Abstract
Agr2 is a putative protein disulfide isomerase (PDI) initially identified as an estrogen-responsive gene in breast cancer cell lines. While Agr2 expression in breast cancer is positively correlated with estrogen receptor (ER) expression, it is upregulated in both hormone dependent and independent carcinomas. Several in vitro and xenograft studies have implicated Agr2 in different oncogenic features of breast cancer; however, the physiological role of Agr2 in normal mammary gland development remains to be defined. Agr2 expression is developmentally regulated in the mammary gland, with maximum expression during late pregnancy and lactation. Using a mammary gland specific knockout mouse model, we show that Agr2 facilitates normal lobuloalveolar development by regulating mammary epithelial cell proliferation; we found no effects on apoptosis in Agr2−/− mammary epithelial cells. Consequently, mammary glands of Agr2−/− females exhibit reduced expression of milk proteins, and by two weeks post-partum their pups are smaller in size. Utilizing a conditional mouse model, we show that Agr2 constitutive expression drives precocious lobuloalveolar development and increased milk protein expression in the virgin mammary gland. In vitro studies using knock down and overexpression strategies in estrogen receptor positive and negative mammary epithelial cell lines demonstrate a role for Agr2 in estradiol-induced cell proliferation. In conclusion, the estrogen-responsive Agr2, a candidate breast cancer oncogene, regulates epithelial cell proliferation and lobuloalveolar development in the mammary gland. The pro-proliferative effects of Agr2 may explain its actions in early tumorigenesis.
Keywords: Agr2, mammary gland, development, proliferation, estrogen-responsive, knockout, overexpression
Introduction
Agr2 is the mammalian homologue of the previously identified Xenopus laevis cement gland protein XAG-2, which promotes cement gland differentiation and ectodermal patterning (Aberger et al., 1998). It is a secreted protein and, based on homology, a putative member of the protein disulfide isomerase family; it was recently shown to bind to nascent proteins and direct them to the endoplasmic reticulum (Higa et al., 2011; Park et al., 2009; Persson et al., 2005; Zhao et al., 2010). The mammalian Agr2 was initially identified in a screen for estrogen-responsive genes in breast cancer cell lines (Fletcher et al., 2003; Thompson and Weigel, 1998). Correspondingly, it is expressed at a higher level in estrogen receptor (ER) positive breast cancers, and within ER positive breast cancers Agr2 expression correlates with poor prognosis (Barraclough et al., 2009; Fletcher et al., 2003; Innes et al., 2006; Thompson and Weigel, 1998). However, in one study where ER status was not taken into account Agr2 was shown to associate with good prognosis (Fritzsche et al., 2006). In vitro work in breast cancer cell lines has implicated Agr2 in transformation, metastasis, and proliferation (Liu et al., 2005; Vanderlaag et al., 2010). Clinical studies demonstrating overexpression of Agr2 in a number of other adenocarcinomas including esophagus, pancreas, ovary, lung and prostate, further support the oncogenic role of Agr2 (Bu et al., 2011; Fritzsche et al., 2007; Maresh et al., 2010; Park et al., 2011; Pizzi et al., 2012; Ramachandran et al., 2008; Riener et al., 2009; Wang et al., 2008; Zhang et al., 2005). Functional in vitro studies point to Agr2’s oncogenic features, also supporting the role of this protein in tumor biology (Brychtova et al., 2011; Dumartin et al., 2011; Fletcher et al., 2003; Maslon et al., 2010; Pohler et al., 2004; Ramachandran et al., 2008; Vanderlaag et al., 2010; Wang et al., 2008).
Despite strong suggestions for a pro-tumorigenic role of Agr2 in breast cancer, no published studies have defined its role in normal mammary gland development; such in vivo studies may provide important clues to the mechanism of action for this candidate breast cancer oncogene. To this end, we generated mammary gland specific Agr2 knockout and conditional overexpression mouse models. We show that Agr2 expression is developmentally regulated in the mammary gland, and that the gene promotes epithelial cell proliferation and milk protein production, facilitating normal alveolar development. Agr2 knockout and constitutive expression mice exhibit decreased and increased mammary epithelial cell proliferation, respectively, without changes in apoptosis rates. This study is the first to utilize transgenic mouse models to show that Agr2 regulates cell proliferation and differentiation in the mammary gland epithelium. We speculate that the pro-proliferative role of Agr2 in normal mammary gland development may mirror its oncogenic role in breast tumorigenesis. Combined with genetically engineered breast tumorigenesis mouse models, the two mouse models developed in the current study will be useful for testing the in vivo role of Agr2 in tumorigenesis and metastasis in breast and other adenocarcinomas.
Materials and Methods
All experiments performed on animals were conducted under approved IACUC protocol # 2001-2239, following strict guidelines as provided by IACUC.
Generation of Inducible Agr2flox/floxMMTV-Cre Mice
Agr2 flox mice were generated as previously described (Zhao et al., 2010). In order to produce inducible deletion of Agr2 we bred Agr2 mice to MMTV-LTR-Cre Transgenic Mice obtained from the Jackson Laboratory (JAX 003553). In the bigenic mice, Agr2 is deleted in Mouse Mammary Tumor Virus Long Terminal Repeat (MMTV-LTR) expressing tissues, including primarily mammary gland (virgin, pregnant and enhanced expression during lactation) as well as salivary gland, seminal vesicles and skin. The specificity of the Cre-induced recombination was confirmed by crossing MMTV-LTR mice to ROSA26 reporter (R26R) mice (Soriano, 1999). Tissues from virgin R26R/MMTV-LTR-Cre female mice were stained with X-Gal to confirm tissue specific expression. We observed abundant staining in the mammary epithelium but not in the brain or heart (Supplemental Figure 2), confirming faithful Cre recombinase expression.
Generation of TRE-hAgr2/MMTV-LTR-rtTA (hAgr2-Tet-On) Mice
pCMV5-Sport6-hAGR2 vector was purchased from ATCC (10701095). Full length cDNA was amplified from the vector template using forward primer (with ClaI restriction sequence) designed to anneal to nucleotides 44-63 and reverse primer to nucleotides (with SpeI restriction sequence) 598-617. The PCR product was then cloned into the pTMILA vector, downstream of an inducible tetracycline promoter (tetop) using ClaI and SpeI sites. Correct insertion of the human Agr2 (hAgr2) transgene into the pTMILA plasmid was verified by sequencing. Tetop-hAgr2-IRES-Firefly-Luc cassette was excised from the vector using PvuII restriction site and injected into fertilized ova from FVB/N background donors. The founders were identified by genotyping. The TRE-hAgr2 homozygous females were crossed to MMTV-LTR-rtTA (Gunther et al., 2002) males to generate bitransgenic TRE-hAgr2/MMTV-LTR-rtTA progeny.
Genotyping
Genomic DNA was extracted from mouse tail using Quick Genotyping DNA Preparation Kit (Bioland Scientific).
TRE-hAgr2/MMTV-LTR
MMTV-LTR forward primer – TGCCGCCATTATTACGACAAGC, reverse primer – ACCGTACTCGTCAATTCCAAGGG; TRE-hAgr2 forward primer – CGTCAGATCGCCTGGAGAC, reverse primer – TTTCTTTAAAGCTTGACTGTGTGG. Agr2flox/flox/MMTV-Cre: Agr2 flox: primer 1 – ATCCAACAAGCATCCACTGA, primer 2 – CTTTGGCCAAGGTACCAGAA, primer 3 – CTGGATCTAATTTGTGCTGAAT. Wild type allele represented by 228 bp product, floxed allele by 335 bp product, and recombined allele by 519 bp product. MMTV-Cre forward primer – TGAGGTTCGCAAGAACCTGAT, reverse primer – GCCGCATAACCAGTGAAACAGC.
