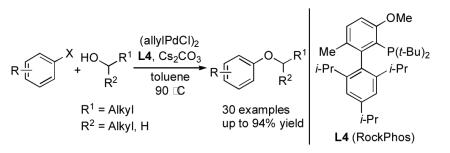
Abstract
An efficient, general palladium catalyst for C–O bond-forming reactions of secondary and primary alcohols with a range of aryl halides has been developed. Use of a catalyst based on a single bulky biarylphosphine ligand L4 (RockPhos, soon to be commercially available) has expanded this chemistry to allow the transformation of a variety of heteroaryl halides, and for the first time, allows for the coupling of electron-rich aryl halides with secondary alcohols. Additionally, this new catalyst system provides the ability to effect these reactions with a diverse set of substrate combinations, while employing a single ligand. Thus the need to survey mutiple ligands, as was previously the case, is obviated.
Keywords: palladium, C–O cross-coupling, aryl alkyl ether, biarylphosphine ligand
Aryl alkyl ethers are present in many naturally occurring and medicinally-relevant compounds.1 Copper2- and palladium-catalyzed C–O bond-forming reactions have become effective strategies for their preparation. Although reasonably efficient Pd catalysts for the coupling reactions of phenols3 and tertiary alcohols,4 which lack β-hydrogens, have been developed, much less progress in the realization of a practical and general system for the analogous coupling of primary and, especially, secondary alcohols5 has, been realized. This is attributed to the competing β-hydride elimination pathway from the LnPdII(Ar)(alkoxide) intermediates leading to significant amounts of arene formation.
Our first report on Pd-catalyzed intermolecular cross-coupling reactions of primary alcohols with unactivated aryl bromides and chlorides6a necessitated the presence of an ortho-substituent or an electron-withdrawing substituent on the aryl halide, which increase the rate of reductive elimination, to achieve satisfactory results. In 2005, we disclosed an efficient protocol for cross-coupling of primary and secondary alcohols with aryl halides that utilized a collection of new (at the time) ligands.6b The ligands employed were carefully chosen to match the steric properties of the substrate combination. Additionally, the analogous reaction of electron-rich aryl halides with secondary alcohols remained a challenge due to the extensive formation of reduced arene.
Further, very few examples of Pd-catalyzed cross-coupling reactions of primary and secondary alcohols with heteroaryl halides have been reported to date.7 Most recently, Beller disclosed that a single catalyst based on a modified version of Singer’s Bippyphos ligand, L2,8 was able to couple primary alcohols with a few types of heteroaryl halides. Examples carried out with this new system were restricted to reactions of primary alcohols with electron-neutral, - deficient, or ortho-substituted aryl halides, i.e., substrates that contain steric and electronic features that are known to facilitate reductive elimination.6a No examples with more challenging electron-rich aryl halides (e.g., p- or o-halo anisole) were described. Importantly, no examples of the successful coupling reactions of secondary alcohols were reported.
Herein, we report a catalyst based on a new ligand that provides a single general system for the coupling of both primary and secondary alcohols and is applicable to the reactions of formerly inaccessible substrates, such as a wider range of heteroaryl and electron-rich aryl halides.
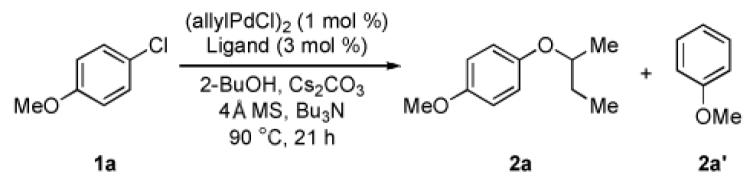
In light of the aforementioned limitations for the Pd-catalyzed coupling reactions of secondary and primary alcohols with aryl halides, we felt that the development of a more general catalyst system for the preparation of (hetero)aryl alkyl ethers was highly desirable. On the basis of our recent observations that a catalyst based on the sterically demanding di-tert-butyl biarylphosphine ligand, L3 (tBuBrettPhos), was able to promote the difficult reductive elimination to form the Ar-F,9a Ar-Br,9b and Ar-O9c bonds, we postulated that for reactions of secondary alcohols this catalyst may accelerate reductive elimination relative to the rate of β-hydride elimination. Use of a catalyst based on L3 for the coupling of 2- butanol and 4-chloroanisole led to only a 20% yield of desired product (2a) and 63% of the reduced arene byproduct (2a’) (Table 1, entry 2).
Table 1.
