Abstract
Pregnancy is an intricately orchestrated process where immune effector cells with fetal specificity are selectively silenced. This requires the sustained expansion of immune suppressive maternal Foxp3+ regulatory T cells (Tregs), because even transient partial ablation triggers fetal-specific effector T cell activation and pregnancy loss1,2. In turn, many idiopathic pregnancy complications proposed to stem from disrupted fetal tolerance are associated with blunted maternal Treg expansion3–5. Importantly however, the antigen-specificity and cellular origin of maternal Tregs that accumulate during gestation remain undefined. Here we show pregnancy selectively stimulates the accumulation of maternal Foxp3+ CD4 cells with fetal-specificity using tetramer-based enrichment that allows the identification of rare endogenous T cells6. Interestingly after delivery, fetal-specific Tregs persist at elevated levels, maintain tolerance to pre-existing fetal antigen, and rapidly re-accumulate during subsequent pregnancy. The accelerated expansion of Tregs during secondary pregnancy was driven almost exclusively by proliferation of fetal-specific Foxp3+ cells retained from prior pregnancy, while induced Foxp3 expression and proliferation of pre-existing Foxp3+ cells each contribute to Treg expansion during primary pregnancy. Furthermore, fetal resorption in secondary compared with primary pregnancy becomes more resilient to partial maternal Foxp3+ cell ablation. Thus, pregnancy imprints Foxp3+ CD4 cells that sustain protective regulatory memory to fetal antigen. We anticipate these findings will spark further investigation on maternal regulatory T cell specificity that unlocks new strategies for improving pregnancy outcomes and novel approaches for therapeutically exploiting regulatory T cell memory.
The accumulation of maternal Tregs during pregnancy parallels the need for expanded tolerance to encompass “non-self” fetal antigens3–5,7,8. However, one consequence of sustained Foxp3+ cell expansion is susceptibility to prenatal infection2. Given the increasingly recognized importance of Treg specificity in regulating the fluid balance between immune activation that maintains host defense and immune suppression that prevents autoimmunity9–14, we reasoned establishing the specificity of maternal Tregs that expand during pregnancy could unravel ways to dissociate their beneficial and detrimental impacts. Furthermore, extending this analysis postpartum may allow regulatory memory recently described for Tregs responsive to an induced self antigen to be investigated in a more physiological context15. To address these questions, we developed a mating strategy where the I-Ab 2W1S52–68 peptide becomes a surrogate fetal antigen using male mice (H-2d; Balb/c or H-2b C57Bl/6 [B6]) engineered to co-express this peptide with β-actin to impregnate non-2W1S-expressing B6 females16. In turn, the high precursor frequency of CD4 cells with 2W1S52–68 specificity allows endogenous maternal Tregs to this surrogate fetal antigen to be identified using MHC class II tetramer enrichment6.
Using this approach, maternal CD4 cells with fetal-2W1S-specificity were found to sharply up-regulate CD44 expression, progressively accumulate throughout pregnancy, and persist at ~10-fold increased levels through day 100 postpartum each compared with non-pregnant controls (Fig. 1a). Maternal 2W1S+ cell expansion was specific to mating with 2W1S-expressing mice because they did not accumulate in females impregnated by non-transgenic Balb/c males (Supplementary Fig. 1). Since seminal fluid also contains cells of paternal origin17, 2W1S+ cells in female mice rendered infertile with low-dose irradiation were also enumerated. We found that although mating without pregnancy stimulated modest 2W1S+ cell expansion and CD44 up-regulation, the magnitude was sharply reduced compared with pregnant mice (Supplementary Fig. 1). Thus, maternal 2W1S+ CD4 cell expansion during pregnancy reflects an antigen-specific response to cells of fetal origin.
