Abstract
The background of this article is that assessment and quantification of skin color is important to health care; color is one indicator of overall health and is linked to oxygenation, tissue perfusion, nutritional status, and injury. The purpose is to describe how skin color varies across racial/ethnic groups so that the information can be applied to clinical practice. The method used is cross-sectional, descriptive design (n = 257). We recorded self-defined race/ethnicity and used a spectrophotometer to measure skin color at two anatomic sites. Skin color variables included L* (light/dark), a* (red/green), and b* (yellow/blue). As regards results, we found significant differences in L*, a*, and b* values by site and race/ethnicity in White, Asian, and Biracial participants. L*: F(3, 233) = 139.04, p < .01 and F(3, 233) = 118.47, p < .01. Black participants had significantly lower mean L* values and wider ranges of L*, a*, and b* as compared with other groups. In regard to application, these findings suggest that clinicians and researchers should plan and provide care based on skin color, rather than race/ethnicity.
Keywords: skin color, injury, protection, CIELAB, spectrophotometry
Skin color matters in health care. Clinicians make decisions based on color assessments multiple times each day as they gauge tissue perfusion and assess for jaundice, pallor, cyanosis, and the blanch response. They evaluate pressure points for early signs of skin breakdown and assess existing wounds for color changes that might indicate healing, worsening, or infection. Clinicians and researchers have published findings relating the importance of skin color in health care. These include differences in the potential for injury when faced with forces associated with trauma (soft tissue injury), friction/shearing (pressure ulcers), and stretching (childbirth; Howard, Davies, DeLancey, & Small, 2000; Robinson, Norwitz, Cohen, McElrath, & Lieberman, 1999; Saladin & Krause, 2009; Sommers et al., 2009). Accurate evaluation of skin color is important in both clinical and research settings (Bennett, 1995; Berardesca, Derigal, Leveque, & Maibach, 1991; Ha et al., 2009; Lindholm et al., 2008). Previously, investigators have stratified skin color based on socially constructed racial or ethnic lines with the untested hypothesis that Black populations have “dark” skin, European populations have “light” skin, and biracial, Asian, and Latino populations have skin tones in the middle ranges (Abbade, Lastoria, & de Almeida Rollo, 2011; Fitzpatrick, 1988). Rigorous methods are easily available to quantify skin color (Andersen & Bjerring, 1990a; Daniel, Heckman, Kloss, & Manne, 2009; Pershing et al., 2008; Pierard, 1998). However, clinicians and researchers often use limited and outdated scales and instruments that perpetuate this faulty stratification. Working from these faulty stratifications impedes nursing’s progress toward the provision of evidence-based care that reflects an individual’s physiological attributes.
Purpose
Our primary purpose was to determine how skin color varies within and differs across populations. We describe empirical determination of skin color values and ranges across several self-defined racial and/or ethnic groups and discuss the practice implications of skin color variation. Additionally, in the “clinical implications” portion of this work, we discuss the use of digital cameras and digital image analysis (DIA) as a viable alternative to using specialized color quantification scales and instruments in clinical settings.
Background
Human skin is the primary interface between nurse and patient, and as such, it is a key area of focus for health care providers (Lott, 1998). During physical assessments, nurses evaluate the color of the patient’s skin as a significant measure of overall health status. They also evaluate the skin for signs of breakdown or other loss of integrity and assess wounds in various stages of formation or healing.
Skin protection and the prevalence of injury have been reported in the literature as varying across populations (Baker, Fargo, Shambley-Ebron, & Sommers, 2010; Shriver & Parra, 2000; Sommers et al., 2008). Investigators describe differences in injury patterns, types, severity, and number between people of different races and/or ethnicities with respect to sexual assault, childbirth-related injury, pressure ulcer formation, and healing (Baumgarten et al., 2004; Fogerty et al., 2008; Saladin & Krause, 2009; Sommers, 2007; Sommers et al., 2008). For example, in a population of women studied after consensual sexual intercourse, Sommers et al. found that not only was injury prevalence significantly higher in White as compared with Black/African American women, but the effect of race/ethnicity became nonsignificant after adding skin color values to the model predicting occurrence of genital injury (Sommers et al., 2008, 2009). These findings indicated the spurious nature of the relationship between race/ethnicity and injury prevalence and demonstrated that skin color rather than race/ethnicity is likely the important variable in injury prevalence.
The importance of skin color in health care research extends beyond its relationship with injury risk or prevalence. For example, the difference in melanin, a significant contributor to visible skin color among different groups of people with varying skin color measurements, may also affect sunburn and skin cancer risk, as well as the synthesis of vitamin D, levels of which are associated with a number of positive and negative health-related implications (Domingo & Matsui, 2009; Osterwalder & Herzog, 2009; Schauber & Gallo, 2008; Weinstock & Moses, 2009). Because of the multiple implications for health care that are inherent in color assessment, researchers and clinicians require an accurate and reliable method to measure skin color.
About Skin Color
We define skin color as the perceived skin pigmentation resulting from the selective absorption and scattering of light from the dermis of the body (Pierard, 1998). Skin color is the product of a combination of anatomical and physiological phenomena within the uppermost layers of the skin. Four pigments contribute to skin color: melanin, carotene, oxygenated hemoglobin, and reduced hemoglobin. Of these, the particle size, shape, and location of melanin contribute most significantly to overall color; the more near the surface melanin is clustered, the darker skin will appear. Carotene gives the skin a yellow hue, while oxygenated and reduced hemoglobin are red and purplish-blue, respectively. In addition to melanin, all light absorbing molecules and particles in the skin, or chromophores, play a part in perceived skin color. Skin color may be described as constitutive or as facultative. Constitutive skin color represents an individual’s baseline, or the color of skin that has not been altered by sun or other types of ultraviolet (UV) exposure; one example of a constitutive skin site in most people is the upper inner arm. Facultative skin color represents skin that has increased melanin production and thus alteration from baseline status after exposure to the sun or other UV sources; most skin sites may be classified as facultative (Choe, Jang, Jo, Ahn, & Youn, 2006).
In research and clinical settings, race has traditionally been considered to be a suitable proxy for skin color. Few researchers have published findings describing physiological phenomena based on a quantitative measure of skin color, and even fewer clinical practice protocols address issues of skin color as a variable of interest when planning care. Caldwell and Popenoe (1995) cautioned practitioners to avoid classifying and treating individuals based on racial descriptions, as entirely different physical or biological characteristics may be found in people grouped within those broad categories.
