Abstract
Detection of microbial products by host inflammasomes is critical for innate immune surveillance. Inflammasomes activate the CASPASE-1 (CASP1) protease, which processes the cytokines interleukin(IL)-1β and -18, and initiates a lytic host cell death called pyroptosis1. To identify novel CASP1 functions in vivo, we devised a strategy for cytosolic delivery of bacterial flagellin, a specific ligand for the NAIP5 (NLR family, apoptosis inhibitory protein 5)/NLRC4 (NLR family, CARD domain containing 4) inflammasome2–4. Here we show that systemic inflammasome activation by flagellin leads to loss of vascular fluid into the intestine and peritoneal cavity, resulting in rapid (< 30 minutes) death in mice. This unexpected response depends on the inflammasome components NAIP5, NLRC4, and CASP1, but is independent of IL-1β/-18 production. Instead, inflammasome activation results, within minutes, in an ‘eicosanoid storm’ – a pathological release of signaling lipids that rapidly initiate inflammation and vascular fluid loss. Mice deficient in cyclooxygenase-1 (COX-1), a critical enzyme in prostaglandin biosynthesis, are resistant to these rapid pathological effects of systemic inflammasome activation by either flagellin or anthrax lethal toxin. Inflammasome-dependent biosynthesis of eicosanoids is mediated by activation of cPLA2 (cytosolic phospholipase A2) in resident peritoneal macrophages, which are specifically primed for production of eicosanoids by high expression of eicosanoid biosynthetic enzymes. Thus, our results identify eicosanoids as a novel cell type-specific signaling output of the inflammasome with dramatic physiological consequences in vivo.
NAIP/NLRC4 inflammasome activation is critical for innate immune detection and defense against multiple bacterial pathogens in mice5–7. Interestingly, this resistance to infection, as well as the inflammasome-dependent response to systemic endotoxin, does not require IL-1β/-185,8,9, suggesting a critical role for pyroptosis and/or other inflammasome functions9–12. Here we sought to identify novel in vivo signaling outputs of the NAIP5/NLRC4 inflammasome. To selectively activate NAIP5/NLRC4, we delivered Legionella pneumophila flagellin (FlaA) to the cytosol by fusion of FlaA to the N-terminal domain of Bacillus anthracis lethal factor (LFn) that mediates cytosolic delivery through the anthrax protective antigen (PA) channel2,3. As expected, PA + LFn-FlaA (here called FlaTox), but not PA or LFn-FlaA alone, activated the NAIP5/NLRC4 inflammasome in bone marrow derived macrophages (BMDM), as indicated by cleavage of CASP1, release of lactate dehydrogenase, and IL-1β secretion (Supplementary Fig. 1). Inflammasome activation was abrogated in Casp1−/−, Naip5−/−, and Nlrc4−/− BMDM (Supplementary Fig. 1b). Importantly, a mutant form of FlaTox (FlaTox(AAA)), which is recognized by Toll-like receptor (TLR)-5 but not NAIP54, did not activate pyroptosis (Supplementary Fig. 1a).
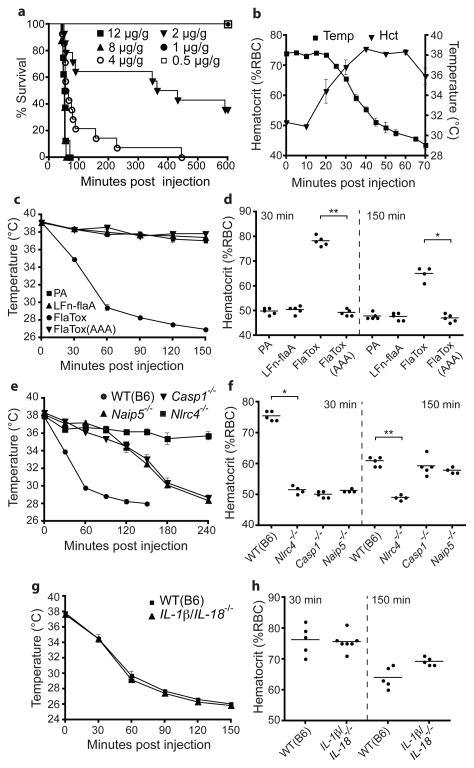
We then determined the effect of FlaTox administration in vivo. Remarkably, intravenous or intraperitoneal delivery of FlaTox rapidly killed mice, causing symptoms within 15 minutes and a mean time to death of ~30 minutes at saturating intravenous doses (Fig. 1a; Supplementary Fig. 1f). For subsequent experiments, we administered a sub-lethal intraperitoneal dose. FlaTox-treated mice rapidly developed diarrhea; however, histological analysis (Supplementary Fig. 2 and data not shown) after 30 minutes revealed no signs of pathology. Instead, we found fluid accumulation in the peritoneal cavity and intestine, but not in the kidneys or lungs (Supplementary Fig. 3). This fluid was lost from the blood, as the percent volume of red blood cells, or hematocrit (Hct), increased to 70–80% (normal 45–50%) within 30–40 minutes after FlaTox injection (Fig. 1b). As Hct rose, body temperature dropped, but with delayed kinetics. Hemoconcentration was the earliest pathological event we detected following FlaTox treatment, and is likely the primary cause of the ensuing circulatory collapse, hypothermia, and death.
Figure 1. Systemic cytosolic delivery of flagellin in vivo induces NAIP5/NLRC4-dependent but IL-1β/IL-18-independent vascular leakage.
(a–f) Mice (wild-type (B6) or indicated genotype) were injected intraperitoneally with FlaTox or indicated proteins (4 μg/g PA; 2 μg/g all others) and monitored for survival (a), hematocrit (b,d,f,h), or rectal temperature (b,c,e,g) at indicated times. Doses in (a) indicate LFn-FlaA. (a) n=5–14; (b) n=3; (c–h) n=4–7. Data shown (± s.e.m.) are pooled from multiple experiments (a) or representative of at least two independent experiments. * p = 0.016; ** p < 0.009 (Mann-Whitney t-test).
