Abstract
Influenza A/Mississippi/03/2001 (H1N1) and A/Hong Kong/2369/2009 (H1N1) viruses containing the neuraminidase gene mutation H275Y (conferring resistance to oseltamivir) were adapted to mice and evaluated for suitability as models for lethal infection and antiviral treatment. The viral neuraminidases were resistant to peramivir and oseltamivir carboxylate but sensitive to zanamivir. Similar pattern of antiviral activity were seen in MDCK cell assays. Lethal infections were achieved in mice with the two viruses. Oral oseltamivir at 100 and 300 mg/kg/day bid for 5 d starting at −2 h gave 30 and 60% protection from death, respectively, due to the A/Mississippi/03/2001 infection. Intraperitoneal treatments with zanamivir at 30 and 100 mg/kg/day starting at −2 h gave 60 and 90% protection, respectively. Neither compound at ≤300 mg/kg/day protected mice when treatments began at +24 h. Amantadine was effective at 10, 30, and 100 mg/kg/day, rimantadine was protective at 10 and 30 mg/kg/day (highest dose tested), and ribavirin was active at 30 and 75 mg/kg/day, with survival ranging from 60–100% for oral treatments initiated at −2 h. For treatments begun at +24 h, amantadine was protective at 30 and 100 mg/kg/day, rimantadine showed efficacy at 10 and 30 mg/kg/day, and ribavirin was active at 75 mg/kg/day, with 60–100% survival per group. In the A/Hong Kong/2369/2009 infection, oral oseltamivir at 100 and 300 mg/kg/day starting at −2 h gave 50 and 70% protection from death, respectively. These infection models will be useful to study newly discovered anti-influenza virus agents and to evaluate compounds in combination.
Keywords: oseltamivir, zanamivir, amantadine, rimantadine, ribavirin
1. Introduction
Virus resistance to neuraminidase inhibitors has become the subject of increasing concern, based upon a higher frequency of isolating such viruses from infected patients (Besselaar et al., 2008; Dharan et al., 2009; Meijer et al., 2009). Prior to the H1N1 pandemic of 2009, the frequency of oseltamivir-resistant viruses was as high as 68% in certain regions of the world (Meijer et al., 2009). Fortunately, the 2009 H1N1 virus was sensitive to oseltamivir, although it was completely resistant to the drugs amantadine and rimantadine (Gubareva et al., 2010; Mossad, 2009). An analysis of treatment benefit during the pandemic indicated that oseltamivir initiated prior to the first 48 h reduced morbidity and mortality in severe cases (Smith et al., 2011). In addition, prophylaxis of individuals in contact with influenza-afflicted persons provided significant protection from infection (Smith et al., 2011). As the pandemic progressed and treatments with oseltamivir were given, some oseltamivir-resistant viruses were isolated and characterized (Chen et al., 2009; Gubareva et al., 2010; Nguyen et al., 2010a). The predominant mutation conferring resistance to oseltamivir was in the viral neuraminidase at position 275 where histidine was replaced by tyrosine (H275Y). However, the overall incidence of oseltamivir-resistant virus isolation was only about 1% of isolates (Thorlund et al., 2011). This situation could change from year to year, based upon finding up to 68% of virus isolates resistant to oseltamivir prior to 2009 (Meijer et al., 2009). The effectiveness of treatment of oseltamivir-resistant H1N1 H275Y virus infections has been shown to be reduced in pediatric clinical settings (Saito et al., 2010). A fatal case of oseltamivir-resistant H1N1 H275Y virus infection was recently reported from Korea (Hong et al., 2011), underscoring the virulence potential of the resistant virus.
Animal models, particularly mice and to a lesser extent ferrets, are routinely used to study treatment of influenza virus infections with existing drugs or new compounds (Barnard, 2009; Sidwell and Smee, 2000). Such studies have primarily entailed the use of neuraminidase inhibitor-sensitive viruses. This is because neuraminidase-resistant viruses have historically been weakly virulent in mice or ferrets (Carr et al., 2002; Ives et al., 2002). More recently this situation has changed. In 2009 Boivin et al. reported a mouse-adapted influenza A/WSN/33 (H1N1) H275Y (referred to as H274Y in the publication) infection model in mice (Baz et al., 2009). Note that prior to 2009 the convention for identifying amino acids in the viral neuraminidase followed H3N2 numbering, which differs in initial length from H1N1 by one amino acid. Thus, all earlier H1N1 viruses with an H275Y mutation were referred to as H274Y. In the present article the viruses are all referred to as H275Y.
The H275Y virus that was used for the studies of Baz et al. (2009) was created by genetic engineering. Oseltamivir was found to be ineffective in treating the infection when administered up to 10 mg/kg/day. However, an experimental neuraminidase inhibitor, A-322278, did show some efficacy in treating this infection. In that study the efficacy of higher doses of oseltamivir were not determined. Yen and colleagues engineered an influenza A/Vietnam/1203/2004 (H5N1) virus with an H275Y mutation that conferred oseltamivir resistance and yet retained lethality in mice (Yen et al., 2007). Antiviral studies with this virus have not been reported. We are not aware of any influenza A (H3N2) or influenza B viruses adapted to mice that are resistant to oseltamivir.
The H1N1 virus that emerged in 2009 was found to be lethal in mice following adaptation (Ilyushina et al., 2010), and some virus isolates were even able to cause mortality without adaptation in certain strains of mice (Otte et al., 2011). The influenza A/California/04/2009 (H1N1) virus that was mouse adapted had the following mutations compared to wild-type virus: 1 in PB2 (E158G), 3 in HA (G155E, S183P, and D222G), and 1 in NP (D101G) genes (Ilyushina et al., 2010). Later, certain oseltamivir-resistant H1N1 viruses were isolated and found to cause disease in mice and ferrets (Hamelin et al., 2010). It is possible that these isolates may be appropriate for antiviral studies in animals.
The purpose of this work was to develop an oseltamivir-resistant virus suitable for chemotherapy experiments in mice, and then to determine its susceptibility in the mouse model to various antiviral agents. Our efforts began prior to the emergence of the 2009 pandemic and before the published work of Baz et al. (2009) with the genetically engineered influenza A/WSN/33 (H1N1) H275Y virus. Because we were aware that mouse-adapted strains might only produce partially lethal infections in mice, several strains of virus were investigated. The viruses that we used were those that became available to us for research purposes. We determined that a mouse-adapted influenza A/Mississippi/03/2001 (H1N1) H275Y (hereafter referred to as A/MS-H275Y) was consistently lethal to BALB/c mice in repeated experiments. A pandemic virus resistant to oseltamivir, A/Hong Kong/2369/2009 (H1N1) H275Y (hereafter referred to as A/HK-275Y), was acquired by us and subsequently adapted for lethality in mice. Another virus strain serially passaged in mice, influenza A/Hawaii/21/2007 (H1N1) H275Y, was found to only be partially lethal to the animals, and no further work was done with that virus.