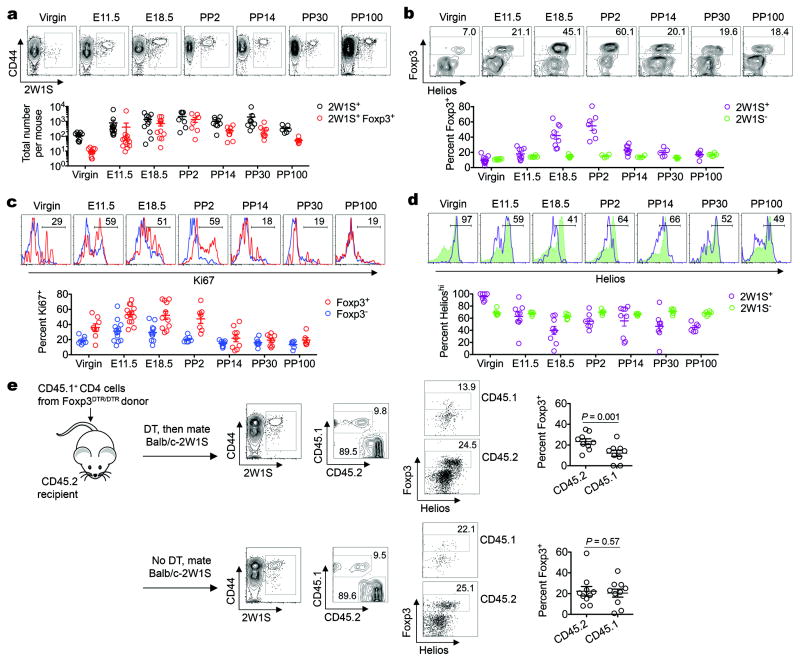
Figure 1. Accumulation of maternal CD4 and Foxp3+ Tregs with fetal-specificity during gestation.
a, Total 2W1S+ or 2W1S+Foxp3+ CD4 cells in B6 females impregnated by Balb/c-2W1S males. b, Percent Foxp3+ among 2W1S+ or 2W1S− CD4 cells. c, Percent Ki67+ among 2W1S+Foxp3+ or 2W1S+Foxp3− CD4 cells. d, Percent Helioshi among 2W1S+Foxp3+or 2W1S−Foxp3+ CD4 cells. e, Percent Foxp3+ among Foxp3DTR/DTR donor (CD45.1+) or Foxp3WT/WT recipient (CD45.2+) 2W1S+ CD4 cells midgestation (E11.5) by Balb/c-2W1S males, with DT treatment (top) or no DT controls (bottom). Bar, mean ± one standard error.
Given the essential requirement for Tregs in maintaining fetal tolerance2,7,18,19, we investigated Foxp3 expression among maternal cells with fetal-2W1S-specificitiy. Beginning midgestation, 2W1S+ compared with 2W1S− CD4 cells became enriched for Foxp3 expression in allogeneic (Fig. 1a,b), as well as syngeneic pregnancy (Supplementary Fig. 2). As pregnancy progressed, Foxp3 expression among 2W1S+ cells became progressively more pronounced peaking at ~50% late gestation through the first 48 hours postpartum (E18.5 to PP2) (Fig. 1a,b; Supplementary Fig. 3). Furthermore, 2W1S+Foxp3+ cells, and to a lesser extent 2W1S+Foxp3− cells, up-regulated the proliferation marker Ki67 that paralleled expanding fetal tissue (Fig. 1c). Reciprocally later after expulsion of the fetus (PP14 to PP100), Ki67 expression among 2W1S+Foxp3+ and 2W1S+Foxp3− cells became reduced (Fig. 1c). However, despite diminished Ki67 levels, Foxp3 expression among 2W1S+ cells was sustained at ~20% through day 100 postpartum (Fig. 1a,b). Accordingly, maternal Tregs with fetal-specificity selectively accumulate during pregnancy and persist following parturition.