Ligand Evaluation[a]
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Ligand | Conv. of 1a [%][b] | Yield of 2a [%][b] | Yield of 2a’ [%][b] |
| 1 | L1 | 100% | 26% | 54% |
| 2 | L3 | 100% | 20 | 63% |
| 3 | L4 | 100% | 70% | 19% |
| 4 | L5 | 70% | trace | 61% |
| 5 | L6 | 67% | 3% | 43% |
| 6 | L7 | 100% | 66% | 26% |
| 7 | L8 | 100% | 64% | 28% |
| 8 | L9 | 100% | 57% | 32% |
| 9 | L10 | 100% | 54% | 31% |
Reaction conditions: 4-chloroanisole (1.0 mmol), 2-BuOH (2.0 mmol), (allyIpdcI)2 (1 mol %), Ligand (3 mol %), Cs2CO3 (1.5 mmol), 4Å molecular sieves (200 mg), Bu3N (1 ml), 90 °C, 21 h.
Determined by GC.
Previous studies from our group have shown that the substituent in the 3-position of our biarylphosphine ligands helps fix the Pd(II) center over the triisopropylphenyl ring, which in turn accelerates reductive elimination (Figure 2).10 Further, we have disclosed, for both C–N and C–O cross-coupling reactions, that a ligand bearing a methoxy in the 3-position led to the most active catalyst systems.9c,11 However, none of our previous studies have focused solely on the effect of the substituent in the 6-position of L3. We previously postulated that the 6-methyl in L1 provided increased conformational rigidity in the ligated Pd(II) complexes, leading to accelerated rates of reductive elimination for cross-coupling reactions of phenols.3d Therefore, replacing the 6-methoxy in L3 with a methyl group, as shown in L4, would provide a hybrid of L1 and L3, which we hypothesized would accelerate the rate of reductive elimination and impede that of β-H elimination for reactions of secondary alcohols (Figure 2).
Figure 2.
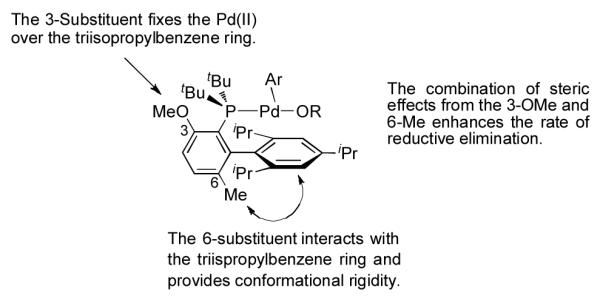
Rationalizing the Substituent Effect on Reductive Elimination.
In support of this hypothesis, a catalyst based on L4 was tested for the coupling of 2-butanol with 4-chloroanisole and gave 70% of the desired product (2a) and only 19% reduced arene (2a’) (Table 1, entry 3). This is the first example for the coupling of a secondary alcohol with an electron-rich aryl halide. Moreover, this result reveals that the substituent in the 6-position of the ligand has a profound effect on the reactivity of the catalyst.12
In an attempt to further probe the effect of the substituents in the 3- and 6-positions of the ligand, catalysts based on L5–L10 were examined for this reaction (Table 1, entries 4 - 9). When the 6- methyl group in L4 was removed (L6), the activity of the derived catalyst dropped off substantially giving only 3% product (Table 1, entry 5). This result again demonstrates that subtle differences in ligand structure have a dramatic effect on these C–O cross-coupling reactions. Utilizing catalysts based on L7, L8, L9, and L10, which contain a 6-ethyl, 6-isopryl, 3-ethoxy, and 3-isopropoxy substituent, respectively, led to a slight reduction in the production of 2a and a modest increase in that of 2a’ formed (Table 1, entries 6 - 9). This indicates that the 3-methoxy and 6-methyl in L4 are optimal for promoting reductive elimination and suppressing β-H elimination.