In the present studies we demonstrate the efficacy of several influenza virus inhibitors over a range of doses in mice lethally infected with the A/MS-H275Y and the A/HK-H275Y viruses. Infections of mice with these novel mouse adapted strains, particularly A/MS-H275Y, will be useful as models for studying treatment regimens that may combat oseltamivir-resistant virus infections.
2. Materials and methods
2.1. Antiviral compounds
Amantadine and rimantadine were purchased from Sigma (St. Louis, MO, USA). Oseltamivir phosphate (hereafter referred to as oseltamivir) was obtained from Tamiflu® capsules that were purchased from a local pharmacy. Oseltamivir carboxylate, the active form of oseltamivir, was obtained from Adamas Pharmaceuticals (Emeryville, CA, USA). Peramivir was kindly provided by BioCryst Pharmaceuticals (Birmingham, AL, USA). Zanamivir was purchased from Haorui Pharma-Chem (Edison, NJ, USA). Ribavirin was obtained from the former ICN Pharmaceuticals (Costa Mesa, CA, USA). All compounds, except zanamivir, were prepared in water for oral gavage treatment of mice. Water served as the oral gavage placebo. Zanamivir was prepared in sterile saline for intraperitoneal injection, and sterile saline served as the placebo for studies with zanamivir in mice. Because oseltamivir was used from pharmaceutical capsules that also contained excipients, the entire contents of 75 mg capsules minus the shell were added to water to make up the highest mg/kg/day dose. Lower doses of oseltamivir were prepared by dilution. We have previously reported that oseltamivir from Tamiflu® capsules performs similar to oseltamivir phosphate in mice (Smee et al., 2010b).
2.2. Viruses
Influenza A/MS-H275Y was obtained from the Neuraminidase Inhibitor Surveillance Network (Melbourne, Australia). The virus was passaged seven times in mice to enhance its virulence, then once in Madin-Darby canine kidney (MDCK) cells (purchased from the American Type Culture Collection, Manassas, VA, USA) to increase its titer. The virus was later titrated in BALB/c mice for lethality. Influenza A/California/07/2009 (H1N1) was obtained from the Centers for Disease Control and Prevention (Atlanta, GA, USA). Influenza A/HK-H275Y virus was provided by Kwok-Yung Yuen, The University of Hong Kong, Hong Kong Special Administrative Region, People’s Republic of China. This was one of the first reported oseltamivir-resistant pandemic viruses (Chen et al., 2009). The virus was adapted to mice by four sequential passages through mouse lungs, followed by one passage in MDCK cells. For passaging, BALB/c mice were infected with a 90-μl suspension of virus at a 1:2 dilution of the previously derived virus pool, starting with virus propagated in MDCK cells. Lungs were harvested from the mice at three days post-infection, homogenized in cell culture medium (MEM, one ml per lung) and frozen for subsequent passage in mice. The mouse-adapted viruses were partially sequenced in the neuraminidase gene and found to contain the H275Y mutation, as did the parental viruses. No other genes were sequenced.
2.3 Viral cytopathic effect (CPE) inhibition assay
MDCK cells in 96-well microplates were infected with approximately 50 cell culture infectious doses (CCID50) of virus. The medium used for assays was MEM, 0.22% sodium bicarbonate, and 10 units/ml of trypsin. Compounds in half-log10 concentrations were applied to cells 5–10 min prior to adding virus-containing medium. Three microwells at each concentration of compound were infected. Two microwells were uninfected and served as toxicity controls. After three days of incubation in 5% CO2 at 37°C, CPE was quantified by neutral red dye uptake (Smee et al., 2001; Smee et al., 2002) using the dye at a 0.011% final concentration for 2 h. Excess dye was rinsed from cells with PBS. The absorbed dye was eluted from the cells with 0.1 ml of 50% Sörensen’s citrate buffer (pH 4.2)/50% ethanol. Plates were read for optical density determination at 560 nm. Readings were converted to percent of uninfected control using an Excel spread sheet developed for this purpose. Fifty percent virus-inhibitory concentrations (EC50 values) and 50% cytotoxic concentrations (CC50 values) were determined by plotting percent CPE versus log10 of inhibitor concentration. Selectivity index (SI) values were calculated as CC50/EC50.
2.4. Viral neuraminidase inhibition assay
The effects of compounds on viral neuraminidase activity were determined using a commercially available kit (NA-Star® Influenza Neuraminidase Inhibitor Resistance Detection Kit, from Applied Biosystems, Foster City, CA, USA) in 96-well opaque white microplates following the manufacturer’s instructions and as has been reported (Smee et al., 2010a). Compounds in half-log dilution increments were incubated with virus (as the source of neuraminidase). The amount of virus in each microwell was approximately 500 CCID50 to achieve an adequate signal for assay quantitation. Plates were pre-incubated for 10 min at 37°C prior to addition of chemiluminescent substrate. Following addition of substrate the plates were incubated for 30 min at 37°C. The neuraminidase activity was evaluated using a Centro LB 960 luminometer (Berthold Technologies, Oak Ridge, TN, USA) for 0.5 sec immediately after addition of NA-Star® accelerator solution. Fifty percent inhibitory concentrations (IC50 values) were determined by plotting percent chemiluminescent counts versus log10 inhibitor concentration.
2.5 Animal experiment design
Female BALB/c mice (18–20 g, Charles River Laboratories, Wilmington, MA, USA) were anesthetized by intraperitoneal injection of ketamine/xylazine (50/5 mg/kg), followed by intranasal infection with a 90-μl suspension of influenza virus. The virus challenge was approximately three 50% mouse lethal infectious doses and equated to approximately 1–3 × 104 50% cell culture infectious doses (CCID50) per mouse. Other investigators use lower (30–50 μl) volumes for virus challenge. A higher volume of inoculum delivers more liquid to the lungs (Southam et al., 2002), which we feel improves the consistency of infection. Treatments were given twice a day (at 12 hours intervals) for 5 days starting either 2 h before or 24 h after virus challenge. Parameters used to assess the infection were survival and body weight changes. Animals were weighed through day 14 of the infection. Animals that lost more than 30% of their weight were euthanized (early euthanasia criterion). There were 10 mice per antiviral compound dosage group. Twenty placebo-treated mice were used in studies with amantadine, oseltamivir, and ribavirin, whereas the rimantadine and zanamivir experiments each had 15 placebos.
For one experiment the lungs from sacrificed mice were assayed for virus titer. This was performed by harvesting the lungs (5 animals per group) on days 3 and 6 after infection. Lungs were weighed, then frozen at −80°C. Later, thawed lungs were homogenized and samples plated in quadruplicate on MDCK cell monolayers in 96-well microplates in 10-fold dilution increments. On day 6 of the infection of cells, wells were observed microscopically for the appearance of viral cytopathic effect. Endpoint dilution titers were calculated (Reed and Muench, 1938), and results converted to log10 cell culture infectious doses per gram of tissue.
