Abstract
Objectives
Describe the association of internal anal sphincter (IAS) relaxation with colonic high amplitude peristaltic contractions (HAPCs).
Methods
Retrospective review of colon manometry tracings of children with constipation to determine the IAS relaxation characteristics associated to HAPC’s (HAPC-IASR) events and compare them to the those seen during the performance of the anorectal manometry (ARM-RAIR) events.
Results
A total of 70 HAPC- IASRs were observed in 15 patients, 65 after bisacodyl, 2 during fasting and 3 after a meal. In 64% of events the IAS relaxation started when the HAPC reached left colon and in 36% as proximal as the hepatic flexure. HAPC propagation seems to be important in HAPC-IASR characteristics; those propagating distal to sigmoid colon demonstrated larger and longer IAS relaxation as well as lower residual pressure but equivalent resting pressure compared to HAPC’s ending proximal to sigmoid colon. While IAS resting pressure was comparable for ARM-RAIRs and HAPC-IASRs, the duration and magnitude of anal relaxation was higher and the anal residual pressure was lower in HAPC-IASRs.
Conclusions
We demonstrated that IAS relaxation in constipated children is associated with HAPCs migrating in the proximal and distal colon; in most cases starting when peristalsis is migrating through left colon and in an important proportion while migrating proximally. We also demonstrated that HAPC-IASRs are different from ARM-RAIRs suggesting a neurally mediated reflex. Lastly, the IAS relaxation characteristics are highly dependent on the degree of propagation of HAPCs, which could have important implications in the understanding of defecation disorders.
INTRODUCTION
Normal defecation is the result of a complex interaction between muscle, nerves and central nervous system. For defecation to occur there needs to be an aboral mass movement of colonic contents associated with internal anal sphincter (IAS) relaxation. These mass movements in the colon have been shown to result from high amplitude propagating contractions (HAPC’s) (1, 2). However their relation to distal IAS relaxation is poorly understood. Limited studies in adults initially by Kamm et. al. (1, 3) and later replicated by Malcolm et. al.(2), reported a probable colo-anal reflex in which IAS relaxation can be induced by and/or be associated to the presence of HAPC’s in the left colon. There are no studies that have determined the relation between right sided colonic motility and IAS relaxation, or if this relaxation occurs in patients in whom HAPC’s do not travel normally in the colon. Furthermore the colo-anal reflex has never been described in children. The main aim of the present study was to describe the relationship between IAS relaxations and HAPC’s in children undergoing colonic motility studies for the evaluation of defecation abnormalities. We also sought out to compare the characteristics between IAS relaxations associated with HAPCs, (HAPC-IASR events) and those IAS relaxations elicited by balloon distention (recto-anal inhibitory responses; RAIR) during anorectal manometry (ARM-RAIR events).
METHODS
This is a retrospective study in which the colonic manometry tracings of children referred for evaluation of intractable constipation to the Center for Motility and Gastrointestinal Disorders at Children’s Hospital in Boston were reviewed. Only those tracings in which the IAS was captured during the colonic motility study were included.
Institutional review board approval was obtained for this retrospective review of the tracings.
Procedures
Colonic motility
Colonic motility (CM) studies were performed as previously reported.(4) All patients underwent a bowel cleanout with electrolyte solution the day before the colonoscopy and an enema on the morning of the catheter placement. A catheter with eight ports with longitudinal staggered sensors spaced by 10–15 cm (according to patient’s size) was utilized, with the most proximal port placed in the cecum and the last in the IAS. The motility catheter was placed during colonoscopy while the children were under anesthesia. The colonic motility study was performed the next day to let the anesthesia wear off. All patients underwent an abdominal radiography the day of the motility study to ascertain correct catheter placement. The motility study consisted in 60 minutes of fasting, followed by a high caloric meal, and a subsequent recording of another 60 minutes to evaluate the post-prandial period. After that patients received crushed bisacodyl 0.25 mg/kg into the right side of the colon via the manometry catheter and recording continued for another 60 minutes. The CM was performed with a continuously non-complaint perfused catheter (Medical Measurement Systems, New Hampshire, US). High-amplitude-propagating contractions (HAPCs) were defined by increase in intraluminal pressure of more than 60 mmHg that did not overlap with other contractions, lasting more than 10 seconds and less than 30 seconds and propagation aborally across 30 cm or more. Low amplitude peristaltic contractions (LAPCs) were defined as those with same characteristics as HAPC’s but with amplitude lower than 60mmHg.(5–9)
Anorectal manometry
All patients also underwent an anorectal manometry (ARM) before undergoing anesthesia for the colon motility catheter placement. The anorectal manometry (ARM) was performed without sedation in those cooperating with the test. In children with anxiety or uncooperative to the study, sedation with oral midazolam at 0.5 mg/k up to a maximum dose of 20mg, or 0.1 mg/kg IV midazolam were used.
Both manometric studies were performed with a continuously perfused catheter using a low compliance pneumo hydraulic system. The manometric catheter was made from polyvinyl tubing and contained 4 recording ports separated by 1 cm at the distal end and staggered at a 90° angle. A latex balloon is attached to the end of the anorectal catheter and its port is connected to the recording system to register the exact time of balloon distention. All baseline measurements were set at atmospheric pressure. Initially a slow pull through was performed until the area of high pressure zone was identified. The balloon was then rapidly inflated with serial volumes that started from to 10 ml and increased by 10 ml up to at least 60 cc. or until maximum IAS relaxation was observed. IAS relaxation was defined as a decrease in the IAS resting pressure of at least 10% (10).