We found that only the complete toxin, and not individual subunits or FlaTox(AAA), induced hypothermia and increased hematocrit (Fig. 1c–d; Supplementary Fig. 4a), demonstrating that TLR signaling activated by flagellin or bacterial contaminants is insufficient to cause pathology. Nlrc4−/− mice were completely protected at all doses and time points tested (Fig. 1e-f, and data not shown), but surprisingly, while Naip5−/− and Casp1−/− mice were completely protected in survival experiments (Supplementary Fig. 4b), they exhibited a delayed hemoconcentration and hypothermic response (Fig. 1e–f). The moderate response of Naip5−/− mice may be due to recognition of flagellin by NAIP6 2,3. Importantly, mice lacking tumor necrosis factor (LTα/LTβ/TNFα−/−) or IL-1β/IL-18 were as sensitive as wild-type (B6) mice (Fig. 1g–h; Supplementary Fig. 4b–d), ruling out an essential role for these cytokines in FlaTox-induced pathologies.
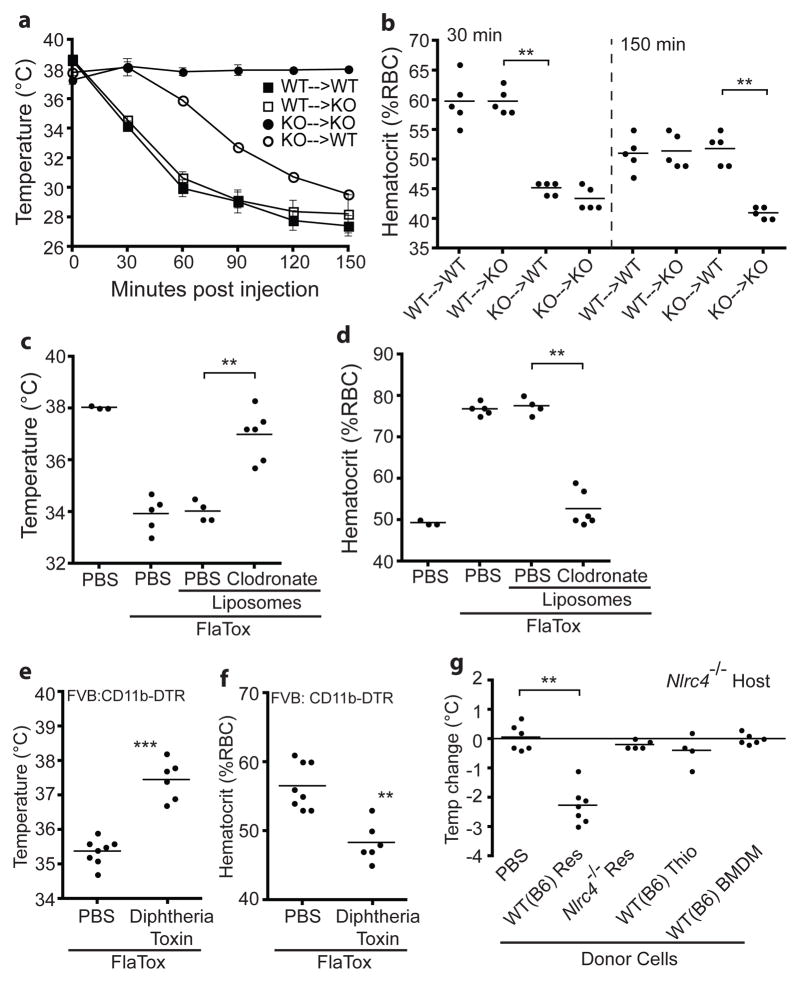
To identify the cell type(s) that respond to FlaTox, we generated bone marrow chimeras using susceptible wild-type (B6) mice and resistant Nlrc4−/− (KO) mice. Nlrc4−/− mice reconstituted with wild-type bone marrow were completely susceptible to FlaTox (Fig. 2a–b). Interestingly, wild-type mice reconstituted with Nlrc4−/− bone marrow also responded to FlaTox, but with delayed kinetics. These results suggest that at least two cell populations respond to FlaTox: (1) radio-sensitive hematopoietic cells that are necessary and sufficient for the early response to FlaTox (0–30 min) and (2) radio-resistant cells that respond after 30 minutes. Hereafter we focused on the early hematopoietic response (EHR).
Figure 2. Resident peritoneal macrophages are critical for the early FlaTox response in vivo.
(a–f) Mice were injected intraperitoneally with FlaTox and rectal temperature (a,c,e) or hematocrit (b,d,f) were measured after 30 minutes or at indicated times. (a–b) Bone marrow chimeric mice (KO = Nlrc4−/−; n=5). (c–d) Macrophage-depleted wild-type (B6) mice (n=3–6). (e–f) CD11b+ cell depleted FVB:CD11b-DTR mice (n=6–7). (g) Nlrc4−/− host mice injected intraperitoneally with 107 resident (Res) or thioglycollate-elicited (Thio) peritoneal cells or BMDM of indicated genotype. Rectal temperature was measured 30 minutes after intraperitoneal FlaTox (8 μg/g PA + 4 μg/g LFn-FlaA) injection. Data shown (± s.e.m.) are pooled from multiple experiments (g) or representative of at least three (a–f) independent experiments. ** p < 0.01; *** p = 0.0007 (Mann-Whitney t-test).