2.6. Statistical analysis of animal studies
Kaplan-Meier survival curves were generated and compared by the Mantel-Cox log-rank test to determine statistical significance. Subsequently, pairwise comparisons were made by the Gehan-Breslow-Wilcoxon test with Bonferroni-corrected threshold for significance. Lung virus titers were analyzed by one-way ANOVA followed by Neuman-Keuls Multiple Comparison Test. Pairwise comparisons were made between drug-treated and placebo groups, and were analyzed using Prism® 5.0 software (GraphPad Software, San Diego, CA).
2.7. Ethics statement for animal studies
The experiments were conducted in accordance with an approved protocol by the Institutional Animal Care and Use Committee of Utah State University. The work was performed in the AAALAC-accredited Laboratory Animal Research Center of the university in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Revision; 2010).
3. Results
3.1. Viral cytopathic effect inhibition studies
Six compounds representing different classes [viral M2 channel blockers (Leonov et al., 2011): amantadine and rimantadine; viral neuraminidase inhibitors (Gubareva et al., 2010): oseltamivir carboxylate, peramivir, and zanamivir; and a viral polymerase inhibitor (Eriksson et al., 1977): ribavirin], were evaluated for anti-influenza virus activity in cell culture against oseltamivir-sensitive influenza A/California/07/2009 (H1N1) virus, and oseltamivir-resistant influenza A/MS-H275Y and A/HK-H275Y viruses (Table 1). Against the sensitive virus, the three neuraminidase inhibitors showed similar inhibitory activity of 0.04–0.08 μM, with ribavirin activity at 28 μM, and amantadine and rimantadine showing no antiviral effect. Against the H275Y viruses, oseltamivir carboxylate was inactive at 100 μM, peramivir exhibited protection at about 8 μM, and zanamivir was inhibitory at 0.24–0.36 μM. Amantadine and rimantadine were both highly active against A/MS-H275Y (although rimantadine exhibited toxicity at 22 μM), but were not active against A/HK-H275Y. Ribavirin was inhibitory at 25–33 μM. These values are comparable to the published values against the non-mouse-adapted influenza A/MS-H275Y virus (Nguyen et al., 2010b).
Table 1.
Antiviral activities of compounds against influenza A (H1N1) virus infections in MDCK cell culture.
| Cytotoxicity | A/MS-H275Y | A/California/07/2009a | A/HK-H275Y | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Compound | CC50b (μM) | EC50c (μM) | SId | EC50 (μM) | SI | EC50 (μM) | SI |
| Amantadine | >100 | 0.30 ± 0.05 | >333 | >100 | 0 | >100 | 0 |
| Rimantadine | 22 ± 1 | 0.01 ± 0.003 | 2200 | >22 | 0 | >22 | 0 |
| Oseltamivir Carboxylate | >100 | >100 | 0 | 0.06 ± 0.1 | >1667 | >100 | 0 |
| Peramivir | >100 | 8.1 ± 5.2 | >12 | 0.04 ± 0.07 | >2500 | 7.9 ± 3.0 | >12 |
| Zanamivir | >100 | 0.24 ± 0.08 | >417 | 0.08 ± 0.07 | >1250 | 0.36 ± 0.58 | >278 |
| Ribavirin | >100 | 25 ± 4.6 | >4 | 28 ± 11 | >3.6 | 33 ± 13 | >3 |
Oseltamivir-sensitive virus.
50% cytotoxic concentration, determined in uninfected confluent cell monolayers by neutral red dye uptake. Data are from five independent assays (mean values ± SD).
50% effective (virus-inhibitory) concentration, determined by CPE inhibition assay and quantified by neutral red dye uptake. Data are from five independent assays (mean values ± SD).
Selectivity index (CC50/EC50).
3.2. Viral neuraminidase inhibition experiments
The three inhibitors of viral neuraminidase were tested for inhibitory activity against one oseltamivir-sensitive and two oseltamivir-resistant (H275Y) viral neuraminidases (Table 2). Oseltamivir carboxylate was ineffective against the two H275Y enzymes at 100 nM. Peramivir showed activity at 29–38 nM. Zanamivir was highly active at 0.9–1 nM against the drug-resistant viruses. Against the wild-type A/California/07/2009 (H1N1) virus, all three compounds were active, although oseltamivir carboxylate was slightly less potent than the other two compounds.
Table 2.
Inhibitory activities of compounds on influenza A (H1N1) neuraminidases.
| IC50a (nM) | |||
|---|---|---|---|
|
| |||
| H1N1 Virus | Oseltamivir Carboxylate | Peramivir | Zanamivir |
| A/MS-H275Y | >100 | 38 ± 1.0 | 1.0 ± 0.2 |
| A/California/07/2009b | 10.5 ± 1.3 | 2.9 ± 0.6 | 3.6 ± 0.5 |
| A/HK- H275Y | >100 | 29 ± 2.3 | 0.9 ± 0.2 |
50% inhibitory concentration, determined by NA-Star® assay. Data are from three independent assays (mean values ± SD).
Oseltamivir-sensitive virus.
3.3. Efficacy of neuraminidase inhibitors in infected mice
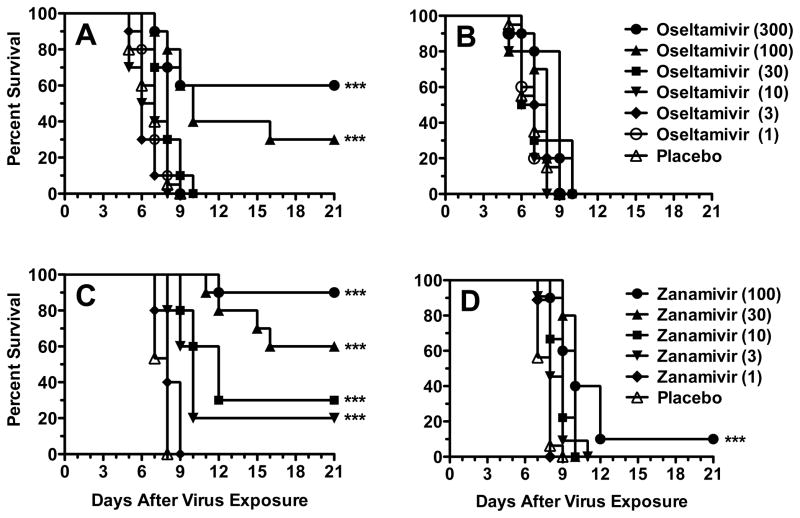
Oseltamivir and zanamivir were evaluated for efficacy against influenza A/MS-H275Y virus infections in mice. Treatments were initiated either 2 h prior to virus challenge or 24 h after infection. In the −2 h infection, oseltamivir was partially protective at 100 and 300 mg/kg/day, with survival rates of 30 and 60%, respectively (Fig. 1A). Lower doses of oseltamivir were not active. When treatment initiation time was delayed to +24 h, all doses of oseltamivir were ineffective (Fig. 1B). Treatment with zanamivir starting at −2 h provided protection of 20, 30, 60, and 90% at doses of 3, 10, 30, and 100 mg/kg/day (Fig. 1C). The 1-mg/kg/day dose of zanamivir was ineffective. Considerably less activity was observed for the +24 h treatments. The 100-mg/kg/day dose of zanamivir delayed the time to death significantly but did little to prevent mortality (Fig. 1D). Thus, neither oseltamivir nor zanamivir were able to treat an established infection.