Data collection and analysis
Colon manometry
The location of the catheter, the quality of propagation of the HAPC’s (partially propagated defined as HAPC’s stopping proximal to the sigmoid colon and fully propagated when reaching the rectosigmoid colon), and the IAS relaxation characteristics during the occurrence of an HAPC (HAPC-IASR event) were obtained. The IAS relaxation characteristics included the IAS resting pressure (RP) in mmHg, residual pressure during the relaxation in mmHg, percent of relaxation from baseline to nadir and duration of relaxation in seconds. We also recorded the colonic segment during which the HAPC was associated with the initiation of the IAS relaxation.
Anorectal manometry
The following characteristics of the rectoanal inhibitory reflex (RAIR) after each balloon distention (ARM-RAIR events) were obtained: IAS resting pressure (RP) in mmHg, residual pressure during the relaxation in mmHg, percent of relaxation from baseline to nadir and duration of relaxation in seconds.
To compare the IAS relaxation characteristics of the ARM-RAIR events with those observed with HAPC-IASR events, we used the measurements obtained after maximum IAS relaxation elicited during ARM with balloon distention.
Statistical analysis
We compared IAS relaxation characteristics between ARM-RAIR and HAPC-IASR events, between HAPC-IASR events associated to partially and fully propagated HAPCs and also between bisacodyl and meal-induced HAPC-IASR events.
Continuous variables are presented as means and standard deviation and medians and range, comparisons were performed using the paired-tests after logarithmic transformation and non-parametric testing (related samples Wilcoxon signed rank test). Proportions were compared using X2.
RESULTS
15 patients were included. Their mean age was 9.9 ± 4.5 years and 10 (66%) were female. From those 15 patients, 13 had idiopathic constipation, 1 had constipation in the setting of controlled celiac disease and one patient had a thoracic myelomeningocele with fecal incontinence.
Colon manometry
The most proximal port of the catheter was located in the right colon (cecum/ascending colon) in 12 patients and at the hepatic flexure in 3 and the most distal port was located in the IAS in all. A total of 70 HAPC- IASR events were noticed in those 15 patients. Sixty five occurred after bisacodyl, 2 occurred spontaneously during fasting, and 3 occurred after the meal.
Bisacodyl induced HAPC-IASR events
From the 65 HAPCs-IASRs 58 started in the right colon (cecum, ascending or hepatic flexure), 3 in the transverse colon and 4 in the left colon (distal to the splenic flexure); 18 HAPCs were fully propagated to the sigmoid colon (See Figure 1) and 47 were partially propagated (See Figure 2) proximal to the sigmoid colon. (See Table 1) IAS relaxation started in 45 (64.3%) events when the HAPC reached left colon. However in 25 (35.7%) we noticed IAS relaxation when HAPC’s were migrating as proximal as the hepatic flexure. (See Figure 3) We also noticed IAS relaxations associated to fully propagated LAPC’s, non-transmitted contractions (See Figure 4) and LAPC’s migrating only on left colon sparing the right and transverse colon. (See Figure 5) In all cases the IAS relaxation persisted until the HAPC’s migrated distally and reached the rectosigmoid.
Figure 1.
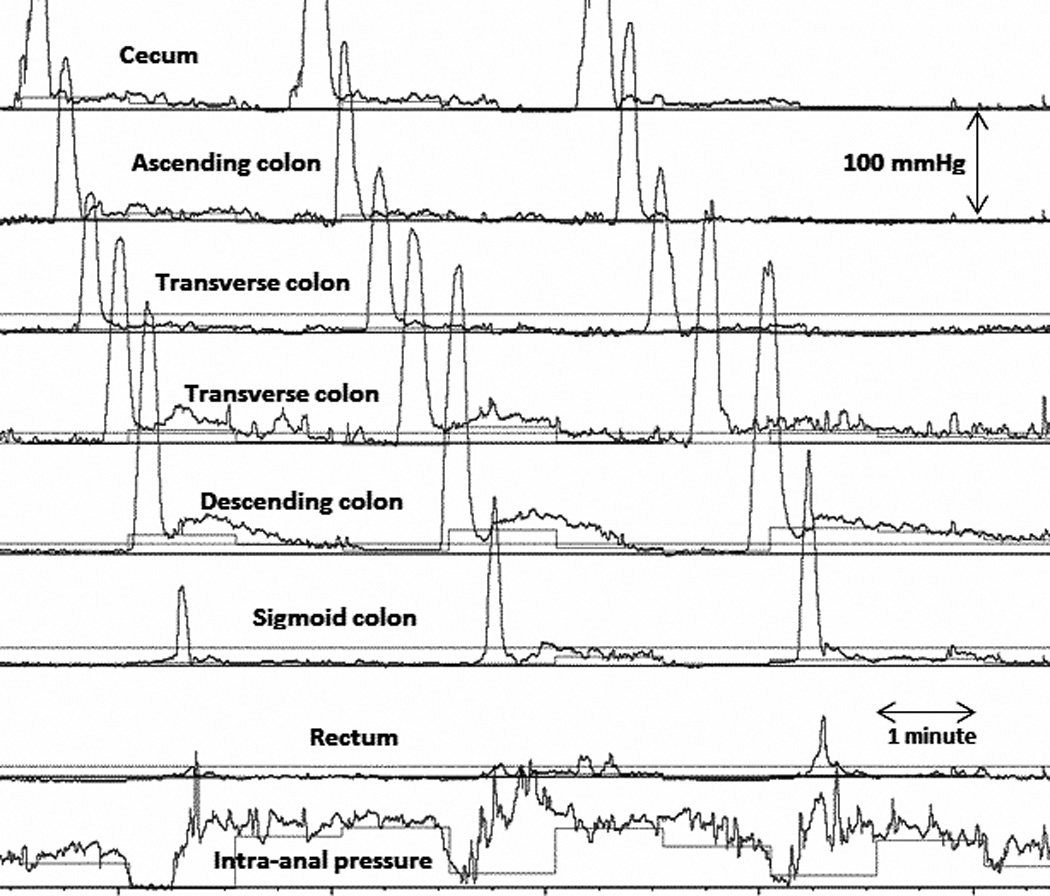
HAPC-IASR events associated to fully propagated bisacodyl-induced HAPC. Note the IAS relaxation starts when the HAPC is migrating through the left colon.