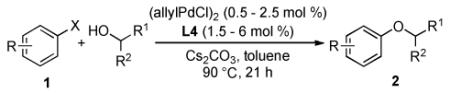
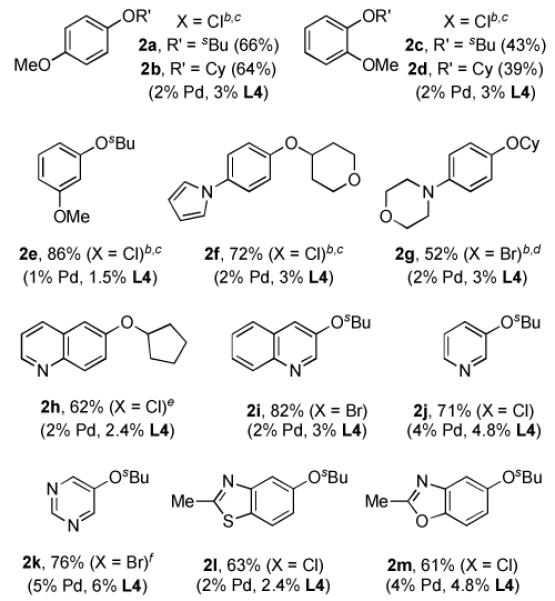
We next explored the scope of the cross-coupling reactions of secondary alcohols with aryl halides (Table 2). Typically these reactions were carried out at 90 °C using 1 mol % (allylPdCl)2. In a few cases, Bu3N was chosen as the solvent due to its ability to suppress the formation of the reduction byproduct.6b A range of electron-rich aryl halides were found to undergo reactions with cyclic and acyclic secondary alcohols to afford aryl alkyl ethers in moderate to good yields (2a-2d, 2f, 2g); these yields are the highest reported to date for this difficult process. For a slightly electron-deficient substrate, 3-chloroanisole, only 0.5 mol % (allylPdCl)2 was required to give 2e in 86% yield. In contrast a 63% yield was obtained with 2 mol % Pd(OAc)2 and 2.4 mol % L12, which was previously the most efficient catalyst system reported for this transformation.6b
Table 2.
Coupling of Aryl Halides with Secondary Alcohols[a]
|
Reaction conditions: ArX (1 mmol), alcohol (2 mmol), Cs2CO3 (1.5 mmol), (allylPdCl)2 (0.5 – 2.5 mol %), L4 (1.5 – 6 mol %), toluene (1 mL), 90 °C, 21 h; isolated yields (average of two or more runs).
200 mg of 4Å molecular sieves was added.
in Bu3N.
cyclohexanol (1.5 mmol) was used.
In Et3N.
24 h.
Furthermore, switching to toluene from Bu3N as solvent did not affect the efficiency of the coupling with basic nitrogen-containing heteroaryl halides as substrates, allowing simplified isolation of the products (2g, 2i-2m). 6-Chloroquinoline, however, was found to be an exception to this trend. In toluene the reaction of 6-chloroquinoline and cyclopentanol resulted in the formation of a significant amount of reduced arene byproduct (see 2h). In this case switching to Et3N as solvent reduced the amount of quinoline formation and resulted in a 62% yield of the desired product. For the coupling of halo-pyridines and -pyrimidines we found it was necessary to premix the (allylPdCl)2, L4, Cs2CO3, and 2-butanol in toluene at 90 °C for 3 minutes, followed by addition of the aryl halide (presumably due to the competitive binding of the substrate’s nitrogen to the Pd center). In this way, 3-chloropyridine and 5- bromopyrimidine were coupled with 2-BuOH in 71% and 76% yields, respectively. Moreover, 5-chlorobenzoisoxazole (see 2m), and 5-chlorobenzothiazole (see 2l) proved to be proficient substrates in these reactions, giving the desired products in 61% and 63%, respectively. Thus, this suggests that the pyridine’s (and related substrates) N-atom interferes with catalyst generation, more than with the catalyst itself.