Fig. 1.
Effects of oseltamivir and zanamivir treatment on survival from an influenza A/MS-H275Y virus infection in mice. Treatments were administered twice a day for 5 days starting 2 h prior to infection (A, C) or 24 h after infection (B, D). Oseltamivir was administered orally (by gavage) and zanamivir was administered intraperitoneally. *** P<0.001, compared to placebo.
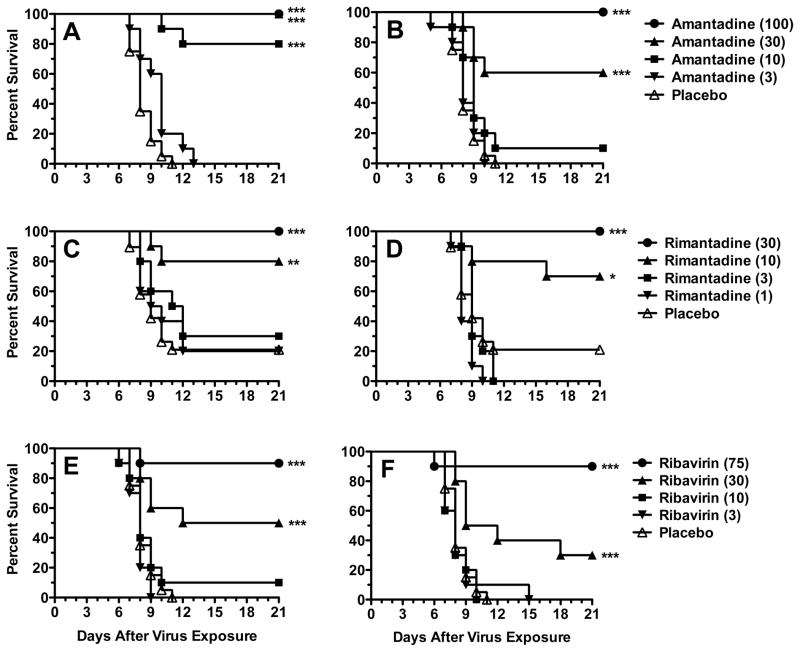
3.4. Efficacy of M2 channel inhibitors in infected mice
Amantadine and rimantadine were tested for their ability to prevent or delay the time to death in mice challenged with the A/MS-H275Y virus. Doses of amantadine (10–100 mg/kg/day) initiated at −2 h were 80–100% protective (Fig. 2A), with no effect seen at 3 mg/kg/day. Some activity was lost by delaying treatment to +24 h (Fig. 2B). The 30 and 100 mg/kg/day doses were 60 and 100% protective, respectively. No significant effect was seen at 3 and 10 mg/kg/day compared to placebo. Rimantadine provided a survival benefit at 10 and 30 mg/kg/day, starting either at −2 or +24 h (Figs. 2C and 2D), with no significant effect at 1 and 3 mg/kg/day. Survival observed was between 70 and 100%, although in these experiments the placebo groups had a survival rate of 20%. Both amantadine and rimantadine were able to treat the infection after its onset.
Fig. 2.
Effects of amantadine, rimantadine, and ribavirin treatment on survival from an influenza A/MS-H275Y virus infection in mice. Oral gavage treatments were administered twice a day for 5 days starting 2 h prior to infection (A, C, and E) or 24 h after infection (B, D, and F). * P<0.05, ** P<0.01, *** P<0.001, compared to placebo.
3.5. Efficacy of ribavirin in infected mice
The protective activity of ribavirin was studied in mice infected with influenza A/MS-H275Y virus. Treatment efficacy was similar for treatments initiated either at −2h (Fig. 2E) or at +24 h (Fig. 2F), although there were more survivors at 30 mg/kg/day in the −2 h group (which was not statistically different from the +24 h group). Ribavirin was not effective at 3 and 10 mg/kg/day. At the higher doses, ribavirin was capable of treating the infection after it was initiated.
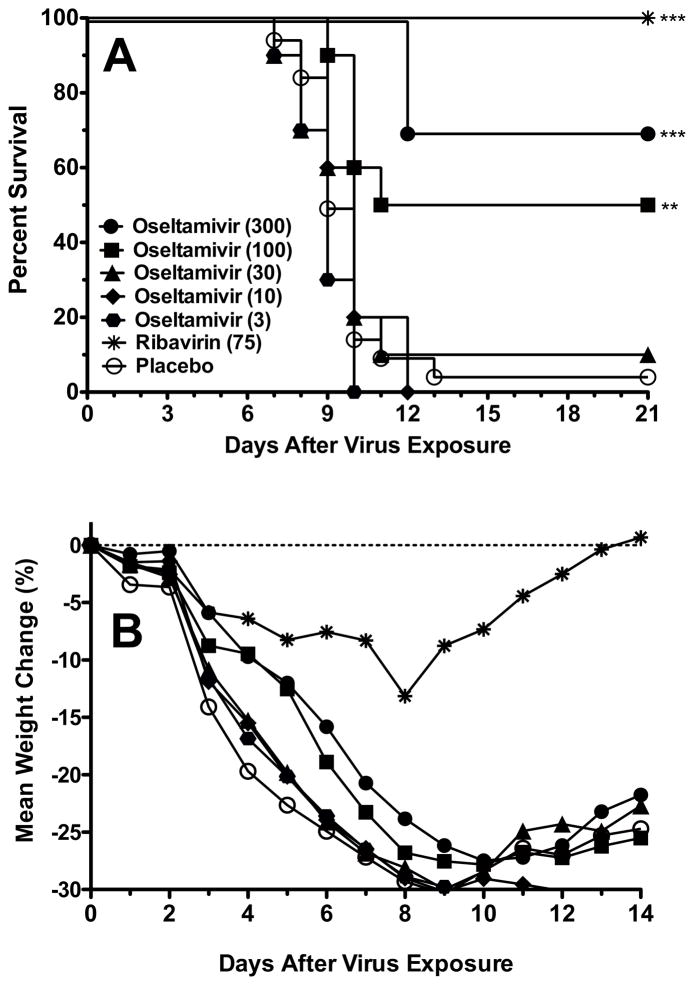
3.6. Efficacy of oseltamivir and ribavirin against influenza A/HK-H275Y virus infection in mice
Oseltamivir and ribavirin were used to treat an A/HK-H275Y virus infection starting treatment at −2 h relative to virus challenge (Fig. 3A). Oseltamivir was partially protective at 100 and 300 mg/kg/day, with survival rates of 50 and 70%, respectively. Doses of 3 and 10 mg/kg/day were not significantly effective compared to placebo. Ribavirin was fully protective at 75 mg/kg/day. These survival results for oseltamivir treatment were similar to those obtained in treating the A/MS-H275Y virus infection (Fig. 1A). Body weights were determined during the A/HK-H275Y virus infection (Fig. 3B). Weight decline was least severe in the ribavirin group, with weight gain starting after day 8 of the infection. Body weights in mice treated with oseltamivir at 100 and 300 mg/kg/day did not fall as rapidly as in the lower dosage and placebo groups. Body weights in these groups began to increase after day 10.