The EHR was intact in mice deficient in mast cells, lymphocytes, and neutrophils (Supplementary Fig. 5). In contrast, wild-type mice depleted of macrophages using clodronate-loaded liposomes were almost completely protected from the EHR (Fig. 2c–d). Similarly, depletion of CD11b+ cells in FVB:CD11b-DTR mice conferred protection (Fig. 2e–f). Flow cytometric analysis revealed near-complete ablation of CD11b/F4–80hi resident peritoneal macrophages by both treatments (Supplementary Fig. 6a), while depletion of splenic and lamina propria macrophages was only partial (clodronate) or not observed (CD11b-DTR) (Supplementary Fig. 6b–c). We therefore hypothesized that resident peritoneal macrophages might mediate responsiveness to FlaTox. Indeed, Nlrc4−/− mice injected with wild-type resident peritoneal cells became sensitive to FlaTox (Fig. 2g). By contrast, spleen or bone marrow cells did not transfer responsiveness, and surprisingly, neither did wild-type thioglycollate-elicited peritoneal macrophages or BMDM (Fig. 2g; Supplementary Fig. 6d). These data demonstrate a unique and critical role for resident peritoneal macrophages in the inflammasome-dependent in vivo response to FlaTox.
We hypothesized that peritoneal macrophages might initiate the EHR through inflammasome-dependent secretion of a previously unidentified factor. Eicosanoids are paracrine signaling lipids critical for activation of inflammation and host defense, and can induce vascular permeability, vasodilation, and leukocyte chemotaxis13–16. Eicosanoids are synthesized when arachidonic acid (AA) released from cell membranes by phospholipases is converted into prostaglandins (PG) and thromboxanes downstream of the cyclooxygenases (COX-1 & COX-2), or into hydroxyeicosatetraenoic acids (HETEs) and leukotrienes (LT) downstream of the lipoxygenases (12/15-LOX & 5-LOX) (Supplementary Fig. 7). Notably, direct intraperitoneal injection of prostaglandins leads to diarrhea and fluid accumulation in the gut17,18, both hallmarks of FlaTox-induced pathology.
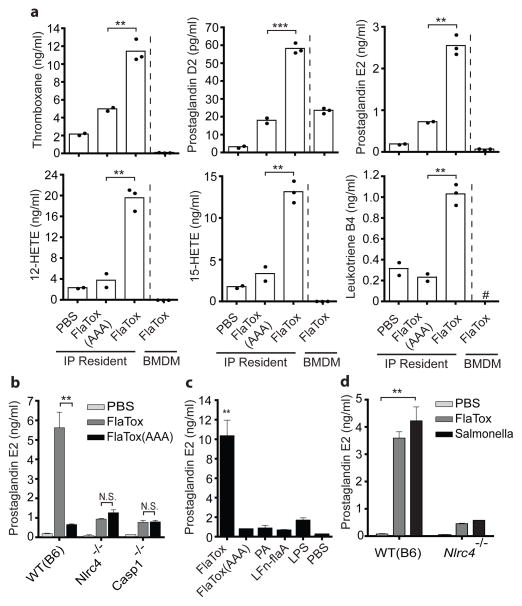
We therefore hypothesized that resident peritoneal macrophages, the specific cells required for the EHR (Fig. 2), produce eicosanoids in response to inflammasome activation. Using liquid chromatography-tandem mass spectrometry (LC/MS/MS) lipidomic analysis and enzyme immunoassay, we detected rapid biosynthesis of numerous COX- and LOX-dependent., PGE2 and cysteinyl leukotrienes in supernatants of peritoneal lavage cells treated ex vivo with FlaTox, whereas the FlaTox(AAA) mutant elicited a much weaker response (Fig. 3; Supplementary Fig. 8a–b). Moreover, eicosanoid induction required NAIP5, NLRC4 and CASP1 (Fig. 3b; Supplementary Fig. 8c). LTB4/PGE2 induction by PA or LFn-FlaA alone was equivalent to FlaTox(AAA) or LPS stimulation, further demonstrating that TLR signaling cannot account for their biosynthesis (Fig. 3c; Supplementary Fig. 8b). Importantly, the flagellated intracellular pathogen Salmonella typhimurium also elicited inflammasome-dependent eicosanoid biosynthesis similarly to FlaTox (Fig. 3d; Supplementary Fig. 8d), demonstrating that this pathway is activated during live infection.
Figure 3. Inflammasome-dependent eicosanoid biosynthesis.
(a) LC/MS/MS-based lipidomicsof wild-type (B6) BMDMor resident peritoneal cells incubated 30 minutes ex vivo with FlaTox (20 μg/ml PA + 10 μg/ml LFn-FlaA). (b–d) PGE2 immunoassay of resident peritoneal macrophages treated ex vivo with FlaTox (b–d), indicated proteins (d: 10 μg/ml PA, 5 μg/ml all others), lipopolysaccharide (d: 1 μg/ml), or S. typhimurium (e: MOI=5) and incubated 30 (b–c) or 180 (d) minutes. Data shown (± s.e.m.) are representative of at least two (d) or three (a,b–c) independent experiments. * p < 0.04; ** p < 0.009 *** p < 0.0005 (Student t-test). # = not detected
Similar to the ex vivo results, LC/MS/MS analysis of peritoneal lavage fluid from mice treated with FlaTox for 20 minutes revealed robust inflammasome-dependent eicosanoid biosynthesis in vivo (Supplementary Fig. 8). Some residual eicosanoid biosynthesis (particularly PGE2) was observed following FlaTox(AAA) treatment. This residual response is likely due to bacterial contaminants (e.g., LPS) activating peritoneal cells other than macrophages, since macrophages did not produce PGE2 in response to 30 minute LPS or FlaTox(AAA) stimulation (Fig. 3c). However, since this residual response did not produce symptoms (Fig. 1c–d) and eicosanoids have only paracrine effects, the FlaTox-induced pathology likely represents the localized response to resident peritoneal macrophages and/or the synergistic effects of multiple eicosanoids. Notably, FlaTox did not result in detectable production of anti-inflammatory lipoxins19. Taken together, our results show that inflammasome activation results in an ‘eicosanoid storm’ ex vivo and in vivo, characterized by broad biosynthesis of both LOX and COX products, with a bias towards pro-inflammatory lipids.