Interestingly, maternal Tregs with fetal-2W1S-specificity also progressively down-regulated Helios expression that nadired to ~40% Helioshi by late gestation, while the few 2W1S+Foxp3+ cells in non-pregnant mice were uniformly Helioshi (Fig. 1d). Comparatively, Helios expression among bulk maternal Tregs did not shift significantly. Although this discordance in Helios expression may suggest conversion of fetal-specific Foxp3− into Foxp3+ cells20, the recent finding that some peripherally induced Tregs also express Helios led us to more definitively investigate the origin of maternal Tregs with fetal-specificity21. In particular, we asked whether mating with 2W1S-expressing males can convert 2W1S+Foxp3− CD4 cells from Foxp3DTR/DTR donors ablated of Tregs with diphtheria toxin (DT)22 into Foxp3+ cells after adoptive transfer into virgin Foxp3WT/WT recipient mice. By midgestation, 2W1S+Foxp3+ among Treg-ablated donor Foxp3DTR/DTR cells were readily recovered illustrating induction of maternal Tregs with fetal-specificity (Fig. 1e). This conversion was pregnancy-specific and not due to incomplete donor Treg ablation because Foxp3+ cells were undetectable among Treg-ablated donor cells in unmated control mice (Supplementary Fig. 4). Importantly however, Foxp3+ among Treg-ablated donor CD4 cells was also consistently reduced (by ~50%) compared with either 2W1S+Foxp3+ donor cells in mice without DT treatment or among recipient CD4 cells not susceptible to DT (Fig. 1e). Thus, Foxp3 induction among Foxp3− precursors and proliferation of pre-existing Foxp3+ cells each contribute to the accumulation of maternal Tregs with fetal-specificity during primary pregnancy.
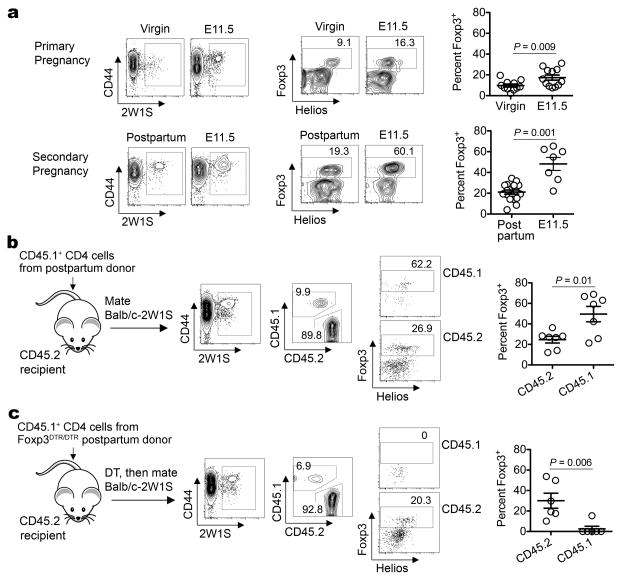
To further characterize maternal Tregs with specificity to pre-existing fetal antigen that persist postpartum, these cells were tracked during subsequent pregnancy. After secondary mating, maternal Foxp3+ cells with fetal-2W1S-specificity accumulated with accelerated kinetics in an antigen-specific fashion (Fig. 2a; Supplementary Fig. 5). The more rapid expansion of maternal Tregs in separate groups of mice was recapitulated within the same mouse by measuring 2W1S+ Treg accumulation among donor CD4 cells from postpartum mice (secondary expansion) adoptively transferred prior to mating with 2W1S-expressing males, compared with cells in virgin recipient mice (primary expansion) (Fig. 2b). By substituting CD4 cells from postpartum Foxp3DTR/DTR mice for adoptive transfer and using DT to eliminate donor Tregs prior to mating, we also addressed whether the accelerated secondary expansion of maternal Tregs with fetal-2W1S-specificity reflects more vigorous induction among Foxp3− cells or proliferation of pre-existing Foxp3+ cells. We found in sharp contrast to primary pregnancy, the ablation of donor Tregs from postpartum Foxp3DTR/DTR mice almost uniformly eliminated their expansion in subsequent pregnancy (Fig. 2c). Thus, recurrent pregnancy primes the accelerated accumulation of maternal Foxp3+ cells that expand from pre-existing Tregs retained from prior pregnancy.
Figure 2. Accelerated expansion of maternal Tregs with fetal-specificity during secondary pregnancy.
a, Percent Foxp3+ among virgin (primary pregnancy) or postpartum (secondary pregnancy) females before mating or midgestation (E11.5) by Balb/c-2W1S males. b, Percent Foxp3+ among postpartum donor (CD45.1+) or naïve recipient (CD45.2+) 2W1S+ CD4 cells midgestation by Balb/c-2W1S males. c, Percent Foxp3+ among Treg-ablated Foxp3DTR/DTR postpartum donor (CD45.1+) or naïve recipient (CD45.2+) 2W1S+ CD4 cells midgestation. Bar, mean ± one standard error.
