Abstract
Objective
Evidence suggests intermuscular adipose tissue (IMAT) may be linked to insulin resistance, whereas thigh subcutaneous adipose tissue (SAT) may be related favorably with indices of metabolic health. However, whether adipose tissue depots of the thigh are differentially related to insulin sensitivity independent of total adiposity and other adipose tissue depots has not been determined. The objective of this study was to identify independent associations of the subcompartments of adipose tissue of the thigh with insulin sensitivity among 97 healthy early postmenopausal women.
Materials/Methods
Computed tomography (CT) scans of the mid-thigh were used to assess Thigh-SAT, Thigh perimuscular adipose tissue (PMAT), and Thigh-IMAT. CT scans at the L4-L5 intervertebral space were used to assess intra-abdominal adipose tissue (IAAT) and Abdominal-SAT. Total body fat was measured by dual-energy X-ray absorptiometry (DXA). The insulin sensitivity index (SI) was assessed by using a frequently sampled intravenous glucose tolerance test with minimal model analysis.
Results
Results indicated SI was positively associated with Thigh-SAT independent of total fat mass and other adipose tissue compartments. Among all women combined, SI was inversely associated with Thigh-IMAT independent of total fat mass. However, the relationship between SI and Thigh-IMAT was independent of IAAT only among women with high levels of Thigh-IMAT and IAAT.
Conclusions
This is the first study to demonstrate independent, opposing relationships of Thigh-SAT and Thigh-IMAT with insulin sensitivity in healthy postmenopausal women. Further research is needed to determine if these associations are causal in nature.
Keywords: Subcutaneous adipose tissue, Intermuscular adipose tissue, Fat distribution, insulin resistance
Introduction
Adipose tissue depots are differentially associated with risk of diseases such as type 2 diabetes and cardiovascular disease. Whereas intra-abdominal adipose tissue (IAAT) is associated with glucose intolerance and insulin resistance, thigh adipose tissue is associated favorably with measures of glucose and lipid metabolism1;2. However, it is unknown whether differences in fat distribution have causal associations with metabolic health or primarily reflect underlying metabolic processes that affect both glucose/lipid metabolism and the location of triglyceride storage.
Further, the nature of the relationship between leg fat and insulin sensitivity is not entirely clear. While some studies demonstrate relationships linking proportionally greater leg fat to favorable fasting insulin and glucose concentrations and blood lipid profile3, other studies have demonstrated an inverse relationship between leg fat and insulin sensitivity4;5. Discrepancies among studies may be explained by differences in adjustment for confounders in statistical models and differences in the specific adipose tissue compartments examined. The thigh region is comprised of multiple adipose tissue compartments including subcutaneous adipose tissue (SAT), perimuscular adipose tissue (PMAT), and intermuscular adipose tissue (IMAT). Lipid is also found within muscle cells as intramyocellular lipid (IMCL). Further, divergent relationships may exist as to the way in which these adipose tissue compartments contribute to metabolic health. Whole body-IMAT is proposed to be an adipose tissue depot similar in size to IAAT6;7. Evidence suggests adipose tissue infiltration in skeletal amuscle, like IAAT, is associated with greater circulating inflammatory markers and may contribute to insulin resistance and other cardio-metabolic disease risk factors8;9. Conversely, Thigh-SAT may be positively related to insulin sensitivity when examined independently of the other adipose tissue depots of the thigh10;11. Thus, use of total thigh fat as an independent variable does not allow for visualization of the opposing effects of the individual compartments, and may lead to discrepant results depending upon the extent to which each of the compartments contributes to the total measure. No study has simultaneously shown independent, opposing relationships among these thigh adipose tissue depots and a robust measure of insulin sensitivity.
