Abstract
AIM
Ankle–foot orthoses are the standard of care for foot drop in cerebral palsy (CP), but may overly constrain ankle movement and limit function in those with mild CP. Functional electrical stimulation (FES) may be a less restrictive and more effective alternative, but has rarely been used in CP. The primary objective of this study was to conduct the first trial in CP examining the acceptability and clinical effectiveness of a novel, commercially available device that delivers FES to stimulate ankle dorsiflexion.
METHOD
Twenty-one individuals were enrolled (Gross Motor Function Classification System [GMFCS] levels I and II, mean age 13y 2mo). Gait analyses in FES and non-FES conditions were performed at two walking speeds over a 4 month period of device use. Measures included ankle kinematics and spatiotemporal variables. Differences between conditions were revealed using repeated measures multivariate analyses of variance.
RESULTS
Nineteen individuals (nine females, 10 males; mean age 12y 11mo, range 7y 5mo to 19y 11mo; 11 at GMFCS level I, eight at level II) completed the FES intervention, with all but one choosing to continue using FES beyond that phase. Average daily use was 5.6 hours (SD 2.3). Improved dorsiflexion was observed during swing (mean and peak) and at foot floor–contact, with partial preservation of ankle plantarflexion at toe-off when using the FES at self-selected and fast walking speeds. Gait speed was unchanged.
INTERPRETATION
This FES device was well accepted and effective for foot drop in those with mild gait impairments from CP.
Cerebral palsy (CP) is the most common neuromuscular disorder among children.1 CP is a group of motor disorders resulting from a non-progressive injury during early brain development leading to impairments of movement and posture.2 Although the clinical presentation is heterogeneous, the ankle is the most commonly affected joint in individuals with CP who are ambulatory.3 Common impairments are insufficient ankle dorsiflexion during swing, or foot drop, and excessive plantarflexion during early to mid-stance. These abnormalities may cause standing and walking instability, and greater risk of tripping and falling.
Positional bracing is the current standard of care for individuals with CP who have limited ankle dorsiflexion. However, many people, particularly those with mild deficits, may choose not to wear orthoses because of the mobility restrictions they impose, or because of issues with comfort or cosmesis. By restricting ankle movement, orthoses may exacerbate muscle weakness and atrophy, leading to further loss of function over time.4 Functional electrical stimulation (FES) may be an effective alternative treatment for this population. In contrast to bracing, FES does not restrict motion, does produce muscle contraction, and thus has the potential to increase strength and motor control through repetitive neural stimulation over time.
The primary objective of this study was to conduct the first trial in CP examining the acceptability and effectiveness of a novel FES foot drop device on ankle motion and gait. We hypothesized that the FES device would be well accepted by children and adolescents with CP. We further hypothesized that it would be effective in increasing ankle dorsiflexion during swing and at initial foot floor–contact compared with the non-FES condition, while preserving ankle plantarflexion at toe-off, and that walking speed would be greater with the device.
METHOD
Study design
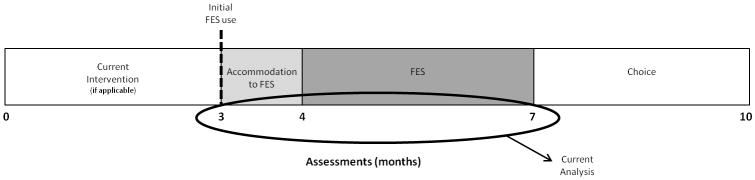
A total of five assessments were conducted for this trial (see Fig. 1 for a diagram of the study design). Data from three assessment points are included here. These include the assessments on the day participants received the device (month 3), after the 1 month accommodation phase (month 4), and after the 3 month FES intervention phase of wearing the device on a daily basis (month 7). Not included here is the initial assessment before a 3 month no-treatment baseline because they had not yet received the device (month 0), and the follow-up assessment 3 months after the FES intervention phase during which participants could choose to wear or not wear the device (month 10).
Figure 1.
Study design. Current analysis includes data obtained at months 3, 4, and 7 when walking trials comparing functional electrical stimulation (FES) with non-FES were obtained in the participants’ own footwear.
