Abstract
H-Dmt-D-Arg-Phe-Lys-NH2 ([Dmt1]DALDA), is a synthetic tetrapeptide with extraordinary selectivity for the mu-opioid receptor and is an extremely potent analgesic. [Dmt1]DALDA is unusual in the way that the greater part of its analgesic potency appears to be produced by its actions in the spinal cord. Furthermore, [Dmt1]DALDA inhibits norepinephrine re-uptake and is a mitochondria-targeted antioxidant. Such characteristics may make [Dmt1]DALDA particularly effective against neuropathic pain. The present study was designed to compare the effects of [Dmt1]DALDA and morphine on thermal hyperalgesia in an experimental neuropathic pain model. Neuropathic pain was induced in rats by surgical ligation of the L5 spinal nerve, and thermal pain thresholds were assessed by latencies of paw withdrawal to radiant heat. The increase in paw withdrawal latency was greater after the administration of [Dmt1]DALDA than that of morphine in neuropathic rats at doses that were equianalgesic in naïve animals. We conclude that [Dmt1]DALDA is more effective than morphine against thermal hyperalgesia in this experimental model of neuropathic pain.
Keywords: Opioid, neuropathic pain, hyperalgesia, [Dmt1]DALDA, morphine
H-Dmt-D-Arg-Phe-Lys-NH2 ([Dmt1]DALDA; Dmt = 2′,6′-dimethyltyrosine), is a synthetic tetrapeptide with extraordinary selectivity for the mu-opioid receptor. Compared to the prototypical mu-opioid, morphine, the affinity of [Dmt1]DALDA for the mu-opioid receptor is 7-fold greater and its potency in the in vitro functional assay (GPI) is 20-fold greater (1). In addition, [Dmt1]DALDA has some very interesting characteristics. As an analgesic, it is very potent especially after spinal administration with a potency 3000 times that of spinal morphine in an acute pain assay, which cannot be explained by its affinity and potency at the mu receptor (2). Furthermore, even after systemic administration [Dmt1]DALDA appears to act predominantly in the spinal cord to produce analgesia while morphine acts both in the brain and the spinal cord (3). [Dmt1]DALDA inhibits norepinephrine re-uptake (2) and it is also a mitochondria-targeted antioxidant (4).
With such characteristics, [Dmt1]DALDA may be superior as an opioid analgesic especially in neuropathic pain states. Firstly, the propensity of [Dmt1]DALDA to act in the spinal cord (3) may be advantageous since the spinal cord is a major site in the mechanisms of neuropathic pain (5). Secondly, norepinephrine reuptake inhibitors have been shown to be effective in neuropathic pain (6). Thirdly, mitochondrial reactive oxygen species (ROS) in spinal cord dorsal horn neurons have been suggested to play a role in the mechanisms of neuropathic pain, and antioxidants are effective against allodynia/hyperalgesia due to neuropathic pain (7, 8, 9). Thus the effect of [Dmt1]DALDA as a mitochondrial antioxidant may also add to its effect in neuropathic pain.
This study was designed to compare the effects of systemic [Dmt1]DALDA and morphine on thermal hyperalgesia due to experimental neuropathic pain in rats. Neuropathic pain in the hind limb was produced by tight ligation of the right L5 spinal nerve (10) and thermal pain threshold was evaluated by the Hargreaves paw withdrawal test (11).
Methods and Materials
Experiments adhered to the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the United States National Institutes of Health and were approved by the Institutional Animal Use Committee of Chiba University Graduate School of Medicine.
Animals
Male SD rats, 7 weeks old at the time of drug testing, were used in the experiments. Rats were caged in groups of 3 prior to surgery and individually after surgery with free access to food and water, and were maintained on a regular light-dark cycle.
Paw withdrawal test
Thermal pain threshold of the hind paw was assessed by measuring paw withdrawal latencies (PWLs) (sec) to radiant heat stimulation (Analgesiometer, IITC, Woodland Hills, CA, USA). Rats were placed in a clear plastic testing chamber on a glass floor and radiant heat was applied to the hind paws from underneath. The intensity of the radiant heat was adjusted so that the PWL of the hind paws of normal rats fell in the range of 10 ± 2 sec. Cut off latency was set at 20 sec. PWL of each paw was determined as a mean of 3 measurements per paw.