Conceptual Framework
The most widely accepted model for color quantification was established by the Commission Internationale d’Eclairage (Baldelli & Paciella, 2008) in 1976 (Fairchild, 2005; Lee, 2005) and is described as a color space, or a three dimensional geometric model that represents color numerically. We chose to use the CIE L*a*b* (CIELAB) color space for several reasons: (a) it represents all colors visible to the human eye (Fairchild, 2005; Ha et al., 2009; Lee, 2005); (b) it represents colors relative to a white reference point; (c) the distances between any two colors are measured proportionally to their space geometrically in the color space; and (d) equal distances in the color space represent equal color differences (Fairchild, 2005; Lee, 2005).
In the CIELAB color space, which is particularly useful in situations where the closeness of colors must be quantified scientifically, the L*, a*, and b* values are plotted at right angles to one another to form a three-dimensional coordinate system (Fairchild, 2005; Ha et al., 2009). Value L* represents lightness/darkness and extends from 0 (black) to 100 (white). Value a* represents the redness/greenness axis; positive a* is red and negative a* is green. Value b* represents the yellowness/blueness axis; positive b* is yellow and negative b* is blue. There are no specific numerical limits for a* and b* (Fairchild, 2005).
Color Measurement
Skin color can be evaluated in several different ways (to be discussed in what follows) with varying degrees of accuracy. Color assessment methods may be categorized as either subjective or objective. Subjective assessment is based on an individual’s interpretation of stimuli, while objective measurements are based strictly on the phenomenon of interest and not subject to judgment or bias. In the past, most skin color assessment has been performed subjectively through the use of visual inspection, comparative color tiles or laminated color cards, written skin-typing guidelines for comparative classification, or by way of qualitative visual estimation by individuals who have received varying levels of training (Roberts, 2009; Taylor, Arsonnaud, & Czernielewski, 2005). The accuracy of these subjective measures for the assessment of skin color could be considered suspect at best, and the repeatability is dependent on a combination of factors, including ease of scale use, degree of rater training, and physical influences such as ambient lighting during the assessment process. UV light and polarized light photography were introduced as a means of assessing the skin for lesions or other pathology, but have not been considered a reasonable means of assessing skin color (Taylor, Westerhof, Im, & Lim, 2006). In 1986, the 6-point Fitzpatrick scale was introduced as a means for classification of different skin types based on their response to ultraviolet or sun exposure (Fitzpatrick, 1986). Though not originally intended to serve this purpose, the Fitzpatrick scale has been and continues to be commonly used as a means of describing skin color.
A noninvasive method, diffuse reflectance spectroscopy (DRS), is considered to be the gold standard for objective quantitative measurement of skin color (Andersen & Bjerring, 1990a; Parra, 2007). The investigator uses the instrument to direct lights of specific wavelengths toward the area of interest and then the wavelength(s) and intensity of light reflected back from the skin back to the instrument’s sensors are measured. DRS records reflectance values from colored surfaces and provides instrument operators with the entire reflectance spectrum as well as specific color measurements within a number of color models or spaces, as programmed by the user. Spectrophotometers vary in cost from approximately US$5,000 to US$15,000 per unit, depending on model capabilities, with further costs depending on the software, accessory hardware, or warranty/service plan options selected (K. Corcoran, personal communication, September 10, 2010).
Decisions as to location for skin color measurement depend entirely on the goal of that measurement. For those evaluating wound progression, the choice is obvious: measurement should occur at and/or near the site of the wound. When assessing skin color as one means to evaluate peripheral tissue perfusion, the measured sites could include the extremities of concern as well as nonaffected sites for the purpose of comparison. If sequential color measurements are called for, then clinicians should take care to assess the same site with the same or similar conditions, particularly as related to ambient light. When researchers or clinicians perform skin color measurements for research or clinical comparative purposes, consideration should be given to the inclusion of constitutive (unexposed or untanned, close to baseline) versus facultative (exposed and tanned) anatomical sites in clinical or research data collection as each will provide different information to investigators.
In the main text of this article, we will discuss the portion of our study that addresses our primary aim: to determine how skin color varies within and differs across populations. After reporting and commenting on our results, we discuss our secondary aim in the “Clinical Implications” section. In the “Clinical Implications” section, we will describe our work applying digital image analysis techniques as a viable proxy for spectrophotometry in clinical settings.
Method
We conducted this prospective, descriptive, cross-sectional study at a large university in an urban environment. We recruited a healthy community convenience sample by posting flyers on a university campus and at women’s health clinics. We collected data from 237 women during two prospective studies (Study 1: n = 152; Study 2: n = 85) using the same procedures, variables, instruments, and skin science measurements to minimize error. The Institutional Review Board of the affiliated university approved both studies. We used the same inclusion and exclusion criteria for each study with respect to key attributes including: self-identification as female, cessation of the use of all topical products on the skin for 24 hr; absence of scars and rashes on the volar forearm and upper inner arm; ability to read, speak, and understand English, and willingness to give informed consent for noninvasive skin testing and for collection of demographic data. We included participants based on self-identification as female as part of a parent study that involved skin mechanics and injury to the skin in women above 21 years of age. Samples across both studies were demographically similar; see Table 1 for total participant demographic information.
Table 1.