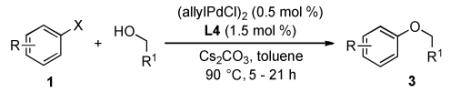
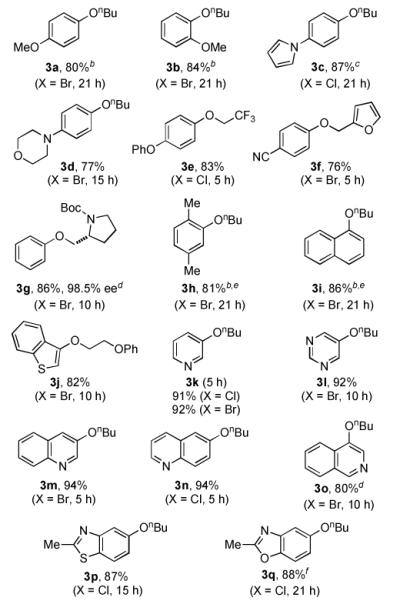
We next decided to explore the application of L4 for the cross-coupling of primary alcohols (Table 3). Excellent yields were obtained for the combination of primary alcohols with electron-rich, -neutral, and -deficient aryl halides using 0.5 mol % (allylPdCl)2 and 1.5 mol % L4. The high efficiency displayed with L4 as the supporting ligand allowed the reactions to be carried out in toluene, rather than in Bu3N as solvent as in our previous method.6b For unactivated substrates, the coupling of aryl chlorides with primary alcohols was generally less efficient than that of aryl bromides and resulted in incomplete conversion of the starting material. For instance, the reaction of n-BuOH with 4-bromoanisole (see 3a) proceeded within 21 hours using only 1 mol % of Pd. However, the analogous reaction with 4-chloroanisole using 2 mol % of Pd resulted in only ~85% conversion in the same time. Interestingly, the less nucleophilic fluorinated primary alcohol was a more efficient coupling partner than n-BuOH.13 Thus, the reaction of trifluoroethanol with 4-chlorodiphenyl ether afforded an 83% yield of the desired product (3e).14 Further, the coupling of N-Boc-D-prolinol gave the desired product 3g with no erosion of enantiopurity (86% yield, 98.5% ee). The catalyst combination of Pd(OAc)2 and the less bulky ligand L3 (tBuBrettPhos) was optimal for the reaction of aryl bromides bearing ortho-alkyl substituents to give 3h and 3i in comparable yields.6b
Table 3.
Coupling of Aryl Halides with Primary Alcohols[a]
|
Reaction conditions: ArX (1 mmol), alcohol (2 mmol), Cs2CO3 (1.5 mmol), (allylPdCl)2 (0.5 mol %), L4 (1.5 mol %), toluene (1 mL), 90 °C, 5 - 21 h; isolated yields (average of two or more runs).
200 mg of 4Å molecular sieves was added.
(allylPdCl)2 (2 mol %) and L4 (4.8 mol %).
alcohol (3 mmol).
Pd(OAc)2 (2 mol %) and L3 (2.4 mol %).
(allylPdCl)2 (1 mol %) and L4 (2.4 mol %).
In contrast to a catalyst based on L2,8 whose application was limited to halo-pyridines and -quinolines, a variety of aryl alkyl ethers derived from five- and six-membered heteroaryl halides could be accessed under our new conditions (3j-3o). For example, 3- bromopyridine, 5-bromopyrimidine, and 4-bromoisoquinoline (see 3k, 3m, and 3o) were all coupled with n-BuOH in good to excellent yields. The conversion of 4-bromoisoquinoline to 3o proved more difficult, but could be efficiently accomplished by using 3 equivalents of n-BuOH and the premixing protocol described above for 3k and 3l.
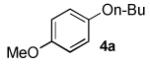
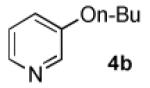
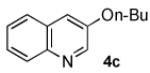
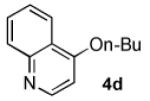
In order to highlight the generality and efficiency of a catalyst based on L4 we directly compared it to several of the previous reported systems. For the reaction of 4-bromoquinoline with a secondary alcohol our new catalyst system gave an 88% isolated yield, whereas a catalyst based on L12 (previously the best reported system for reactions of secondary alcohols)6b gave no desired product (Scheme 1). Further, for the reaction of a primary alcohol with an electron-rich aryl halide a catalyst based on L4 gave an 84% GC yield; for the same reaction a catalyst based on the recently reported L28 afforded no desired product and a catalyst based on L1 gave a 73% GC yield (Table 4, 4a). Switching to reactions of primary alcohols with heteroaryl bromides further displayed the superiority of a catalyst based on L4 compared to previous catalyst systems (Table 4, 4b-4d).
Scheme 1.
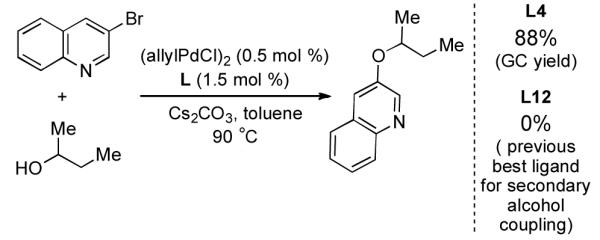
Comparison of Catalysts Based on L4 and L12 for the Coupling of a Secondary Alcohol.
Table 4.