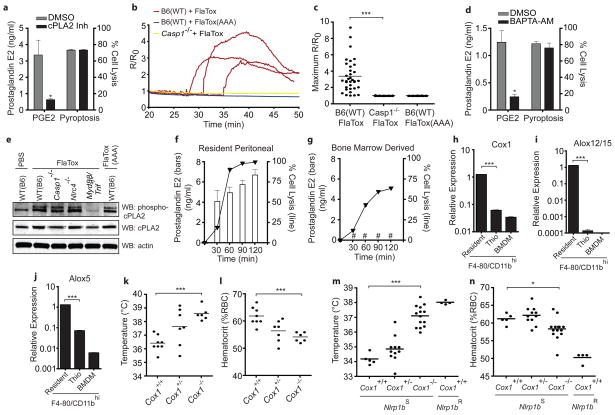
How does inflammasome activation induce eicosanoid biosynthesis? Although cells express several initiating A2 phospholipases, the calcium-dependent cytosolic phospholipase cPLA2 accounts for nearly all eicosanoid biosynthesis in peritoneal macrophages treated with a calcium ionophore20. Inhibition of cPLA2 also blocked LTB4/PGE2 production but not pyroptosis in response to FlaTox (Fig. 4a; Supplementary Fig. 8e). An increase in intracellular calcium (Ca2+) is both necessary and sufficient for cPLA2 activation21; notably, the earliest detectable CASP1-dependent events in S. typhimurium infected BMDM are formation of plasma membrane pores and the influx of Ca2+ 22,23. We observed that CASP1-dependent Ca2+ influx, comparable in magnitude to an ionomycin control (Supplementary Fig. 10), preceded cell lysis (79sec ± 29sec before onset of membrane blebbing) in resident peritoneal macrophages (Fig. 4b–c). This Ca2+ influx appears critical for eicosanoid biosynthesis, but dispensable for pyroptosis in response to FlaTox, as the intracellular Ca2+ chelator BAPTA-AM inhibited PGE2/LTB4 production without blocking LDH release (Fig. 4d; Supplementary Fig. 8f). Macrophages treated with glycine to inhibit pyroptosis23 still produced PGE2 in response to FlaTox, whereas cells lysed by digitonin or H2O2 did not, further indicating that eicosanoid production and cell lysis are separable events (Supplementary Fig. 11). Taken together, our results suggest a model (Supplementary Fig. 12) in which CASP1 activation results in rapid Ca2+ flux (Fig. 4b) that leads to cPLA2 activation and downstream eicosanoid biosynthesis. Identification of a CASP1 substrate required for Ca2+ influx is an important, but technically challenging area for future research as proteomic studies to identify novel substrates of CASP124–26 have so far yielded limited insight.
Figure 4. Mechanism and in vivo role of eicosanoid production.
(a,d) PGE2 immunoassay (30 min) or LDH (2 h) on supernatants from wild-type (B6) resident peritoneal macrophages. Pre-treated 45 min with DMSO, cPLA2 inhibitor (a: pyrrophenone; 0.2 μM) or BAPTA-AM (d: 10μM) then FlaTox + DMSO/inhibitor. (b–c) Wild-type (B6) or Casp1−/− resident peritoneal macrophages treated with FlaTox or FlaTox(AAA) and calcium indicator (Fluo-4; 2.5 mM) fluorescence/background (R/R0) quantified over time. (b) Representative cell, traces. (c) Maximum R/R0 for each cell. (e) Resident peritoneal cells of indicated genotype treated 30 min as indicated. Cell lysates were probed for indicated proteins by Western blot. (f–g) resident peritoneal macrophages (f) or BMDM (g) treated with FlaTox. PGE2 (bars) and cell lysis (line) measured over time as in (a). (h–j) Expression of indicated genes measured by quantitative RT-PCR in BMDM, and resident peritoneal macrophages or thioglycollate-elicited peritoneal cells sorted for CD11b/F4-80hi. (k–n) Mice were injected intraperitoneally with FlaTox (k–l) or anthrax lethal toxin (m–n; 200 μg PA + 100 μg lethal factor) and rectal temperature (k,m) and hematocrit (l,n) were measured after 30 (k–l) or 45 (m–n) minutes. (k–l) B6;129P2-Cox1−/− mice and littermate controls. (m–n) Cox-1−/− mice and littermate controls expressing a lethal toxin sensitive (S) or resistant (R) Nlrp1b allele. Data shown (± s.e.m.) are pooled from multiple experiments (k–n) or representative of at least two (b–c, h–j) or three (a, d–g) independent experiments. * p < 0.04; ** p < 0.006 *** p < 0.0007 (k–n: Mann-Whitney t-test; a,c,d,h–j: Student t-test). # = not detected.
Although cPLA2 activity is regulated primarily by Ca2+ influx, its activity can be enhanced by mitogen activated protein kinase (MAPK)-dependent phosphorylation21. We wondered if TLR signaling activated by FlaTox (FlaA or bacterial contaminants) might enhance cPLA2 activity through downstream MAPKs. Indeed, cPLA2 in resident peritoneal cells was phosphorylated following treatment with FlaTox, and this was inflammasome-independent, but Myd88/Trif-dependent (Fig 4e). Accordingly, LTB4 production in response to FlaTox was partially attenuated in Myd88/Trif−/− cells, and Myd88/Trif−/− mice were partially protected in vivo (Supplementary Fig. 13). Thus, although inflammasome-dependent Ca2+ flux is both sufficient and necessary for eicosanoid production in response to FlaTox, TLR signaling, which is expected to accompany natural infection, can synergize with inflammasome activation to produce maximal responses.