Participant Demographic and Skin Color (L*, a*, and b*) Data in Females (N = 237)
| Self-defined race (n) | Age Mean (SD) | Volar forearm Mean L* (SD) | Upper inner arm Mean L* (SD) | Volar forearm Mean a* (SD) | Upper inner arm Mean a* (SD) | Volar forearm Mean b* (SD) | Upper inner arm Mean b* (SD) |
|---|---|---|---|---|---|---|---|
| Asian (13) | 23.7 (4.4) | 59.47 (7.33) | 59.30 (8.37) | 8.55 (1.53) | 9.17 (1.56) | 19.60 (2.17) | 19.41 (2.99) |
| Black/African American (101) | 33.8 (10.3) | 47.26 (7.66) | 46.49 (8.07) | 9.44 (1.12) | 9.84 (1.13) | 20.07 (2.07) | 20.13 (2.31) |
| White (118) | 29.0 (9.5) | 65.00 (4.45) | 65.22 (4.88) | 7.14 (1.63) | 7.67 (1.58) | 17.37 (2.55) | 17.18 (2.96) |
| Biracial/more than one race (5) | 29.1 (7.9) | 59.73 (12.45) | 59.28 (12.19) | 7.79 (1.68) | 8.25 (2.20) | 18.09 (1.52) | 18.62 (1.60) |
Instruments
For skin color measurements, we used a hand-held ColorTec® spectrophotometer that was calibrated to CIELAB standards to measure color via reflectance. The instrument is designed to obtain reflectance values from colored surfaces and return the entire reflectance spectrum as well as L*, a*, and b* values. This type of spectrophotometer is recognized as the gold standard for skin color measurements and has been tested extensively in the cosmetics industry as well as by scientists investigating ultraviolet ray exposure to the skin (Takiwaki, 1998; Weatherall & Coombs, 1992). The technique has been found accurate and reliable in both in vitro studies with standardized color charts (Clarys, Alewaeters, Lambrecht, & Barel, 2000) and in vivo studies in experimentally induced ultraviolet erythema (Andersen & Bjerring, 1990a, 1990b). We performed external calibrations every 7 to 14 days during the study period with standardized white and black tiles provided by the manufacturer. The results were within calibration standards (L* value error < 5%) during each instance, requiring no further actions or manufacturer recalibration.
We controlled for ambient temperature to explain the variation in surface blood flow among participants. Skin color is directly affected by surface blood flow changes that occur as functions in the skin’s role of systemic thermoregulation; vasoconstriction routes blood away from the skin’s surface in ambient cold, and vasodilation increases surface blood flow as a cooling mechanism in warmer environments. To monitor ambient temperatures, we used an Electro-Tech Systems, Inc.® (ETS) Model 5640 humidity and temperature meter to record temperature and humidity within the imaging laboratory. The Humidity/Temperature/Dew Point Meter is a handheld meter with a fixed probe that measures temperature in Fahrenheit or Celsius with an accuracy of ±0.9°F and ±0.5°C. Relative humidity of 0% to 99% is also measured with an accuracy of ±3% (ElectroTech, 2008). The ETS Model 5640 is International Organization for Standards (ISO) certified for accuracy (ElectroTech, 2008).
Procedures
Following recruitment and informed consent procedures, we completed data collection for all participants within a controlled environment, with temperatures ranging between 68°F and 76°F; humidity was monitored but not controlled. Study staff measured and recorded laboratory temperature using the ETS Model 5640 humidity and temperature meter prior to data collection for each participant. We maintained control over ambient light in our skin imaging laboratory and data collection location by the use of light-blocking shades, portable room partitions, and a consistent location for measurements. After obtaining informed consent, trained study staff collected demographic and other study-related information; staff were asked to follow a scripted dialogue and to avoid prompting participants on what might be a “better” response for them. Thus all responses from all participants were self-reported demographics, characteristics, health histories, and behaviors.
After participants rested for 10 min to acclimate to the environmental temperature and humidity, we obtained skin color measurements by way of spectrophotometry. Using a plastic template for consistency, we used soft-tipped, washable colored markers to define a 2″ × 2″ square measurement area 1″ distal to the antecubital space on the volar forearm (a facultative, or exposed, site) and 2″ distal to the axilla on the upper inner arm (a constitutive, or unexposed, site). Taking care to avoid the colored markings or any obvious aberrations in the participant’s “typical” skin color such as tattoos or moles, we obtained three spectrophotometric measurements at each anatomical location and recorded the L*, a*, and b* values for each reading.
Data Management and Analysis Plan
All data were de-identified for participant confidentiality and then double entered into an encrypted database for security and stored on secured and encrypted research drives. We completed data cleaning using EpiInfo™ software version 3.5.1 to compare databases for discrepancies, which were then reviewed and corrected. Original physical (paper) data collection forms were used to resolve errors; these were stored in locked cabinets in a locked office, with access limited to study principal investigators (PIs). Missing data were evaluated on a case-by-case basis, with every attempt to include each participant unless we were missing critical data points. The PI for the parent study (Sommers) and for the nested study (Everett) retained final authority regarding error correction, data handling, and data management issues.
We used descriptive statistics such as measures of central tendency, dispersion and ranges to characterize the study variables and the characteristics of the sample. We used one-way analyses of variance (ANOVAs) to examine differences in L*, a*, and b* values among the four self-identified racial groups included in the study (Asian, Black/African American, White, and Biracial). We completed post hoc tests using Tukey’s Least Significant Difference criteria to assess where specific differences existed between the groups. We conducted all statistical analyses with SPSS® software version 18.
Findings
Of the 237 women who participated in the study, 52% self-identified as White (n = 118), 44% self-identified as Black/African American (n = 101), 6% self-identified as Asian (n = 13), and 2% self-identified as Biracial/More than one race (n = 5). Mean L*, a*, and b* values and standard deviations for each anatomical site (volar forearm vs. upper inner arm) stratified by race (Black, White, Asian, and Biracial) may be found in Table 1. We conducted a series of one-way ANOVAs to determine whether significant racial differences existed in L* values. The ANOVA for volar forearm L* values was significant, F(3, 233) =118.47, p < .01, with respect to race. Post hoc tests revealed that Black participants had significantly lower L* values (M = 47.24, SD = 7.66) than Asian (M = 59.47, SD = 7.33), White (M = 64.67, SD = 5.71), and Biracial participants (M = 56.85, SD = 10.82). We found similar results when conducting the series of one-way ANOVAs with upper inner arm L* value data, F(3, 233) = 139.04, p < .01. Post hoc tests again revealed that Black participants had significantly lower L* values (M = 46.49, SD = 8.07) than Asian (M = 59.30, SD = 8.37), White (M = 65.22, SD = 4.88) and Biracial participants (M = 59.28, SD = 12.19).
One-way ANOVAs indicated significant racial differences in a* values for both volar forearm, F(3, 233) = 47.43, p < .01 and upper inner arm measurements, F(3, 233) = 42.99, p < .01. Post hoc tests revealed that for both volar forearm and upper inner arm measurements, Black participants had significantly higher a* values than Asian, White, and Biracial participants (see Table 1). For b* values, one-way ANOVAs also revealed significant racial differences on both volar forearm, F(3, 233) = 25.49, p < .01 and upper inner arm measurements, F(2,233) = 22.39, p = .01. For both sites, post hoc analysis indicated that Black participants had significantly higher b* values than only White participants, but no significant differences existed between Black participants and those identifying as Asian or Biracial. Additionally, Asian participants had significantly higher b* values (at both anatomical locations) than White participants but not significantly higher than those identifying as White or Biracial.