Figure 2.
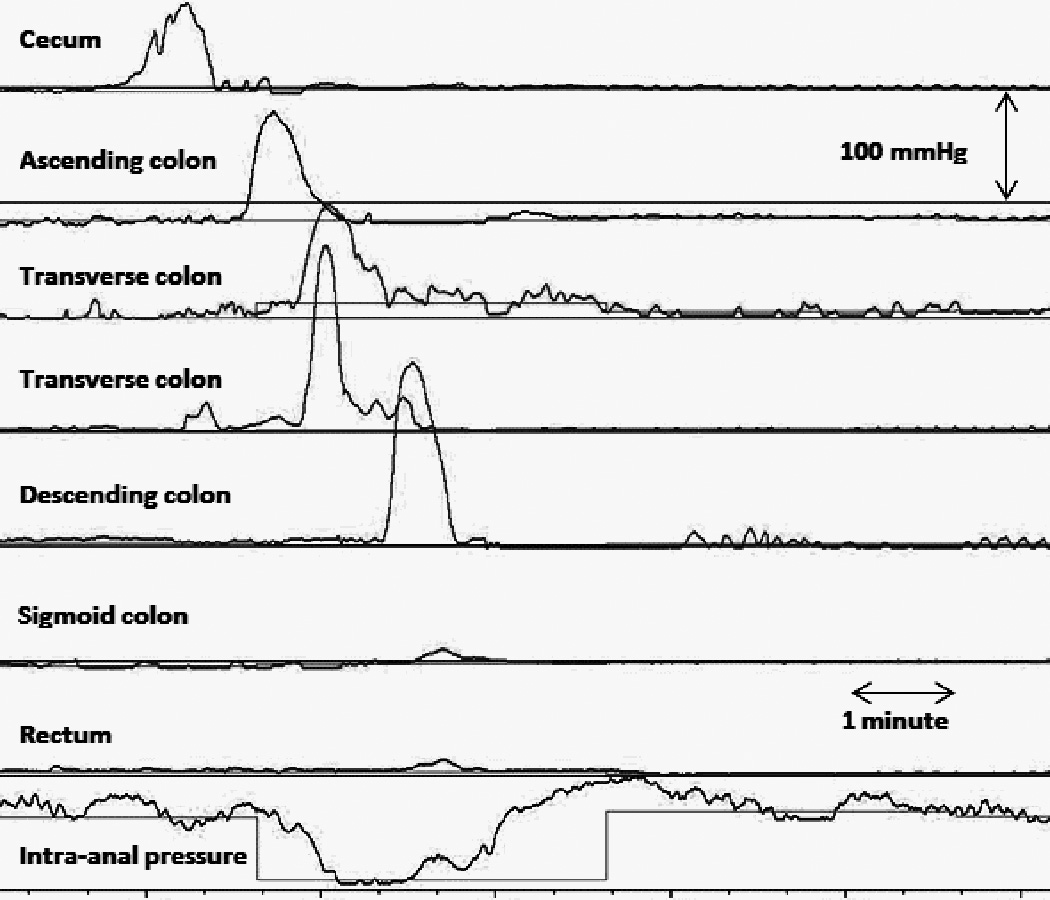
HAPC-IASR events associated to partially propagated bisacodyl-induced HAPC. Please note there is internal anal sphincter relaxation despite lack of propagation of the HAPC through the sigmoid colon.
Table 1.
Quality of the HAPC’s.
| HAPC Quality | Frequency | Percent |
|---|---|---|
| Fully propagated to rectosigmoid colon | 23 | 32.9 |
| Partially propagated in right colon then simultaneous in left colon | 31 | 44.3 |
| Partially propagated in left colon then becoming non-transmitted | 1 | 1.4 |
| Partially propagated in right then non-transmitted in left colon | 13 | 18.6 |
| Partially propagated in left colon then becoming simultaneous | 2 | 2.9 |
| Total | 70 | 100.0 |
Figure 3.