Fig. 3.
Effects of oseltamivir and ribavirin treatment on survival (A) and body weight change (B) during an influenza A/HK-H275Y virus infection in mice. Oral gavage treatments were administered twice a day for 5 days starting 2 h prior to infection. The SD for weight change was ±10% or less. ** P<0.01, *** P<0.001, compared to placebo.
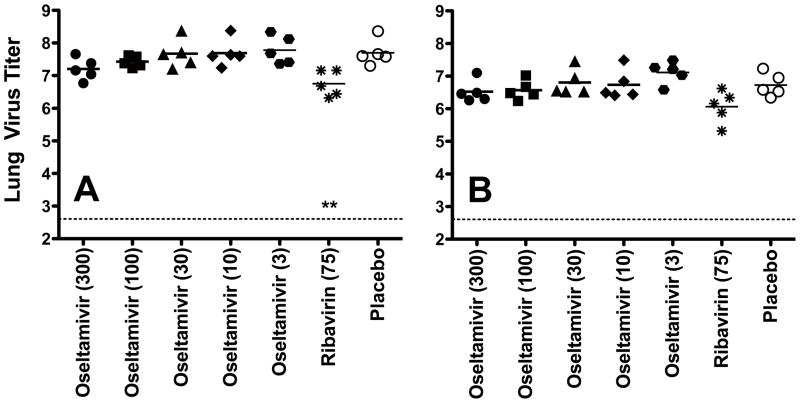
Lung virus titers from infected mice were determined on days 3 and 6 of the infection. On day 3, only ribavirin treatment significantly reduced virus titers compared to placebo (Fig. 3A). Virus titers in the ribavirin treatment group were not significantly lower than placebo on day 6, however (Fig. 4B).
Fig. 4.
Effects of oseltamivir and ribavirin treatment on lung virus titer from mice determined on days 3 (A) and 6 (B) of the infection with A/HK-H275Y virus. Oral gavage treatments were administered twice a day for 5 days starting 2 h prior to infection. The dotted line on each figure indicates the limit of virus detection. Virus titer units are log10 CCID50/g. ** P<0.01, compared to placebo.
4. Discussion
In this report we demonstrated that two different influenza A viruses possessing the H275Y mutation in the neuraminidase gene could be mouse adapted to cause fatal infections in mice. The H275Y mutation was stable during the mouse passages, as determined by genetic analysis of the relevant region of the neuraminidase gene. The viruses were also evaluated in cell culture and neuraminidase assays to demonstrate that they were phenotypically resistant to oseltamivir. The mouse infection models were then used to demonstrate antiviral activities of several known-active antiviral agents. This is not the first time that antiviral studies in mice were performed with oseltamivir-resistant viruses. The first study that we are aware of was that of Baz et al. (2009) using a recombinant influenza A/WSN/33 (H1N1) H275Y virus. In that report, they evaluated oseltamivir and a novel inhibitor, A322278. Oseltamivir was only used up to 10 mg/kg/day, giving a mortality rate of 75%. We used higher doses of the drug and saw survival rates of 30–50% at 100 mg/kg/day and 60–70% at 300 mg/kg/day. It is not known whether the A/WSN/33 (H1N1) H275Y virus reported by Baz et al. (2009) would perform the same as our viruses in infected mice if treated with higher doses of oseltamivir. This report differs from that of Baz and colleagues in showing antiviral activities of amantadine, rimantadine, ribavirin, and zanamivir, which to our knowledge has never been shown before in a mouse model of oseltamivir-resistant virus infection.
The cell culture studies demonstrated that the A/MS-H275Y and A/HK-H275Y viruses were resistant to oseltamivir, showed decreased sensitivity to peramivir, and were highly sensitive to zanamivir. The A/MS-H275Y virus was also inhibited by amantadine and rimantadine whereas the A/HK-H275Y was resistant to the adamantanes. Ribavirin was the least potent in cell culture of the compounds tested. The relative potencies identified for these compounds in vitro are similar to published reports for oseltamivir (Smee et al., 2001), peramivir (Smee et al., 2001), zanamivir (Smee et al., 2001), amantadine (Furuta et al., 2002), rimantadine (Valette et al., 1993), and ribavirin (Selvam et al., 2010; Selvam et al., 2006; Sleeman et al., 2010), against these or other wild-type influenza A (H1N1) virus strains. Resistance of the pandemic 2009 H1N1 viruses to adamantanes has been previously described (Gubareva et al., 2010; Mossad, 2009), and is attributable to an S31N mutation in the M2 gene.
Using a neuraminidase inhibition assay method, the relative inhibitory concentrations of oseltamivir carboxylate, peramivir, and zanamivir compared well with published H1N1 virus results (Gubareva et al., 2010; Nguyen et al., 2010a). Nguyen et al. (2010a) tested the same strain of influenza A/HK-H275Y virus that we used with the NA-Star® assay, and reported IC50 values of 78, 9.2, and 0.43 μM for oseltamivir carboxylate, peramivir, and zanamivir, respectively (Nguyen et al., 2010a). This compares to our data of >100, 29, and 0.9 μM, respectively. The same pattern of antiviral potency exhibited by the compounds (i.e., zanamivir > peramivir > oseltamivir) was evident for both the A/MS-H275Y and A/HK-H275Y viruses. This was not unexpected since both viruses contain the same oseltamivir-resistance mutation.