Among the studies that have aimed to characterize the multiple adipose tissue compartments of the thigh and their contributions to metabolic health, differences in the definition of Thigh-IMAT, study populations, and scan location and modality may have lead to discrepant results. Although Thigh-IMAT is often considered as all adipose tissue deposited beneath the fascia lata within and adjacent to skeletal muscle, sub-compartments within Thigh-IMAT have been identified5. Goodpaster et al described the adipose tissue compartments of the thigh as Thigh SAT, Thigh-PMAT (also described as subfascial adipose tissue), and Thigh-IMAT5. With this characterization, Thigh-IMAT was inversely associated, Thigh-PMAT tended to be inversely associated, and Thigh-SAT was not associated with insulin sensitivity in subjects with obesity and type 2 diabetes5. These relationships were not independent of total body fat. Other study populations within which Thigh-IMAT and insulin resistance are associated include elderly men or those with at least one risk factor for diabetes9. No study has examined the relationship of insulin sensitivity with thigh adipose tissue distribution in a healthy, relatively homogeneous study population, specifically early postmenopausal women. These associations may be important considering that changes occur in both disease risk12;13 and body fat distribution14;15 following menopause.
The primary purpose of this study was to investigate independent associations of Thigh-SAT, Thigh-PMAT, and Thigh-IMAT with insulin sensitivity in healthy early postmenopausal women. A secondary aim was to identify associations between Thigh-IMAT and other adipose tissue compartments such as IAAT, Thigh-PMAT, and Thigh-SAT. We hypothesized that Thigh-SAT would be positively associated with, and Thigh-IMAT and Thigh-PMAT would be inversely associated with, insulin sensitivity, and furthermore, that Thigh-IMAT would be positively associated with IAAT.
Methods and Procedures
Subjects were 97 healthy postmenopausal women aged 45-60 years who participated in one of two studies at the University of Alabama at Birmingham (UAB). Metabolic testing and body composition assessment took place under controlled conditions during an in-patient visit to the Department of Nutrition Sciences and the General Clinical Research Center (GCRC) at UAB. Women who experienced a natural menopause, with the time of cessation of menstruation of at least 6 months, or hysterectomy, and FSH level >30 IU/mL (FSH ranged 44–138 IU/mL) were included in the study. Fifty-two percent of the women recruited for the study were using hormone replacement therapy (HRT) and had been using HRT for an of average 2.8 yrs. Among the women recruited, 9% were African American (n=9) and 91% European American (n=78). Five of the African American women were HRT users. None of the women smoked. The protocol was approved by the Institutional Review Board for Human Use at UAB, and all subjects signed an informed consent prior to testing.
Body composition and fat distribution
Mid-thigh and abdominal cross-sectional tissue areas were analyzed by computed tomography (CT) scanning using a HiLight/Advantage scanner (General Electric, Milwaukee, WI). Thigh muscle and thigh adipose tissue areas were determined using a one slice mid-thigh (between the superior border of the patella and the inferior anterior iliac crest) CT scan. A scout scan was conducted to identify the L4-L5 intervertebral spaces and was followed by a 5-mm scan of the abdomen at the identified site. Scans were later analyzed for cross-sectional area (cm2) of adipose tissue and muscle tissue using SliceOmatic image analysis software (version 4.2: Tomovision, Montreal, Canada). The abdomen scan was used to analyze IAAT and Abdominal-SAT. Thigh-IMAT and Thigh-PMAT were separated from Thigh-SAT by manually drawing a line along the fascia lata surrounding the thigh muscle. Subsequently, Thigh-IMAT was partitioned from Thigh-PMAT by manually drawing a line around the muscle itself to capture adipose tissue located directly between and within muscle groups.
Total and regional body composition were measured by dual-energy X-ray absorptiometry (DXA) using a Lunar DPX-L densitometer (LUNAR Radiation Corp., Madison, WI). Subjects were scanned in light clothing while lying flat on their backs with arms at their sides. DXA scans were performed and analyzed with adult software version 1.5g. The software provided measures of total fat (kg), total lean (kg), and leg fat (kg).