Mammary gland whole mounts and quantification of lobuloalveolar development
The estrous cycle was synchronized by putting male bedding in female cages. Synchronization of estrous cycle was confirmed by vaginal smears prior to any analysis of virgin mice. Fourth or ninth inguinal mammary glands were spread on a glass slide. Mammary glands were fixed in carnoy’s fixative, and stained in carmine alum overnight. The mammary glands were dehydrated by gradual change in graded ethanol and were cleared in xylene. After clearing in xylene the glands were mounted in permount. The images were captured on Nikon SMZ1500 dissecting microscope. Mammary glands number 4 and 9 were analyzed for quantification of lobuloalveolar development. Quantification was performed by calculating the percent of area filled with epithelial tissue in ImageJ and by counting the number of alveolar buds per millimeter of duct. To count the number of alveolar buds per mm of duct: for each mammary gland five ducts were selected at random and for each duct the number of alveolar buds were counted in ImageJ for at least 5 mm of duct tissue. This number was normalized to the length of the duct (measured in ImageJ), and the mean number of alveolar buds per mm of duct tissue is reported for each mouse. A minimum one mammary gland from at least three mice were used to calculate the mean and SEM, where each mouse represents one biological replicate.
Quantitative Real-Time PCR (qPCR) analysis
RNA was extracted from fourth or ninth inguinal mammary gland using trizol Reagent (Invitrogen). cDNA was synthesized using iScript cDNA reverse transcriptase (Bio-Rad Laboratories). Agr2 expression in Agr2 KO and over-expression mice was measured in triplicates using pre-designed taqman assay for mouse (TaqMan Probe #4331182, Applied Biosystems) and human Agr2 (TaqMan probe #4331182, Applied Biosystems) respectively. The expression level was normalized to GAPDH expression measured using GAPDH TaqMan probe (TaqMan probe #4331182, Applied Biosystems) or Keratin 8 (Krt8) expression. The relative gene expression level was calculated using delta-delta-cT method (Livak and Schmittgen, 2001). Primers for mouse Agr3: forward – CATGCTCGCAAAAGTAACAAACC, reverse – GAAGGGTCAACAAACATGATCCT; human Agr3: forward – ATCACCTGATGGGCAATATGTG, reverse – GAGTATCTTCCAGCTATGTCAGC; mouse Krt8: forward – GAAGTTCGTGCCCAGTACGAG, reverse – CGGTTGATGTTGCGGTTCAT; mouse - casein: forward - GCCCTTCCCACAAATCTTCCA, reverse – GGGAGTAAGGTACTGCATATCCT; mouse -casein: forward - GGCACAGGTTGTTCAGGCTT, reverse - AAGGAAGGGTGCTACTTGCTG; mouse - casein: forward - AACTGCCGTGGTGAGAAGAAT, reverse - AAAGATGGCCTGTAGTGGTAGTA; and mouse lactalbumin: forward – GTTCCTTTGTTCCTGGTGTGT, reverse – TGCCTTGATAGCCATCTATGTCT; human AREG: forward – GTGGTGCTGTCGCTCTTGATA, reverse – CCCCAGAAAATGGTTCACGCT; mouse AREG: forward – GGTCTTAGGCTCAGGCCATTA, reverse – CGCTTATGGTGGAAACCTCTC.
Pup growth study and statistics
Mouse pups from wild type and Agr2flox/floxMMTV-Cre dams were weighed every day for 14 days. Litter sizes were culled to five pups (the maximal litter size from knockout dams) to ensure that there was no added variation due to differences in litter size. The mean pup weight for four wild type litters and seven Agr2flox/floxMMTV-Cre litters were analyzed by repeated measures ANOVA with type III SS using R (http://www.R-project.org). Mouse total lipid extracts were collected using the Bligh and Dyer method (Bligh and Dyer, 1959). Free fatty acids were quantified using BioVision Free Fatty Acid Quantification Kit (cat# K612-100). Triglycerides were quantified using Cayman Chemical Triglyceride Calorimetric Assay Kit (cat# 10010303).
Brdu labeling proliferation assay (in vivo) and Immunohistochemistry
To assess proliferation, mice were injected 2 hours before sacrifice with BrdU labeling reagent (Roche Inc., USA) as per manufacturer’s protocol. After lymph node removal, the fourth or ninth inguinal mammary gland was dissected in half. Half of the mammary gland was utilized for RNA extraction and the other half was fixed in formalin (10%), paraffin embedded, and cut into 8 micron thick sections on Probe-on-PlusTMslides (Fisher). For immunohistochemistry, sections were deparaffinized in xylene and hydarated in graded ethanol, followed by antigen retrieval in .01M citrate buffer (pH6.0). Sections were blocked for 60 minutes in protein block (cat# 2013-02, Dako) as per manufacturer’s instructions, and incubated overnight at 4°C with primary antibody at the following dilutions: Anti-Agr2 (Imgenex, IMG-5279A, 1:100), Anti-pH3(cat# 06-570, Milipore, 1:500), Anti-SMA(cat#, company, and 1:1000), Anti-BrdU (cat# 1893, Abcam, and 1:500). After primary antibody incubation, corresponding biotinylated secondary antibody (Vector) 1:500 was added for 30 minutes followed by detection with Vectastain ABC reagent (Dako) and development with DAB chromogen (Dako) according to manufacturer’s recommendations. Slides were counterstained with hematoxylin, dehydrated, and mounted with permount mounting medium. Images were captured on Nikon Eclipse 50i microscope. Three slides from each mammary gland were used for quantification of BrdU and pH3-positve cells, and ten to fifteen fields of view were used for each slide. The number of pH3 positive cells was normalized to 10,000 mm2 of epithelial area, and the number of BrdU positive cells is reported as the percentage of total epithelial cells per field of view. Quantification was performed in ImageJ. The mean ± SEM were calculated for three mice with at least one mammary gland per mouse, where each mouse represents one biological replicate.
Western blot
Specificity of the Agr2 antibody (Imgenex, IMG-5279A) was determined by western blot analysis. 5 g each of Agr2 and Agr3 overexpression lysates were loaded. Anti-Agr3 primary antibody (Cat# TA308177, OriGene) was used to detect Agr3, and Anti-GAPDH (cat# AM4300, Applied Biosystems) was used to detect GAPDH as a loading control.
Luciferase assay
Total protein was extracted from ninth mammary gland tissue utilizing passive lysis buffer (Promega) with protease inhibitor cocktail (cat# 04693159001, Roche). Protein concentration was measured using BCA reagent (Thermo scientific). Luciferase activity was measured in equal amount of protein extracted from Agr2 WT and hAgr2+/MMTVrtTA+ bigenic mice according to manufacturer’s instructions (Promega).
Cell lines, small interfering RNA (siRNA) transfection, and Agr2 overexpression
MCF10A cells were cultured in DMEM/F12 supplemented with 5% equine serum, insulin (10 g/ml), EGF (20ng/ml), cholera toxin (100 ng/ml), hydrocortisone (500 ng/ml), and antibiotics (penicillin – streptomycin at 100 g/ml). MCF7 cells were cultured in DMEM without phenol red and supplemented with 10% charcoal-stripped FBS and antibiotics. Cells were cultured either with or without estradiol (1×10−8 M). For knockdown of Agr2, three siRNAs were selected from Ambion’s Silencer Select pre-designed siRNA library (siRNA ID# s20692, s2o693, and s20694, Life Technologies). Cells were also transfected with Silencer Select Negative Control #1 siRNA (cat# 4390843, Life Technologies). Three Agr2 siRNAs were pooled and reverse transfections were performed with Lipofectamine RNAiMAX reagent at a final siRNA concentration of 25nM (Life Technologies). Transient Agr2 overexpression in MCF10A cells was achieved via pCMV5-Sport6-hAGR2 vector (cat# 10701095, ATCC) transfection with X-tremeGENE HP DNA transfection reagent (Roche) using a 8:1 ratio of transfection reagent to DNA (μg).