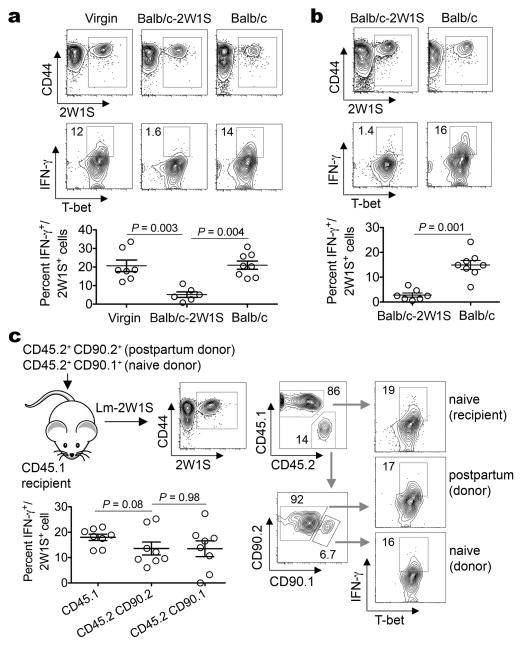
Expanding this model, the responsiveness of maternal CD4 cells with fetal-specificity was also investigated. We found 2W1S+ cells recovered from mice midgestation or postpartum each compared with non-pregnant controls did not produce appreciable IFN-γ ex vivo following stimulation, consistent with previously described anergy of maternal cells with fetal-specificity (Supplementary Fig. 6)2,23. Therefore, to more fully evaluate the responsiveness of maternal T cells with fetal-2W1S-specificity, we measured their in vivo response to Listeria monocytogenes engineered to express 2W1S52–68 peptide (Lm-2W1S) that potently stimulates Th1-differentiation in other contexts24,25. We found 2W1S+ cells expand and up-regulate T-bet expression each in an antigen-specific fashion in naïve and mice impregnated by 2W1S-expressing males after Lm-2W1S inoculation similar to other intracellular pathogens (Supplementary Fig. 7)26. Interestingly however, 2W1S+ cells in pregnant mice where 2W1S represents a surrogate fetal antigen produced only background levels of IFN-γ and other effector cytokines, with reciprocal accumulation of Foxp3+ cells (Fig. 3a; Supplementary Fig. 8). Comparatively, >15% of 2W1S+ cells in Lm-2W1S inoculated virgin mice were IFN-γ+ (Fig. 3a). This hypo-responsiveness was specific to fetal-2W1S stimulation because 2W1S+ CD4 cells in mice impregnated with non-2W1S-expressing males produced IFN-γ comparable to non-pregnant controls (Fig. 3a; Supplementary Fig. 8). Given the sustained enrichment of fetal-specific Tregs after delivery (Fig. 1a,b), these studies were extended to investigate whether diminished IFN-γ production among maternal CD4 cells with specificity to pre-existing fetal antigen is similarly maintained. Remarkably, IFN-γ production remained anemic in postpartum mice previously exposed to 2W1S as a fetal antigen, while postpartum mice without prior fetal-2W1S exposure produce IFN-γ comparable to non-pregnant controls (Fig. 3b). Accordingly, pregnancy imprints functional anergy for maternal CD4 cells with fetal-specificity that is sustained postpartum.
Figure 3. The postpartum environment maintains anergy for maternal CD4 cells with pre-existing fetal-specificity.
a, IFN-γ producing 2W1S+ CD4 cells five days after Lm-2W1S inoculation in virgin, or pregnant females midgestation by Balb/c-2W1S or Balb/c males. b, IFN-γ producing 2W1S+ CD4 cells five days after Lm-2W1S inoculation in postpartum mice previously impregnated by Balb/c-2W1S or Balb/c males. c, IFN-γ producing postpartum donor (CD45.2+CD90.2+), naive donor (CD45.2+CD90.1+), or naive recipient (CD45.1+) CD4 cells five days after Lm-2W1S inoculation and stimulation with PMA/ionomycin. Bar, mean ± one standard error.