We further investigated the basis for the cell-type specificity of inflammasome-dependent eicosanoid biosynthesis. Consistent with the inability of BMDM to transfer responsiveness to FlaTox in vivo (Fig. 2g), we observed no eicosanoid production by LC/MS/MS in BMDM treated 30 min with FlaTox (Fig. 3a). Even two hours post-treatment, when most BMDM have undergone pyroptosis, we could not detect PGE2/LTB4 production (Fig. 4g–h; Supplementary Fig. 8g–h). Interestingly, Cox1, Alox12/15, and Alox5, which encode key enzymes required for eicosanoid biosynthesis, are expressed at much (10- to 1,000-fold) higher levels in CD11b/F4-80hi resident peritoneal macrophages than in BMDM or thioglycollate-elicited CD11b/F4-80hi cells (Fig. 4i–k). Resident peritoneal macrophages are therefore uniquely primed for eicosanoid responses. Most characterization of inflammasomes has relied on BMDM, perhaps explaining why a link to eicosanoid biosynthesis has not been described. We speculate that the primed state of resting peritoneal macrophages may be a general characteristic of resident macrophages guarding sites of pathogen entry; it will be important to further explore lineage-specific regulation of inflammasome function in vivo.
To test whether eicosanoids cause FlaTox-induced pathology, we injected B6;129P2.Cox1−/− mice and littermate controls with FlaTox, and found that the EHR was significantly attenuated in these mice (p<0.0007; Fig. 4l–m) despite intact pyroptosis (Supplementary Fig. 14). As expected, given that FlaTox induces broad biosynthesis of both COX-1-dependent and -independent eicosanoids, the role of COX-1 was masked at high doses of FlaTox or at later time points (data not shown), suggesting a contribution of COX-1-independent eicosanoids or other unidentified factors. Confirming the results with Cox1−/− mice, chemical inhibition of COX-1 protected mice from a low dose of FlaTox (Supplementary Fig. 15). Inhibition of the inducible enzyme COX-2 or genetic deletion of Alox5 had no effect, although their contribution may be masked by functional redundancy of downstream eicosanoids (Supplementary Fig. 15). These data link production of COX-1-dependent eicosanoids to the pathology associated with in vivo delivery of FlaTox, consistent with earlier reports that purified prostaglandins are sufficient to cause fluid accumulation and diarrhea17,18.
We hypothesized that other inflammasomes might also lead to eicosanoid production in vivo. For example, anthrax lethal toxin (LT) activates the NLRP1B inflammasome27, resulting in a rapid (but transient and non-lethal) hypothermic response28. Since C57BL/6 mice express a non-functional allele of Nlrp1b (Nlrp1bR), we injected Cox1+/+ mice expressing the sensitive 129 allele of Nlrp1b (Nlrp1bS). These mice developed diarrhea, decreased body temperature, and an inflammasome-dependent increase in hematocrit (Fig. 4n–o). As in FlaTox-treated mice, deletion of Cox1 attenuated these early pathologies of lethal toxin (Fig. 4n–o), indicating that this naturally occurring bacterial toxin activates a similar, though non-lethal, inflammasome pathway in mice.
While many cellular immune responses require de novo transcription, the NAIP5/NLRC4 and NLRP1B inflammasomes assemble from preformed protein components to activate a proteolytic cascade. As such, these inflammasomes are ideally positioned to mediate rapid responses to infection. Initiated within minutes of flagellin detection, the inflammasome-dependent eicosanoid production described here represents one of the most rapid innate immune cellular responses known in vivo. Although carried out in vivo, the results presented here rely on systemic administration of toxins. It will be important to explore the role of inflammasome-dependent eicosanoids during live infection. When restricted to the site of infection, such eicosanoids may play a beneficial role in host defense, for example, by rapidly increasing local vascular permeability, allowing influx of antibody, complement, and immune cells. Future studies should also evaluate a role for eicosanoids in other inflammasome-dependent phenotypes previously described in vivo. Indeed, our results suggest that the signaling outputs of inflammasomes may be much broader than has been previously appreciated.
METHODS SUMMARY
Toxin Delivery & Pathology
Recombinant proteins (PA, LFn-FlaA, LFn-FlaA(AAA), and LF) were purified from E. coli as previously described,29 and endotoxin removed using Detoxi-Gel (Pierce). LFn-FlaA(AAA) was generated by mutating the three C-terminal leucines of L. pneumophila flagellin to alanine4. Unless otherwise noted, standard toxin doses were 2 μg/g body weight LFn-FlaA in vivo (200 μl intraperitoneally) and 5 μg/ml LFn-FlaA in vitro. PA dose was always 2x LFn-FlaA. Rectal temperature was measured using a MicroTherma 2T thermometer (Braintree Scientific). Blood for hematocrit was collected by retroorbital-bleed into StatSpin microhematocrit tubes (Fisher Scientific).
Mice
For bone marrow chimeras, mice were irradiated 2x with 600 Rad 4 h apart, injected with 5×106 donor cells, and analyzed after 12 weeks. Macrophages were depleted 48 h with liposome encapsulated Clodronate (N. van Rooijen) (500 μl intraperitoneally and 200 μl intravenously). CD11b+ cells were depleted 24 h in FVB-Tg(CD11b/EGFP)34Lan/J mice with 25 ng/g of body weight (intraperitoneally) diphtheria toxin (Sigma-Aldrich).
Lipidomics
Lipid autacoids were extracted by solid phase with SampliQ ODS-C18 cartridges (Agilent Technologies). Eicosanoids and docosanoids were identified and quantified using a triple-quadrupole linear ion trap LC/MS/MS system (MDS SCIEX 3200 QTRAP) equipped with a Kinetex C18 mini-bore column.