Neuropathic pain model
Nerve injury was produced by a surgical ligation of the L5 spinal nerve described by Kim and Chung (10). Each rat was anesthetized with sevoflurane (2–3%) and was placed in a prone position. Right paraspinal muscles were separated from spinous processes at the L5–L6 level and retracted. The right L6 transverse process was carefully removed with small forceps to visualize the L4 and L5 spinal nerves. The L5 spinal nerve was tightly ligated with an 8-0 silk ligature proximal to the confluence of the spinal nerves and distal to the dorsal root ganglion. PWLs were determined prior to and at 7 days after ligation procedure to observe the development of thermal hyperalgesia. In control animals, the same procedure up to the visualization of the spinal nerves was performed but the right L5 nerve was not ligated
Drug testing and data analysis
[Dmt1]DALDA was synthesized by methods described elsewhere (1). Morphine hydrochloride was obtained from Takeda Pharmaceuticals, Osaka, Japan. Drugs were dissolved in saline and were delivered subcutaneously (s.c.) at a volume of 0.1 ml/ 100 g rat weight. First, dose response studies were performed in naïve rats to determine equianalgesic doses of morphine and [Dmt1]DALDA in the paw withdrawal test. PWLs were determined prior to (baseline latency) and 30 or 120 min after the administration of morphine or [Dmt1]DALDA, respectively (response latency). The timing of testing was at peak effects of the compounds determined in previous studies (3). Maximum possible effect (%MPE) was calculated by the following equation.
A value of 20 (sec) was used for the cut-off latency value. Dose-response curves were constructed using the GraphPad Prism computer software (version 5).
Drug testing was performed in neuropathic and control rats 7 days after surgery. ED30 and ED90 doses determined from dose-response curves of morphine and [Dmt1]DALDA were tested. PWLs of the right hind paws were determined prior to and 30 or 120 min after the administration of morphine or [Dmt1]DALDA, respectively. Normal saline was given as vehicle control and tested 30 min after administration. Data were analyzed using the two-way analysis of variance for repeated measures followed by the Student-Newman-Keuls’ test. A p value less than 0.05 was considered significant.
Results and Discussions
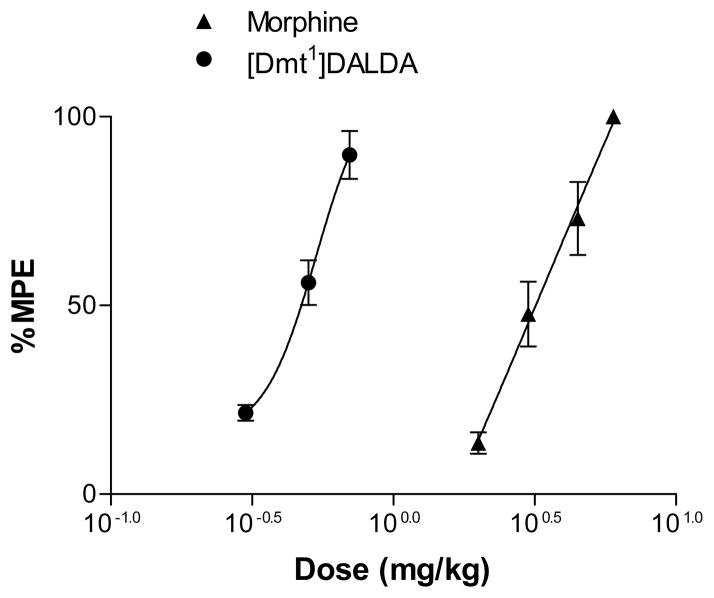
Morphine (2, 3, 4.5, 6 mg/kg) and [Dmt1]DALDA (0.3, 0.5, 0.7 mg/kg) showed dose-dependent analgesic effects in the paw withdrawal test in naïve rats (Fig. 1). ED30 and ED90 doses determined from the dose-response curves were 2.5 and 5.4 mg/kg for morphine, and 0.37 and 0.71 mg/kg for [Dmt1]DALDA, respectively.
Fig. 1.