Participants
The initial goal was to enroll 20 children and adolescents over the age of 5 years with CP in this clinical trial. All participants were required to demonstrate unilateral foot drop, particularly the absence of initial heel contact. All participants could ambulate independently, and were therefore classified at Gross Motor Function Classification System (GMFCS) levels I or II.5 Participants were referred from the study group physiatrist’s (KEA) clinical practice, and through word of mouth from other participants.
Exclusion criteria were passive range of ankle motion less than 0° dorsiflexion with the knee extended, botulinum toxin injection to the plantar or dorsiflexor muscle groups within the 4 months before the study, orthopedic surgery to the legs in the previous year, or seizure in the previous 6 months. The study was approved by an institutional review board at the National Institutes of Health in Bethesda, MD, USA. Written informed consent was obtained from participants over 18 years of age and parents of minors. Written assent was obtained from participants under 18 years of age.
FES intervention
The FES device used (WalkAide, Innovative Neurotronics, Austin, TX, USA) delivers asymmetrical biphasic surface electrical stimulation to the common fibular (formerly common peroneal) nerve, triggered by an individually programmed tilt sensor, to improve foot clearance during the swing phase of gait. The major dorsiflexor of the ankle is the tibialis anterior muscle, which lifts and inverts the foot. The fibular (formerly peroneal) muscle group primarily everts the foot, with some contribution to plantarflexion.6 Minor adjustments in placement of the stimulating electrodes can more selectively activate the tibialis anterior and/or the fibular muscles to achieve the desired motion in both the sagittal and frontal planes.
In addition to the user-controlled amplitude dial, the stimulation parameters that are adjustable by the clinician include pulse frequency (16.7–33 pulses per second), pulse width (25–300μs), tilt angles to trigger stimulation off and on, presence of ramps up or down, minimum and maximum stimulation times, and a wait time in between consecutive stimulations. Software provided with the device is able to record walking data to guide decision making around these parameters.
Initial setup of the FES device occurred at the month 3 assessment after the baseline phase. A 1 month accommodation period of gradually increased use followed, with participants instructed to increase wear time from 30 minutes per day to 6 hours per day. During the 3 month FES phase, participants were asked to wear the device daily for at least 6 hours, during the times when they walked the most. After the FES phase, participants had the option to continue wearing the device for the final 3 months of the study.
Several strategies were used to increase tolerance and acceptability, including provision of family support and individualized modification of the stimulation settings. The accommodation phase allowed time to become comfortable with the stimulation and the device may have been important in promoting user acceptance. In addition, electrode placement and the customized control settings were reevaluated at each assessment and modified as needed to remain optimal. Participants experiencing difficulty had the option to return for assistance between assessments or seek phone support.
All devices were initially programmed with a low (25 or 50μs) pulse width to improve comfort. In addition, electrode placement that extended over the tibial crest was often uncomfortable and was avoided, while using the largest possible size electrodes to distribute the stimulation over a larger cutaneous area. Stimulation amplitude was initially low and gradually increased with tolerance during the initial visit and throughout the accommodation phase to optimize effectiveness. Finally, a short ramp up of the stimulation often improved comfort if needed, and decreased the elicitation of a stretch response from the plantarflexors in cases of high spasticity.
The number of stimulations delivered during walking per day (up to 71 days) could be recorded by the device used, and was one measure of acceptability. The percentage of those who chose to continue using FES after the month 7 assessment was a second measure of acceptability.