Discussion
In our sample, we found that those participants self-identifying as Black/African American had overall darker and more variable skin lightness/darkness (L*) values than any other self-defined group. Those identifying as Asian had darker skin as compared with White, but lighter than Black/African American participants. Participants identifying as White had overall lighter skin values and far less variability among the group than any other group of participants. These findings were consistent for both the constitutive skin site (upper inner arm) and the facultative skin site (the volar forearm). The other color variables, a* and b*, varied less dramatically between and among racially stratified groups. This lack of variation was likely due to the tendency for human skin to group in the same general ranges for a* and b* color values in the yellow and red hues. In contrast, some variation exists among those with either increased superficial melanin or decreased carotenoids, which typically leads to lower b* values, indicating a trend toward more blue and away from more yellow coloring (Stamatas, Zmudzka, Kollias, & Beer, 2004; Stephen, Law Smith, Stirrat, & Perrett, 2009). As observed in our sample, standard deviations within each group for those values were nearly identical across groups. Our findings replicated those of Shriver and Parra (2000), although it should be noted that our sample size was larger, included a greater representation of those identifying as Black/African American, and included those of Hispanic/Latina origin, whose data were incorporated within the coidentified groups (Black, White, Asian, or Bi/Multiracial) during analysis.
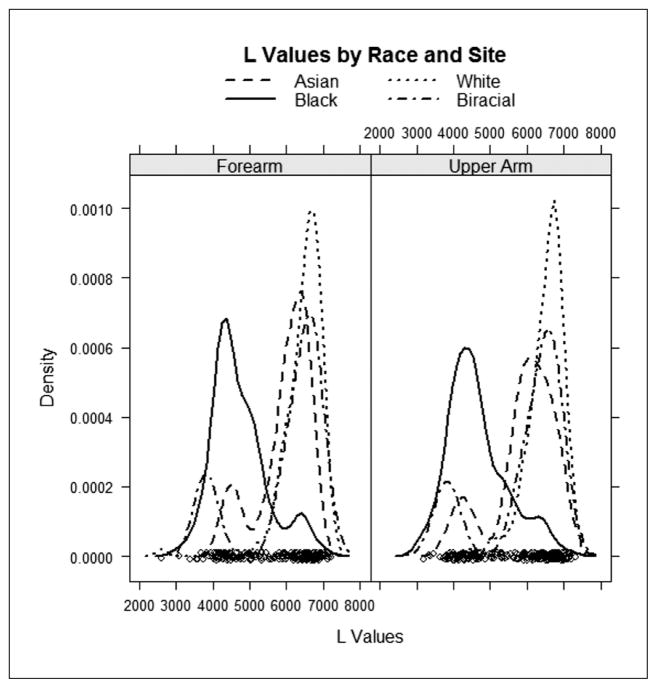
For those interested in skin color quantification, but find that spectrophotometry is not a viable option because of cost, implementation of digital cameras and digital image analysis offer a more readily accessible and typically less-expensive option for skin color analysis (see “Clinical Implications”). Regardless of objective color measurement technique employed (spectrophotometry or digital image analysis), our data as well as those of Shriver and Parra (2000) indicated that there are distinct differences in skin color among groups of individuals. With the widest range of L* values associated with those people identifying as Black/African American (see Figure 1), measurement strategies should allow for the most variation in skin color for African Americans as compared to other populations.
Figure 1.
Density plot of L* values by race and anatomical site in females (N = 237)
Society and health care personnel tend to ascribe characteristics based on the socially constructed categorizations of race, rather than on an individual’s physiology and phenotype. Recently, Torres and Kittles, (2007), Santos et al. (2009), Parra, Kittles, and Shriver (2004), and Shields et al. (2005) implored researchers and clinicians to look toward physiology rather than ascribing characteristics to individuals based on racial or ethnic identification.
With regard to individualized care, clinicians should consider many care issues related to skin color, including (but not limited to) gauging tissue perfusion; assessing for jaundice, pallor, cyanosis, and the blanch response; evaluating pressure points for early signs of skin breakdown; and assessment of existing wounds for color changes that might indicate healing, worsening, or infection. Although more research is needed, clinicians should note differences of early signs of skin breakdown among those with lighter versus darker skin. While patients with light skin may have the blanch response that demonstrates adequate tissue perfusion, dark-skinned patients rarely have the same response to light skin pressure. Thus it may be difficult to determine when dark-skinned patients are at risk for pressure ulcers. For patients with dark skin, pressure ulcer assessment includes applying light pressure and looking for an area that is darker than the surrounding skin or that is taut, shiny, or indurated (Sommers, 2011).
With respect to protection from sun and other UV exposures, light-skinned individuals have been documented to have an increased risk of skin cancer as compared with darker skinned individuals (Rees, 1999; Rees et al., 1999). Darker skinned individuals, on the other hand, have an increased risk of decreased vitamin D levels (Chen et al., 2007), which may affect risk for multiple chronic conditions including cancer, autoimmune diseases, and cardiovascular disease (Chen et al., 2007; Giovannucci, 2005; Holick, Chen, Lu, & Sauter, 2007; Lappe, Travers-Gustafson, Davies, Recker, & Heaney, 2007). Teaching regarding protection from sun and other UV exposure should vary based on the needs of each individual depending, at least in part, on skin color. Those with dark skin are not immune to sun damage, despite the fact that there is a not-uncommon belief that “Black skin doesn’t burn.” Thus patient teaching is required to reinforce the need for sun protection in everyone, regardless of skin color (Berwick, Fine, & Bolognia, 1992; Robinson, Rademaker, Sylvester, & Cook, 1997). At the same time, clinicians should be attentive to the increased potential for Vitamin D deficiency among those with dark skin so as to balance skin protection, sun exposure, and potential supplementation to address deficiencies (Dawson-Hughes, 2004; Finkelstein et al., 2002; Holick, 2007; Lee, O’Keefe, Bell, Hensrud, & Holick, 2008).