BrdU labeling proliferation assay (in vitro)
Transfected cells were cultured for 48 hours and pulsed with BrdU (20 M) for 30 min at 37°C. Cells were trypsinized and collected for analysis of BrdU incorporation by flow cytometry. Cells were washed twice in PBS supplemented with 1% FBS, and fixed for one hour in 70% cold ethanol in PBS at -20°C. After one wash in cold PBS cells were permeabilized for one hour in 2M HCl with 0.2% Pepsin at room temperature. Cells were washed three times with cold PBS and counted. At least 5x105 cells were labeled with primary antibody in 50 l PBS with 0.5% Tween-20 and 0.5% BSA for 45 minutes at room temperature. Primary antibodies used were FITC-conjugated -BrdU (cat# 347583, BD Biosciences) at 1:10 dilution, and FITC-conjugated mouse IgG1, K isotype control (cat# 11-4714-81, eBioscience) at 1:50 dilution. Following primary antibody incubation, cells were washed twice with cold PBS and resuspended in 1ml PBS with 2.6 g propidium iodide. Cells were analyzed within 30 minutes on the BD FACSCalibur Flow Cytometry System. Further validation of cell proliferation results was performed by quantifying total ATP content via the ApoSENSOR ATP Luminescence luciferase reporter assay (cat# K254-1000, BioVision Inc.).
Statistical analysis
All experiments were performed on at least three biological replicates from which the mean and SEM were calculated. Statistical analysis was performed utilizing student’s un-paired t test unless otherwise specified.
Results
Agr2 expression is developmentally regulated in the mouse mammary gland
To gain insights into the role of Agr2 in mammary gland biology, we assessed its expression in mouse mammary glands during the many stages of development. Using qPCR and immunohistochemistry (IHC) we found that Agr2 is expressed at low levels in virgin mammary glands (Figure 1A and 1B), and increases during pregnancy, reaching a peak in lactation, followed by a drop during involution. While expression of AGR2 protein by IHC is most prominent in late pregnancy and lactation day 10 (Figure 1B), the pattern of mRNA expression is not precisely reproduced. This could be due to various post-transcriptional events leading to stabilization of AGR2 during pregnancy and/or the non-quantitative nature of IHC. Agr3 was consistently expressed at low levels throughout development, suggesting Agr2 may play a more prominent role than Agr3 in normal mammary gland biology. Within the mammary gland, AGR2 is primarily expressed in ductal and alveolar epithelial cells (Figure 1B). The AGR2 antibody used for IHC is specific as it detects only AGR2 and not AGR3 by western blot (Supplemental Figure 1). We conclude that mammary gland expression of Agr2, but not Agr3, is developmentally regulated, and that based on the temporal expression pattern, its normal function is likely carried out during pregnancy and lactation.
Figure 1. Agr2, but not Agr3, expression is developmentally regulated in the mammary gland.
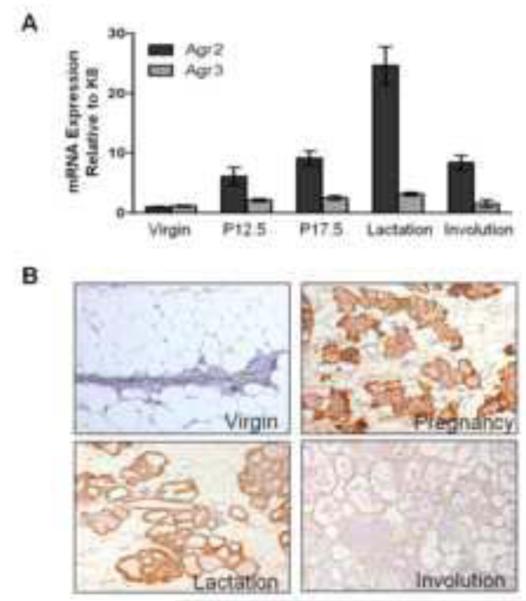
(A) Agr2 and Agr3 mRNA expression were measured in the mammary glands of virgin, pregnant (day 12.5 and day 17.5), lactating (day 10) and involuting (day 2) wild type mice. Expression was normalized to Keratin 8 (Krt8) mRNA expression levels to account for expansion of the epithelial compartment during development, and is represented as relative expression compared to virgin mice. The data was collected from at least one mammary gland from a minimum of three mice and is represented as the mean ± SEM. (B) AGR2 protein expression was detected with immunohistochemistry on formalin fixed paraffin embedded (FFPE) sections of mammary glands. Representative images from triplicate experiments done on at least two mice are shown. The images represent virgin, pregnancy (day 17.5), lactation (day 10), and involution (day 2) stages of mammary gland development.
Agr2 knockout mice exhibit decreased alveologenesis
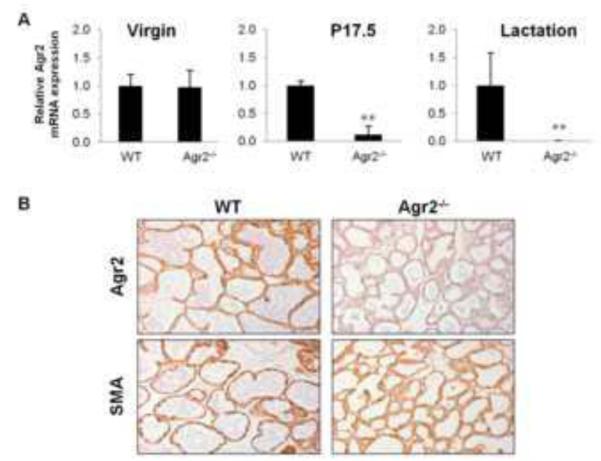
To determine the role of Agr2 in normal mammary gland development we generated mammary specific Agr2 knockout mice utilizing the Cre-loxP strategy. To generate mammary specific Agr2 knockout mice, Agr2flox/flox mice were interbred with another transgenic line that expresses cre-recombinase under the control of the MMTV promoter/enhancer (MMTV-Cre). The MMTV promoter directs cre-recombinase expression to mammary epithelium as well as epididymis, salivary gland and other secretory tissues (Wagner et al., 1997). MMTV-Cre expression in mammary gland was confirmed by interbreeding the MMTV-Cre line with the Rosa-26 line. The -gal staining of the R26R/MMTV-LTR-Cre bigenic line confirmed MMTV-Cre expression in mammary gland and not in brain and heart (Supplemental Figure 2). Agr2 deletion in mammary glands of Agr2flox/floxMMTV-Cre mice was confirmed by genotyping mammary tissue and liver tissue, using specific primer sequences as previously described (Zhao et al., 2010). By genotyping, the homozygous and heterozygous Agr2 deletion was evident in mammary tissue of Agr2flox/floxMMTV-Cre and Agr2wt/floxMMTV-Cre mice, respectively, but not in liver tissue, indicating mammary gland-specific deletion of Agr2. Agr2 deletion was also confirmed by analyzing mRNA and protein expression in mammary glands from virgin, pregnant (day 17.5), and lactational (day 10) mice (Figure 2A and 2B). We did not observe changes in Agr2 mRNA or protein expression in Agr2flox/floxMMTV-Cre virgin mice, which could be explained by the low expression of Agr2 and/or ineffective deletion in virgin mice. In contrast, Agr2 mRNA expression was decreased more than 75% in Agr2flox/floxMMTV-Cre mice at pregnancy day17.5 (Figure 2A), likely due to the developmental MMTV-Cre expression which tends to be expressed highest during late pregnancy and lactation (Munoz and Bolander, 1989; Wagner et al., 2001). By immunohistochemistry, Agr2 deletion exhibits a mosaic pattern in the pregnant mammary gland, consistent with the mosaic expression of MMTV-Cre in the mammary epithelium (Wagner et al., 2001; Wagner et al., 1997). During lactation, Agr2 levels were decreased 100-fold in Agr2flox/floxMMTV-Cre mice (Figure 2A), and immunohistochemistry confirmed the successful deletion of Agr2 in almost all alveoli in the lactational mammary gland (Figure 2B). Collectively, these data indicate that this is an efficient mammary gland-specific knock out mouse model for the study of Agr2 in normal mammary gland development and breast cancer.