The choice of doses used for animal studies was based largely upon our previous experience in animal models, as well as anticipated outcomes based upon the cell culture and neuraminidase assay results. With oseltamivir, we wanted to administer high enough doses to find activity. In our animal models we rarely exceed 300 mg/kg/day with any compound, since such high doses in humans may not be tolerated. There are also problems with solubility, availability, and/or cost, depending upon the compound. Zanamivir was administered only up to 100 mg/kg/day because it showed better in vitro activity than oseltamivir carboxylate, and it was expected to perform better than oseltamivir. Our experience with amantadine indicated that it should be very effective at 100 mg/kg/day against the amantadine-sensitive virus (Smee et al., 2009). We had no experience with rimantadine, but knew from these studies that it was more potent but also more toxic than amantadine, thus, the highest dose of 30 mg/kg/day was selected. Finally, ribavirin was used at a high dose of 75 mg/kg/day because many published studies indicated that this dose was highly protective (Sidwell et al., 2005; Sidwell et al., 2001; Smee et al., 2004) as well as safe to administer. Ribavirin begins to cause toxicity in mice if used at 100 mg/kg/day. Significant antiviral activity was seen in mice treated with oseltamivir at 100 and 300 mg/kg/day, although only up to 60% protection. The 100-mg/kg/day dose of zanamivir (90% protective) proved to be more effective than oseltamivir at the same dose. This is not surprising since the virus was oseltamivir-resistant and zanamivir-sensitive. Amantadine’s 100% protective activity at the highest dose against the A/MS-H275Y virus infection was similar to that seen previously (Smee et al., 2009). Lower doses of rimantadine were as effective as the higher doses of amantadine, which correlated with the in vitro potency of each compound. The 75-mg/kg/day dose of ribavirin protected 90–100% of mice from death. In addition, weight loss data for treatments were largely dose-responsive (Fig. 3B), as have been reported in other influenza virus infections (Bantia et al., 2010; Smee et al., 2010a).
It has only been the more recent 2009 strains of oseltamivir-resistant influenza virus that have been reported to be lethal to mice (Hamelin et al., 2010). Prior to this, only the genetically engineered A/WSN/33 (H1N1) and A/Vietnam/1203/2004 (H5N1) H275Y viruses had been reported to lethally infect mice (Baz et al., 2009; Yen et al., 2007). In this report we indicate that the A/HK-H275Y 2009 virus described by Chen et al. (2010) could be adapted for lethality in mice. In addition, we found the older A/MS-H275Y virus isolated in 2001 was also adapted and found lethal to mice. The genetic changes acquired by these viruses during mouse adaptation that make then lethal to mice are not presently known. The wild type neuraminidases for the A/HK-H275Y and A/MS-275Y viruses were sequenced and reported in GenBank. An analysis of their sequences using the BLAST software available on PubMed indicated 82% identical homology and 90% functional homology (i.e., certain amino acid changes were not predicted to dramatically alter the structure). Ilyushina and colleagues (2010) reported five amino acid changes following adaptation of influenza A/California/04/2009 (H1N1) wild-type virus to mice. These changes were in PB2, HA and NP, with none in NA. One of the HA mutations, D225G, was associated with increased virulence in mice (Zheng et al., 2010). No other comparisons between influenza A/HK/2369/2009 (H1N1) and A/Mississippi/03/2001 (H1N1) viruses could be made due to lack of sequence data for the latter virus.
Experimental data suggest that fitness of influenza viruses carrying neuraminidase mutations conferring drug resistance may differ (Baz et al., 2010; Herlocher et al., 2002; Yen et al., 2007; Yen et al., 2005). Virulence in a mouse model may vary depending upon several factors such as location of the mutation, virus genetic background, existence of permissive secondary neuraminidase mutations, degree of neuraminidase functional loss, and an appropriate balance between neuraminidase and hemagglutinin. This cannot be determined in advance without going through the process of mouse adaptation and lethality determinations, as was done for the present studies.
Because antiviral protection was seen with oseltamivir at high (100 and 300 mg/kg/day) doses in the mouse models of A/MS-H275Y and A/HK-H275Y virus infections, we are not suggesting that this could translate into protection against oseltamivir-resistant virus infections in humans. This is because the doses that were used in mice exceed the approved human dose of oseltamivir. In work performed at Adamas Pharmaceuticals (Nguyen et al., 2012) a dose of 25 mg/kg/day in mice gave plasma levels of oseltamivir similar to that of humans. At this dose in mice, oseltamivir provided no benefit against the A/MS-H275Y and A/HK-H275Y virus infections. In addition, the efficacy in mice was observed only when the first treatment of oseltamivir was given prophylactically. Patients will be initiating treatment after infection. An example of this was a study of children infected with oseltamivir-resistant influenza A H1N1 H275Y virus who showed reduced benefit from treatment compared to children infected with sensitive virus (Saito et al., 2010). Thus, our results in mice treated with low doses of oseltamivir correlate with clinical findings. As a side note, ribavirin at 27 mg/kg/day and amantadine at 46 mg/kg/day in mice gave approximate human equivalent doses (Nguyen et al., 2012). In general, a dose in mice is 12 times that of a human for equivalency, based upon body surface area (Reagan-Shaw et al., 2007).
Delaying treatment by even one day is somewhat analogous to infecting with virus at a higher multiplicity of infection, thus making the infection more difficult to treat. Influenza virus strains vary in their ability to be treated after the infection has been initiated. Oseltamivir treatment of an influenza A/NWS/33 (H1N1) virus infection [not to be confused with the A/WSN/33 (H1N1) virus] can be effective even when initiated up to three days after infection (Sidwell et al., 1998). Treatment of influenza H3N2 and H5N1 virus infections did not provide sufficient protection with even a one-day delay of treatment initiation (Smee et al., 2010a; Smee et al., 2006), as occurs with the A/MS-H275Y and A/HK-H275Y virus infections. We have been investigating possible reasons for these differences but have not yet come to any conclusions. Other researchers have investigated differences among H5N1 virus strains in their ability to be treated by oseltamivir (Govorkova et al., 2009). The investigators suggested that multiple factors can contribute to the efficacy of neuraminidase inhibitors against highly pathogenic H5N1 influenza viruses in vivo, such as virus virulence, virus neurotropic potential, high levels of pulmonary expression of pro-inflammatory chemokines and cytokines, and pre-existing minor populations of drug-resistant clones.
Much work has been performed to evaluate compounds used in combination (Govorkova and Webster, 2010), some of which involved testing of drug-resistant viruses. However, these efforts have primarily focused on using amantadine-resistant viruses that are readily obtainable and many of which were easily mouse adapted (Ilyushina et al., 2007; Smee et al., 2009). From the results of these studies, the infection of mice with mouse-adapted influenza A/MS-H275Y virus appears to represent a useful model for studying the treatment of oseltamivir-resistant virus infections with newly discovered antiviral substances and compounds used in combination. The A/HK-H275Y model is also useful but we found that variable mortality occurred from experiment to experiment (the studies presented here were acceptable, but other unreported experiments where we tested different inhibitors had too few deaths in placebo groups for adequate statistical interpretation). Since the A/HK-H275Y virus is resistant to oseltamivir and to adamantanes, it offers more limited possibilities for combination treatment than the A/MS-H275Y virus. The A/MS-H275Y virus has already shown to be synergistically inhibited in vitro by the combination of oseltamivir carboxylate, amantadine, and ribavirin (Nguyen et al., 2010b). It will be important to investigate such combinations in mouse models of oseltamivir-resistant virus infection.