Insulin sensitivity testing
Glucose tolerance and insulin sensitivity were measured by frequently sampled intravenous glucose tolerance (FSIGT) test16 and details were described elsewhere17;18. In brief, the FSIGT test involves intravenous glucose administration (11.4 g/m2)at time “0”, and subsequent tolbutamide injection(125 mg/m2) or insulin infusion (0.02 units/kg over 5 min) 20 minutes later. For both tests, three samples were collected prior to glucose administration, and glucose and insulin values averaged to determine mean fasting values. For the tolbutamide-modified test, 29 additional blood samples (2 ml each) were collected at time points +2 to +180 minutes relative to the initiation of glucose administration. For the insulin-modified test, 31 additional blood samples (2 ml each) were collected at time points +2 to +240 minutes. All samples were analyzed for insulin and glucose concentrations. The insulin sensitivity index (SI) was calculated using MINMOD computer software (version 3.0)19;20. In this study, the tolbutamide-modified method was used for 81 women and the insulin-modified method was used for 16 women. SI values from tolbutamide-modified tests are reported to be approximately 16% higher than those from insulin-modified tests21. Between-study differences in FSIGT were accounted for statistically by including a variable for FSIGT method (“test type” variable) in analyses as previously described22.
Assay of glucose and insulin
Glucose was measured in 10 μl sera using an Ektachem DT II System (Johnson and Johnson Clinical Diagnostics, Rochester, NY). For the majority of the postmenopausal women (those who had the tolbutamide-modified FSIGT), insulin was assayed in duplicate 200 μl aliquots with “Coat-A-Count” kits (Diagnostic Products Corporation, Los Angeles, CA). In our laboratory, this assay has a sensitivity of 1.9 μIU/ml, a mean intra-assay coefficient of variation of 5%, and a mean interassay coefficient of variation of 6%. For the remainder of the women (those who had the insulin-modified FSIGT), insulin was assayed in duplicate 100 μl aliquots with reagents from Linco Research Products Inc. (St. Charles. MO). In our laboratory, this assay has a sensitivity of 3.35 μIU/ml, a mean intra-assay coefficient of variation of 3.49%, and a mean interassay coefficient of variation of 5.57%. Commercial quality control sera of low, medium, and high insulin concentration are included in every assay to monitor variation over time. Difference in methodology was accounted for by inclusion of a “test type” variable in analyses.
Statistical methods
Descriptive statistics were computed for all study variables of interest. Variables known to deviate from a normal distribution, such as SI, were log 10 transformed prior to statistical analysis. All statistical tests were two-sided and were performed using a type I error rate of 0.05. Statistical analyses were performed using SAS (version 9.1; SAS Institute, Inc., Cary, NC). Pearson correlation coefficients were calculated to determine associations of SI with Thigh-SAT, Thigh-PMAT, Thigh-IMAT, IAAT, Abdominal -SAT, and total fat mass. Partial correlation coefficients were calculated to determine associations of SI with Thigh-SAT, Thigh-PMAT, Thigh-IMAT, IAAT, and Abdominal-SAT adjusted for total fat mass. Thigh-IMAT was further adjusted for thigh muscle area. Multiple linear regression analysis was conducted to determine independent associations of SI with Thigh-SAT, Thigh-IMAT, total fat, and IAAT. This model was also adjusted for thigh muscle area. All final models were adjusted for “test type.” HRT use was also tested as in all models as a confounding variable; however, HRT use was not significant and subsequently was excluded from models.
To further explore the interrelationships among IAAT, Thigh-SAT, and SI, subjects were characterized into four fat distribution phenotype groups based on IAAT and Thigh-SAT. The median IAAT (103.2 cm2) and median Thigh-SAT (238.5 cm2) were used to classify subjects into 1 of 4 groups including 1) high IAAT/high Thigh-SAT, 2) high IAAT/low Thigh-SAT, 3) low IAAT/high Thigh-SAT, and 4) low IAAT/low Thigh-SAT. Analysis of covariance (ANCOVA) was used to compare group differences in SI adjusted for total fat. Additionally, because IAAT has a profound effect on hepatic SI that may mask other contributions to whole-body insulin sensitivity, subjects were dichotomized into 2 groups, low (<103.2 cm2) or high (>103.2 cm2) IAAT using the median IAAT value of the entire study population. Multiple linear regression analysis was conducted to determine independent associations of SI and Thigh-IMAT, % fat, and IAAT within each group.