Statistical Analysis
Statistical differences were calculated with an unpaired two-tailed Student’s t-test (in vitro/ex vivo) or two-tailed non-parametric Mann-Whitney test (in vivo) using GraphPad Prism 4.0b.
METHODS
Mice & Cell Culture
Except bone marrow chimeras (see below), all mice were sex and age-matched at 5–8 weeks old. C57BL/6J (B6) and B6.cKitwsh/wsh mice were purchased from Jackson Labs. B6;129P2-Ptgs1tm1Unc (Cox1) mice were purchased from Taconic. B6.Nlrc4−/− mice1 were from S. Mariathasan and V. Dixit. B6.Casp1−/− mice2 were a gift from A. Van der Velden and M. Starnbach. B6.MyD88/Trif−/− and FVB-Tg(CD11b/EGFP)34Lan/J were a gift from G. Barton. B6.Rag1−/− mice were a gift from D. Raulet. B6.IL-1β/IL-18−/− mice were a gift from D. Portnoy. B6.Alox5−/− mice were provided by C. Brown. B6.Naip5−/− mice were described previously3. For bone marrow chimeras, mice were irradiated 2x with 600 Rad 4 h apart and reconstituted with 5×106 donor cells by tail vein injection. Chimeric mice were assayed >12 weeks post irradiation. Bone marrow macrophages were differentiated from bone-marrow-derived precursor cells using macrophage colony stimulating factor as described previously3. For thioglycollate-elicited macrophages, mice were injected intraperitoneally with 2 ml of 4% aged thioglycollate media (BD Diagnostics) 4 days before peritoneal lavage. Cell lysis was measured by lactate dehydrogenase (LDH) release assay (Promega) according to the manufacturer’s protocol. Animal experiments were approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (Fig. 1a; Supplementary Fig. 1f) and the UC Berkeley Animal Care and Use Committee (all other figures).
FlaTox Injections & Pathology
Recombinant proteins (PA, LFn-FlaA, LFn-FlaA(AAA), and LF) were purified from E. coli as previously described4. Endotoxin was removed from these proteins using Detoxi-Gel (Pierce) according to the manufacturer’s protocol. LFn-FlaA(AAA) was generated by mutating the three C-terminal leucines of L. pneumophila flagellin to alanine3. Toxin was injected intraperitoneally or intravenously (tail vein) in 200 μl PBS. Unless otherwise noted, standard doses were 2 μg/g body weight LFn-FlaA in vivo (intraperitoneally) and 5 μg/ml LFn-FlaA in vitro. PA dose was always 2x LFn-FlaA. Rectal temperature was measured using a MicroTherma 2T thermometer (Braintree Scientific) with a lubricated RET-3 probe. For hematocrit measurement, mice were briefly anesthetized with isofluorane and blood was collected in a heparinized StatSpin 40 mm tube (Fisher Scientific) by retroorbital-bleed. The tube was sealed at one end with StatSpin sealant (Fisher Scientific) and centrifuged 10 minutes at 9,000g. The percentage of red blood cells was quantified using a StatSpin card hematocrit reader.
Fluid Loss
Peritoneal fluid was collected with a 1-ml insulin syringe (BD Biosciences) and quantified by weight. Intestines (small intestine + cecum + colon) were harvested and immediately weighed. Harvested tissues were then dried uncovered overnight at 37°C and weighed again. Fluid volume was calculated as wet weight - dry weight.
Cell Depletion & Transfer
Neutrophils were depleted by injecting B6 mice with 200 μg (intraperitoneal; 36 h before treatment) and 150 μg (intravenous; 6 h before treatment) of RB6-8C5 antibody (α-GR1; gift from D. Portnoy5). Macrophages were depleted by injecting B6 mice intraperitoneally with 500 μl and intravenously with 200 μl of liposome encapsulated Clodronate (N. van Rooijen) 48 h before FlaTox treatment. Clodronate liposomes were prepared as previously described6. CD11b+ cells were depleted by injecting FVB:CD11b-DTR mice intraperitoneally with 25 ng/g of body weight diphtheria toxin (Sigma-Aldrich) 24 h before FlaTox treatment.
For macrophage cell transfer (Fig. 2g), peritoneal cells were lavaged from naive or thioglycollate-treated mice and BMDM were derived in culture. ~107 macrophages (estimated by counting large cells in lavage) were injected intraperitoneally into host mice in 500 μl PBS. After 2 h, mice were injected with FlaTox and monitored for changes in rectal temperature. For spleen transfer, a single-cell suspension was generated by pushing spleen through mesh followed by ACK lysis of red blood cells. Bone marrow cells were collected from femurs and tibias followed by ACK lysis of red blood cells. Entire spleen or bone marrow from one mouse was transferred into one host by intraperitoneal injection and analyzed as above.