Dose-response curves of the effects of morphine and [Dmt1]DALDA in the paw withdrawal test of the rat. Radiant heat was applied to the right hind paws of naïve rats, and paw withdrawal latencies were measured. Paw withdrawal latency of each paw was determined as a mean of 3 measurements per paw. Paw withdrawal latencies were determined prior to (baseline latencies) and 30 or 120 min after subcutaneous administration of morphine or [Dmt1]DALDA, respectively (response latencies). The number of animals tested for each dose was 6. Data are shown as maximum possible effect (%MPE) (see text).
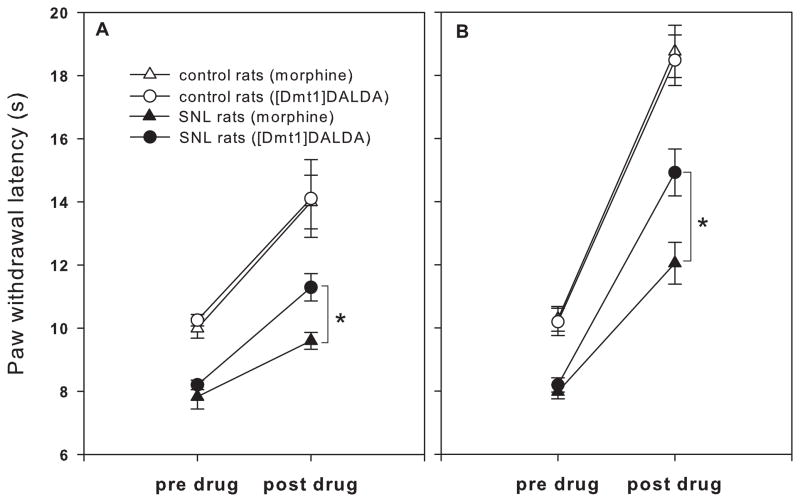
Spinal nerve ligation resulted in a significant reduction in PWL from 10.10±0.17 s to 8.01±0.17 (mean±SEM) s in the right hind paw at day 7 post-ligation, demonstrating the development of thermal hyperalgesia (Fig. 2). No significant change in PWL was observed in the right hind paw of control rats.
Fig. 2.
The effects of morphine and [Dmt1]DALDA at (A) ED30 and (B) ED90 doses on paw withdrawal latencies in spinal nerve ligated (SNL) and control rats. Radiant heat was applied to the right hind paw, and paw withdrawal latencies were measured. Paw withdrawal latency of each paw was determined as a mean of 3 measurements per paw. Paw withdrawal latencies were determined prior to (pre-drug) and 30 or 120 min after subcutaneous administration of morphine or [Dmt1]DALDA, respectively (post-drug). The number of animals in each group was 8 to 9.
*Significantly different between treatment groups (p<0.05).
Drug testing in control rats showed similar increases in PWL produced by morphine at 2.5 mg/kg and [Dmt1]DALDA at 0.37 mg/kg (Fig. 2A), and by morphine at 5.4 mg/kg and [Dmt1]DALDA at 0.71 mg/kg (Fig. 2B), confirming that the ED30 as well as ED90 doses of morphine and [Dmt1]DALDA were equianalgesic in the paw withdrawal test. In the hyperalgesic paw of the neuropathic rat, morphine and [Dmt1]DALDA both produced increases in PWL but the increase was significantly greater in rats that were given [Dmt1]DALDA compared to those given equianalgesic doses of morphine (Fig. 2A,B). [Dmt1]DALDA was just as effective in neuropathic rats as in control rats whereas morphine was less effective. The administration of saline control had no effects in both control and neuropathic rats (data not shown).
In the present study we showed that [Dmt1]DALDA was more effective than morphine in increasing thermal pain threshold in thermal hyperalgesia due to neuropathic pain produced by spinal nerve ligation in rats. Studies using Chinese hamster ovary (CHO) cells expressing the human mu-opioid receptor (hMOR)(12) or rat brain membranes for the [35S]GTPgammaS assay(13) showed that morphine has an intrinsic efficacy (e) of 86% and 85%, respectively, relative to [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) (e = 100%). Further two studies have shown that [Dmt1]DALDA also has slightly reduced efficacy at the MOR as compared to DAMGO (e = 100%): a study using calf striatal- or whole mouse brain membranes (e = 75 – 85%)(14), and a study using CHO expressing hMOR (e = 91%)(15). Taken together, these results indicate that [Dmt1]DALDA and morphine have comparable efficacies (75 – 91%, which are slightly reduced as compared to DAMGO (e = 100%). Thus the superior effectiveness shown in this study cannot be explained by the difference in efficacies between the two opioids.