Figure 2. Agr2 is specifically deleted in mammary glands of Agr2flox/floxMMTV-Cre mice.
(A) Agr2 mRNA expression in wild type (WT) and Agr2flox/floxMMTV-Cre (Agr2−/−) mammary glands at virgin, pregnancy (day 17.5) and lactational (day 10) stages. Agr2 expression was normalized to Krt8 expression and is represented as the mean ± SEM from two experiments with at least three biological replicates in each experiment. ** indicates a p-value < 0.01 (B) AGR2 protein expression was detected by immunohistochemistry in FFPE sections of mammary glands excised from WT and Agr2flox/floxMMTV-Cre (Agr2−/−) mice at lactational day 10 (upper panels). Smooth Muscle Actin (SMA) expression was detected as a control for staining (lower panels). The experiment was repeated three times with at least two biological replicates in each experiment.
To determine the role of Agr2 during mammary gland development, whole mount staining of the fourth and ninth inguinal mammary glands from virgin (4wk, 7 wk and 10wk), pregnant (day 12.5 and day 17.5) and lactational (day 2 and day 10) Agr2flox/floxMMTV-Cre and littermate control mice were compared. As expected, based on the lack of Agr2 expression and/or ineffective deletion in virgin glands, we did not observe significant differences in terminal end buds, branching morphogenesis, or elongation of the epithelial tree in virgin Agr2flox/floxMMTV- Cre mice. In contrast, whole mounts from Agr2flox/floxMMTV-Cre mice at day 12.5 and at day 17.5 of pregnancy showed significantly fewer lobuloalveolar epithelial structures than control mice, suggesting a role for Agr2 in alveologenesis during pregnancy (Figure 3A-D). Moreover, even though Agr2 is expressed at its highest level during lactation we did not observe significant changes by whole mount staining or histology in lactational Agr2flox/floxMMTV-Cre mice. The Agr2flox/floxMMTV-Cre females were able to nurse their pups; however, only to a limited extent. The mean weight gain of pups per litter began to decrease significantly from their wild type counterparts after approximately one week postnatal (Figure 3E). By postnatal week two the difference in mean pup weight per litter between the two genotypes was determined to be statistically significant by a repeated measures ANOVA, and the rate of pup growth as determined by the interaction between genotype and time is significantly reduced in the litters nursed by Agr2flox/floxMMTV-Cre mice (ANOVA table reported in Supplemental Table 1). Based on these data, we conclude that Agr2 facilitates normal lobuloalveolar development during pregnancy.
Figure 3. Agr2 deletion causes reduced alveologenesis during pregnancy, resulting in decreased expression of milk proteins and reduced pup growth rate.
Whole mount staining on fourth and ninth inguinal mammary gland from WT and Agr2flox/floxMMTV-Cre (Agr2−/−) mice at pregnancy (A) day 12.5 and (B) day 17.5. Images were captured at 0.75X and 4X (inserts) magnifications. (C) The number of alveolar buds per millimeter of duct is significantly reduced in Agr2−/− mammary glands. (D) The percentage area filled with epithelial tissue in whole mounts was significantly reduced in Agr2−/− mice as calculated using ImageJ software on the same mammary glands used in (C). The data in C and D represent mean ± SEM from three mice using two mammary glands from each mouse. ** indicates a p-value < 0.01. (E) Pups were weighed daily for 14 postnatal days from four WT litters and seven Agr2−/− litters. Litter sizes were culled to five pups to prevent variation in weight due to variable litter size. The data represents the mean pup weight for each litter ± SEM. The p-values were calculated from a repeated measures ANOVA, where ‘Genotype’ represents the WT and Agr2−/− between-subjects main effect, ‘Day’ represents the within-subjects main effect, and ‘Genotype × Day’ represents the interaction between the two. (F) Gene expression analysis of milk proteins in the mammary gland at pregnancy day 12.5 and 17.5, and lactation day 2 (LD2). Expression was normalized to Krt8 to account for changes in epithelial compartment size. Fold changes are relative to expression at P12.5. (G-I) Expression of milk proteins in Agr2−/− mammary glands is significantly reduced during pregnancy and lactation. Gene expression levels were normalized as in panel F. Data represents mean ± SEM for three mice, one mammary gland was analyzed per mouse. Lalba: Lactalbumin. ** indicates a p-value < 0.01. * indicates a p-value < 0.05. NS: Not Significant
To gain insights into the possible causes for reduced weight gain in litters from Agr2flox/floxMMTV-Cre females, we analyzed milk protein expression at the mRNA level by qPCR during pregnancy (day 12.5 and 17.5) and lactation (day 2). Relative to expression at pregnancy day 12.5, -casein, -casein, -casein, and lactalbumin (Lalba) mRNA levels each increase two to four-fold by late pregnancy in wild type mice. The caseins increase another two to three-fold by lactation, while Lalba increases approximately 22-fold from mid-pregnancy to lactation (Figure 3F). The mRNA levels for these milk proteins are consistently down-regulated at each of these developmental time points in Agr2flox/floxMMTV-Cre mice (Figure 3G-I), suggesting that one possible explanation for the reduced rate of pup growth in these mice is decreased milk protein content in Agr2flox/floxMMTV-Cre mammary glands.
Agr2 overexpression mice exhibit precocious lobuloalveolar development
To further test the role of Agr2 in promotion of mammary gland epithelial development, we generated an Agr2
overexpression mouse model with temporally and spatially regulated expression of Agr2. To do this we took advantage of the Tet-on system where human Agr2 (hAgr2) cDNA linked to a firefly luciferase is expressed under the control of the TRE promoter, which is active only in the presence of rtTA activator with stimulation by tetracycline or its analog doxycycline (Figure 4A) (Furth et al., 1994; Gossen and Bujard, 1992; Kistner et al., 1996). To generate a mouse line overexpressing Agr2 in a mammary gland specific manner, the hAgr2 transgenic line was interbred with MMTV-rtTA mice (Whisenhunt et al., 2006). To confirm transgene expression we measured luciferase activity in total protein lysates prepared from mammary gland tissues of transgenic virgin mice fed with doxycycline (2mg/ml) for 3 weeks. hAgr2+/MMTVrtTA+ bigenic mice showed a 300-400 fold higher expression of luciferase in comparison to hAgr2− /MMTVrtTA−, hAgr2−/MMTVrtTA+, or hAgr2+/MMTVrtTA− mice, suggesting that Agr2 expression can be induced in a specific and robust fashion in the hAgr2+/MMTVrtTA+ transgenic line (Figure 4B). As there is no significant difference in the basal level of transgene expression between hAgr2−/MMTVrtTA−, hAgr2−/MMTVrtTA+ and hAgr2+/MMTVrtTA− mice, any of these three genotypes were utilized as control mice in further experiments. Doxycycline-induced Agr2 overexpression in the mammary glands of hAgr2+/MMTVrtTA+ bigenic mice was also confirmed by mRNA and protein expression utilizing qPCR and immunohistochemistry (Figure 4C and 4D).
Figure 4. Agr2 expression is spatially and temporally regulated in hAgr2+/MMTVrtTA+ transgenic mice.
(A) A diagrammatic representation of Agr2 overexpression mouse model. (B) The transgene expression in Agr2 overexpression mice was measured by luciferase assay. Littermate control, monogenic and bigenic virgin mice were fed with doxycycline (2mg/ml) for three weeks. At the end of the treatment period, luciferase activity was measured in equal amount of total protein lysates extracted from their mammary gland, utilizing a luciferase assay kit (Promega) according to the manufacturer’s instructions. The data is represented as Relative Luciferase Units (RLU)/second. (C) Agr2 mRNA expression was measured in total RNA extracted from mammary glands of control (wild type and monogenic) and Agr2 overexpression (hAgr2+/MMTVrtTA+ bigenic) mice (TG) induced with doxycycline for three weeks. The data is represented as relative expression of Agr2 in comparison to control mice. Gapdh expression was used for normalization. Data for B and C represent the mean ± SEM from three biological replicates. (D) AGR2 protein expression in mammary glands of doxycycline induced control and TG mice were measured by immunohistochemistry. The experiment was performed on three biological replicates.