Comparison of Catalysts Based on L4 and the Previously Reported Ligands for the C–O Cross-Coupling Reactions of Primary Alcohols.[a]
| L4 | L2 | L11 | L1 | |
|---|---|---|---|---|
| (previous best ligands for coupling primary alcohols) |
(primary alcohols with electron-rich arylhalides) |
|||
|
84% | 0% | NA[d] | 73% |
|
96% | 0%[b] | 26% | NA[e] |
|
98% | 57%[c] | 12% | NA[e] |
|
83% | 29% | 39% | NA[e] |
Corrected GC yields.
No desired product was obtained under our conditions or the conditions reported by Beller.8
Using the conditions reported by Beller. 8
A catalyst based on this ligand was reported to not be efficient for coupling electron-rich aryl halides.
A catalyst based on this ligand was reported to only be efficient for reactions of electron-rich aryl halides.
In summary, we have developed a general system for the palladium-catalyzed C–O cross-coupling reactions of aryl halides with secondary and primary alcohols. We found that the substituent in the 6-position of the biarylphosphine ligand scaffold has a profound effect on the catalytic activity of these systems and that a catalyst based on L4 (RockPhos, soon to be commercially available), which contains a methyl group in the 6-position, displays the highest reactivity reported to date for these reactions. We postulate that the introduction of 6-methyl, rather than a 6-methoxy, in the ligand provides increased conformational rigidity in the LPd(Ar)(alkoxide) complexes and, therefore, accelerates the rate of reductive elimination while preventing β-hydride elimination. Thus, the utilization of catalyst based on L4 allows for the synthesis of an array of aryl alkyl ethers with unprecedented substrate scope of both the aryl halide and alcohol coupling partners.
Supplementary Material
Figure 1.
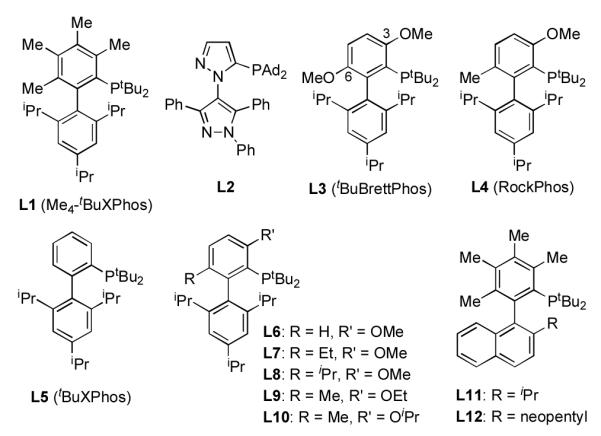
Ligands for Pd-catalyzed C–O cross-coupling reactions.
Footnotes
We are grateful to the National Institutes of Health for financial support (grant GM-58160). This activity is supported by an educational donation provided by Amgen. BPF thanks Bristol-Myers Squibb for a graduate fellowship. The Varian 300 MHz instrument used in this work was purchased with funding from the National Institutes of Health (GM 1S10RR13886-01).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- [1].Buckingham J. Dictionary of Natural Products. University Press; Cambridge, MA: 1994. [Google Scholar]
- [2](a).For Cu-catalyzed C–O coupling of aliphatic alcohols, see: Fagan PJ, Hauptman E, Shapiro R, Casalnuovo A. J. Am. Chem. Soc. 2000;122:5043.; Wolter M, Nordmann G, Job GE, Buchwald SL. Org. Lett. 2002;4:973. doi: 10.1021/ol025548k.; Hosseinzadeh R, Tajbakhsh M, Mohadjerani M, Alikarami M. Synlett. 2005:1101.; Shafir A, Lichtor PA, Buchwald SL. J. Am. Chem. Soc. 2007;129:3490. doi: 10.1021/ja068926f.. Zhang H, Ma D, Cao W. Synlett. 2007:243.. Altman RA, Shafir A, Choi A, Lichtor PA, Buchwald SL. J. Org. Chem. 2008;73:284. doi: 10.1021/jo702024p.. Naidu AB, Sekar G. Tetrahedron Lett. 2008;49:3147.. Naidu AB, Jaseer EA, Sekar G. J. Org. Chem. 2009;74:3675. doi: 10.1021/jo900438e.. Niu J, Guo P, Kang J, Li Z, Xu J, Hu S. J. Org. Chem. 2009;74:5075. doi: 10.1021/jo900600m.