Acknowledgments
This work was supported in part by contract N01-AI-15435 and contract N01-AI-30063 (awarded to Southern Research Institute), both from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. The contents of this article do not necessarily reflect the policy of the government and no official endorsement shall be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bantia S, Kellogg D, Parker CD, Babu YS. Combination of peramivir and rimantadine demonstrate synergistic antiviral effects in sub-lethal influenza A (H3N2) virus mouse model. Antiviral Res. 2010;88:276–280. doi: 10.1016/j.antiviral.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Barnard DL. Animal models for the study of influenza pathogenesis and therapy. Antiviral Res. 2009;82:A110–122. doi: 10.1016/j.antiviral.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baz M, Abed Y, Nehme B, Boivin G. Activity of the oral neuraminidase inhibitor A-322278 against the oseltamivir-resistant H275Y (A/H1N1) influenza virus mutant in mice. Antimicrob Agents Chemother. 2009;53:791–793. doi: 10.1128/AAC.01276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baz M, Abed Y, Simon P, Hamelin ME, Boivin G. Effect of the neuraminidase mutation H275Y conferring resistance to oseltamivir on the replicative capacity and virulence of old and recent human influenza A(H1N1) viruses. J Infect Dis. 2010;201:740–745. doi: 10.1086/650464. [DOI] [PubMed] [Google Scholar]
- Besselaar TG, Naidoo D, Buys A, Gregory V, McAnerney J, Manamela JM, Blumberg L, Schoub BD. Widespread oseltamivir resistance in influenza A viruses (H1N1), South Africa. Emerg Infect Dis. 2008;14:1809–1810. doi: 10.3201/eid1411.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J, Ives J, Kelly L, Lambkin R, Oxford J, Mendel D, Tai L, Roberts N. Influenza virus carrying neuraminidase with reduced sensitivity to oseltamivir carboxylate has altered properties in vitro and is compromised for infectivity and replicative ability in vivo. Antiviral Res. 2002;54:79–88. doi: 10.1016/s0166-3542(01)00215-7. [DOI] [PubMed] [Google Scholar]
- Chen H, Cheung CL, Tai H, Zhao P, Chan JF, Cheng VC, Chan KH, Yuen KY. Oseltamivir-resistant influenza A pandemic (H1N1) 2009 virus, Hong Kong, China. Emerg Infect Dis. 2009;15:1970–1972. doi: 10.3201/eid1512.091057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, St George K, Epperson S, Brammer L, Klimov AI, Bresee JS, Fry AM. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301:1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- Eriksson B, Helgstrand E, Johansson NG, Larsson A, Misiorny A, Noren JO, Philipson L, Stenberg K, Stening G, Stridh S, Oberg B. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother. 1977;11:946–951. doi: 10.1128/aac.11.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Watanabe Y, Narita H, Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova EA, Ilyushina NA, McClaren JL, Naipospos TS, Douangngeun B, Webster RG. Susceptibility of highly pathogenic H5N1 influenza viruses to the neuraminidase inhibitor oseltamivir differs in vitro and in a mouse model. Antimicrob Agents Chemother. 2009;53:3088–3096. doi: 10.1128/AAC.01667-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova EA, Webster RG. Combination chemotherapy for influenza. Viruses. 2010;2:1510–1529. doi: 10.3390/v2081510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva LV, Trujillo AA, Okomo-Adhiambo M, Mishin VP, Deyde VM, Sleeman K, Nguyen HT, Sheu TG, Garten RJ, Shaw MW, Fry AM, Klimov AI. Comprehensive assessment of 2009 pandemic influenza A (H1N1) virus drug susceptibility in vitro. Antivir Ther. 2010;15:1151–1159. doi: 10.3851/IMP1678. [DOI] [PubMed] [Google Scholar]
- Hamelin ME, Baz M, Abed Y, Couture C, Joubert P, Beaulieu E, Bellerose N, Plante M, Mallett C, Schumer G, Kobinger GP, Boivin G. Oseltamivir-resistant pandemic A/H1N1 virus is as virulent as its wild-type counterpart in mice and ferrets. PLoS Pathog. 2010;6:e1001015. doi: 10.1371/journal.ppat.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlocher ML, Carr J, Ives J, Elias S, Truscon R, Roberts N, Monto AS. Influenza virus carrying an R292K mutation in the neuraminidase gene is not transmitted in ferrets. Antiviral Res. 2002;54:99–111. doi: 10.1016/s0166-3542(01)00214-5. [DOI] [PubMed] [Google Scholar]
- Hong SD, Park SH, Kang SJ, Kwon YS, Kee SJ, Park KH, Jung SI, Jang HC. First Fatal Oseltamivir-Resistant 2009 Pandemic Influenza A (H1N1) Case in an Adult in Korea. Chonnam Med J. 2011;47:127–129. doi: 10.4068/cmj.2011.47.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyushina NA, Hoffmann E, Salomon R, Webster RG, Govorkova EA. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir Ther. 2007;12:363–370. [PubMed] [Google Scholar]
- Ilyushina NA, Khalenkov AM, Seiler JP, Forrest HL, Bovin NV, Marjuki H, Barman S, Webster RG, Webby RJ. Adaptation of pandemic H1N1 influenza viruses in mice. J Virol. 2010;84:8607–8616. doi: 10.1128/JVI.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives JA, Carr JA, Mendel DB, Tai CY, Lambkin R, Kelly L, Oxford JS, Hayden FG, Roberts NA. The H275Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo. Antiviral Res. 2002;55:307–317. doi: 10.1016/s0166-3542(02)00053-0. [DOI] [PubMed] [Google Scholar]
- Leonov H, Astrahan P, Krugliak M, Arkin IT. How do aminoadamantanes block the influenza M2 channel, and how does resistance develop? J Am Chem Soc. 2011;133:9903–9911. doi: 10.1021/ja202288m. [DOI] [PubMed] [Google Scholar]
- Meijer A, Lackenby A, Hungnes O, Lina B, van-der-Werf S, Schweiger B, Opp M, Paget J, van-de-Kassteele J, Hay A, Zambon M. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis. 2009;15:552–560. doi: 10.3201/eid1504.081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossad SB. The resurgence of swine-origin influenza A (H1N1) Cleve Clin J Med. 2009;76:337–343. doi: 10.3949/ccjm.76a.09047. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Sheu TG, Mishin VP, Klimov AI, Gubareva LV. Assessment of pandemic and seasonal influenza A (H1N1) virus susceptibility to neuraminidase inhibitors in three enzyme activity inhibition assays. Antimicrob Agents Chemother. 2010a;54:3671–3677. doi: 10.1128/AAC.00581-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JT, Hoopes JD, Le MH, Smee DF, Patick AK, Faix DJ, Blair PJ, de Jong MD, Prichard MN, Went GT. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS One. 2010b;5:e9332. doi: 10.1371/journal.pone.0009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JT, Smee DF, Barnard DL, Julander JG, Gross M, de Jong MD, Went GT. Efficacy of combined therapy with amantadine, oseltamivir, and ribavirin in vivo against susceptigble and amantadine-resistnt influenza A viruses. PLoS One. 2012;7:e31006. doi: 10.1371/journal.pone.0031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte A, Sauter M, Alleva L, Baumgarte S, Klingel K, Gabriel G. Differential host determinants contribute to the pathogenesis of 2009 pandemic H1N1 and human H5N1 influenza A viruses in experimental mouse models. Am J Pathol. 2011;179:230–239. doi: 10.1016/j.ajpath.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–498. [Google Scholar]