To analyze the effect of Agr2 overexpression in virgin mice, Agr2 expression was induced in control and hAgr2+/MMTVrtTA+ bigenic littermate female mice starting at the age of 12 weeks. Doxycycline was fed to the mice through the drinking water (2mg/ml) continuously for six months, and at the end of the treatment period mammary glands were analyzed by whole mount staining and histology. To avoid variation in side branching due to estrous stage, the estrous cycle for all mice was synchronized. hAgr2+/MMTVrtTA+ bigenic virgin mice on doxycycline treatment showed increased branching and developed lobuloalveolar like structures, while littermate control mice on doxycycline treatment did not show any alterations (Figure 5A-D). In the absence of doxycycline, hAgr2+/MMTVrtTA+ bigenic mice did not show defects in lobuloalveolar development indicating a working conditional system. Analysis of milk protein expression revealed an increase in expression of milk proteins at the mRNA level (Figure 5E). From these experiments, we conclude that Agr2 overexpression leads to an advanced differentiation phenotype with increased lobuloalveolar development and milk protein expression, features reminiscent of early pregnancy.
Figure 5. Agr2 constitutive expression induces precocious lobuloalveolar development and increased milk protein expression in virgin mice.
Littermate control and hAgr2+/MMTVrtTA+ bigenic (TG) mice were induced by doxycycline (2mg/ml) for six months. At the end of the treatment period mammary glands were analyzed by (A) whole mount staining and (B) by histology using hematoxylin and eosin staining. The images of whole mounts were captured at 0.75X and 4X (inserts) magnifications. The images of histology were captured at 10X and 40 X magnifications (inserts). Quantification of lobuloalveolar development (C) and epithelial coverage (D) in TG mice revealed significant increases in both measurements. Quantification represents the mean ± SEM for three mice, using two mammary glands from each mouse. ** indicates a p-value < 0.01. (E) Increased expression of milk proteins at the mRNA level as determined by qPCR. Expression was normalized to Krt8 to account for changes in epithelial compartment size. Data represents mean ± SEM for three biological replicates. ** indicates a p-value < 0.01. *** indicates a p-value < 0.001. D: Duct, A: Adipose, Lalba: Lactalbumin.
Agr2 promotes epithelial cell proliferation in the mammary gland
To investigate the cellular mechanism underlying decreased lobuloalveolar development in the Agr2flox/floxMMTV-Cre mice and the increased alveologenesis in hAgr2+/MMTVrtTA+ bigenic mice, we investigated the proliferation rate in mammary glands from the two mouse models. Formalin fixed paraffin embedded mammary gland tissues from Agr2flox/floxMMTV-Cre mice were stained for expression of the mitosis marker phosho-histone H3 (pH3). The number of pH3 positive cells was significantly reduced in Agr2flox/floxMMTV-Cre mice compared to littermate controls at pregnancy days 12.5 and 17.5 (Figure 6A and 6B). Furthermore, no difference in apoptosis between Agr2flox/floxMMTV-Cre and control mammary glands was detected by TUNEL staining (data not shown), suggesting that decreased alveologenesis observed in Agr2flox/floxMMTV-Cre mice is due to reduced proliferation rather than increased cell death.
Figure 6. Agr2 knockout mice exhibit reduced proliferation during pregnancy, and Agr2 overexpression mice exhibit increased proliferation.
Formalin Fixed Paraffin Embedded (FFPE) sections of mammary glands from WT and Agr2−/− mice at (A) pregnancy day 12.5 and (B) at pregnancy day 17.5 were stained for pH3, a mitotic marker. Total pH3 positive cells and total epithelial area was calculated from 10 to 15 fields of view using Image J. The number of pH3 positive cells/10,000 square mm of epithelial area in WT and Agr2−/− mice at P12.5 and P17.5 is represented in the far right panels of (A) and (B), respectively, as the mean ± SEM from three biological replicates. (C-D) Littermate control and Agr2 overexpression (TG) 12 week old virgin female mice were fed with doxycycline (2mg/ml in water) for six months. To mark the mitotic cells, at the end of the treatment period mice were injected with BrdU. The mice were sacrificed two hours after BrdU injection and the mammary glands were fixed and embedded for sectioning. The sections of mammary gland were stained for (C) pH3 and (D) BrdU. The number of pH3 positive cells in control and TG mice was calculated as described for panels (A) and (B) from three WT and three TG mice. The number of BrdU positive cells and total number of cells was counted from 10 to 15 fields of view using ImageJ software. The chart represents percentage of BrdU positive cells in three WT control and three TG mice. * represents p-value < 0.05 and ** represents p-value < 0.01.
To test the effect of Agr2 overexpression on epithelial cell proliferation, hAgr2+/MMTVrtTA+ bigenic and control mice were given doxycycline for 6 months starting from 12 weeks of age. The mice were injected with BrdU two hours prior to euthanasia, and mammary glands were sectioned and stained for BrdU and pH3. Both BrdU and pH3 staining showed a significant increase in number of dividing cells in hAgr2+/MMTVrtTA+ bigenic mice in comparison to littermate controls, indicating that Agr2 overexpression can drive increased rates of mammary epithelial cell proliferation (Figure 6C and 6D).
Agr2 is required for estradiol-induced cell proliferation in vitro
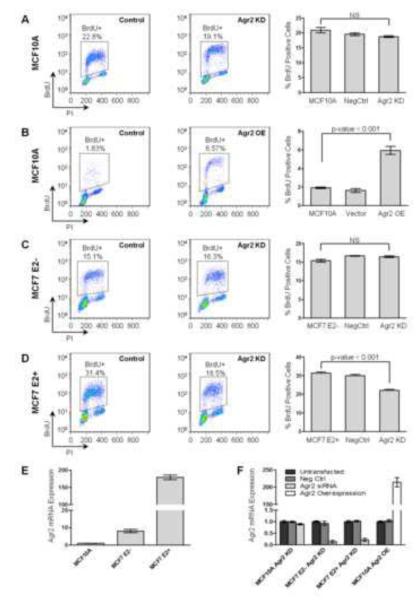
In order to dissect the effects of estradiol and Agr2 on cell proliferation in normal mammary epithelial and breast cancer cell lines, we used the ER-negative MCF10A, which does not express Agr2, and the ER-positive MCF7 cell lines to knock down and overexpress Agr2. Expectedly, knock down of Agr2 expression in MCF10A cells resulted in no change in proliferation status (Figure 7A) as there is no Agr2 expression in this cell line (Figure 7E). However, transient overexpression of Agr2 in MCF10A cells resulted in an approximately 3-fold increase in BrdU incorporation (Figure 7B). Agr2 knock down and overexpression was validated by qPCR (Figure 7F), and the changes in cell proliferation were also demonstrated by quantifying the amount of ATP present after each treatment via the ApoSENSOR ATP Luminescence luciferase reporter assay (Supplemental Figure 3).
Figure 7. Agr2 knock down results in decreased proliferation, while Agr2 overexpression results in increased proliferation of breast epithelial cells.
(A) MCF10A cells were transfected with either scrambled siRNA (left panel) or siRNA targeting Agr2 mRNA (middle panel). After 48 hours, cells were treated with BrdU for 30 minutes and percentage of proliferating cells that incorporated BrdU was assessed by flow cytometry (right panel). (B) Assessment of proliferation was also performed in MCF10A cells that were either transfected with a vector control (left panel) or an Agr2 overexpression plasmid (middle panel), and BrdU incorporation quantified by flow cytometry (right panel). (C – D) Cell proliferation in MCF7 cells was assessed after siRNA knockdown of Agr2 in the absence of estradiol (C), and in the presence of estradiol (D) the same way as explained in (A). Each experiment was performed in triplicate and percent BrdU-positive cells is reported as the mean ± SEM. Isotype controls for flow cytometry are shown in Supplemental Figure 6. (E) Agr2 mRNA expression relative to expression in MCF10A cells was determined by qPCR and normalized to Gapdh. (F) Validation of Agr2 mRNA knockdown and overexpression by qPCR. E2: estradiol, NS: not significant, KD: knock down, OE: overexpression.
