Bain J, et al. The selectivity of protein kinase inhibitors: A further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curran T, Bravo R, Müller R. Transient induction of c-fos and c-myc in an immediate consequence of growth factor stimulation. Cancer Surv. 1985;4:655–681. [PubMed] [Google Scholar]
- 36.Rollins BJ, Stiles CD. Regulation of c-myc and c-fos proto-oncogene expression by animal cell growth factors. In Vitro Cell Dev Biol. 1988;24:81–84. doi: 10.1007/BF02623883. [DOI] [PubMed] [Google Scholar]
- 37.Johnson GR, Kannan B, Shoyab M, Stromberg K. Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. J Biol Chem. 1993;268:2924–2931. [PubMed] [Google Scholar]
- 38.Chrisman CA. Care of chronic wounds in palliative care and end-of-life patients. Int Wound J. 2010;7:214–235. doi: 10.1111/j.1742-481X.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markova A, Mostow EN. US skin disease assessment: Ulcer and wound care. Dermatol Clin. 2012;30:107–111, ix. doi: 10.1016/j.det.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–870. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 43.Vogel V, Sheetz MP. Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr Opin Cell Biol. 2009;21:38–46. doi: 10.1016/j.ceb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolahi KS, Mofrad MR. Mechanotransduction: A major regulator of homeostasis and development. Wiley Interdiscip Rev Syst Biol Med. 2010;2:625–639. doi: 10.1002/wsbm.79. [DOI] [PubMed] [Google Scholar]
- 45.Cai Y, Sheetz MP. Force propagation across cells: Mechanical coherence of dynamic cytoskeletons. Curr Opin Cell Biol. 2009;21:47–50. doi: 10.1016/j.ceb.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard J. Mechanical signaling in networks of motor and cytoskeletal proteins. Annu Rev Biophys. 2009;38:217–234. doi: 10.1146/annurev.biophys.050708.133732. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: The important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sachs F. Stretch-activated ion channels: What are they? Physiology (Bethesda) 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu Y, Forostyan T, Sabbadini R, Rosenblatt J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J Cell Biol. 2011;193:667–676. doi: 10.1083/jcb.201010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belacortu Y, Paricio N. Drosophila as a model of wound healing and tissue regeneration in vertebrates. Dev Dyn. 2011;240:2379–2404. doi: 10.1002/dvdy.22753. [DOI] [PubMed] [Google Scholar]
- 52.Gorfinkiel N, Schamberg S, Blanchard GB. Integrative approaches to morphogenesis: Lessons from dorsal closure. Genesis. 2011;49:522–533. doi: 10.1002/dvg.20704. [DOI] [PubMed] [Google Scholar]
- 53.Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol. 2000;10:831–838. doi: 10.1016/s0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- 54.Tambe DT, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trepat X, Fredberg JJ. Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol. 2011;21:638–646. doi: 10.1016/j.tcb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 57.Yoshioka J, et al. Cardiomyocyte hypertrophy and degradation of connexin43 through spatially restricted autocrine/paracrine heparin-binding EGF. Proc Natl Acad Sci USA. 2005;102:10622–10627. doi: 10.1073/pnas.0501198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higashiyama S, et al. Membrane-anchored growth factors, the epidermal growth factor family: Beyond receptor ligands. Cancer Sci. 2008;99:214–220. doi: 10.1111/j.1349-7006.2007.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bindschadler M, McGrath JL. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J Cell Sci. 2007;120:876–884. doi: 10.1242/jcs.03395. [DOI] [PubMed] [Google Scholar]
- 60.Ouaknin GY, Bar-Yoseph PZ. Stochastic collective movement of cells and fingering morphology: No maverick cells. Biophys J. 2009;97:1811–1821. doi: 10.1016/j.bpj.2009.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mark S, et al. Physical model of the dynamic instability in an expanding cell culture. Biophys J. 2010;98:361–370. doi: 10.1016/j.bpj.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Angelini TE, et al. Glass-like dynamics of collective cell migration. Proc Natl Acad Sci USA. 2011;108:4714–4719. doi: 10.1073/pnas.1010059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.