Assessment procedures
Three-dimensional lower extremity spatiotemporal and kinematic data were collected with a ten-camera motion capture system (Vicon MX, Vicon, Lake Forest, CA, USA) using a custom 6 degree of freedom gait model with a 34-cluster-based reflective marker set. Three foot markers were placed on the shoes by palpating the second and fifth metatarsals through the shoes, and then aligning the heel marker with the second metatarsal. Markers remained in place for all four conditions. Participants walked overground in their own shoes (and lift and/or supramalleolar orthosis, if applicable) at self-selected and fast speeds. They were instructed to walk at a ‘normal, comfortable pace’ and ‘as fast as possible,’ respectively. Three to six walking trials were completed for each speed. These procedures were then repeated while the participants wore the FES device with their footwear. Most participants (15/19) wore athletic shoes at each visit. One brought backless sandals to the month 7 session, so her gait data were not used for that visit. The other four wore other types of shoes with a back for one or two sessions. Owing to scheduling needs, gait data were collected at the month 3 assessment before receipt and setup of the FES device in six participants, therefore FES condition gait data were not obtained.
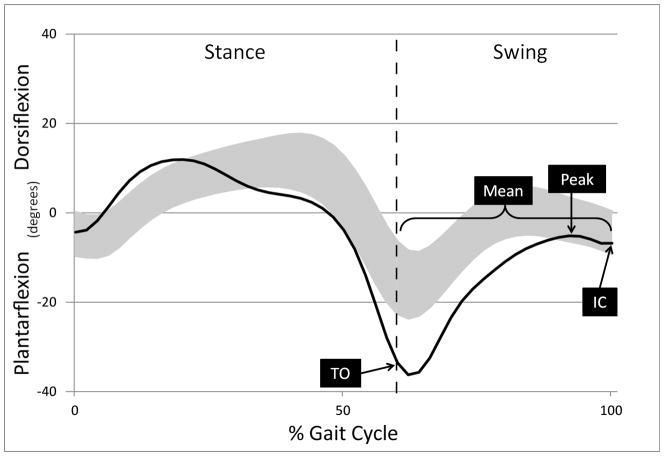
Five right and five left gait cycles were extracted for analysis and averaged from each of the four conditions (FES and non-FES at two speeds each) using Visual3D software (C-motion, Gaithersburg, MD). The spatiotemporal variables chosen to represent general gait function were walking speed, cadence, and step length. Walking speed and step length were normalized to height.7 The kinematic variables chosen to represent ankle-specific function were peak and mean dorsiflexion angle in swing, ankle angle at toe-off, and ankle angle at initial foot–floor contact. See Figure 2 for kinematic variables labeled on representative data. Barefoot walking conditions and measures of muscle architecture, surface electromyography, and kinetics were also collected and will be reported elsewhere.
Figure 2.
Kinematic gait variables are noted on representative mean ankle angle data from one participant (black line) during the gait cycle (foot contact, 0%, to subsequent foot contact, 100%). The gray band represents one standard deviation around the mean for typical gait. Stance phase is the period when the limb is in contact with the ground, swing phase is the period when the limb is not in contact with the ground and is advancing forward. The transition from stance to swing phase (toe-off) is noted by the dashed vertical line. TO, ankle angle at toe-off; Peak, maximum dorsiflexion in swing; Mean, mean ankle angle in swing; IC, ankle angle at initial floor contact.
Statistical analysis
Data were tested for normality and repeated measures multivariate analyses of variance (α-level=0.05) were conducted on each group of variables (spatiotemporal and kinematic) at each speed (self-selected and fast) to determine if differences existed between the two conditions (FES and non-FES). Use of multivariate analyses of variance on independent groups of variables reduced the likelihood of type I errors that would be risked with multiple univariate analyses on related variables. When indicated, post hoc tests were performed to identify which specific gait variables were affected by the FES (α-level=0.05).
RESULTS
Acceptability
The first hypothesis was supported. Nineteen of the 21 participants who enrolled successfully completed the accommodation phase and entered the FES phase, and 18 (86%) of those chose to continue using the FES after the 3 month FES phase. The two individuals who did not continue to the FES phase included one child (9y old) who was unable to tolerate the stimulation and one adolescent (14y old) in whom the stimulation triggered dystonic posturing of the foot. Both wore the device several times after the initial setup, but neither experienced an improvement and therefore neither continued to the month 4 assessment or the FES phase. The remaining 19 participants (nine females, 10 males) had a mean age of 12 years 11 months (range 7y 5mo to 19y 11mo). Eleven were classified at GMFCS level I and eight were at level II. The more affected side was the right side in 12 participants and the left side in seven participants.