Eicosanoid Analysis
For ex vivo analysis, total resident peritoneal cells were lavaged from euthanized mice and macrophage numbers estimated by counting only large cells. 1–2×106 macrophages (~2–4×106 total cells) were aliquoted to 1.5 ml eppendorf tubes. Toxin treatments were carried out in 1 ml pre-warmed Dulbecco’s PBS with Ca2+ and Mg2+. After 30 minutes, cells were pelleted by centrifugation and the supernatant was immediately transferred to 2 ml cold methanol for storage at −80°C. For in vivo analysis, mice were injected intraperitoneally with FlaTox. After 20 minutes, mice were euthanized and the peritoneum was lavaged with 1 ml cold PBS, which was immediately transferred to 2 ml cold methanol for storage at −80°C. Before analysis, 400 pg each of the deuterated internal standards prostaglandin E2 (PGE2-d4), 15(S)-hydroxyeicosatetraenoic acid [15(S)-HETE-d8], and leukotriene B4 (LTB4-d4) were added to each sample to calculate the recovery of different classes of oxygenated fatty acid. Lipid autacoids were extracted by solid phase with SampliQ ODS-C18 cartridges (Agilent Technologies). Eicosanoids and docosanoids were identified and quantified by LC/MS/MS-based lipidomics7–10. In brief, we analyzed extracted samples by a triple-quadrupole linear ion trap LC/MS/MS system (MDS SCIEX 3200 QTRAP) equipped with a Kinetex C18 mini-bore column. The mobile phase was a gradient of A [water/acetonitrile/acetic acid (72:28:0.01, v:v:v)] and B [isopropanol/acetonitrile (60:40, v:v)] with a 450 μl/min flow rate. MS/MS analyses were performed in negative ion mode and prominent fatty acid metabolites were quantified by multiple reaction monitoring (MRM mode) using established transitions (2,4,5,6) for PGE2/PGD2 (351→271, 351→189 m/z), TXB2 (369→169 m/z), PGF2α (353→193m/z), 5-HETE (319→115 m/z), 12-HETE (319→179 m/z), 15-HETE (319→175 m/z), 5,12-DiHETE/LTB4 (335→195 m/z), LXA4 (351→115 m/z), PGE2-d4 (335→275 m/z), LTB4-d4 (339→197 m/z), and 15-HETE-d8 (327→182 m/z). Calibration curves (1 to 1000 pg) and specific LC retention times for each compound were established with synthetic standards (Cayman Chemical, Ann Arbor, MI). Structures were confirmed for selected autacoids by MS/MS analyses using enhanced product ion mode with appropriate selection of the parent ion in quadrupole 1.
For enzyme immunoassay (EIA) of LTB4, PGE2, and cysteinyl leukotrienes, total resident peritoneal cells were collected by lavage as above. 2×105 macrophages were seeded into 96-well plates and incubated 4 h in RPMI, 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine. Before assay, cells were washed 1x with PBS to select for adherent macrophages. Cells were treated for 30 minutes in 100 μl DPBS with Ca2+ and Mg2+. PGE2, LTB4, and cysteinyl leukotrienes in supernatants were measured by EIA (Cayman Chemicals).
Salmonella Infections
Resident peritoneal macrophages were selected from total peritoneal lavage by overnight plating on petri dishes followed by rinsing with PBS prior to replating into 96-well plates (2×105 cells/well). Salmonella enterica serovar Typhimurium strain LT2 was grown in 5 ml LB in a shaking incubator at 37°C overnight. The next morning, the cultures were diluted 1:66 in LB and grown for 3 h (standing culture, 37°C). Bacteria were added to cells at MOI=5 in DPBS + Ca2+ + Mg2+, followed by centrifugation at 400g for 10 min. After 3 h, eicosanoid production and cell lysis were measured by EIA and LDH assay, respectively, as described above.
Flow Cytometry
Leukocytes were collected from the peritoneal cavity by lavage with 7 ml PBS, from spleen passed through a nylon mesh (BD Falcon) to establish a single cell suspension, or from the lamina propria as previously described11. Cells were stained with α-F4-80-APC (eBiosciences; BM8; 1:200), α-CD11b-PE (eBiosciences; M1/70; 1:400), and α-CD11c-PE-Cy7 (eBiosciences; N418; 1:100), and analyzed using standard flow cytometry protocols. F4-80/CD11bhi cells were isolated by fluorescence-activated cell sorting.
Quantitative RT-PCR
CD11b/F4-80hi cells were sorted from total peritoneal cells lavaged from naive or thioglycollate-injected (2 ml injected intraperitoneally four days in advance) mice. Bone marrow derived macrophages are >95% CD11b/F4-80hi and were used without sorting. RNA was isolated with the RNeasy kit (Qiagen) according to the manufacturer’s protocol. RNA samples were treated with RQ1 DNase (Promega) prior to reverse transcription with Superscript III (Invitrogen). cDNA reactions were primed with poly dT for measurement of mature transcripts. Quantitative PCR was performed as described12 using the Step One Plus RT PCR System (Applied Biosystems) with Platinum Taq DNA polymerase (Invitrogen) and EvaGreen (Biotium). Transcript levels were normalized to Rps17. The following primers were used in this study. Rps17 sense: CGCCATTATCCCCAGCAAG; Rps17 antisense: TGTCGGGATCCACCTCAATG; Ptgs1(Cox1) sense: ATGAGTCGAAGGAGTCTCTCG; Ptgs1(Cox1) antisense: GCACGGATAGTAACAACAGGGA; Alox5 sense: ACTACATCTACCTCAGCCTCATT; Alox5 antisense: GGTGACATCGTAGGAGTCCAC; Alox12/15 sense: GGCTCCAACAACGAGGTCTAC; Alox12/15 antisense: AGGTATTCTGACACATCCACCTT
Western Blotting
Secreted proteins from FlaTox-treated cells were collected and probed with α-CASP1 p10 (Santa Cruz; sc-514) as previously described2. Cell lysates from ~2×106 resident peritoneal cells were probed with α-cPLA2 (Cell Signaling; 2832), α-phospho-cPLA2 (Cell Signaling; 2831), andα-beta-actin (Santa Cruz; sc-47778).