In order to define the proliferation role of Agr2 in the ER-positive MCF7 cell line, we cultured these cells in the absence of estradiol (E2-; phenol red-free DMEM supplemented with charcoal stripped FBS) as well as in media supplemented with estradiol (E2+; phenol red-free DMEM supplemented with charcoal-stripped FBS and 1x10−8 M estradiol). In the absence of estradiol approximately 15% of MCF7 cells were BrdU-positive (Figure 7C, left panel), while stimulation with estradiol alone resulted in a two-fold increase in BrdU-positive cells (Figure 7D, left panel). Stimulation of MCF7 cells with estradiol also induced Agr2 expression by about 20-fold (Figure 7E). Interestingly, knock down of Agr2 expression in estradiol-negative conditions had no effect on cell proliferation (Figure 7C, middle panel), while knockdown of Agr2 in estradiol-positive conditions (Figure 7D, middle panel) restricted proliferation to the basal proliferation levels observed in MCF7 cells grown the absence of estradiol (Figure 7C, left panel). These data suggest that Agr2 is sufficient to drive proliferation in ER-negative MCF10A cells and is required for full estradiol-stimulated proliferation in ER-positive MCF7 cells.
Discussion
Agr2 is a putative member of the protein disulfide isomerase family (Higa et al., 2011; Park et al., 2009; Zhao et al., 2010) known to be up-regulated in a number of epithelial neoplasms including breast, prostate, pancreas, and ovarian cancers. In breast tumors its expression positively correlates with ER status (Barraclough et al., 2009; Innes et al., 2006; Maresh et al., 2010; Park et al., 2011; Ramachandran et al., 2008; Vivekanandan et al., 2009; Wu et al., 2008; Zhang et al., 2005). Functional studies involving cancer cell lines and engraftment experiments point to the involvement of Agr2 in a broad spectrum of pro-oncogenic features including promotion of proliferation, metastasis and decreased apoptosis (Vanderlaag et al., 2010). Despite the presence of significant clinical data and suggestive in vitro models, information on the role of Agr2 in normal mammary gland biology is lacking. Our study, introduces the first mammary gland specific deletion and overexpression Agr2 mouse models, demonstrating that Agr2 facilitates normal lobuloalveolar development in pregnancy through positive regulation of mammary epithelial cell proliferation. This work suggests that promotion of cell proliferation could be an early oncogenic feature of Agr2 overexpression.
Developmentally, the expression of Agr2 is low in virgin mammary glands, increasing during pregnancy and reaching its peak at lactation. Within 48 hours of weaning, Agr2 expression in the mammary gland decreases back to early pregnancy levels, suggesting a role during pregnancy and lactation. In contrast, Agr3 is minimally expressed in comparison to Agr2 throughout mammary gland development. While this suggests Agr3 may not play a prominent role in development, it has been reported to be overexpressed in ER-positive breast cacrcinomas (Fletcher et al., 2003), and its function in normal mammary gland development and breast cancer remains to be elucidated.
To understand the role of Agr2 during post-natal mammary gland development we created mammary specific Agr2 knockout and overexpression mice. Consistent with low levels of Agr2 expression in virgin mice, Agr2 knockout virgin mice appear to undergo normal branching morphogenesis and elongation. However, Agr2 knockout pregnant mice (P12.5 and P17.5) have decreased lobuloalveolar mass in comparison to control animals, indicating a role for Agr2 in alveologenesis during pregnancy. In addition, we consistently observed a reduction in milk protein expression during late pregnancy and lactation in Agr2 knockout mice. Consistent with the pregnancy and lactational developmental defects in the Agr2 knockout mice, the rate of pup growth was significantly, although not dramatically, decreased after two weeks post-partum. One possible explanation for a moderate effect on pup growth is the short duration of weight monitoring experiments performed in this study. In addition, these moderate changes in weight could be due to the small litter sizes used in the experiments (5 pups each, the maximal litter size from knockout dams), and consequently greater overall amount of milk available per pup, providing nearly sufficient protein content for growth of pups from knockout dams. Also, other milk proteins and lipids may compensate for the decrease in casein and lactalbumin expression. For example, free fatty acid and triglyceride levels showed a trend toward increased levels in Agr2flox/floxMMTV-Cre mice, although this did not reach statistical significance (Supplemental Figure 4).
Interestingly, the gene expression pattern for milk proteins follows the expression pattern for Agr2 through pregnancy and lactation. This is especially the case for lactalbumin and Agr2, both of which are moderately expressed by mid-pregnancy, increase slightly by late-pregnancy, and increase sharply throughout lactation. Recently, it has been shown that AGR2 binds to nascent proteins and directs them to the endoplasmic reticulum lumen for folding and post-translational modifications (Higa et al., 2011). We speculate that the tremendous amount of new milk proteins synthesized during lactation and the concurrent up-regulation of Agr2 during lactation may be in response to newly synthesized proteins as a part of the unfolded protein response machinery.
Consistent with Agr2 function in cell proliferation during pregnancy, induced overexpression of Agr2 caused increased branching and precocious lobuloalveolar development in virgin mice. This result is suggestive of a pro-oncogenic function of Agr2 since increased branching is often linked to pro-tumorigenic pathways (Fata et al., 2004), possibly because the underlying mechanisms are partially shared with those causing pathological hyperplasia and invasiveness, features associated with pro-tumorigenic activity (Wang et al., 2007). Moreover, Agr2 overexpression mice exhibit significantly increased proliferation, as measured by both BrdU incorporation and phospho-histone H3 staining. However, it is interesting to note that no tumors were observed during the study (the longest doxycycline treatment experiment lasted six months), indicating that Agr2 overexpression alone is either insufficient for tumorigenesis, requires longer induction, or plays a more prominent role in tumor progression and metastasis. Consistent with the overexpression, hyper-proliferative phenotype in hAgr2+/MMTVrtTA+ mice, Agr2 knockout pregnant mice exhibit a significantly reduced number of mitotic cells (as measured by pH3 staining), indicating that Agr2 physiologically regulates mammary epithelial cell proliferation.
In conjunction with our in vivo studies, we found that Agr2 is a regulator of cell proliferation in vitro. We demonstrate that Agr2 overexpression is sufficient to stimulate proliferation in ER-negative MCF10A cells. In ER-positive MCF7 cells stimulated with estradiol, Agr2 expression as well as overall cell proliferation is increased. Importantly, the estradiol-mediated increase in proliferation depends on Agr2 expression since knockdown of Agr2 significantly diminishes this effect.
Although the mechanisms by which Agr2 is involved in regulating proliferation are currently unknown, two recent studies provide potential clues. In an in vitro system utilizing breast cancer cell lines, Vanderlaag and coworkers showed that CyclinD1 is downstream of Agr2 either directly or via positive regulation of ER-alpha (Vanderlaag et al., 2010). Most recently, utilizing esophageal and lung adenocarcinoma cell lines, Dong et al. showed that Agr2 affects cell proliferation and anchorage independent growth in adenocarcinomas by regulating the expression of amphiregulin (Areg), a mitogenic ligand of the Epidermal Growth Factor Receptor (EGFR) pathway (Dong et al., 2011). Both pathways implicated in these studies can be highly relevant to Agr2 induced proliferation in the normal mammary gland. For example, Sternlicht et al. has shown that Areg plays a prominent role during branching morphogenesis by activating EGFR signaling (Sternlicht et al., 2005). While we show no effect of Agr2 deletion on branching morphogenesis, the induction of Areg expression by Agr2 may be one possible mechanism by which Agr2 affects cell proliferation. We found Areg to be significantly down-regulated in the Agr2flox/floxMMTV-Cre mammary gland at pregnancy day 12.5, but not day 17.5, and modestly decreased during lactation (Supplemental Figure 5A). In contrast there was a significant increase of Areg expression in the mammary glands of hAgr2+/MMTVrtTA+ mice and when Agr2 is overexpressed in MCF10A cells (Supplemental Figure 5). However, knockdown of Agr2 expression in MCF7 cells showed no affect on Areg expression (Supplemental Figure 5B). Thus, Agr2 may in part influence cell proliferation by influencing Areg expression and downstream EGFR signaling.