To dissociate whether pregnancy-induced T cell anergy was cell-intrinsic or imposed by features associated with the postpartum environment, we measured IFN-γ production by CD4 cells from postpartum or virgin mice each after adoptive transfer into naive recipient mice. We found IFN-γ production by donor postpartum and each group of naïve (donor and recipient) 2W1S+ CD4 cells were similar, and sharply increased compared with 2W1S+ cells in un-manipulated postpartum mice following Lm-2W1S inoculation (Fig. 3b,c). Thus, anergy among maternal CD4 cells with specificity to pre-existing fetal antigen is not cell-intrinsic, but maintained by the postpartum environment.
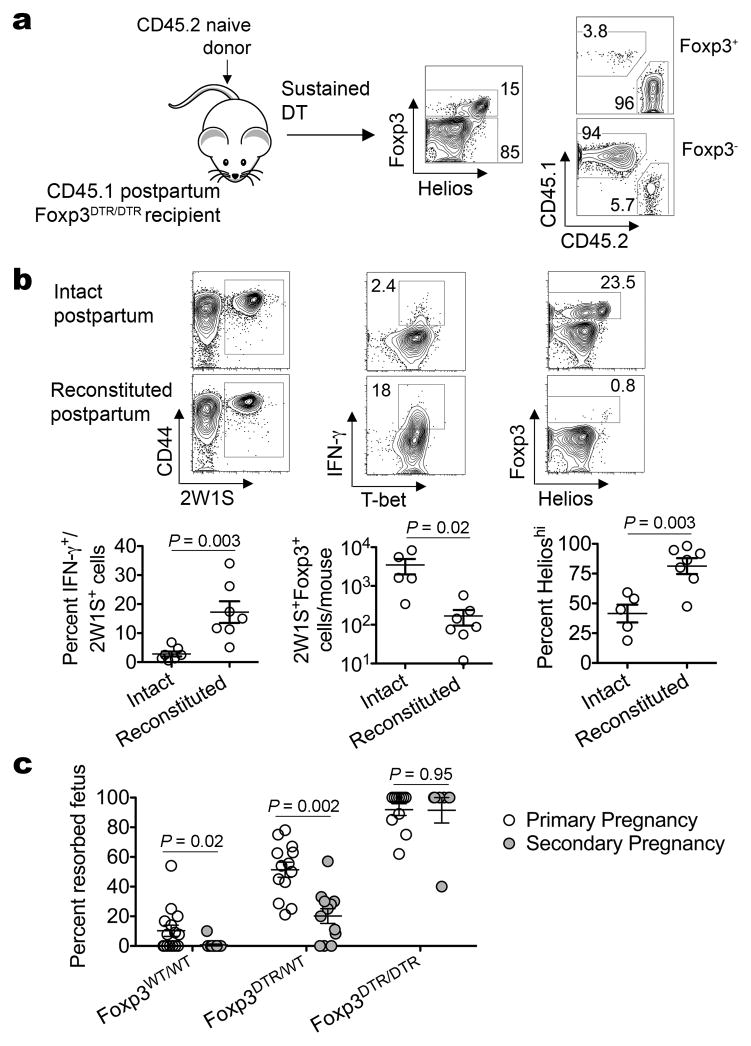
In complementary studies we addressed the importance of maternal Tregs in sustaining anergy to cells with specificity to pre-existing fetal antigen by investigating the impacts of replacing the entire Treg compartment in postpartum mice previously exposed to fetal-2W1S with naïve Foxp3+ cells from virgin mice. Consistent with recent studies using adoptively transferred Foxp3WT/WT CD4 cells to refill the cellular compartment in Foxp3DTR/DTR mice sustained on DT treatment2, Tregs from naïve mice efficiently reconstituted Treg-ablated Foxp3DTR/DTR postpartum mice (Fig. 4a). Using this approach, we found replacing maternal Foxp3+ cells in postpartum mice with Tregs from naïve mice restored IFN-γ production for 2W1S+ CD4 cells (Fig. 4b). Furthermore, while only rare 2W1S+Foxp3+ cells that were Helioshi were found among postpartum mice reconstituted with naïve Tregs, a significant proportion of 2W1S+ cells expanded in response to Lm-2W1S in intact postpartum mice remained Foxp3+ (~20%) and Helioslo (~40%) (Fig. 4b). Thus, the muted expansion of naïve Foxp3+ CD4 cells with Lm infection is overcome by pregnancy-induced Treg activation (Fig. 4b; Supplementary Fig. 8)24. By extension, the restored responsiveness of postpartum 2W1S+ cells after adoptive transfer into Treg-sufficient naïve mice most likely represents dilution of co-transferred maternal Tregs with fetal-2W1S-specificity (Fig. 3c).