- [3](a).For Pd-catalyzed C–O coupling of phenols, see: Aranyos A, Old DW, Kiyomori A, Wolfe JP, Sadighi JP, Buchwald SL. J. Am. Chem. Soc. 1999;121:4369.; Mann G, Incarvito C, Rheingold AL, Hartwig JF. J. Am. Chem. Soc. 1999;121:3224.; Harkal S, Kumar K, Michalik D, Zapf A, Jackstell R, Rataboul F, Riermeier T, Monsees A, Beller M. Tetrahedron Lett. 2005;46:3237.; Burgos CH, Barder TE, Huang X, Buchwald SL. Angew. Chem. Int. Ed. 2006;45:4321. doi: 10.1002/anie.200601253.; Hu T, Schulz T, Torborg C, Chen X, Wang J, Beller M. J. Huang, Chem. Commun. 2009:7330. doi: 10.1039/b915249k.
- [4](a).For Pd-catalyzed intermolecular C–O coupling of tertiary alcohols, see: Mann G, Hartwig JF. J. Am. Chem. Soc. 1996;118:13109.; Watanabe M, Nishiyama M, Koie Y. Tetrahedron Lett. 1999;40:8837.; Shelby Q, Kataoka N, Mann G, Hartwig JF. J. Am. Chem. Soc. 2000;122:10718.; Parrish CA, Buchwald SL. J. Org. Chem. 2001;66:2498. doi: 10.1021/jo001426z.
- [5](a).For Pd-catalyzed intramolecular C–O coupling of primary and secondary alcohols, see: Palucki M, Wolfe JP, Buchwald SL. J. Am. Chem. Soc. 1996;118:10333.; Torraca KE, Kuwabe S.-i., Buchwald SL. J. Am. Chem. Soc. 2000;122:12907. doi: 10.1021/ja012046d.; Kuwabe S.-i., Torraca KE, Buchwald SL. J. Am. Chem. Soc. 2001;123:12202. doi: 10.1021/ja012046d.
- [6](a).Torraca KE, Huang X, Parrish CA, Buchwald SL. J. Am. Chem. Soc. 2001;123:10770. doi: 10.1021/ja016863p. [DOI] [PubMed] [Google Scholar]; (b) Vorogushin AV, Huang X, Buchwald SL. J. Am. Chem. Soc. 2005;127:8146. doi: 10.1021/ja050471r. [DOI] [PubMed] [Google Scholar]
- [7].Only three examples of Pd-catalyzed C–O coupling of alcohols with heteroaryl halides have been previously described (See footnote 6).
- [8].Gowrisankar S, Sergeev AG, Anbarasan P, Spannenberg A, Neumann H, Beller M. J. Am. Chem. Soc. 2010;132:11592. doi: 10.1021/ja103248d. [DOI] [PubMed] [Google Scholar]
- [9](a).Watson DA, Su M, Teverovskiy G, Zhang Y, García-Fortanet J, Kinzel T, Buchwald SL. Science. 2009;325:1661. doi: 10.1126/science.1178239. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shen X, Hyde AM, Buchwald SL. J. Am. Chem. Soc. 2010;132:14076. doi: 10.1021/ja107481a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Maimone TJ, Buchwald SL. J. Am. Chem. Soc. 2010;132:9990. doi: 10.1021/ja1044874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10](a).Ikawa T, Barder TE, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2007;129:13001–13007. doi: 10.1021/ja0717414. [DOI] [PubMed] [Google Scholar]
- [11](a).Dooleweerdt K, Fors BP, Buchwald SL. Org. Lett. 2010;12:2350. doi: 10.1021/ol100720x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hicks JD, Hyde AM, Cuezva AM, Buchwald SL. J. Am. Chem. Soc. 2009;131:16720. doi: 10.1021/ja9044357. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fors BP, Buchwald SL. J. Am. Chem. Soc. 2009;131:12898. doi: 10.1021/ja905768k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Fors BP, Watson DA, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2008;130:13552. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].See supporting information for further studies on the effect of the substituent in the 6-position of the ligand.
- [13](a).For Cu-mediated or catalyzed C–O coupling with fluorinated alcohols, see: Suzuki H, Matuoka T, Ohtsuka I, Osuka A. Synthesis. 1985:499.; Vuluga D, Legros J, Crousse B, Bonnet-Delpon D. Eur. J. Org. Chem. 2009:3513. doi: 10.1021/jo9012699.
- [14].The conversion of 4-chlorodiphenyl ether, which coupled with n-BuOH using 2 mol% Pd in 24 h, was less than 80% (determined by GC) using variety of bases or at elevated temperature.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.