Pearson correlation coefficients were calculated to determine associations among fat distribution variables: Thigh-SAT, Thigh-PMAT, Thigh-IMAT, IAAT, and Abdominal-SAT. Partial correlation coefficients were also calculated to determine associations among Thigh-SAT, Thigh-PMAT, Thigh-IMAT, IAAT, and Abdominal-SAT independent of total fat mass.
Results
Subject characteristics are shown in Table 1. Pearson simple correlation analysis indicated all fat distribution variables were significantly inversely associated with SI and positively associated with HOMA-IR (Table 2). Partial correlation analysis indicated that Thigh-IMAT and IAAT were inversely associated with SI independent of total fat mass. Partial correlation analysis also indicated that Thigh-SAT was positively associated with SI independent of total fat (Table 2). Multiple linear regression analysis indicated that SI was positively associated with Thigh-SAT and inversely associated with IAAT independent of total fat mass and other leg fat variables (Table 3).
Table 1.
Subject characteristics (n=97)
| Variable | Mean±SD |
|---|---|
| Age (yrs) | 50.8 ± 2.8 |
| BMI (kg/m2) | 26.2 ± 4.7 |
| Fasting Insulin (μIU/ml) | 9.9 ± 5.1 |
| Fasting Glucose (mg/dL) | 94.4 ± 8.7 |
| SI [×10−4min−1/(μIU/ml)] | 5.7 ± 3.7 |
| Total Fat (kg) | 27.3 ± 9.1 |
| Total Lean (kg) | 38.9 ± 4.4 |
| Thigh Muscle (cm2) | 213.5 ± 31.1 |
| Thigh-SAT (cm2) | 256.4 ± 91.1 |
| Thigh-PMAT (cm2) | 15.9 ± 6.2 |
| Thigh-IMAT (cm2) | 10.9 ± 5.3 |
| IAAT (cm2) | 114.1 ± 51.4 |
| Abdominal-SAT (cm2) | 316.8 ± 122.4 |
BMI, body mass index; SI, insulin sensitivity index; SAT, subcutaneous adipose tissue; PMAT, perimuscular adipose tissue; IMAT, intermuscular adipose tissue; IAAT, intra-abdominal adipose tissue.
Table 2.
Pearson simple and partial correlation analysis with SI as the dependent variables
| SI | ||
|---|---|---|
| Simple r | Partial r | |
| Thigh-SAT | −0.36*** | 0.34*** |
| Thigh-PMAT | −0.37*** | 0.07 |
| Thigh-IMAT | −0.54*** | −0.24* |
| IAAT | −0.40*** | −0.36*** |
| Abdominal-SAT | −0.53*** | 0.03 |
| Total fat | −0.61*** | --- |
All partial correlations adjusted for total fat mass. Thigh-IMAT is also adjusted for thigh muscle area; SI, insulin sensitivity index; SAT, subcutaneous adipose tissue; PMAT, perimuscular adipose tissue; IMAT, intermuscular adipose tissue; IAAT, intra-abdominal adipose tissue;
p<0.001;
p<0.01;
p<0.05
Table 3.
Multiple linear regression models with SI as the dependent variables
| Variable estimate ± SEE | Std β | P | |
|---|---|---|---|
| Thigh-SAT | 0.96 ±0.30 | 0.48 | <0.01 |
| Thigh-IMAT | 0.00 ± 0.01 | 0.05 | 0.72 |
| Total fat | −1.42 ± 0.41 | −0.71 | <0.001 |
| IAAT | −0.00 ± 0.00 | −0.40 | <0.01 |
Model adjusted for thigh muscle area and test type; data reported as standardized β; SI, insulin sensitivity index; SAT, subcutaneous adipose tissue; IMAT, intermuscular adipose tissue; IAAT, intra-abdominal adipose tissue.