Estradiol/estrogen plays a critical role in proliferation of mammary epithelial cells during ductal elongation and branching as well as during lobuloalveolar development in pregnancy (Bocchinfuso et al., 2000; Olsson et al., 1996; Shyamala et al., 2002; Zeps et al., 1998). Anti-estrogens such as tamoxifen inhibit proliferation in ER-positive breast cancers by inhibiting the binding of estrogen to its receptor, affirming the role of estrogen during physiological as well as pathological proliferation in the mammary gland (Dorssers et al., 2001). Agr2 expression is positively correlated with ER expression in breast cancer and Agr2 itself is an estradiol responsive gene (Innes et al., 2006). Our data suggests that some of the effects of ER on proliferation in mammary gland are mediated through Agr2.
A recent study utilized ChIP-Seq to identify differential ER binding events in both good and poor prognosis ER-positive tumors, as well as metastases derived from ER-positive tumors (Ross-Innes et al., 2012). Interrogating ER binding events reported in this study using a 20-kilobase window surrounding the Agr2 gene, an optimal size for searching for ER binding sites associated with target gene expression (Fullwood et al., 2009), we located ER binding events near Agr2 in only one of the eight ER-positive tumors associated with good prognosis, while four of the seven poor prognosis tumors and all three of the metastases had multiple ER binding events surrounding the gene. These observations are consistent with previous studies linking increased Agr2 expression to poor prognosis in ER-positive tumors (Barraclough et al., 2009; Hrstka et al., 2010; Innes et al., 2006), and suggest that Agr2 may be playing a more prominent role in difficult to treat ER-positive tumors that escape the current regimens of hormonal therapy. Interestingly, none of the ER-negative tumors used as controls in this study exhibited ER binding near the Agr2 gene, supporting previous experiments pointing to Agr2 as an ER-responsive gene.
We believe our study provides an important initial step in characterizing the role of Agr2 in normal mammary gland biology as a positive regulator of lobuloalveolar development in pregnancy and as a pro-proliferative factor in response to ER signaling. Furthermore, the knockout and overexpression models utilized in our study will serve as integral tools that will help elucidate the molecular mechanism by which Agr2 influences proliferation in both normal tissue homeostasis and cancer.
Supplementary Material
Supplemental Figure 4: Triglyceride and free fatty acid analysis in milk. Triglyceride (A) and free fatty acid (B) levels were measured in milk collected from nursing wild type (control) and Agr2flox/floxMMTV-Cre (Agr2 KO) mice. NS = no statistical significance as determined by a student’s t-test.
Supplemental Figure 5: Amphiregulin (Areg) expression in mouse and cell culture studies. (A) Expression of Areg in wild type and knockout mice during pregnancy (days 12.5 and 17.5), lactation (day 10), and in mice overexpressing Agr2 six months after induction of expression (Agr2 OE 6mo). Expression was normalized to Keratin 8 (K8). (B) Expression of Areg in the same in vitro studies described in Figure 7. * represents a p-value < 0.05. NS = not significant.
Supplemental Figure 6: Isotype controls of flow cytometry. FITC-conjugated mouse IgG isotype controls for detection of BrdU-positive cells by flow cytometry are shown for (A) MCF10A cells following siRNA knockdown of Agr2, (B) MCF10A cells overexpressing Agr2, and following siRNA knockdown of Agr2 in (C) MCF7 cells without estradiol, and (D) MCF7 cells with estradiol.
Supplemental Figure 1: Agr2 antibody specifically detects Agr2 protein. Specificity of the Agr2 antibody was determined by western blot analysis on Agr2 and Agr3 overexpression protein lysates (5μg each). Agr2 antibody detected AGR2 protein, but not AGR3 protein, while the Agr3 antibody detected AGR3, but not AGR2.
Supplemental Figure 2: Expression of MMTV-cre is localized to the mammary gland. MMTV-Cre expression in mammary gland was confirmed by interbreeding the MMTV-Cre line with the Rosa-26 line. The -gal staining of the R26R/MMTV-LTR-Cre bigenic line confirmed MMTV-Cre expression in mammary gland and not in heart and brain.
Supplemental Figure 3: Validation of in vitro cell proliferation assays by ATP luminescence assay. BrdU incorporation was validated by ATP luminescence assays for (A) MCF10A cells following siRNA knockdown of Agr2, (B) MCF10A cells overexpressing Agr2, and following siRNA knockdown of Agr2 in (C) MCF7 cells without estradiol, and (D) MCF7 cells with estradiol.
Supplemental Table 1: ANOVA table for repeated measures ANOVA with type III sum of squares on the pup growth data presented in Figure 3E.
Highlights.
Agr2, previously implicated in breast cancer, regulates mammary gland development.
Estrogen-induced Agr2 expression is developmentally regulated in mammary gland.
Over-expression induces epithelial cell proliferation in virgin mammary glands.
Over-expression induces precocious lobuloalveolar development in virgin mice.
Agr2 deletion impairs cell proliferation and alveologenesis during pregnancy.