Figure 4. Maternal postpartum Tregs mitigate IFN-γ responsiveness and mediate resiliency to fetal resorption in secondary pregnancy.
a, Representative plots illustrating the majority (>96%) of Foxp3+ cells are derived from adoptively transferred CD4 in DT treated Foxp3DTR/DTR mice. b, IFN-γ producing 2W1S+ cells among CD45.1+CD45.2− cells, accumulation of 2W1S+Foxp3+ cells, and Helios expression among 2W1S+Foxp3+ Tregs five days after Lm-2W1S inoculation. c, Percent fetal resorption during primary (open) or secondary (shaded) allogeneic pregnancy for Foxp3WT/WT, Foxp3DTR/WT, or Foxp3DTR/DTR females five days after DT initiation beginning midgestation. Bar, mean ± one standard error.
Lastly, to establish how maternal Tregs with fetal-specificity retained postpartum impacts subsequent pregnancy outcomes, the frequency of fetal resorption triggered by partial maternal Foxp3+ cell ablation using Foxp3DTR/WT mice was compared between secondary and primary pregnancy2. We found secondary pregnancy became significantly more resilient to partial Treg ablation because fetal resorption was reduced by ~60% compared with primary pregnancy (Fig. 4c). In turn, fetal resorption in Treg-sufficient secondary pregnancy was also significantly reduced from background levels with primary allogeneic pregnancy. Maternal Tregs were essential for these protective effects because wholesale Foxp3+ cell ablation using Foxp3DTR/DTR mice triggered pervasive fetal resorption equally in secondary and primary allogeneic pregnancy (Fig. 4c). Importantly, fetal wastage with maternal Treg ablation in this context was driven by antigen heterogeneity, and not poor maternal health, because the frequency of resorption was sharply reduced with Treg ablation in mice bearing syngeneic pregnancy (Supplementary Fig. 9).
Together, these findings establish a model whereby pregnancy primes the selective accumulation and activation of maternal Tregs with fetal-specificity (Supplementary Fig. 10), and extend the role of antigen experienced Tregs from primary into subsequent pregnancies2,7,18. In this regard while maternal Tregs have been described to expand up to 2-fold when examined in a non-antigen-specific fashion2–5, our results demonstrate Foxp3+ cells with fetal-specificity expand >100-fold through parturition (Fig. 1a; Supplementary Fig. 3). After delivery, maternal Tregs with fetal-specificity are sustained at enriched levels, and are functionally distinct as they re-accumulate with accelerated kinetics and out compete “naïve” Tregs during secondary pregnancy. Similar to discordant functional properties of naïve and activated effector T cells27, these results uncover the exciting possibility of exploiting antigen-specific “memory” Tregs to dissociate detrimental and beneficial immune responses. Applied to human pregnancy, these data may explain why rates of preeclampsia, and other complications associated with disrupted fetal tolerance, are reduced in secondary compared with primary pregnancy28. However, given the increased risk of preeclampsia in recurrent human pregnancy when the inter-pregnancy interval is extended, waning Treg memory similar to other CD4 subsets would not be unexpected25,29. Therefore, establishing the durability of pregnancy-induced regulatory memory and characterizing whether this response can be sustained with boosting represent next-steps with exceptional scientific importance.
Methods Summary
Mice
2W1S-expressing and Foxp3DTR mice have each been described2,16,22. Mated females and the timing of pregnancy was determined by visualization of a copulation plug (E0.5). For infection, Lm-2W1S (104 CFUs, see Methods)24 was intravenously inoculated. Experiments were performed in accordance with University of Minnesota IACUC approved protocols.
Tetramer enrichment and flow cytometry
I-Ab 2W1S52–68 tetramer staining and enrichment have been described6. Lymphoid cells from the spleen and lymph nodes were enriched with 2W1S52–68 tetramer prior to surface, intracellular, or intranuclear staining. For stimulation, cells were cultured with PMA/ionomycin (5 hours) and Brefeldin A before staining.