Limitations
We used a spectrophotometer to quantify skin color variables at constitutive and facultative anatomic locations of 237 participants in a university setting within an urban location in the northeastern United States. Our findings cannot be generalized to populations from other geographical locations at other latitudes as typical sun exposure levels may vary. Future work should include participants from additional geographic locations.
Measurement error occurs during any biophysiological measurement. Random error may have resulted from incorrect instrument placement over skin markings, incorrect placement on the skin causing the blanch response, or transcription errors of skin measurements. Nonrandom error may have occurred because of incorrect calibration procedures. Other limitations include the use of multiple individuals to collect demographic and spectrophotometric data. While training and quality assurance checks were performed regularly with all individuals involved in data collection, adherence to technique may have varied during data collection. Finally, lower numbers of participants who identified within groups other than Black or White limited our ability to explicate the differences and ranges in other groups, particularly those identifying as Asian and those of Hispanic origin.
Conclusion
Color quantification in clinical settings can provide both immediate and long-term benefits. Rather than exercising “color blindness” when assessing and treating patients, practitioners should exercise “color awareness” by adjusting interventions and assessment techniques using the patients’ physiological characteristics rather than depending on racial or ethnic categorization to guide care (Sommers, 2011). As our attention as clinicians (and researchers) shifts toward this goal of color awareness, we will be able to address gaps in knowledge regarding more specific needs of our patient populations and provide better individualize teaching, prevention, and care to improve outcomes for all patient populations.
Implementation in Clinical Care (“Clinical Implications”)
While spectrophotometry can be used for highly accurate and reliable data on human skin color, in many clinical settings the use of spectrophotometers may be impractical. Spectrophotometers are sensitive instruments that may be damaged in the day-to-day rush of clinical care. They also represent a significant financial investment that may be prohibitive in many settings. Digital cameras are easily accessible, inexpensive to maintain, and may present an acceptable alternative. In this section, we discuss the potential of and methods for using digital image analysis as a substitute for spectrophotometry for the measurement of skin color in clinical settings.
Digital image capture is already used to document burns, and sequential imaging shows the progression of pressure ulcers (Korber, Cesko, & Dissemond, 2009; Nelson, Boyle, Taggart, & Watson, 2006; Parry, Walker, Niszczak, Palmieri, & Greenhalgh, 2010). We propose that digital image analysis (DIA) may be used clinically to provide data for nursing diagnoses and outcomes of nursing care and is particularly useful to better understand skin injury across the continuum of skin color. When used properly, digital image processing programs, such as Adobe Photoshop®, provide detailed color data in a relatively short period of time.
Images intended for color quantification should be obtained with a camera capable of recording images with minimal in-camera processing and with no color changes to files after the image is obtained. Experts choose cameras that allow them to save images in the RAW file format, which eliminates all in-camera color and light adjustments to images. Additionally, the ability to program the camera for consistent exposure over multiple image captures helps the photographer to achieve consistency across images. Several midrange digital cameras currently available meet these guidelines, including (but not limited to) the Canon Powershot® G12 or S100, the Olympus® XZ-1, and the Panasonic Lumix® DMC-LX5; as of February 2012, market prices for these cameras range from approximately US$400 to US$500. When obtaining images, one should preserve consistent, predetermined exposure settings, with any “color correction” choices turned off. Use full-spectrum, bright, ambient light (avoiding use of the camera’s flash) to illuminate the subject of the image and, keeping the lighting the same, take an image of a color card prior to image capture of skin or a wound.
An industry standard color card, such as the Munsell® Color Mini Colorchecker (Munsell, 2007), is useful when quantifying skin color because it is referenced to known color values, thereby allowing image color correction to “near-true” color. Color correction is a process by which an image of the reference standard, in this case the color card, is used to establish what, if any, color balance and white balance adjustments are needed to bring an image closer to “true color.” In the case of the Mini Colorchecker card, each of the 24 colors on the card are sampled and analyzed so that the photographer is able to adjust that image and subsequent images from the same session, with the same camera, and in the same lighting conditions through the use of software. We use a software plug-in (a small computer program designed to do a very specific task within a larger software program) within Photoshop® to create a customized color profile for each photographic session, thus allowing for color-correction of all images to “near-true” color prior to sampling for L*, a*, and b* values within Photoshop®.
We recommend two sampling methods for DIA. In the first, a user-defined shape is selected within the image using Photoshop’s® “lasso” tool; the number of pixels selected may vary greatly (up to the hundreds of thousands or more pixels) depending on the size of the selection, but generally we analyze areas of 120,000 to 150,000 pixels to obtain a consistent and representative area for skin color analysis. The second method of obtaining color data from digital images is to use a Photoshop’s® “eye-dropper color sampler” tool to select several smaller points within the image if no large area is available for color measurement. This technique would be useful for areas that have large amounts of hair or many skin markings such as tattoos or moles. In this case, the number of pixels is clearly defined, and may be as small as a single pixel up to a 101 × 101 (10,201 pixels) average. In both methods, color values obtained within the software’s expanded histogram window represent the means based on the total number of selected pixels.
While these two methods are not new to those experienced in color quantification, we could find no published literature outlining how one method might be different from the other, nor if they could be considered comparable for use in clinical or research settings. To attempt to answer this question, we had three independent raters use these DIA techniques to collect color data from multiple representative skin images of the volar forearm and upper inner arm of women from the principal investigators’ image repository. All procedures were approved by the university’s IRB. After training staff members on color correction and each of the DIA methods, three individual raters each completed DIA on 200 skin images. Each rater was blinded to the findings of the other raters. For the “lasso” method, each rater used the following procedure: they (a) opened the color card image and created a color profile using the color card associated with that image; (b) assigned that color profile to the image being analyzed (thus performing color correction, bringing the image to nearly true color); (c) changed the color mode within Photoshop® to CIELAB; and (d) used the lasso tool to select a large area (between 120,000 and 150,000 pixels) within the defined anatomical location captured on the image. Raters avoided tattoos, scars, and obvious aberrations in the participant’s “typical” skin color. Raters then recorded the mean color value (L*, a*, and b*) displayed within the expanded histogram from Photoshop® along with the standard deviation and number of pixels. After all values were recorded, the rater repeated the process twice more, for a total of three sets of “lasso” color values. For the same image, the rater then performed a similar process, this time using the “eye-dropper color sampler” technique set for a 5 × 5 (25 pixels) selection within the defined measurement area. This process was completed after the rater recorded the mean L*, a*, and b* values for each of 9 of these 25 pixel selections. To summarize, for each of the 200 images, each rater obtained three sets of L*, a*, and b* “lasso” and nine sets of “eye-dropper color sampler” values.