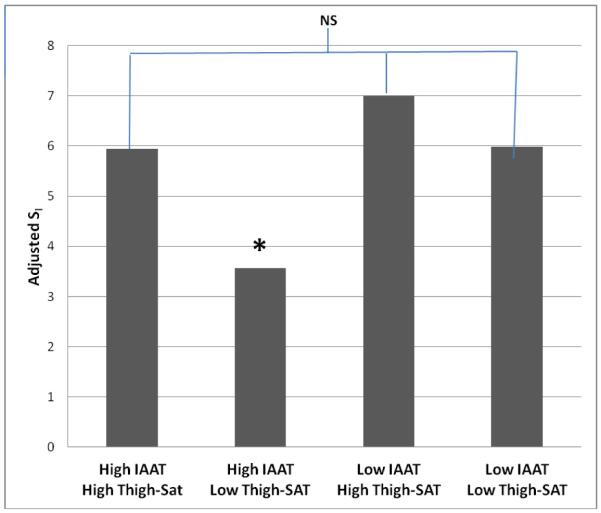
Subgroup analysis by fat distribution phenotype (Figure 1) indicated subjects in the high IAAT/low Thigh-SAT group had significantly lower adjusted SI when compared with all other subgroups (high IAAT/high Thigh-SAT; p=0.01, low IAAT/high Thigh-SAT; p<0.001, low IAAT/low Thigh-SAT; p=0.005). There were no other significant between-group differences in adjusted SI. Multiple linear regression analysis by subgroup (“high” or “low” IAAT) indicated that SI was inversely associated Thigh-IMAT independent of %fat and IAAT amongst subjects with >130 cm2 IAAT, and SI was inversely associated with IAAT and %fat amongst subjects with <130 cm2 IAAT (Table 4).
Figure 1.
SI adjusted means by fat distribution phenotype group (SI adjusted for total fat and FSIGT method). Subjects with high IAAT/low Thigh-SAT had significantly lower adjusted SI than all other groups. There were no other significant between-group differences. NS; not significant, *P<0.01
Table 4.
Multiple linear regression models with SI as the dependent variable
| High IAAT (>103.2 cm2) | Low IAAT (<103.2 cm2) | |||
|---|---|---|---|---|
|
| ||||
| Std β | P | Std β | P | |
| Thigh-IMAT | −0.81 | 0.01 | 0.12 | 0.48 |
| IAAT | −0.05 | 0.80 | −0.33 | 0.05 |
| % Fat | 0.28 | 0.24 | −0.37 | 0.03 |
Models also adjusted for test type. Data reported as standardized β; SI, insulin sensitivity index; IMAT, intermuscular adipose tissue; IAAT, intra-abdominal adipose tissue
Simple correlation coefficients among all fat distribution variables are shown in Table 5a. All variables were significantly positively associated. The highest correlation coefficients calculated were between Abdominal-SAT and thigh SAT (r=0.73), IAAT and Thigh-IMAT (r=0.69), and IAAT and Abdominal-SAT (0.69). Partial correlation coefficients for adipose tissue compartments of the thigh and IAAT adjusted for total fat mass are shown in Table 5b. Thigh-IMAT was significantly positively associated with IAAT and Thigh-PMAT. Thigh-SAT was significantly inversely associated with IAAT independent of total fat.
Table 5.
Pearson simple (a) and partial (b) correlation analysis among fat distribution variables
| a. Simple (r) | Thigh-IMAT | Thigh-PMAT | Thigh-SAT | Abdominal-SAT |
|---|---|---|---|---|
| IAAT | 0.69*** | 0.52*** | 0.54*** | 0.69*** |
| Abdominal-SAT | 0.63*** | 0.55*** | 0.73*** | |
| Thigh-SAT | 0.49*** | 0.52*** | ||
| Thigh-PMAT | 0.62*** |
| b. Partial (r) | Thigh-IMAT | Thigh-PMAT | Thigh-SAT | Abdominal-SAT |
|---|---|---|---|---|
| IAAT | 0.25** | <0.01 | −0.29*** | 0.18 |
| Abdominal-SAT | 0.20 | −0.01 | 0.09 | ----- |
| Thigh-SAT | −0.09 | 0.02 | ----- | ----- |
| Thigh-PMAT | 0.38*** |
Data for partial analysis are adjusted for total fat mass; SAT, subcutaneous adipose tissue; PMAT, perimuscular adipose tissue; IMAT, intermuscular adipose tissue; IAAT, intra-abdominal adipose tissue;
p <0.001;
p<0.01;
p<0.05
Discussion
The major finding of this study is that Thigh-SAT was positively and independently associated with SI, whereas Thigh-IMAT was inversely associated with SI. This is the first study to demonstrate opposing relationships between thigh adipose tissue compartments of the thigh and SI among postmenopausal women. Our findings suggest maintenance of greater Thigh-SAT and lesser Thigh-IMAT may promote or reflect favorable metabolic health among early postmenopausal women.