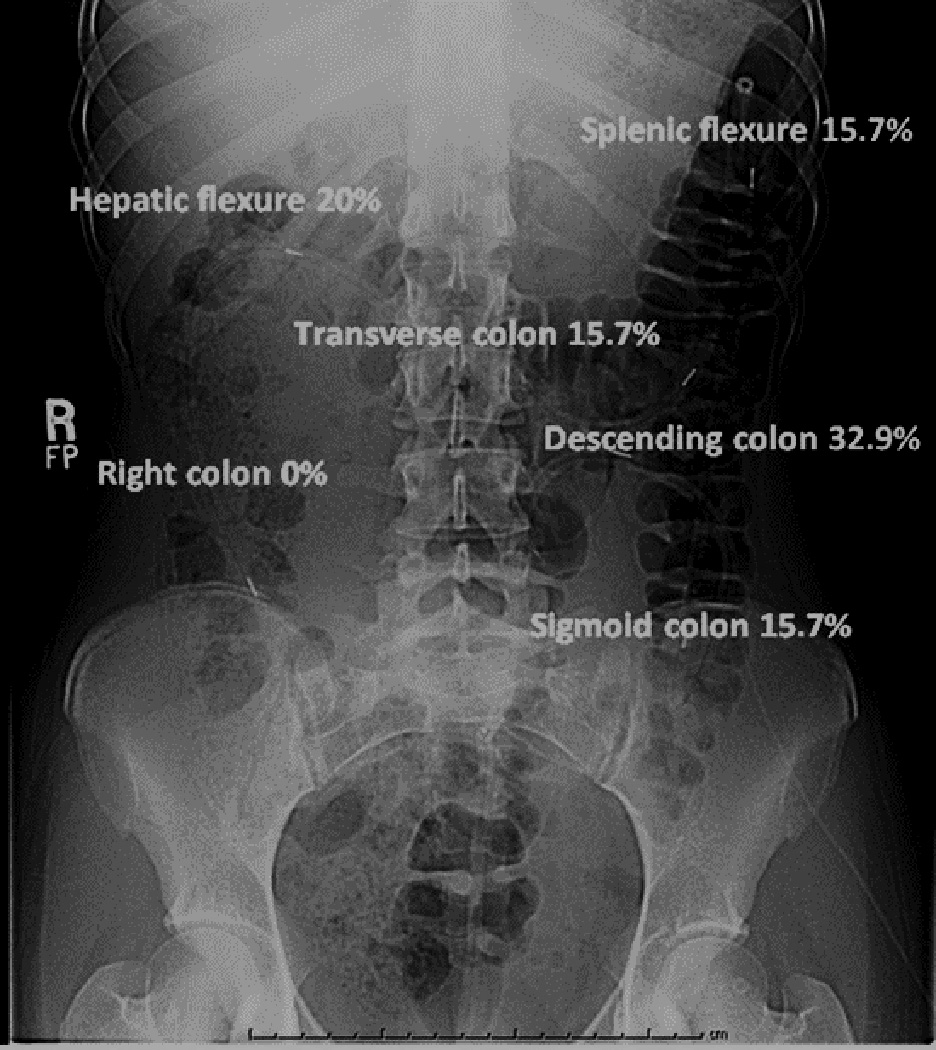
Colonic segment of HAPC migration at the initiation of the IAS relaxation. This plain abdominal film demonstrates the typical position of the colon motility catheter and the colonic segment where the HAPC is migrating at the initiation of the IAS relaxation.
Figure 4.
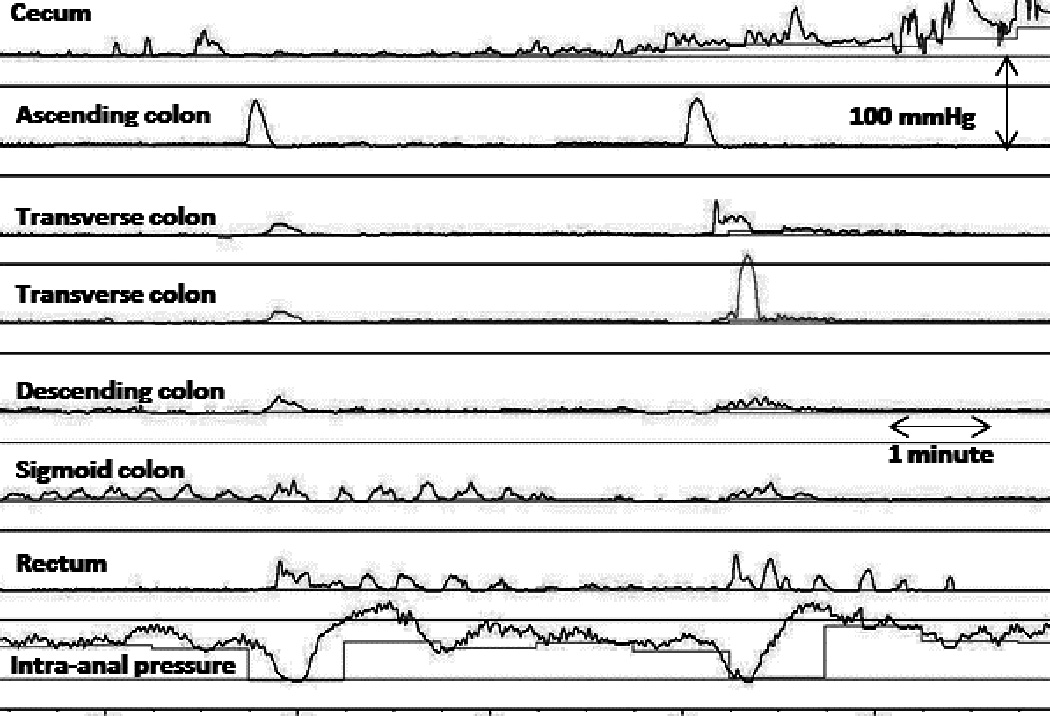
IAS relaxation associated to propagated LAPC’s and non-transmitted contractions. Note IAS relaxation occurred both with transmitted as well non-transmitted contractions through left colon.