One participant (17y) withdrew from the study midway through the FES phase after deciding that the benefit for him was not worth the inconvenience to don and wear the device, and therefore was the sole participant who chose not to continue using it after the FES phase. The other 18 participants, on average, used the FES device 5.6 hours per day (SD 2.3) and took an average of 2087 steps (SD 1039) with the affected leg per day during the FES phase. Ten participants used the device at least 6 hours per day, four used it 4 to 6 hours per day, three used it 2 to 4 hours per day, and one used it less than 2 hours per day. See Table I for use data.
Table I.
Participant functional electrical stimulation use during the 3-month treatment phase
| Stimulations per daya | Hours per day | |
|---|---|---|
| Participants who chose to continue after the month 7 assessment (n=18)
|
||
| Mean (SD) | 2087 (1039) | 5.6 (2.3) |
| Minimum | 485 | 1.5 |
| Maximum | 3999 | 9.4 |
|
|
||
| Participant who withdrew before the month 7 assessment (n=1)
|
||
| Mean | 369 | 1.2 |
One stimulation per step on the more affected side. Multiply by 2 to calculate the number of total steps taken when wearing the functional electrical stimulation device.
One participant who completed the study demonstrated significant plantarflexor spasticity on a visit to his physiatrist (KEA) during the FES phase, and an injection of botulinum toxin was medically indicated to decrease the spasticity. He continued using FES and attended all planned study assessments, but gait data collected at the month 7 assessment were not included in the analysis due to the confounding factor of the injection.
Effect on ankle kinematics and gait function
The improvements in ankle kinematics observed when the participants used FES supported the second hypothesis. At both speeds, significant increases in dorsiflexion were observed at initial contact (p=0.017 for self-selected and 0.032 for fast) and for peak (p=0.015 for both speeds) and mean (p=0.011 for self-selected and 0.014 for fast) dorsiflexion values during swing compared with the non-FES condition. Additionally, some plantarflexion motion was preserved at toe-off with the use of FES. There was a significant mean shift towards greater dorsiflexion at toe-off of 2.1° and 2.6° across all time points, for self-selected and fast speeds respectively, in the FES compared with the non-FES condition (p=0.033 and 0.038 respectively). However, the ankle remained in a plantarflexed position at toe-off at both speeds with the FES (mean of −3.5° for self-selected and −7.2° for fast across all time points). This is presumably more plantarflexion than would be allowed at toe-off when wearing ankle orthoses, which typically are designed to block plantarflexion beyond a neutral ankle angle (0°). See Table II for ankle kinematic data.
Table II.
Group values for gait spatiotemporal and kinematic variables for non-functional electrical stimulation (FES) and FES conditions
| Self-selected walking | Month 3 | Month 4 | Month 7 | |
|---|---|---|---|---|
| Walking speed | Non-FES | 0.30 (0.04) | 0.31 (0.03) | 0.31 (0.03)a |
| FES | 0.30 (0.03)b | 0.32 (0.03) | 0.31 (0.03)a | |
| Mean difference | 0.00 | 0.00 | 0.00 | |
| (95% CI) | (−0.02 to 0.01)b | (0.00 to 0.01) | (−0.01 to 0.01)a | |