In this study, Thigh-SAT, after adjustment for total adiposity, was positively and independently associated with insulin sensitivity in all women combined. When women were divided based on their fat distribution pattern, those with low Thigh-SAT/high IAAT had lower SI. These findings agree with other studies linking greater Thigh-SAT, relative to other fat depots, to favorable metabolic indices10;11;23. However, few other studies have used well-accepted measures of insulin sensitivity. Among obese HIV-positive women, Thigh-SAT was positively associated with S from FSIGT10 I. However, to our knowledge, this is the first study to observe an independent association of Thigh-SAT with SI among healthy postmenopausal women.
The physiological basis for the positive association between Thigh-SAT and SI is not clear. Subcutaneous adipose tissue under normal conditions sequesters nonesterified fatty acids released from adipose tissue, and fatty acids from dietary sources24. It has been suggested that under certain pathological conditions, such as obesity, there is a down regulation of lipid storage to subcutaneous depots resulting from a maladaptive response to postprandial increases in fatty acids25. A reduction in the uptake of triglyceride to subcutaneous adipose tissue depots may lead to greater lipid storage to ectopic depots known to be adversely associated with both hepatic and peripheral insulin sensitivity24;25. It is possible that other factors involved in regulating insulin sensitivity, such as genotype, dietary intake, physical activity, and inflammation, are also responsible for the degree of triglyceride storage to subcutaneous depots, thus mediating the association between insulin sensitivity and subcutaneous adipose tissue. Although in the present study we cannot draw cause–and-effect conclusions, we believe our findings support the hypothesis that Thigh-SAT either exhibits protective effects on insulin sensitivity, or reflects a metabolic state compatible with maintenance of relatively high insulin sensitivity among
In our study, greater Thigh-IMAT was associated with lower insulin sensitivity, independent of total body fat. Few studies have examined the relationship between Thigh-IMAT and insulin sensitivity using appropriate measures of insulin sensitivity, such as clamp or FSIGT, and appropriately adjusting for other confounding variables. In a study involving middle-aged men and women, calf IMAT was significantly inversely associated with glucose infusion rate from the hyperinsulinemic euglycemic clamp4. Among subjects with obesity and type 2 diabetes, an inverse association was observed between insulin sensitivity assessed by hyperinsulinemic euglycemic clamp and Thigh-IMAT5. However, neither study considered whether the relationship was independent of total fat mass. Similarly in a study of premenopausal women, whole-body IMAT was inversely associated with insulin sensitivity in both African Americans and Caucasians; however this relationship was not adjusted for total adiposity26. Thus, our study is the first to demonstrate that Thigh-IMAT is associated with a robust measure of insulin sensitivity after accounting for total body adiposity.
Although our observed association between Thigh-IMAT and SI was independent of total fat, it was not independent of IAAT. In all women combined, IAAT but not Thigh-IMAT was independently associated with SI. However, when women were divided based on their degree of intra-abdominal adiposity, Thigh-IMAT was independently associated with SI among those women with high, but not low, IAAT. In this group, IAAT was not independently associated with SI, possibly because all women had levels of IAAT above the threshold for metabolic dysfunction26;27. It has been suggested that IAAT specifically impairs hepatic insulin sensitivity by increasing exposure of the liver to fatty acids. In contrast, due to its location, Thigh-IMAT is likely to affect skeletal muscle insulin sensitivity. Because the SI index we used captures both hepatic and peripheral insulin sensitivity, it may be difficult to isolate relationships that are specific to skeletal muscle in subjects with high volumes of IAAT. Thus, when variance attributable to IAAT was minimized, the association of Thigh-IMAT with insulin sensitivity was apparent. However, it is also possible that the greater Thigh-IMAT in the women with greater IAAT played a role in the stronger association of Thigh-IMAT with SI in this group.