Figure 5.
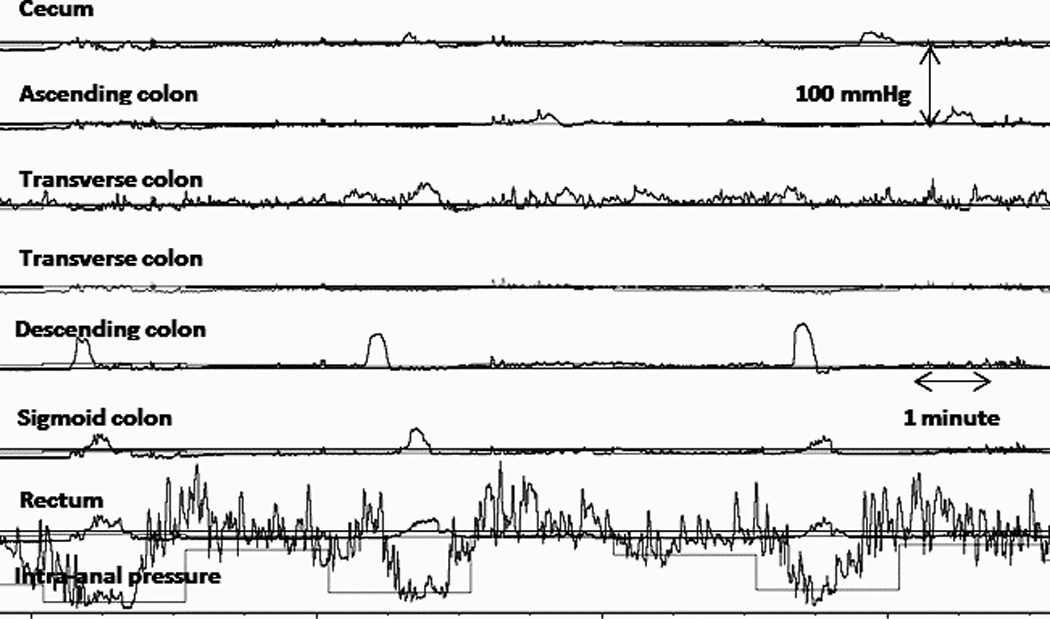
IAS relaxation associated to distally propagating LAPC’s. Note the IAS relaxation started when the HAPC reached the left colon even when there was no migration through right colon.
Fasting and meal induced HAPC-IASR events
Two occurred during fasting: one starting in hepatic flexure and other in the proximal sigmoid colon and both ending in the distal sigmoid colon, both on same patient who had no other HAPC-IASR events. These 2 events occurred in a single patient. Three HAPC-IASRs occurred after a meal: one starting in the cecum, another in the transverse colon and the other in the descending colon and all 3 ended in the distal sigmoid colon, all on same patient who also had 4 bisacodyl induced HAPC-IASR events.
As can be seen in Table 2, we found a significant difference in the mean percent and duration of IAS relaxation as well as the IAS residual pressure in the bisacodyl induced HAPC-IASR events between those associated with partially vs. fully propagated HAPC’s, but no difference between the IAS resting pressure. We also compared the HAPC-IASRs events between bisacodyl and meal induced HAPC’s. We found that bisacodyl-induced HAPC-IASR events had a lower IAS resting pressure, lower residual pressure and higher percent of relaxation but no difference on the duration of relaxation. (See Table 3) Since the only 3 meal induced HAPC’s noticed during the study corresponded to the same patient, we then compared those 3 meal induced with the 4 bisacodyl induced HAPC-IASRs characteristics recorded from the same patient and we found no difference on any parameter. (Data not shown).
Table 2.
Comparison of IAS relaxation characteristics between bisacodyl-induced HAPC-IASR events associated to fully and partially propagated HAPC’s
| IAS resting pressure (mmHg) |
IAS residual pressure (mmHg) |
IAS relaxation (%) |
IAS relaxation duration (seconds) |
|
|---|---|---|---|---|
| HAPC quality | Median (range) | Median (range) | Median (range) | Median (range) |
| Fully propagated (n=23) |
64 (30–90) | 0 (0–49) | 100 (46–100) | 50 (19–165) |
| Partially propagated (n=47) |
49 (21–95) | 5.5 (0–70) | 86 (18–100) | 34.5 (12–192) |
| P value | 0.057 | 0.001 | 0.001 | 0.009 |
Table 3.