Cell transfer and ablation
One mouse equivalent of purified CD4 cells was intravenously transferred into recipient mice one day before mating/infection. Donor Tregs from Foxp3DTR mice were ablated in recipient Foxp3WT mice using purified DT (two doses 8 hours apart, 0.5 μg/dose). For ablation of endogenous Tregs, DT was administered daily (0.5 μg first dose, followed by 0.1 μg/dose daily thereafter) beginning midgestation or immediately following donor cell transfer.
Statistics
Data was analyzed using the unpaired (separate groups of mice) or paired (cell subsets within the same mice) Student’s t test.
Methods
Mice
Balb/c (H-2d/d), and C57Bl/6 (B6; H-2b/b) or CD45.1 (H-2b/b) and CD90.1 (H-2b/b) mice each on the B6 background were purchased from The Jackson Laboratory or The National Cancer Institute. Transgenic mice expressing 2W1S52–68 antigen behind the β-actin promoter in all cells, and Foxp3DTR/DTR mice where Tregs become susceptible to ablation with low dose diphtheria toxin (DT) have each been described16,22. Foxp3DTR/DTR mice on the B6 background were intercrossed with CD45.1 mice to generate CD45.1+/+ Foxp3DTR/DTR mice. For mating, 2W1S-expressing males were used either on the B6 background, or backcrossed 5 generations to Balb/c mice, and verified to be H-2d/d. Males were introduced to either virgin or postpartum (>14 days) females for 24 hours, and mated mice visualized by a copulation plug representing E0.5. In each experiment, pups were removed within 24 hours after delivery to prevent the potential transfer of fetal antigen through suckuling. For sterilization, female mice were sub-lethally irradiated (100 rads) prior to mating.
Tetramer enrichment and flow cytometry
PE or APC conjugated MHC class II I-Ab 2W1S52–68 tetramer, and their use with anti-fluorophore conjugated magnetic beads (Miltenyi Biotec) for enrichment have been described6,30. For enumerating total 2W1S+ cells, all nucleated cells from secondary lymphoid tissue (spleen and axillary, brachial, cervical, inguinal, mesenteric, pancreatic, paraaortic/uterine lymph nodes) were collected, enriched using I-Ab 2W1S52–68 tetramer, and stained for cell-surface CD4, CD8α, CD25, CD45.1, CD45.2, CD90.1, CD90.2, CD44, CD11b, CD11c, B220, F4/80, intracellular IFN-γ, IL-4, IL-10, IL-17A, or intranuclear Foxp3, Helios, Ki67, or T-bet expression using commercially available antibodies and cell permeabilization reagents (BD PharMingen or eBioscience). For stimulation, cells were cultured with PMA/ionomycin for 5 hours in media supplemented with Brefeldin A before tetramer staining.
Cell transfer and ablation
For adoptive transfer, CD4 cells in the spleen and lymph nodes were first purified by negative selection (Miltenyi Biotech), and one mouse equivalent donor cells intravenously transferred into recipient mice one day before mating and/or infection. For ablation of donor Foxp3DTR/DTR cells, recipient Foxp3WT/WT mice were treated with purified DT (Sigma Chemicals, two doses 8 hours apart, 0.5 μg/dose). For ablation of endogenous Tregs in Foxp3DTR/WT or Foxp3DTR/DTR mice during pregnancy, purified DT was administered daily (0.5μg first dose, followed by 0.1 μg/dose thereafter beginning midgestation) for 4 consecutive days. For Foxp3+ cell reconstitution requiring sustained ablation of endogenous Tregs in Foxp3DTR/DTR recipient mice, purified DT was administered daily (0.5 μg first dose, followed by 0.1 μg/dose thereafter) immediately following transfer of purified donor CD4 cells as described2.