| Cadence (steps/min) | Non-FES | 115.8 (9.8) | 117.0 (8.4) | 115.7 (8.4)a |
| FES | 117.2 (7.7)b | 118.3 (8.7) | 113.5 (7.3)a | |
| Mean difference | 0.6 | 1.2 | −2.1 | |
| (95% CI) | (−2.7 to 4.0)b | (−0.7 to 3.2) | (−4.7 to 0.4)a | |
| Step length | Non-FES | 0.40 (0.04) | 0.41 (0.03) | 0.41 (0.04)a |
| FES | 0.41 (0.05)b | 0.42 (0.04) | 0.42 (0.04)a | |
| Mean difference | 0.00 | 0.00 | 0.01 | |
| (95% CI) | (−0.01 to 0.01) | (0.00 to 0.01) | (−0.01 to 0.02)a | |
| c Ankle angle at initial contact (°, p=0.017) | Non-FES | −4.6 (3.7) | −6.7 (3.6) | −5.9 (4.4)a |
| FES | −3.7 (3.2)b | −4.3 (3.6) | −1.9 (5.3)a | |
| Mean difference | 0.4 | 2.4 | 4.0 | |
| (95% CI) | (−1.5 to 2.2)b | (1.2–3.6) | (1.6–6.4)a | |
| c Peak DF angle in swing (°, p=0.015) | Non-FES | 0.7 (3.8) | −1.6 (3.9) | −0.3 (4.8)a |
| FES | 2.9 (4.9)b | 1.1 (3.4) | 3.5 (5.3)a | |
| Mean difference | 1.5 | 2.6 | 3.8 | |
| (95% CI) | (0.0–3.0)b | (1.6–3.7) | (1.4–6.1)a | |
| c Mean DF angle in swing (°, p=0.011) | Non-FES | −4.5 (5.0) | −7.5 (5.6) | −5.7 (6.2)a |
| FES | −2.4 (5.7)b | −4.3 (4.5) | −2.0 (5.7)a | |
| Mean difference | 1.8 | 3.2 | 3.7 | |
| (95% CI) | (−0.4 to 4.0)b | (2.1–4.4) | (1.7–5.6)a | |
| c Ankle angle at toe-off (°, p=0.033) | Non-FES | −5.0 (5.1) | −7.4 (5.5) | −5.4 (4.9)a |
| FES | −2.5 (4.7)b | −4.7 (3.8) | −3.2 (5.3)a | |
| Mean difference | 1.4 | 2.7 | 2.1 | |
| (95% CI) | (−0.1 to 2.9)b | (1.2–4.2) | (0.3–4.0)a | |
|
| ||||
| Fast walking
| ||||
| Walking speed | Non-FES | 0.42 (0.05) | 0.42 (0.05) | 0.44 (0.07)a |
| FES | 0.41 (0.04)b | 0.43 (0.05) | 0.44 (0.07)a | |
| Mean difference | 0.00 | 0.00 | 0.00 | |
| (95% CI) | (−0.02 to 0.01)b | (−0.01 to 0.02) | (−0.01 to 0.01)a | |
| Cadence (steps/min) | Non-FES | 140.0 (18.7) | 141.5 (17.3) | 142.5 (21.4)a |
| FES | 140.1 (15.1)b | 142.0 (17.1) | 142.5 (21.7)a | |
| Mean difference | 2.9 | 0.5 | 0.0 | |
| (95% CI) | (−1.7 to 7.4)b | (−4.0 to 4.9) | (−3.5 to 3.6)a | |
| Step length | Non-FES | 0.46 (0.04) | 0.46 (0.04) | 0.46 (0.05)a |
| FES | 0.46 (0.04)b | 0.46 (0.04) | 0.47 (0.05)a | |
| Mean difference | −0.01 | 0.00 | 0.01 | |
| (95% CI) | (−0.03 to 0.00)b | (−0.01 to 0.01) | (−0.01 to 0.02)a | |
| c Ankle angle at initial contact (°, p=0.032) | Non-FES | −5.0 (3.3) | −6.1 (3.1) | −6.0 (4.2)a |
| FES | −3.9 (3.4)b | −4.6 (3.7) | −3.9 (5.3)a | |
| Mean difference | 0.8 | 1.5 | 2.1 | |
| (95% CI) | (−1.0 to 2.5)b | (0.3–2.8) | (0.4–3.8)a | |
| c Peak DF angle in swing (°, p=0.015) | Non-FES | 0.3 (4.2) | −1.1 (3.7) | −0.8 (5.2)a |
| FES | 1.9 (5.3)b | 0.8 (4.1) | 3.1 (5.1)a | |
| Mean difference | 1.0 | 2.0 | 3.9 | |
| (95% CI) | (−0.8 to 2.8)b | (1.0–2.9) | (2.1–5.7)a | |
| c Mean DF angle in swing (°, p=0.014) | Non-FES | −5.8 (5.2) | −7.3 (5.6) | −6.3 (6.0)a |
| FES | −4.2 (6.0)b | −4.9 (5.3) | −2.1 (5.7)a | |
| Mean difference | 1.4 | 2.4 | 4.2 | |
| (95% CI) | (−0.7 to 3.5)b | (1.5–3.3) | (2.4–6.1)a | |
| c Ankle angle at toe-off (°, p=0.038) | Non-FES | −9.2 (6.2) | −10.9 (6.2) | −10.1 (7.0)a |
| FES | −7.0 (6.3)b | −8.5 (6.2) | −6.1 (8.1)a | |
| Mean difference | 1.5 | 2.4 | 4.0 | |
| (95% CI) | (−0.4 to 3.3)b | (1.2–3.6) | (2.0–6.0)a | |
Values are means (SD). Walking speed and step length are dimensionless values, normalized to subject height. Unless otherwise indicated, the full data set is represented (n=19);
n=16;
n=13.