Comparison of IAS relaxation characteristics between meal and bisacodyl-induced HAPC-IASR events
| IAS resting pressure (mmHg) |
IAS residual pressure (mmHg) |
IAS relaxation (%) |
IAS relaxation duration (seconds) |
|
|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | Median (range) | |
| Meal-induced HAPC (n=3) |
90 (83–90) | 22 (21–22) | 76 (74–76) | 40 (25–84) |
| Bisacodyl-induced HAPC (n=65) |
54 (21–95) | 0 (0–70) | 100 (18–100) | 39 (12–192) |
| P value | 0.01 | 0.01 | 0.04 | 0.83 |
Excluding the colonic motility findings of the 2 patients with non-idiopathic conditions (myelomeningocele or controlled celiac disease) does not alter any of the results.
Anorectal manometry
The procedure was performed without any sedation in 8 patients and with midazolam in 7 ( oral in 5 and intravenous midazolam in 2). We only found a difference on the median IAS resting pressure between sedated and un-sedated patients (62.5 range 36–101mmHg vs. 50 range 36–70mmHg respectively, p=0.03) but no difference on the rest of the ARM parameters.
Comparison between anorectal manometry and colonic manometry
One patient underwent a sphinctermyectomy 1 year before the study so his tracing was included in the descriptive report of HAPC-IASR events (this patient had the only 2 spontaneous HAPC-IASR events during fasting included in this report) but was not included in the comparison between the HAPC-IASR and ARM-RAIR events.
IAS resting pressures prior to HAPC-IASR and during ARM-RAIR events were not significantly different. Also, we did not find a significant effect of sedation on the IAS characteristics between HAPC-IASR’s and ARM-RAIR’s. We found a significant difference in the residual pressure, median percent and duration of IAS relaxation with the HAPC-IASR events showing larger and more prolonged relaxation when compared with the ARM-RAIR events. (See Table 4) Excluding ARM-RAIR events recorded under sedation does not alter any of the results.
Table 4.
Comparison of IAS relaxation characteristics between bisacodyl-induced HAPC-IASR and ARM-RAIR events
| IAS resting pressure (mmHg) |
IAS residual pressure (mmHg) |
IAS relaxation (%) |
IAS relaxation duration (seconds) |
|
|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | Median (range) | |
| HAPC-RAIR (n=70) |
54 (21–95) | 0 (0–70) | 100 (18–100) | 39 (12–192) |
| ARM-RAIR (n=45) |
54 (36–101) | 25 (7–45) | 57 (22–87) | 8 (1–21) |
| P value | 0.280 | <0.001 | <0.001 | <0.001 |
DISCUSSION
We have shown that the colon has a characteristic pattern of reflex relaxation of the IAS associated to the presence of HAPC’s in the proximal colon, akin to esophageal peristaltic events during swallowing. This is an interesting and novel finding of colorectal physiology that may be important in the understanding of normal and abnormal defecation. This reflex has never been shown in children, and has been described in very limited samples of healthy and constipated adults associated to HAPC’s only in the distal colon.
We have been able to demonstrate that even though most of the HAPC-IASR events (64.3%) are elicited when the HAPC was progressing through the left colon as previously described,(2, 3) a third occurred while the HAPCs were still in the right colon. We documented that IAS relaxation started with the HAPC migrating through the hepatic flexure and the transverse colon in 20% and 15.7% of cases, respectively. We also noticed IAS relaxations associated with non-transmitted HAPC’s as well as LAPC’s. Previous reports in adults and healthy volunteers have described IAS relaxation related to left sided HAPC’s only (2, 3) and with amplitude >75mmHg, so our findings are important as they suggest this reflex may start when the HAPC’s originate proximal to the splenic flexure or when there are LAPCs. The use of water perfusion system and catheters with 8 ports may have limitations since current evidence reports solid state catheters are more sensitive in detecting contractions than water perfusion systems(11) and increasing the number of sensors identifies contraction profiles not previously identified.(12, 13) Another potential explanation to the observed IAS relaxations not associated to HAPC’s is pre-defecatory augmentation (including propagated as well as not propagated colonic activity up to an hour prior to spontaneous defecation) reported by Dinning and colleagues.(14)
It was interesting that the percent and duration of IAS relaxation were significantly larger in HAPC-IASRs as compared with ARM-RAIRs, suggesting that this colo-anal reflex may have physiologic importance. This may support the importance of the HAPC-IASRs events in defecation by allowing more transit of stools through the anal canal compared to the shorter IAS relaxation seen with the ARM even after distention with larger balloon volume, possibly more related to the inflation reflex. Furthermore the fact that the HAPC’s that propagate to the rectum are associated with higher IAS percent and duration of relaxation compared to those that are only partially propagated may support the importance of HAPC’s in eliciting IAS relaxation to aid in normal defecation and may in part explain the constipation that is noted in otherwise healthy volunteers with only partially propagated HAPC’s.
The exact mechanism by which this IAS relaxation occurs is not known. Our findings and those from Malcolm et al(2) document that IAS relaxations associated with HAPCs are more closely associated with the onset of the HAPC and not with the arrival of the HAPC to the rectum, suggesting a neurally mediated reflex is at play, rather than a phenomenon associated with rectal distention, as is noted with the RAIR. One of the patients included in this study had a thoracic myelomeningocele and the reflex was no different from other neurologically intact patients, supporting the notion that this reflex is generated by intramural nerves. More studies in well-defined population of patients with different levels of spinal cord lesions are obviously needed to truly understand this relationship. Previous studies have also shown that these HAPC-IASRs were not modulated by alpha2 adrenergic mechanisms (2). We also found that the degree and duration of IAS relaxation were larger in the HAPC-IASR events than those elicited by maximum balloon rectal distention during the ARM, again suggesting a neurally mediated reflex. In fact previous studies of the RAIR in both humans (15) and rats (16), have shown that the segment of rectum that is distended seems to be important in the quality of the RAIR. A larger response is noted when distention is elicited closer to the anal canal, further supporting a neurally mediated HAPC-IASR theory.