Due to differences in the assignment of color values within Adobe’s Photoshop® software, each of the color values obtained by the “lasso” method was converted to match the official CIELAB color space. To transform Adobe’s values to match those within CIELAB color space, the following mathematical transformations were made prior to statistical analysis: (a) (Photoshop®-obtained L* value mean 100)/255 = mean L* value; (b) Photoshop®-obtained a* value mean – 128 = mean a* value; and (c) Photoshop®-obtained b* value mean – 128 = mean b* value. Once these values were converted, the results of all color quantification were ready for the data analysis process (Rochester Institute of Technology [RIT], n.d.).
We wanted to understand how each rater’s values compared to those of other raters (intrarater reliability) for each method (“lasso” and “eyedropper”). Intraclass correlation (ICC; Molinari, Fato, De Leo, Riccardo, & Beltrame, 2005) coefficients for this phase captured each rater’s consistency from one measurement to the next. All raters had high ICCs (ranging from 0.98 to 1.00, with confidence intervals from 0.97 to 1.00), indicating a high level of consistency among all three independent raters for all color values for both techniques. We also wanted to compare the two methods (“lasso” vs. “eye-dropper color sampler”) for each color variable. We found a high level of agreement between the “lasso” and “eye-dropper color sampler” methods for all color values with ICC of 0.99 (CI [0.98, 1.00]) and correlation r = .99 for each of the L*, a*, and b* values. We also found that after training, students reached a high level of agreement between the “lasso” and “eye-dropper color sampler” method for each rater across the L*, a*, and b* variables (intraclass correlation coefficients [ICC] = 0.99 for each method and rater).
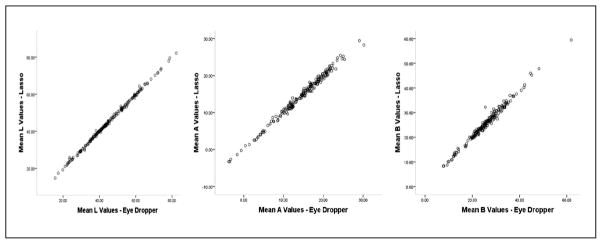
Finally, we wanted to compare the two methods (lasso and eyedropper) to see if they could be used interchangeably. Each of the final mean L*, a*, and b* values in this phase represented the average of the multiple measurements across the three raters so that we were left with one L*, a*, and b* value per method per image. ICCs were again calculated under the assumption of a two-way mixed model with consistency. The results of this phase indicate a high level of agreement between the “lasso” and “eye-dropper color sampler” methods for all color values with ICC of .99 (CI [.98, 1.00]) and correlation r = .99 for each of the L*, a*, and b* values. Figure 2 illustrates the linear relationship between the two methods for each skin color value.
Figure 2.
Scatterplots for the lasso and eye-dropper color sampler methods: L*, a*, and b* values in females (N = 200)
In summary, we learned that raters could be trained to complete DIA and their measurements had high levels of agreement. We also learned that the two methods are interchangeable; small areas of color obtained by the “eyedropper” approach can be compared to larger areas of color obtained by the “lasso” approach. Both techniques are useful in images captured in clinical situations.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by the National Institute of Nursing Research (F31NR011106; Janine Everett, Principal Investigator; 2R01NR005352; Marilyn Sommers, Principal Investigator; 1R01NR011 589; Marilyn Sommers, Principal Investigator; T32NR007100; Marilyn Sommers, Principal Investigator), The American Nurses Foundation, The Emergency Nurses Association, The International Association of Forensic Nurses, and the Lillian S. Brunner Professorship of Medical-Surgical Nursing, University of Pennsylvania.
Biographies
Janine S. Everett, PhD, RN was a Postdoctoral Research Fellow at the University of Pennsylvania School of Nursing (Philadelphia, PA) on submission of this manuscript. She is currently a Research Investigator at the University of Pennsylvania School of Nursing and an Adjunct Assistant Professor of Biology and Public Health at Franklin and Marshall College, Department of Biology, 415 Harrisburg Ave, Lancaster, PA 17603. She can be reached at Janine.Everett@fandm.edu.
Mia Budescu, PhDc, is a doctoral candidate at the Temple University, Philadelphia, PA, USA.