Acknowledgements
This work is funded by grants from the NIH Biomedical Informatics Training Grant T15LM0744 to MLS, California Breast Cancer Research Program (CBCRP) Grant 16FB-0028 to SV, CBRCP Fellowship 14GB-0163 to MG, NIH Grant AR44882 to BA, and a generous donation by Matthew Bell to SML. We thank Dr. Lewis A. Chodosh from the University of Pennsylvania School of Medicine for providing us with MMTV-LTR-rtTA mice. We also thank the members of the Andersen laboratory for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberger F, et al. Anterior specification of embryonic ectoderm: the role of the Xenopus cement gland-specific gene XAG-2. Mech Dev. 1998;72:115–30. doi: 10.1016/s0925-4773(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Barraclough DL, et al. The metastasis-associated anterior gradient 2 protein is correlated with poor survival of breast cancer patients. Am J Pathol. 2009;175:1848–57. doi: 10.2353/ajpath.2009.090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso WP, et al. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology. 2000;141:2982–94. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- Brychtova V, et al. Anterior gradient 2: a novel player in tumor cell biology. Cancer Lett. 2011;304:1–7. doi: 10.1016/j.canlet.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Bu H, et al. The anterior gradient 2 (AGR2) gene is overexpressed in prostate cancer and may be useful as a urine sediment marker for prostate cancer detection. Prostate. 2011;71:575–87. doi: 10.1002/pros.21273. [DOI] [PubMed] [Google Scholar]
- Dong A, et al. The human adenocarcinoma-associated gene, AGR2, induces expression of amphiregulin through Hippo pathway co-activator YAP1 activation. J Biol Chem. 2011;286:18301–10. doi: 10.1074/jbc.M110.215707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorssers LC, et al. Tamoxifen resistance in breast cancer: elucidating mechanisms. Drugs. 2001;61:1721–33. doi: 10.2165/00003495-200161120-00004. [DOI] [PubMed] [Google Scholar]
- Dumartin L, et al. AGR2 Is a Novel Surface Antigen That Promotes the Dissemination of Pancreatic Cancer Cells through Regulation of Cathepsins B and D. Cancer Res. 2011;71:7091–7102. doi: 10.1158/0008-5472.CAN-11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, et al. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GC, et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br J Cancer. 2003;88:579–85. doi: 10.1038/sj.bjc.6600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche FR, et al. Expression of AGR2 in non small cell lung cancer. Histol Histopathol. 2007;22:703–8. doi: 10.14670/HH-22.703. [DOI] [PubMed] [Google Scholar]
- Fritzsche FR, et al. Prognostic relevance of AGR2 expression in breast cancer. Clin Cancer Res. 2006;12:1728–34. doi: 10.1158/1078-0432.CCR-05-2057. [DOI] [PubMed] [Google Scholar]
- Fullwood MJ, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth PA, et al. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994;91:9302–6. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther EJ, et al. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–92. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- Higa A, et al. Role of the pro-oncogenic Protein Disulfide Isomerase (PDI)-family member Anterior Gradient 2 (AGR2) in the control of Endoplasmic Reticulum homeostasis. J Biol Chem. 2011 doi: 10.1074/jbc.M111.275529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrstka R, et al. The pro-metastatic protein anterior gradient-2 predicts poor prognosis in tamoxifen-treated breast cancers. Oncogene. 2010;29:4838–47. doi: 10.1038/onc.2010.228. [DOI] [PubMed] [Google Scholar]
- Innes HE, et al. Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients. Br J Cancer. 2006;94:1057–65. doi: 10.1038/sj.bjc.6603065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner A, et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:10933–8. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, et al. Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Res. 2005;65:3796–805. doi: 10.1158/0008-5472.CAN-04-3823. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maresh EL, et al. Differential expression of anterior gradient gene AGR2 in prostate cancer. BMC Cancer. 2010;10:680. doi: 10.1186/1471-2407-10-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslon MM, et al. A Divergent Substrate-Binding Loop within the Pro-oncogenic Protein Anterior Gradient-2 Forms a Docking Site for Reptin. J Mol Biol. 2010;404:418–38. doi: 10.1016/j.jmb.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Munoz B, Bolander FF., Jr. Prolactin regulation of mouse mammary tumor virus (MMTV) expression in normal mouse mammary epithelium. Mol Cell Endocrinol. 1989;62:23–9. doi: 10.1016/0303-7207(89)90109-3. [DOI] [PubMed] [Google Scholar]
- Olsson H, et al. Proliferation of the breast epithelium in relation to menstrual cycle phase, hormonal use, and reproductive factors. Breast Cancer Res Treat. 1996;40:187–96. doi: 10.1007/BF01806214. [DOI] [PubMed] [Google Scholar]
- Park K, et al. AGR2, a mucinous ovarian cancer marker, promotes cell proliferation and migration. Exp Mol Med. 2011;43:91–100. doi: 10.3858/emm.2011.43.2.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A. 2009;106:6950–5. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, et al. Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36:734–40. doi: 10.1016/j.ympev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Pizzi M, et al. Anterior gradient 2 overexpression in lung adenocarcinoma. Appl Immunohistochem Mol Morphol. 2012;20:31–6. doi: 10.1097/PAI.0b013e3182233f9f. [DOI] [PubMed] [Google Scholar]
- Pohler E, et al. The Barrett’s antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol Cell Proteomics. 2004;3:534–47. doi: 10.1074/mcp.M300089-MCP200. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, et al. Anterior gradient 2 is expressed and secreted during the development of pancreatic cancer and promotes cancer cell survival. Cancer Res. 2008;68:7811–8. doi: 10.1158/0008-5472.CAN-08-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riener MO, et al. Prognostic significance of AGR2 in pancreatic ductal adenocarcinoma. Histol Histopathol. 2009;24:1121–8. doi: 10.14670/HH-24.1121. [DOI] [PubMed] [Google Scholar]
- Ross-Innes CS, et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481:389–93. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala G, et al. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. 2002;80:137–48. doi: 10.1016/s0960-0760(01)00182-0. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, et al. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–33. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem Biophys Res Commun. 1998;251:111–6. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- Vanderlaag KE, et al. Anterior gradient-2 plays a critical role in breast cancer cell growth and survival by modulating cyclin D1, estrogen receptor-alpha and survivin. Breast Cancer Res. 2010;12:R32. doi: 10.1186/bcr2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekanandan P, et al. Anterior gradient-2 is overexpressed by fibrolamellar carcinomas. Hum Pathol. 2009;40:293–9. doi: 10.1016/j.humpath.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, et al. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 2001;10:545–53. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- Wagner KU, et al. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–30. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, et al. The LIM-only factor LMO4 regulates expression of the BMP7 gene through an HDAC2-dependent mechanism, and controls cell proliferation and apoptosis of mammary epithelial cells. Oncogene. 2007;26:6431–41. doi: 10.1038/sj.onc.1210465. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68:492–7. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- Whisenhunt TR, et al. Disrupting the enzyme complex regulating O-GlcNAcylation blocks signaling and development. Glycobiology. 2006;16:551–63. doi: 10.1093/glycob/cwj096. [DOI] [PubMed] [Google Scholar]
- Wu ZS, et al. [Expression of a novel metastasis-inducing protein human anterior gradient-2 (AGR2) in breast cancer and its clinical and prognostic significance] Zhonghua Bing Li Xue Za Zhi. 2008;37:109–13. [PubMed] [Google Scholar]
- Zeps N, et al. Estrogen receptor-negative epithelial cells in mouse mammary gland development and growth. Differentiation. 1998;62:221–6. doi: 10.1046/j.1432-0436.1998.6250221.x. [DOI] [PubMed] [Google Scholar]
- Zhang JS, et al. AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes Chromosomes Cancer. 2005;43:249–59. doi: 10.1002/gcc.20188. [DOI] [PubMed] [Google Scholar]
- Zhao F, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol. 2010;338:270–9. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 4: Triglyceride and free fatty acid analysis in milk. Triglyceride (A) and free fatty acid (B) levels were measured in milk collected from nursing wild type (control) and Agr2flox/floxMMTV-Cre (Agr2 KO) mice. NS = no statistical significance as determined by a student’s t-test.
Supplemental Figure 5: Amphiregulin (Areg) expression in mouse and cell culture studies. (A) Expression of Areg in wild type and knockout mice during pregnancy (days 12.5 and 17.5), lactation (day 10), and in mice overexpressing Agr2 six months after induction of expression (Agr2 OE 6mo). Expression was normalized to Keratin 8 (K8). (B) Expression of Areg in the same in vitro studies described in Figure 7. * represents a p-value < 0.05. NS = not significant.
Supplemental Figure 6: Isotype controls of flow cytometry. FITC-conjugated mouse IgG isotype controls for detection of BrdU-positive cells by flow cytometry are shown for (A) MCF10A cells following siRNA knockdown of Agr2, (B) MCF10A cells overexpressing Agr2, and following siRNA knockdown of Agr2 in (C) MCF7 cells without estradiol, and (D) MCF7 cells with estradiol.
Supplemental Figure 1: Agr2 antibody specifically detects Agr2 protein. Specificity of the Agr2 antibody was determined by western blot analysis on Agr2 and Agr3 overexpression protein lysates (5μg each). Agr2 antibody detected AGR2 protein, but not AGR3 protein, while the Agr3 antibody detected AGR3, but not AGR2.
Supplemental Figure 2: Expression of MMTV-cre is localized to the mammary gland. MMTV-Cre expression in mammary gland was confirmed by interbreeding the MMTV-Cre line with the Rosa-26 line. The -gal staining of the R26R/MMTV-LTR-Cre bigenic line confirmed MMTV-Cre expression in mammary gland and not in heart and brain.
Supplemental Figure 3: Validation of in vitro cell proliferation assays by ATP luminescence assay. BrdU incorporation was validated by ATP luminescence assays for (A) MCF10A cells following siRNA knockdown of Agr2, (B) MCF10A cells overexpressing Agr2, and following siRNA knockdown of Agr2 in (C) MCF7 cells without estradiol, and (D) MCF7 cells with estradiol.
Supplemental Table 1: ANOVA table for repeated measures ANOVA with type III sum of squares on the pup growth data presented in Figure 3E.