Another potential explanation for the presence of HAPC-IASRs is that the HAPC is inducing air and/or stool transit into the rectosigmoid colon causing distention and subsequently a RAIR. We found that HAPC’s that propagate to the rectum are associated with higher IAS relaxations compared to those that are partially propagated, suggesting that this may be a possibility. However all our patients as in previous studies(2) underwent a complete bowel cleanout with an electrolyte solution 24 hours before the performance of the colonoscopy for the motility catheter placement. Therefore we find it unlikely that the migration of stool contents distally in the colon results in rectal distention and a secondary IAS relaxation. In a previous study by Kamm et al in which left sided colonic manometry and scintigraphy to evaluate colonic transit were simultaneously performed it was shown that the presence of these HAPC-IASRs occurred at the onset of the HAPCs, and before colonic contents reached the rectum.(1)
It is important to mention that this study is limited by its retrospective nature. Additionally the IAS port was not measured with a sleeve so it is possible that some of the observed relaxations could have been the result of catheter movement. To control for the latter, the catheter was secured to the perianal area, and the initial position of the catheter in the IAS was documented by an abdominal radiograph. Furthermore the IAS in the tracing demonstrated a clear relaxation that would return to baseline after the HAPC occurring in multiple occasions within each patient also confirms the relaxations observed were related to sphincter relaxation.
An important limitation is the fact that we studied only children with severe constipation referred for colonic manometry. However our findings are similar to those obtained in healthy adult volunteers. Additionally, by studying constipated patients we have also shown that the IAS relaxation also occurs in those patients with HAPC’s that are not transmitted to the sigmoid, a finding that may explain why many patients with sigmoid dysmotility still respond to medical therapy.
Another potential limitation is that most of the HAPC’s were induced by the administration of bisacodyl into the right colon, unlike in the study by Malcolm et. al.(2) However, in previous studies it has been shown that the administration of bisacodyl is a good marker of neuro-enteric integrity and it has been used to induce HAPC’s and shorten study duration.(17) Although the exact mechanism of action of bisacodyl has not been fully elucidated, bisacodyl has been reported to increase myoelectrical propagating activity, (18) transit in the ascending colon(19) and rectal tone(20) in humans. It has also been shown to decrease expression of aquaporin-3 resulting in inhibition of water passage from lumen to vascular space in rats, producing diarrhea.(21) There is still contradicting data on the bisacodyl effect towards inhibiting(22) or inducing(23) nitric oxide synthase in rats. Although we found a significant difference in the RAIR characteristics between meal and bisacodyl induced HAPC-IASR when all patients were combined, our sample size for meal induced HAPC-IASR was very limited. More importantly there was no difference in any of the parameters within the same patients in which both meal induced and bisacodyl induced HAPC-IASR occurred.
In conclusion we are describing in children the presence of a colo-anal reflex in which the presence of HAPC’s are associated with IAS relaxations. This reflex seems to be neurally mediated. Even though its physiologic and pathophysiologic importance needs to be studied further, it may have important implications for the understanding of defecation and colonic motility disorders.
ACKNOWLEDGEMENTS
Financial support: Dr Nurko is supported by NIH grant K24DK082792A and Dr Siddiqui was supported by Schneider Charitable Trust Research Fellowship; Spina Bifida Association
Footnotes
Guarantor of the article: Leonel Rodriguez MD, MS
Author contributions:
Conception and design of the study: Dr Rodriguez and Dr Nurko
Analysis and interpretation of the data: Dr Rodriguez, Dr Siddiqui and Dr Nurko
Drafting of the article: Dr Rodriguez, Dr Siddiqui and Dr Nurko
Critical revision of the article: Dr Rodriguez, Dr Siddiqui and Dr Nurko
Final approval of the article: Dr Rodriguez, Dr Siddiqui and Dr Nurko
Disclosures: “None.”