- Saito R, Sato I, Suzuki Y, Baranovich T, Matsuda R, Ishitani N, Dapat C, Dapat IC, Zaraket H, Oguma T, Suzuki H. Reduced effectiveness of oseltamivir in children infected with oseltamivir-resistant influenza A (H1N1) viruses with His275Tyr mutation. Pediatr Infect Dis J. 2010;29:898–904. doi: 10.1097/INF.0b013e3181de9d24. [DOI] [PubMed] [Google Scholar]
- Selvam P, Chandramohan M, Hurst BL, Smee DF. Activity of isatine-sulfadimidine derivatives against 2009 pandemic H1N1 influenza virus in cell culture. Antivir Chem Chemother. 2010;20:143–146. doi: 10.3851/IMP1471. [DOI] [PubMed] [Google Scholar]
- Selvam P, Murugesh N, Chandramohan M, Sidwell RW, Wandersee MK, Smee DF. Anti-influenza virus activities of 4-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)amino]-N-(4,6-dimethyl-2-pyrimidi n-2-yl)benzenesulphonamide and its derivatives. Antivir Chem Chemother. 2006;17:269–274. doi: 10.1177/095632020601700504. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Bailey KW, Wong MH, Barnard DL, Smee DF. In vitro and in vivo influenza virus-inhibitory effects of viramidine. Antiviral Res. 2005;68:10–17. doi: 10.1016/j.antiviral.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Huffman JH, Barnard DL, Bailey KW, Wong MH, Morrison A, Syndergaard T, Kim CU. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antiviral Res. 1998;37:107–120. doi: 10.1016/s0166-3542(97)00065-x. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Smee DF. In vitro and in vivo assay systems for study of influenza virus inhibitors. Antiviral Res. 2000;48:1–16. doi: 10.1016/s0166-3542(00)00125-x. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Smee DF, Huffman JH, Barnard DL, Bailey KW, Morrey JD, Babu YS. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RJW-270201. Antimicrob Agents Chemother. 2001;45:749–757. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman K, Mishin VP, Deyde VM, Furuta Y, Klimov AI, Gubareva LV. In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A(H1N1) viruses. Antimicrob Agents Chemother. 2010;54:2517–2524. doi: 10.1128/AAC.01739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Huffman JH, Morrison AC, Barnard DL, Sidwell RW. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob Agents Chemother. 2001;45:743–748. doi: 10.1128/AAC.45.3.743-748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, Bailey KW, Morrey JD. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob Agents Chemother. 2009;53:2120–2128. doi: 10.1128/AAC.01012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, Bailey KW, Tarbet EB, Morrey JD, Furuta Y. Effects of the combination of favipiravir (T-705) and oseltamivir on influenza A virus infections in mice. Antimicrob Agents Chemother. 2010a;54:126–133. doi: 10.1128/AAC.00933-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, Tarbet EB, Babu YS, Klumpp K, Morrey JD. Combinations of oseltamivir and peramivir for the treatment of influenza A (H1N1) virus infections in cell culture and in mice. Antiviral Res. 2010b;88:38–44. doi: 10.1016/j.antiviral.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Morrison AC, Barnard DL, Sidwell RW. Comparison of colorimetric, fluorometric, and visual methods for determining anti-influenza (H1N1 and H3N2) virus activities and toxicities of compounds. J Virol Methods. 2002;106:71–79. doi: 10.1016/s0166-0934(02)00137-4. [DOI] [PubMed] [Google Scholar]
- Smee DF, Wandersee MK, Wong MH, Bailey KW, Sidwell RW. Treatment of mannan-enhanced influenza B virus infections in mice with oseltamivir, ribavirin and viramidine. Antivir Chem Chemother. 2004;15:261–268. doi: 10.1177/095632020401500505. [DOI] [PubMed] [Google Scholar]
- Smee DF, Wong MH, Bailey KW, Sidwell RW. Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza A (H1N1) and B viruses. Antivir Chem Chemother. 2006;17:185–192. doi: 10.1177/095632020601700403. [DOI] [PubMed] [Google Scholar]
- Smith JR, Rayner CR, Donner B, Wollenhaupt M, Klumpp K, Dutkowski R. Oseltamivir in seasonal, pandemic, and avian influenza: a comprehensive review of 10-years clinical experience. Adv Ther. 2011;28:927–959. doi: 10.1007/s12325-011-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam DS, Dolovich M, O’Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L833–839. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- Thorlund K, Awad T, Boivin G, Thabane L. Systematic review of influenza resistance to the neuraminidase inhibitors. BMC Infect Dis. 2011;11:134. doi: 10.1186/1471-2334-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valette M, Allard JP, Aymard M, Millet V. Susceptibilities to rimantadine of influenza A/H1N1 and A/H3N2 viruses isolated during the epidemics of 1988 to 1989 and 1989 to 1990. Antimicrob Agents Chemother. 1993;37:2239–2240. doi: 10.1128/aac.37.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HL, Ilyushina NA, Salomon R, Hoffmann E, Webster RG, Govorkova EA. Neuraminidase inhibitor-resistant recombinant A/Vietnam/1203/04 (H5N1) influenza viruses retain their replication efficiency and pathogenicity in vitro and in vivo. J Virol. 2007;81:12418–12426. doi: 10.1128/JVI.01067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HL, Monto AS, Webster RG, Govorkova EA. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J Infect Dis. 2005;192:665–672. doi: 10.1086/432008. [DOI] [PubMed] [Google Scholar]
- Zheng B, Chan KH, Zhang AJ, Zhou J, Chan CC, Poon VK, Zhang K, Leung VH, Jin DY, Woo PC, Chan JF, To KK, Chen H, Yuen KY. D225G mutation in hemagglutinin of pandemic influenza H1N1 (2009) virus enhances virulence in mice. Exp Biol Med (Maywood) 2010;235:981–988. doi: 10.1258/ebm.2010.010071. [DOI] [PubMed] [Google Scholar]