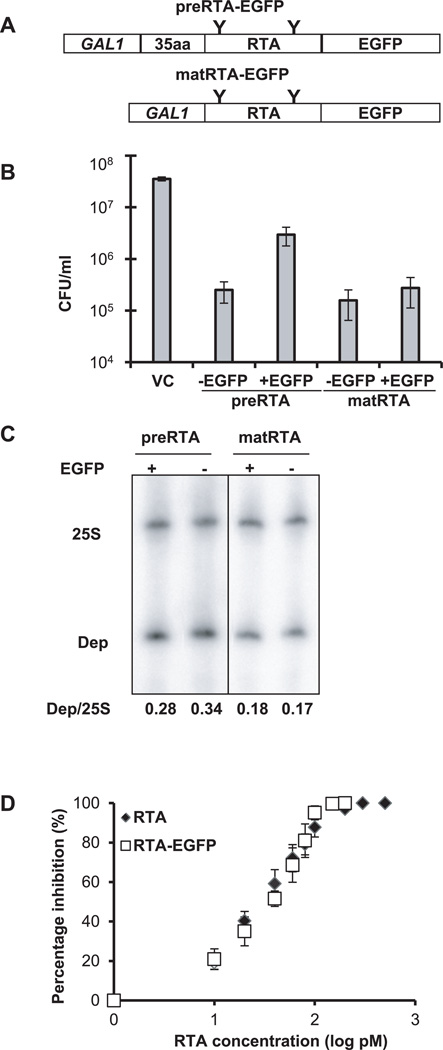
Figure 1. Cytotoxicity and enzymatic activity of RTA-EGFP fusions.
(A) Schematic representation of the C-terminal EGFP fusions with the precursor (preRTA-EGFP) and mature form of RTA (matRTA-EGFP). PreRTA contains the 35-residue leader and the 267-residue mature RTA, while mature RTA contains only the 267-residue RTA downstream of the GAL1 promoter. “Y” indicates the glycosylation sites. (B) Viability of yeast harboring the empty vector (VC), preRTA, preRTA-EGFP, mature RTA and mature RTA-EGFP. The CFU/ml was calculated based on the analysis of eleven different transformants at 10 hpi. (C) Ribosome depurination in yeast expressing the precursor and mature form of RTA with and without the EGFP tag. Total RNA isolated after 10 h of growth on galactose was analyzed by dual primer extension analysis using two different end-labeled primers, the depurination primer (Dep), which was used to measure the extent of depurination, and the 25S rRNA primer (25S), which was used to measure the total amount of 25S rRNA (22). The depurination was quantified by calculating the ratio between the intensity of depurination product (Dep) relative to the intensity of the 25S rRNA (25S) and is shown below each lane. (D) Translation inhibition by recombinant RTA-EGFP and recombinant RTA without EGFP. Purified RTA-EGFP and RTA were added to the Flexi Rabbit Reticulocyte Lysate System at 0, 10, 20, 40, 60, 80, 100, 200, 500, 1000 pM. The reaction was incubated at 30 °C for 30 min and the luciferase activity was measured. Data are the mean ± SE of at least 3 individual experiments.