Bacteria
Listeria monocytogenes (Lm) was engineered to stably express and secrete 2W1S52–68 antigen by sub-cloning the hly promoter, signal sequence, 2W1S52–68 and ovallbumin coding sequence from the pAM401-based expression contstruct24,31 into the temperature sensitive plasmid pKSV732. This pKSV7-based plasmid containing the hly promoter, signal sequence, 2W1S52–68 and ovallbumin coding sequence was used to electroporate Lm-OVA33, and transformants selected by resistance to chloramphenicol (10 μg/ml final concentration) at room temperature. Individual clones were then passaged five times at 40oC in brain heart infusion media (Becton Dickinson) with chloramphenicol selection (plasmid integration into the Lm genome), followed by passage without antibiotic selection (plasmid excision from the Lm genome). Lm clones where double homologous recombination had occurred were initially screened by replica plating for sensitivity to chloramphenicol, and a single clone (Lm-2W1S) verified by PCR and DNA sequencing using previously described methods34. For infection, Lm-2W1S was grown to early log phase (OD600 0.1) in brain heart infusion media, washed and diluted with sterile saline, and then intravenously inoculated via the lateral tail vein (104 CFUs per mouse). Five days thereafter, the antigen-specific CD4 cell response in the spleen and lymph nodes was investigated with I-Ab 2W1S52–68 tetramer staining.
Data acquisition and analysis
Cells stained with fluorochrome-conjugated tetramer and antibody were acquired on a FACSCanto cytometer (Becton Dickinson), and analyzed using FlowJo (TreeStar) software. The number and percent cells were then analyzed and found to be normally distributed, and thereafter the difference between separate groups of mice were analyzed using an unpaired Student’s t test, while differences between individual cell subsets within the same mouse were analyzed using the paired Student’s t test (Prism, GraphPad). For all analysis, P < 0.05 was taken as statistical significance.
Supplementary Material
Acknowledgments
We thank Marc Jenkins for providing 2W1S-expressing mice, and Alexander Rudensky for providing Foxp3DTR mice. This research was supported by NIH-NIAID awards R01AI087830 and R01AI100934 (SSW), and NIH-NIDDK award F30DK084674 (JHR). SSW holds an Investigators in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund.
Footnotes
Author Contributions
J.H.R., J.M.E, L.X. and S.S.W. designed and performed the experiments, and wrote the paper.
Author Information
The authors declare no competing financial interests.
References
- 1.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) Regulatory T Cell Expansion Required for Sustaining Pregnancy Compromises Host Defense against Prenatal Bacterial Pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prins JR, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;28:300–311. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- 4.Santner-Nanan B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki Y, et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Molecular human reproduction. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 6.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nature immunology. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 8.Andersen KG, Nissen JK, Betz AG. Comparative Genomics Reveals Key Gain-of-Function Events in Foxp3 during Regulatory T Cell Evolution. Frontiers in immunology. 2012;3:113. doi: 10.3389/fimmu.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. The Journal of experimental medicine. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature immunology. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nature reviews. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 14.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. The Journal of experimental medicine. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblum MD, et al. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon JJ, et al. Quantitative impact of thymic selection on Foxp3+ and Foxp3− subsets of self-peptide/MHC class II-specific CD4+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson SA. Immune regulation of conception and embryo implantation-all about quality control? Journal of reproductive immunology. 2010;85:51–57. doi: 10.1016/j.jri.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Kallikourdis M, Andersen KG, Welch KA, Betz AG. Alloantigen-enhanced accumulation of CCR5+ ‘effector’ regulatory T cells in the gravid uterus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:594–599. doi: 10.1073/pnas.0604268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunological reviews. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 20.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188:976–980. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 22.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 23.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. The Journal of clinical investigation. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ertelt JM, et al. Selective priming and expansion of antigen-specific Foxp3− CD4+ T cells during Listeria monocytogenes infection. J Immunol. 2009;182:3032–3038. doi: 10.4049/jimmunol.0803402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepper M, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature immunology. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nature immunology. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakim LM, Bevan MJ. From the thymus to longevity in the periphery. Current opinion in immunology. 2010;22:274–278. doi: 10.1016/j.coi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trupin LS, Simon LP, Eskenazi B. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiology (Cambridge, Mass. 1996;7:240–244. doi: 10.1097/00001648-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Conde-Agudelo A, Belizan JM. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. BMJ (Clinical research ed. 2000;321:1255–1259. doi: 10.1136/bmj.321.7271.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon JJ, et al. Tracking epitope-specific T cells. Nature protocols. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirth R, An FY, Clewell DB. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. Journal of bacteriology. 1986;165:831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith K, Youngman P. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie. 1992;74:705–711. doi: 10.1016/0300-9084(92)90143-3. [DOI] [PubMed] [Google Scholar]
- 33.Foulds KE, et al. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 34.Rudd BD, et al. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.