A secondary aim of this study was to characterize the associations of Thigh-IMAT and Thigh-PMAT with other adipose tissue depots. Our results suggested a positive, independent, relationship between Thigh-IMAT and IAAT, in agreement with other studies6;7. These results support the concept of coordinated accumulation of ectopic adipose tissue. While the mechanisms leading to this coordinate deposition are unclear, lipid deposition to both IAAT and Thigh-IMAT may result from the downregulation of lipid uptake by the subcutaneous adipose tissue depots. An estrogenic hormone profile is also thought to direct lipid deposition to the subcutaneous depots28. Therefore, among postmenopausal women, decline in circulating estrogen may further contribute to a shift in lipid deposition from subcutaneous depots to ectopic depots. Our results also indicated that Thigh-IMAT and Thigh-PMAT are highly correlated. However, only Thigh-IMAT was independently associated with SI. Therefore we recommend that these adipose tissue compartments be separated when conducting analyses concerning thigh fat distribution and metabolic health.
This study has several strengths. Measures of fat distribution and body composition were obtained using DXA and CT scanning. To our knowledge, this is the only study identifying independent relationships of the adipose tissue compartments of the thigh with SI among healthy early postmenopausal women. Limitations to this study include the cross-sectional study design and relatively small number of subjects. We did not have statistical power to examine potential race/ethnicity differences in the relationships of interest. We did not assess hepatic fat content or liver function; therefore, we were unable to identify independent relationships of hepatic fat with other ectopic lipid depots and insulin sensitivity, or the extent to which observed associations of IAAT, Thigh-SAT, and Thigh-IMAT with metabolic outcomes were independent of hepatic fat. Further, we did not assess IMCL which may also adversely affect insulin sensitivity.
In conclusion, results suggested that among healthy early postmenopausal women, maintenance of greater Thigh-SAT may exhibit protective effects on insulin sensitivity, or may reflect a fat distribution pattern synonymous with good metabolic health. Thigh-IMAT was inversely and independently associated with insulin sensitivity among women with high but not low levels of IAAT. These results identify independent, opposing relationships of adipose tissue depots of the thigh with metabolic health among a relatively homogenous population of healthy postmenopausal women, thus emphasizing the importance of considering fat distribution phenotype in identifying risk of development of metabolic disease among otherwise healthy, aging women. Further studies are needed to determine whether a cause-and-effect association exists between Thigh-SAT and insulin sensitivity, and to also determine whether adipose tissue infiltration in skeletal muscle independently influences peripheral insulin sensitivity.
Acknowledgements
The authors wish to acknowledge Tena Hilario for subject recruitment, and the help of the GCRC staff. This work was supported by NIA K01AG00740 (Gower), the General Clinical Research Center (M01-RR-00032), the Center for Clinical and Translational Science (UL 1RR025777), the Nutrition Obesity Research Center (P30 DK56336), and the Diabetes Research and Training Center (P60 DK079626).
Funding This work was supported by K01AG00740, M01-RR-00032, UL 1RR025777, P30 DK56336, and P60 DK079626.
List of abbreviations
- ANCOVA
Analysis of covariance
- CT
Computed tomography
- DXA
Dual-energy x-ray absorptiometry
- FSIGT
Frequently sampled intravenous glucose tolerance
- GCRC
General Clinical Research Center
- IAAT
Intra-abdominal adipose tissue
- IMAT
Intermuscular adipose tissue
- IMCL
Intramyocellular lipid
- PMAT
Perimuscular adipose tissue
- SAT
Subcutaneous adipose tissue
- SI
Insulin sensitivity index
- UAB
University of Alabama at Birmingham
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors had no conflicts of interest to disclose.
Disclosure statement The authors had no conflicts of interest to disclose.
Authors’ contributions The author’s responsibilities were as follows – AMG and BAG: designed the research project, analyzed the data, wrote the manuscript, and had primary responsibility for final content.