Significant differences (p<0.05) between non-FES and FES conditions. DF, dorsiflexion.
The third hypothesis, that the device would increase walking speed, was not supported, nor were any differences in other spatiotemporal gait parameters observed at either self-selected (p=0.137) or fast (p=0.106) speeds.
DISCUSSION
This study reports improvements in ankle dorsiflexion with partly preserved ankle plantarflexion during FES use in independently ambulatory individuals with CP. There was good acceptability of the FES device in this sample. Despite all participants having inadequate dorsiflexion during swing and at foot–floor contact without the device, few used an ankle–foot orthosis, suggesting that positional bracing is not necessarily a well-accepted treatment option in this population. FES may be a more acceptable and more effective treatment alternative in individuals with CP who have mild gait impairments.
FES has not traditionally been considered a viable long-term treatment option in children because of concerns about tolerance, feasibility, and effectiveness. However, the current results dispute these concerns. The results here are generally consistent with a descriptive study by Durham and colleagues8 who reported that nine of 12 children with CP accepted the Odstock stimulator. Three of the nine did not use the device as often as suggested, but still wanted to continue wearing it after the study ended. The Odstock is another FES device for foot drop that includes a controller worn on the waist with lead wires to the stimulating electrodes on the leg and to a footswitch worn in the shoe. Factors limiting wear in their study were that the device was large, and the wires difficult for children to manage at school. The self-contained design of the device used in the current study may explain our slightly better acceptance rate. Our strategy of providing a high-level family support as well as individually modifying the stimulation parameters may have also contributed to the high tolerance and acceptability. In fact, two participants who had trialed this same device in the past and did not use it long-term were successful with this comprehensive approach to encourage acceptability.
The current tolerance results are superior to the outcomes of Postans et al.9 who reported that six of 21 children did not tolerate electrical stimulation during their trial. However, they stimulated two large antigravity muscle groups in the lower extremities, which typically require higher amounts of stimulation to produce functional contraction.
To our knowledge, this is the only trial so far in this population evaluating this novel device. Use of FES increased dorsiflexion during the swing phase of gait, as intended. The decrease in plantarflexion at toe-off, although not to a neutral ankle position as with an ankle–foot orthosis, may reduce push-off power and raises the question of whether the stimulation was perhaps delivered too early or too strongly at that point. The tibialis anterior typically becomes active before toe-off10 and stimulation is designed to mimic that. This observation of FES affecting ankle angle at toe-off may also reflect differences between the onset of muscle contraction from artificial electrical stimulation compared with preparatory activation from the central nervous system. Clinicians should be attentive to onset timing so as not to interfere with propulsion more than needed, recognizing the challenge of determining the optimal balance of force and timing for each patient.
Activation of the tibialis anterior and peroneals is the only option with this commercially available device. Others have investigated and reported some benefits of plantarflexor stimulation, with and without accompanying dorsiflexor stimulation, in those with CP or after stroke.11–16 The combination should be studied in larger trials with more rigorous study designs.