REFERENCES
- 1.Kamm MA, van der Sijp JR, Lennard-Jones JE. Observations on the characteristics of stimulated defaecation in severe idiopathic constipation. Int J Colorectal Dis. 1992;7:197–201. doi: 10.1007/BF00341220. [DOI] [PubMed] [Google Scholar]
- 2.Malcolm A, Camilleri M. Coloanal motor coordination in association with high-amplitude colonic contractions after pharmacological stimulation. Am J Gastroenterol. 2000;95:715–719. doi: 10.1111/j.1572-0241.2000.01840.x. [DOI] [PubMed] [Google Scholar]
- 3.Kamm MA, van der Sijp JR, Lennard-Jones JE. Colorectal and anal motility during defaecation. Lancet. 1992;339:820. doi: 10.1016/0140-6736(92)91957-a. [DOI] [PubMed] [Google Scholar]
- 4.Di Lorenzo C, Flores AF, Reddy SN, et al. Use of colonic manometry to differentiate causes of intractable constipation in children. J Pediatr. 1992;120:690–695. doi: 10.1016/s0022-3476(05)80229-x. [DOI] [PubMed] [Google Scholar]
- 5.Narducci F, Bassotti G, Gaburri M, et al. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987;28:17–25. doi: 10.1136/gut.28.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassotti G, Gaburri M. Manometric investigation of high-amplitude propagated contractile activity of the human colon. Am J Physiol. 1988;255:G660–G664. doi: 10.1152/ajpgi.1988.255.5.G660. [DOI] [PubMed] [Google Scholar]
- 7.Bassotti G, Clementi M, Antonelli E, et al. Low-amplitude propagated contractile waves: a relevant propulsive mechanism of human colon. Dig Liver Dis. 2001;33:36–40. doi: 10.1016/s1590-8658(01)80133-x. [DOI] [PubMed] [Google Scholar]
- 8.Bassotti G, Chistolini F, Nzepa FS, et al. Colonic propulsive impairment in intractable slow-transit constipation. Arch Surg. 2003;138:1302–1304. doi: 10.1001/archsurg.138.12.1302. [DOI] [PubMed] [Google Scholar]
- 9.Bassotti G, Chistolini F, Marinozzi G, et al. Abnormal colonic propagated activity in patients with slow transit constipation and constipation-predominant irritable bowel syndrome. Digestion. 2003;68:178–183. doi: 10.1159/000075554. [DOI] [PubMed] [Google Scholar]
- 10.Chumpitazi BP, Fishman SJ, Nurko S. Long-term clinical outcome after botulinum toxin injection in children with nonrelaxing internal anal sphincter. Am J Gastroenterol. 2009;104:976–983. doi: 10.1038/ajg.2008.110. [DOI] [PubMed] [Google Scholar]
- 11.Liem O, Burgers RE, Connor FL, et al. Solid-state vs water-perfused catheters to measure colonic high-amplitude propagating contractions. Neurogastroenterol Motil. 2012;24 doi: 10.1111/j.1365-2982.2011.01870.x. 345-e167. [DOI] [PubMed] [Google Scholar]
- 12.Arkwright JW, Underhill ID, Maunder SA, et al. Design of a high-sensor count fibre optic manometry catheter for in-vivo colonic diagnostics. Opt Express. 2009;17:22423–22431. doi: 10.1364/OE.17.022423. [DOI] [PubMed] [Google Scholar]
- 13.Davidson JB, O'Grady G, Arkwright JW, et al. Anatomical registration and three-dimensional visualization of low and high-resolution pan-colonic manometry recordings. Neurogastroenterol Motil. 2011;23:387–390. e171. doi: 10.1111/j.1365-2982.2010.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bampton PA, Dinning PG, Kennedy ML, et al. Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am J Gastroenterol. 2000;95:1027–1035. doi: 10.1111/j.1572-0241.2000.01839.x. [DOI] [PubMed] [Google Scholar]
- 15.Musial F, Enck P, Kalveram KT, et al. The effect of loperamide on anorectal function in normal healthy men. J Clin Gastroenterol. 1992;15:321–324. doi: 10.1097/00004836-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Radomirov R, Ivancheva C, Itzev D, et al. Locality-dependent descending reflex motor activity in the anal canal--cholinergic and nitrergic contributions in the rat model. Acta Pharmacol Sin. 2009;30:1276–1282. doi: 10.1038/aps.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamid SA, Di Lorenzo C, Reddy SN, et al. Bisacodyl and high-amplitude-propagating colonic contractions in children. J Pediatr Gastroenterol Nutr. 1998;27:398–402. doi: 10.1097/00005176-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Schang JC, Hemond M, Hebert M, et al. Changes in colonic myoelectric spiking activity during stimulation by bisacodyl. Can J Physiol Pharmacol. 1986;64:39–43. doi: 10.1139/y86-005. [DOI] [PubMed] [Google Scholar]
- 19.Manabe N, Cremonini F, Camilleri M, et al. Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers. Aliment Pharmacol Ther. 2009;30:930–936. doi: 10.1111/j.1365-2036.2009.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosselink MJ, Hop WC, Schouten WR. Rectal tone in response to bisacodyl in women with obstructed defecation. Int J Colorectal Dis. 2000;15:297–302. doi: 10.1007/s003840000259. [DOI] [PubMed] [Google Scholar]
- 21.Ikarashi N, Baba K, Ushiki T, et al. The laxative effect of bisacodyl is attributable to decreased aquaporin-3 expression in the colon induced by increased PGE2 secretion from macrophages. Am J Physiol Gastrointest Liver Physiol. 2011;301:G887–G895. doi: 10.1152/ajpgi.00286.2011. [DOI] [PubMed] [Google Scholar]
- 22.Karmeli F, Stalnikowicz R, Rachmilewitz D. Effect of colchicine and bisacodyl on rat intestinal transit and nitric oxide synthase activity. Scand J Gastroenterol. 1997;32:791–796. doi: 10.3109/00365529708996536. [DOI] [PubMed] [Google Scholar]
- 23.Gaginella TS, Mascolo N, Izzo AA, et al. Nitric oxide as a mediator of bisacodyl and phenolphthalein laxative action: induction of nitric oxide synthase. J Pharmacol Exp Ther. 1994;270:1239–1245. [PubMed] [Google Scholar]