Calcium Flux
Resident peritoneal macrophages were selected from total peritoneal lavage by overnight plating on untreated petri dishes followed by 1x rinse with PBS and replating onto 8-chamber glass slides (Thermo Scientific) coated with poly-D-lysine (Sigma-Aldrich). After overnight incubation, cells were incubated 45 min at 37°C with 2.5 mM Fluo-4 (Invitrogen) + 0.02% pluronic (Invitrogen) in Ringer’s buffer containing 2 mM Ca2+. Cells were washed twice and incubated for 45 min in Ringer’s buffer at 37°C before transfer to microscope for imaging. Cells were maintained at 37°C with CO2 throughout imaging. Fluorescent (470/520) and differential interference contrast images were collected every 10 seconds on a Nikon Eclipse TE 2000-E microscope and analyzed using NIS Elements AR 3.2 software. At least 30 cells were analyzed for each replicate.
Supplementary Material
Acknowledgments
Work in R.E.V.’s laboratory is supported by Investigator Awards from the Burroughs Wellcome Fund and the Cancer Research Institute and by NIH grants AI075039, AI080749 and AI063302. K.G.’s laboratory is supported by NIH grant EY016136. J.v.M. is supported by a grant from the Cancer Research Coordinating Committee of the University of California. We thank S. Mariathasan and V. Dixit for Nlrc4−/− mice. We thank I. Bergin and S. Griffey for pathology reports. We thank D. Crown for help with survival experiments, L. Lopez for support in our animal facility, and D. Bautista and R. Nichols for help with calcium imaging. We thank M. Fontana and members of the Barton and Vance laboratories for discussions.
Footnotes
AUTHOR CONTRIBUTIONS
J.v.M. and R.E.V. conceived the study. J.v.M., R.E.V. and K.G. designed the experiments and wrote the paper. J.v.M. performed the experiments with help from N.J.T. M.M. performed experiments in Fig. 1a and Supplementary Fig. 1f. J.v.M., N.J.T., M.M., S.B.W., K.G. and R.E.V. analyzed the results. A.F.K., B.A.K., C.R.B., S.H.L. and N.v.R. provided mice and/or reagents.
References
- 1.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140 (6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011 doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011 doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 4.Lightfield KL, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9 (10):1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Case CL, Shin S, Roy CR. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun. 2009;77 (5):1981–1991. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutterwala FS, et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204 (13):3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107 (7):3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11 (12):1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamkanfi M, et al. Inflammasome-dependent release of the alarminHMGB1 in endotoxemia. J Immunol. 2010;185 (7):4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126 (6):1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Amer A, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281 (46):35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 12.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 isa regulator of unconventional protein secretion. Cell. 2008;132 (5):818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294 (5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 14.Samuelsson B. From studies of biochemical mechanism to novel biological mediators: prostaglandin endoperoxides, thromboxanes, and leukotrienes. Nobel Lecture, 8 December 1982. Biosci Rep. 1983;3 (9):791–813. doi: 10.1007/BF01133779. [DOI] [PubMed] [Google Scholar]
- 15.Tobin DM, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140 (5):717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan CN, ZHJ . Lipid Mediators in Acute Inflammation and Resolution: Eicosanoids, PAF, Resolvins and Protectins. In: Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. Cambridge University Press; New York: 2011. pp. 153–175. [Google Scholar]
- 17.Robert A, Nezamis JE, Lancaster C, Hanchar AJ, Klepper MS. Enteropooling assay: a test for diarrhea produced by prostaglandins. Prostaglandins. 1976;11 (5):809–828. doi: 10.1016/0090-6980(76)90189-1. [DOI] [PubMed] [Google Scholar]
- 18.Riviere PJ, Farmer SC, Burks TF, Porreca F. Prostaglandin E2-induced diarrhea in mice: importance of colonic secretion. J Pharmacol Exp Ther. 1991;256 (2):547–552. [PubMed] [Google Scholar]
- 19.Serhan CN, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21 (2):325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonventre JV, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390 (6660):622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 21.Gijon MA, Spencer DM, Leslie CC. Recent advances in the regulation of cytosolic phospholipase A(2) Adv Enzyme Regul. 2000;40:255–268. doi: 10.1016/s0065-2571(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 22.Bergsbaken T, Fink SL, den Hartigh AB, Loomis WP, Cookson BT. Coordinated host responses during pyroptosis: caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation. J Immunol. 2011;187 (5):2748–2754. doi: 10.4049/jimmunol.1100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8 (11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 24.Lamkanfi M, et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7 (12):2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282 (50):36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 26.Agard NJ, Maltby D, Wells JA. Inflammatory stimuli regulate caspase substrate profiles. Mol Cell Proteomics. 2010;9 (5):880–893. doi: 10.1074/mcp.M900528-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38 (2):240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 28.Terra JK, et al. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184 (1):17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krantz BA, et al. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science. 2005;309 (5735):777–781. doi: 10.1126/science.1113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
METHOD REFERENCES
- 1.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430 (6996):213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 2.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80 (3):401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 3.Lightfield KL, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9 (10):1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krantz BA, et al. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science. 2005;309 (5735):777–781. doi: 10.1126/science.1113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glomski IJ, Decatur AL, Portnoy DA. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect Immun. 2003;71 (12):6754–6765. doi: 10.1128/IAI.71.12.6754-6765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174 (1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 7.Sapieha P, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3(69):69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liclican EL, Nguyen V, Sullivan AB, Gronert K. Selective activation of the prostaglandin E2 circuit in chronic injury-induced pathologic angiogenesis. Invest Ophthalmol Vis Sci. 2010;51 (12):6311–6320. doi: 10.1167/iovs.10-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leedom AJ, Sullivan AB, Dong B, Lau D, Gronert K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol. 2010;176 (1):74–84. doi: 10.2353/ajpath.2010.090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan IR, Gronert K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J Immunol. 2009;182 (5):3223–3232. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- 11.Annacker O, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202 (8):1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monroe KM, McWhirter SM, Vance RE. Identification of host cytosolic sensors and bacterial factors regulating the type I interferon response to Legionella pneumophila. PLoS Pathog. 2009;5 (11):e1000665. doi: 10.1371/journal.ppat.1000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.