Marilyn S. Sommers, PhD, RN, FAAN, is the Lillian S. Brunner professor of medical surgical nursing at the University of Pennsylvania School of Nursing, Philadelphia, PA, USA.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abbade LP, Lastoria S, de Almeida Rollo H. Venous ulcer: Clinical characteristics and risk factors. International Journal of Dermatology. 2011;50:405–411. doi: 10.1111/j.1365-4632.2010.04654.x. [DOI] [PubMed] [Google Scholar]
- Andersen PH, Bjerring P. Noninvasive computerized analysis of skin chromophores in vivo by reflectance spectroscopy. Photodermatology, Photoimmunology & Photomedicine. 1990a;7:249–257. [PubMed] [Google Scholar]
- Andersen PH, Bjerring P. Spectral reflectance of human skin in vivo. Photodermatology, Photoimmunology & Photomedicine. 1990b;7(1):5–12. [PubMed] [Google Scholar]
- Baker RB, Fargo JD, Shambley-Ebron D, Sommers MS. A source of healthcare disparity: Race, skin color, and injuries after rape among adolescents and young adults. Journal of Forensic Nursing. 2010;6:144–150. doi: 10.1111/j.1939-3938.2010.01070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli P, Paciella M. Creation and implementation of a pressure ulcer prevention bundle improves patient outcomes. American Journal of Medical Quality. 2008;23(2):136–142. doi: 10.1177/1062860607313145. [DOI] [PubMed] [Google Scholar]
- Baumgarten M, Margolis D, van Doorn C, Gruber-Baldini AL, Hebel JR, Zimmerman S, Magaziner J. Black/White differences in pressure ulcer incidence in nursing home residents. Journal of the American Geriatrics Society. 2004;52:1293–1298. doi: 10.1111/j.1532-5415.2004.52358.x. [DOI] [PubMed] [Google Scholar]
- Bennett MA. Report of the task force on the implications for darkly pigmented intact skin in the prediction and prevention of pressure ulcers. Advances in Skin & Wound Care. 1995;8(6):34–35. [PubMed] [Google Scholar]
- Berardesca E, Derigal J, Leveque JL, Maibach HI. In vivo biophysical characterization of skin physiological differences in races. Dermatologica. 1991;182(2):89–93. doi: 10.1159/000247752. [DOI] [PubMed] [Google Scholar]
- Berwick M, Fine JA, Bolognia JL. Sun exposure and sunscreen use following a community skin cancer screening. Preventive Medicine. 1992;21:302–310. doi: 10.1016/0091-7435(92)90029-H. [DOI] [PubMed] [Google Scholar]
- Caldwell SH, Popenoe R. Perceptions and misperceptions of skin color. Annals of Internal Medicine. 1995;122:614–617. doi: 10.7326/0003-4819-122-8-199504150-00010. [DOI] [PubMed] [Google Scholar]
- Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, Holick MF. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Archives of Biochemistry and Biophysics. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. S0003-9861(06)00508-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y, Jang J, Jo S, Ahn K, Youn J. The difference between constitutive and facultative skin color does not reflect skin phototype in Asian skin. Skin Research and Technology. 2006;12:68–72. doi: 10.1111/j.0909-725X.2006.00167.x. [DOI] [PubMed] [Google Scholar]
- Clarys P, Alewaeters K, Lambrecht R, Barel AO. Skin color measurements: Comparison between three instruments: the Chromameter(R), the DermaSpectrometer(R) and the Mexameter(R) Skin Research and Technology. 2000;6:230–238. doi: 10.1034/j.1600-0846.2000.006004230.x. [DOI] [PubMed] [Google Scholar]
- Daniel LC, Heckman CJ, Kloss JD, Manne SL. Comparing alternative methods of measuring skin color and damage. Cancer Causes Control. 2009;20:313–321. doi: 10.1007/s10552-008-9245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Hughes B. Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. American Journal of Clinical Nutrition. 2004;80:1763S–1766S. doi: 10.1093/ajcn/80.6.1763S. [DOI] [PubMed] [Google Scholar]
- Domingo D, Matsui M. Photoprotection in non-Caucasian skin. In: Baron E, editor. Light-based therapies for skin of color. London, UK: Springer; 2009. pp. 111–134. [Google Scholar]
- ElectroTech. Humidity/temperature/dew point indicators: Series 5600. Glenside, PA: Author; 2008. [Google Scholar]
- Fairchild MD. Color appearance models. Hoboken, NJ: Wiley; 2005. [Google Scholar]
- Finkelstein JS, Lee M-LT, Sowers M, Ettinger B, Neer RM, Kelsey JL, Greendale GA. Ethnic Variation in Bone Density in Premenopausal and Early Perimenopausal Women: Effects of Anthropometric and Lifestyle Factors. Journal of Clinical Endocrinological Metabolism. 2002;87:3057–3067. doi: 10.1210/jc.87.7.3057. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TB. Ultraviolet-induced pigmentary changes: Benefits and hazards. Current Problems in Dermatology. 1986;15:25–38. doi: 10.1159/000412090. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Archives of Dermatology. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Fogerty M, Abumrad N, Nanney L, Arbogast P, Poulose B, Barbul A. Risk factors for pressure ulcers in acute care hospitals. Wound Repair and Regeneration. 2008;16(1):11–18. doi: 10.1111/j.1524-475X. 2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States) Cancer Causes and Control. 2005;16(2):83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- Ha S, Lee M, Lee O, Lee G, Kim J, Moon J, Oh C. A study of a method for distribution analysis of skin color. Skin Research and Technology. 2009;15:200–213. doi: 10.1111/j.1600-0846.2009.00355.x. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: A D-lightful story. Journal of Bone and Mineral Research. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- Howard D, Davies PS, DeLancey JO, Small Y. Differences in perineal lacerations in black and white primiparas. Obstetrics and Gynecology. 2000;96:622–624. doi: 10.1016/S0029-7844(00)00956-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber A, Cesko E, Dissemond J. Visual wound-assessment by population groups with different educational backgrounds. Phlebologie. 2009;38:219–225. [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. American Journal of Clinical Nutrition. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- Lee H. Introduction to color imaging science. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency: An important, common, and easily treatable cardiovascular risk factor? Journal of the American College of Cardiology. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- Lindholm C, Sterner E, Romanelli M, Pina E, Torra y Bou J, Hietanen H, Dealey C. Hip fracture and pressure ulcers: The Pan-European pressure ulcer study: Intrinsic and extrinsic risk factors. International Wound Journal. 2008;5:315–328. doi: 10.1111/j.1742-481X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott JW, Hoath SB. Neonatal skin: The ideal nursing interface. Journal of Pediatric Nursing. 1998;13:302–306. doi: 10.1016/S0882-5963(98)80016-X. [DOI] [PubMed] [Google Scholar]