The observations of increased dorsiflexion during swing and improved foot floor–contact, with no change in spatiotemporal gait characteristics, are consistent with several other reports of FES in smaller samples of children with CP.8,9,13,14,17,18 The magnitude of change in dorsiflexion between the FES and non-FES conditions is also similar to that reported by Kesar and colleagues in a group of adults with post-stroke hemiplegia.19
However, two different groups of adults with central nervous system lesions who used this same device demonstrated increases in walking speed, contrasting with our observations in the current sample.20 These increases in walking speed were 5.0% in the group with non-progressive lesions and 5.7% in the group with progressive lesions. A significant difference in walking speed between the FES and non-FES conditions was not present in earlier work by the same group in a smaller sample.21 All participants in those studies initially walked at a speed slower than 1.2m/s, indicating perhaps greater gait impairment than participants in the current sample. When normalizing velocity for height, five of our 19 participants would not have met the inclusion criteria for the Stein studies because they walked too fast. Changes in walking speed might be less likely to occur in our sample because many already walked at a more functional speed.
Common factors in this population that may limit the amount of dorsiflexion achieved with stimulation, such as gastrocnemius muscle contracture and spasticity, should be considered when determining if FES could benefit individual patients. Further investigation should address several remaining questions. No direct comparison with an orthosis condition could be made as was originally planned in the current study because only one individual wore an ankle–foot orthosis at the time of initial FES use. However, this comparison would be valuable, and is recommended at least on an individual basis for clinical decision making with those individuals who do use a brace. The fact that only one participant was wearing an ankle–foot orthosis at the time of initial FES use, despite all having a history of previous orthosis prescription, suggests that ankle–foot orthoses may not be the best solution for this population. FES may be most appropriate for those children with mild CP, as in this study, who may feel overly constrained by an ankle orthosis, or for those who choose not to wear a brace because of issues with comfort or cosmesis.
We excluded those who had recent botulinum toxin injections because this would confound the study results, but the combination of FES with botulinum toxin injections may be particularly advantageous in this population, and should be investigated for added benefit. Additionally, it should be determined if optimal dosage parameters can be predicted based on individual characteristics, if FES can lead to increases in muscle size and strength, and if FES has neuroplastic effects on the central nervous system in children with brain injuries as has been shown in adults.22
What this paper adds.
This study is the first trial in cerebral palsy (CP) demonstrating the acceptability and clinical effectiveness of a novel, commercially available device that delivers functional electrical stimulation to improve ankle dorsiflexion.
The device studied here quantifies compliance, showing it to be very high in children and adolescents with mild CP.
This device improves swing phase dorsiflexion while allowing plantarflexion and may prove superior to traditional bracing in those with mild gait impairments.
Acknowledgments
We acknowledge Chris Stanley, Cristiane Zampieri-Gallagher, and Laurie Ohlrich, for their assistance with data collection and entry, and Sara Sadeghi for her assistance with scheduling and protocol management. This research was supported by the Intramural Research Program of the National Institutes of Health Clinical Center.