Reference List
- 1.Van Pelt RE, Jankowski CM, Gozansky WS, et al. Lower-body adiposity and metabolic protection in postmenopausal women. J.Clin.Endocrinol.Metab. 2005;90:4573–4578. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams MJ, Hunter GR, Kekes-Szabo T, et al. Regional fat distribution in women and risk of cardiovascular disease. Am.J.Clin.Nutr. 1997;65:855–860. doi: 10.1093/ajcn/65.3.855. [DOI] [PubMed] [Google Scholar]
- 3.Hunter GR, Chandler-Laney PC, Brock DW, et al. Fat distribution, aerobic fitness, blood lipids, and insulin sensitivity in African-American and European-American women. Obesity.(Silver.Spring) 2010;18:274–281. doi: 10.1038/oby.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boettcher M, Machann J, Stefan N, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J.Magn Reson.Imaging. 2009;29:1340–1345. doi: 10.1002/jmri.21754. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am.J.Clin.Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am.J.Clin.Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song MY, Ruts E, Kim J, et al. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am.J.Clin.Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 8.Yim JE, Heshka S, Albu JB, et al. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J.Appl.Physiol. 2008;104:700–707. doi: 10.1152/japplphysiol.01035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoico E, Rossi A, Di F, V, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J.Gerontol.A Biol.Sci.Med.Sci. 2010;65:295–299. doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albu JB, Kenya S, He Q, et al. Independent associations of insulin resistance with high whole-body intermuscular and low leg subcutaneous adipose tissue distribution in obese HIV-infected women. Am.J.Clin.Nutr. 2007;86:100–106. doi: 10.1093/ajcn/86.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pigeon E, Couillard E, Tremblay A, et al. Mid-thigh subcutaneous adipose tissue and glucose tolerance in the Quebec family study. Obes.Facts. 2008;1:310–318. doi: 10.1159/000177047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen I, Powell LH, Crawford S, et al. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch.Intern.Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch.Intern.Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gower BA, Munoz J, Desmond R, et al. Changes in intra-abdominal fat in early postmenopausal women: effects of hormone use. Obesity.(Silver.Spring) 2006;14:1046–1055. doi: 10.1038/oby.2006.120. [DOI] [PubMed] [Google Scholar]
- 15.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J.Clin.Endocrinol.Metab. 2007;92:895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr.Rev. 1985;6:45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 17.Munoz J, Derstine A, Gower BA. Fat distribution and insulin sensitivity in postmenopausal women: influence of hormone replacement. Obes.Res. 2002;10:424–431. doi: 10.1038/oby.2002.59. [DOI] [PubMed] [Google Scholar]
- 18.Pacini G, Tonolo G, Sambataro M, et al. Insulin sensitivity and glucose effectiveness: minimal model analysis of regular and insulin-modified FSIGT. Am.J.Physiol. 1998;274:E592–E599. doi: 10.1152/ajpendo.1998.274.4.E592. [DOI] [PubMed] [Google Scholar]
- 19.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J.Clin.Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput.Methods Programs Biomed. 1986;23:113–122. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 21.Welch S, Gebhart SS, Bergman RN, et al. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J.Clin.Endocrinol.Metab. 1990;71:1508–1518. doi: 10.1210/jcem-71-6-1508. [DOI] [PubMed] [Google Scholar]
- 22.Gower BA, Ard JD, Hunter GR, et al. Elements of the metabolic syndrome: association with insulin sensitivity and effects of ethnicity. Metab Syndr.Relat Disord. 2007;5:77–86. doi: 10.1089/met.2006.0027. [DOI] [PubMed] [Google Scholar]
- 23.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 24.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 25.McQuaid SE, Hodson L, Neville MJ, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60:47–55. doi: 10.2337/db10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am.J.Clin.Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter GR, Snyder SW, Kekes-Szabo T, et al. Intra-abdominal adipose tissue values associated with risk of possessing elevated blood lipids and blood pressure. Obes.Res. 1994;2:563–568. doi: 10.1002/j.1550-8528.1994.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 28.Elbers JM, Asscheman H, Seidell JC, et al. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am.J.Physiol. 1999;276:E317–E325. doi: 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]