- Molinari E, Fato M, De Leo G, Riccardo D, Beltrame F. Simulation of the biomechanical behavior of the skin in virtual surgical applications by finite element method. IEEE Transactions on Biomedical Engineering. 2005;52:1514–1521. doi: 10.1109/TBME.2005.851529. [DOI] [PubMed] [Google Scholar]
- Munsell . ColorChecker image reproduction targets. Grand Rapids, MI: X-Rite; 2007. pp. 1–4. [Google Scholar]
- Nelson L, Boyle M, Taggart I, Watson S. Are burns photographs useful? Burns. 2006;32:876–879. doi: 10.1016/j.burns.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Osterwalder U, Herzog B. Sun protection factors: World wide confusion. British Journal of Dermatology. 2009;161:13–24. doi: 10.1111/j.1365-2133.2009.09506.x. [DOI] [PubMed] [Google Scholar]
- Parra EJ. Human pigmentation variation: Evolution, genetic basis, and implications for public health. American Journal of Physical Anthropology. 2007:85–105. doi: 10.1002/ajpa.20727. [DOI] [PubMed] [Google Scholar]
- Parra EJ, Kittles RA, Shriver MD. Implications of correlations between skin color and genetic ancestry for biomedical research. Nature Genetics. 2004;36(Suppl 11):S54–60. doi: 10.1038/ng1440. [DOI] [PubMed] [Google Scholar]
- Parry I, Walker K, Niszczak J, Palmieri T, Greenhalgh D. Methods and tools used for the measurement of burn scar contracture. Journal of Burn Care & Research. 2010;31:888–903. doi: 10.1097/BCR.0b013e3181f9354. [DOI] [PubMed] [Google Scholar]
- Pershing LK, Tirumala VP, Nelson JL, Corlett JL, Lin AG, Meyer LJ, Leachman SA. Reflectance spectrophotometer: The dermatologists’ sphygmomanometer for skin phototyping? Journal of Investigative Dermatology. 2008;128:1633–1640. doi: 10.1038/sj.jid.5701238. [DOI] [PubMed] [Google Scholar]
- Pierard GE. EEMCO guidance for the assessment of skin colour. Journal of the European Academy of Dermatology and Venereology. 1998;10(1):1–11. doi: 10.1016/s0926-9959(97)00183-9. [DOI] [PubMed] [Google Scholar]
- Rees J. Understanding barrier function of the skin. Lancet. 1999;354:1491–1492. doi: 10.1016/S0140-6736(99)00281-0. [DOI] [PubMed] [Google Scholar]
- Rees JL, Birch-Machin M, Flanagan N, Healy E, Phillips S, Todd C. Genetic studies of the human melanocortin-1 receptor. Annals of the New York Academy of Sciences. 1999;885:134–142. doi: 10.1111/j.1749-6632.1999.tb08670.x. [DOI] [PubMed] [Google Scholar]
- Roberts WE. Skin type classification systems old and new. Dermatologic Clinics. 2009;27:529–533. doi: 10.1016/j.det.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Epidural analgesia and third- or fourth-degree lacerations in nulliparas. Obstetrics and Gynecology. 1999;94:259–262. doi: 10.1016/s0029-7844(99)00259-8. S0029-7844(99)00259-8. [DOI] [PubMed] [Google Scholar]
- Robinson JK, Rademaker AW, Sylvester JA, Cook B. Summer sun exposure: Knowledge, attitudes, and behaviors of midwest adolescents. Preventive Medicine. 1997;26:364–372. doi: 10.1006/pmed.1997.0156. [DOI] [PubMed] [Google Scholar]
- Rochester Institute of Technology. Imaging FAQ. Munsell Color Science Laboratory; n.d. Retrieved from http://www.cis.rit.edu/mcsl/faq3#312. [Google Scholar]
- Saladin LK, Krause JS. Pressure ulcer prevalence and barriers to treatment after spinal cord injury: Comparisons of four groups based on race-ethnicity. Neurorehabilitation. 2009;24(1):57–66. doi: 10.3233/nre-2009-0454. [DOI] [PubMed] [Google Scholar]
- Santos RV, Fry PH, Monteiro S, Maio MC, Rodrigues JC, Bastos-Rodrigues L, Pena SD. Color, race, and genomic ancestry in Brazil: dialogues between anthropology and genetics. Current Anthropology. 2009;50:787–819. doi: 10.1086/644532. [DOI] [PubMed] [Google Scholar]
- Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. Journal of Allergy and Clinical Immunology. 2008;122:261–266. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields AE, Fortun M, Hammonds EM, King PA, Lerman C, Rapp R, Sullivan PF. The use of race variables in genetic studies of complex traits and the goal of reducing health disparities: A transdisciplinary perspective. American Psychologist. 2005;60(1):77–103. doi: 10.1037/0003-066X.60.1.77. [DOI] [PubMed] [Google Scholar]
- Shriver MD, Parra EJ. Comparison of narrow-band reflectance spectroscopy and tristimulus colorimetry for measurements of skin and hair color in persons of different biological ancestry. American Journal of Physical Anthropology. 2000;112(1):17–27. doi: 10.1002/(SICI)1096-8644(200005)112:1<17::AID-AJPA3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Sommers MS. Defining patterns of genital injury from sexual assault: A review. Trauma, Violence, and Abuse. 2007;8:270–280. doi: 10.1177/1524838007303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers MS. Color awareness: A must for patient assessment. American Nurse Today. 2011;6(1):6. [Google Scholar]
- Sommers MS, Fargo JD, Baker RB, Fisher BS, Buschur C, Zink TM. Health disparities in the forensic sexual assault examination related to skin color. Journal of Forensic Nursing. 2009;5:191–200. doi: 10.1111/j.1939-3938.2009.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers MS, Zink TM, Fargo JD, Baker RB, Buschur C, Shambley-Ebron DZ, Fisher BS. Forensic sexual assault examination and genital injury: Is skin color a source of health disparity? American Journal of Emergency Medicine. 2008;26:857–866. doi: 10.1016/j.ajem.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatas GN, Zmudzka BZ, Kollias N, Beer JZ. Non-invasive measurements of skin pigmentation in situ. Pigment Cell Research. 2004;17:618–626. doi: 10.1111/j.1600-0749.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Stephen I, Law Smith M, Stirrat M, Perrett D. Facial skin coloration affects perceived health of human faces. International Journal of Primatology. 2009;30:845–857. doi: 10.1007/s10764-009-9380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiwaki H. Measurement of skin color: Practical aplication and theoretical considerations. Journal of Medical Investigation. 1998;44(3):121–126. [PubMed] [Google Scholar]
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor Hyperpigmentation Scale: A new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270–274. [PubMed] [Google Scholar]
- Taylor SC, Westerhof W, Im S, Lim J. Noninvasive techniques for the evaluation of skin color. Journal of the American Academy of Dermatology. 2006;54(5 Suppl 2):S282–290. doi: 10.1016/j.jaad.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Torres J, Kittles R. The relationship between “race” and genetics in biomedical research. Current Hypertension Reports. 2007;9:196–201. doi: 10.1007/s11906-007-0035-1. [DOI] [PubMed] [Google Scholar]
- Weatherall IL, Coombs BD. Skin color measurements in terms of CIELAB color space values. Journal of Investigative Dermatology. 1992;99:468–473. doi: 10.1111/1523-1747.ep12616156. [DOI] [PubMed] [Google Scholar]
- Weinstock MA, Moses AM. Skin cancer meets vitamin D: The way forward for dermatology and public health. Journal of the American Academy of Dermatology. 2009;61:720–724. doi: 10.1016/j.jaad.2009.04.016. [DOI] [PubMed] [Google Scholar]