ABBREVIATIONS
- FES
Functional electrical stimulation
References
- 1.Pakula AT, Van Naarden Braun K, Yeargin-Allsopp M. Cerebral palsy: classification and epidemiology. Phys Med Rehabil Clin N Am. 2009;20:425–52. doi: 10.1016/j.pmr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum P, Paneth N, Leviton A, et al. A report: The definition and classification of cerebral palsy, April 2006. Dev Med Child Neurol. 2007;109 (Suppl):8–14. [PubMed] [Google Scholar]
- 3.Fowler EG, Staudt LA, Greenberg MB. Lower-extremity selective voluntary motor control in patients with spastic cerebral palsy: increased distal motor impairment. Dev Med Child Neurol. 2010;52:264–9. doi: 10.1111/j.1469-8749.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 4.Psatha M, Wu Z, Gammie FM, et al. A longitudinal MRI study of muscle atrophy during lower leg immobilization following ankle fracture. J Magn Reson Imaging. 2012;35:686–95. doi: 10.1002/jmri.22864. [DOI] [PubMed] [Google Scholar]
- 5.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 6.Kendall FP, McCreary EK, Kendall HO. Muscles, Testing and Function. 3. Baltimore, MD: Williams & Wilkins; 1983. [Google Scholar]
- 7.Hof A. Scaling gait data to body size. Gait Posture. 1996;4:222–3. doi: 10.1016/s0966-6362(01)00097-2. [DOI] [PubMed] [Google Scholar]
- 8.Durham S, Eve L, Stevens C, Ewins D. Effect of functional electrical stimulation on asymmetries in gait of children with hemiplegic cerebral palsy. Physiotherapy. 2004;90:82–90. [Google Scholar]
- 9.Postans NJ, Granat MH. Effect of functional electrical stimulation, applied during walking, on gait in spastic cerebral palsy. Dev Med Child Neurol. 2005;47:46–52. doi: 10.1017/s0012162205000083. [DOI] [PubMed] [Google Scholar]
- 10.Craik R, Oatis CA. Gait Analysis: Theory and Application. 1. St. Louis, MO: Mosby; 1995. [Google Scholar]
- 11.Kesar TM, Reisman DS, Perumal R, et al. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture. 2011;33:309–13. doi: 10.1016/j.gaitpost.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesar TM, Perumal R, Reisman DS, et al. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: effects on poststroke gait. Stroke. 2009;40:3821–7. doi: 10.1161/STROKEAHA.109.560375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlin MN, Pierce SR, Stackhouse CL, et al. Immediate effect of percutaneous intramuscular stimulation during gait in children with cerebral palsy: a feasibility study. Dev Med Child Neurol. 2005;47:684–90. doi: 10.1017/S0012162205001398. [DOI] [PubMed] [Google Scholar]
- 14.Pierce SR, Laughton CA, Smith BT, Orlin MN, Johnston TE, McCarthy JJ. Direct effect of percutaneous electric stimulation during gait in children with hemiplegic cerebral palsy: a report of 2 cases. Arch Phys Med Rehabil. 2004;85:339–43. doi: 10.1016/s0003-9993(03)00473-8. [DOI] [PubMed] [Google Scholar]
- 15.Carmick J. Managing equinus in children with cerebral palsy: electrical stimulation to strengthen the triceps surae muscle. Dev Med Child Neurol. 1995;37:965–75. doi: 10.1111/j.1469-8749.1995.tb11951.x. [DOI] [PubMed] [Google Scholar]
- 16.Comeaux P, Patterson N, Rubin M, Meiner R. Effect of neuromuscular electrical stimulation during gait in children with cerebral palsy. Pediatr Phys Ther. 1997;9:103–9. [Google Scholar]
- 17.van der Linden ML, Hazlewood ME, Hillman SJ, Robb JE. Functional electrical stimulation to the dorsiflexors and quadriceps in children with cerebral palsy. Pediatr Phys Ther. 2008;20:23–9. doi: 10.1097/PEP.0b013e31815f39c9. [DOI] [PubMed] [Google Scholar]
- 18.Ho CL, Holt KG, Saltzman E, Wagenaar RC. Functional electrical stimulation changes dynamic resources in children with spastic cerebral palsy. Phys Ther. 2006;86:987–1000. [PubMed] [Google Scholar]
- 19.Kesar TM, Perumal R, Jancosko A, et al. Novel patterns of functional electrical stimulation have an immediate effect on dorsiflexor muscle function during gait for people poststroke. Phys Ther. 2010;90:55–66. doi: 10.2522/ptj.20090140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein RB, Everaert DG, Thompson AK, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010;24:152–67. doi: 10.1177/1545968309347681. [DOI] [PubMed] [Google Scholar]
- 21.Stein RB, Chong S, Everaert DG, et al. A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair. 2006;20:371–9. doi: 10.1177/1545968306289292. [DOI] [PubMed] [Google Scholar]
- 22.Everaert DG, Thompson AK, Chong SL, Stein RB. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. 2010;24:168–77. doi: 10.1177/1545968309349939. [DOI] [PubMed] [Google Scholar]