Neuropathic pain involves multiple spinal mechanisms (5), and [Dmt1]DALDA is a potent mu-receptor selective opioid peptide with several unusual properties that might make it superior to morphine for neuropathic pain. [Dmt1]DALDA is especially potent after spinal administration, with potency 3000 times that of spinal morphine while its potency is only 30 times that of morphine after systemic administration in acute pain testing (2). This finding suggests that there are specific spinal analgesic mechanisms that may be unique to [Dmt1]DALDA that produces its potent spinal analgesic effects. Furthermore, the observation in our prior study that systemic administration of [Dmt1]DALDA was more potent in the tail flick test than the hot plate test while morphine was equally effective in the two tests also supports the theory that [Dmt1]DALDA has a propensity to act in the spinal cord (3). The specific spinal mechanism involved in the potent action of [Dmt1]DALDA, at least in part, may likely be its ability to inhibit norepinephrine reuptake. [Dmt1]DALDA has a norepinephrine reuptake-inhibiting potency that is more than 100-fold higher than morphine (2). A synergistic interaction between the mu-opioid receptor and norepinephrine receptor in the spinal cord has long been recognized (16,17), and [Dmt1]DALDA’s potent spinal analgesic effect may be produced by such interaction. Furthermore, it has been shown that norepinephrine-reuptake inhibitors by themselves are effective in neuropathic pain (6).
In addition, ROS are involved in the development and maintenance of neuropathic pain, and large doses of free radical scavengers have been shown to reduce neuropathic pain (7). Recent studies suggest that mitochondria are the primary source of ROS in spinal cord neurons. Mitochondrial superoxide was increased 60–100% in dorsal horn neurons of rats following L5 spinal nerve ligation (8). A subsequent study showed that intrathecal injection of inhibitors of the mitochondrial electron transport complexes produced increase in mitochondrial superoxide in the dorsal horn and long-lasting mechanical hyperalgesia (9). Although free radical scavengers have been shown to reduce spinal cord ROS and neuropathic pain, extremely high doses are required (9). This is probably due to the poor delivery of most antioxidants to mitochondria. [Dmt1]DALDA selectively targets and concentrates ~1000-fold on the inner mitochondrial membrane and is therefore extremely potent in reducing mitochondrial oxidative stress (4,18). As a result, the effective dose of [Dmt1]DALDA (~0.7 mg/kg) is 100–300 fold less than the dose of 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) used in other neuropathic pain models (9, 19).
In summary, [Dmt1]DALDA was more effective than morphine in increasing thermal pain threshold in thermal hyperalgesia due to experimental neuropathic pain in the rat. The superior effect may be due to the multiple functions of [Dmt1]DALDA that likely enhance its opioid action in neuropathic pain states.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Cancer Research (Study Group on Palliative Care and Psycho-oncology in Cancer Treatment) from the Ministry of Health and Welfare, Japan (N.S.), by the National Institute on Drug Abuse, NIH, USA (Multi-Center Program Project Grant DA08924 (H.H.S., P.W.S.) and grant DA004443 (P.W.S.)).
Footnotes
Conflict of Interest Disclosure
The peptide described in this article is licensed for commercial research and development to Stealth Peptides Inc, a clinical stage biopharmaceutical company, in which Hazel H. Szeto and Peter W. Schiller have financial interests.
References
- 1.Schiller PW, Nguyen TMD, Berzowska I, Dupuis S, Weltrowska G, Chung NN, Lemieux C. Synthesis and in vitro activity profiles of DALDA analogues. Eur J Med Chem. 2000;35:895–901. doi: 10.1016/s0223-5234(00)01171-5. [DOI] [PubMed] [Google Scholar]
- 2.Shimoyama M, Shimoyama N, Zhao GM, Schiller PW, Szeto HH. Antinociceptive and respiratory effects of intrathecal H-Tyr-D-Arg-Phe-Lys-NH2 (DALDA) and [Dmt1]DALDA. J Pharmacol Exp Ther. 2001;297:364–371. [PubMed] [Google Scholar]
- 3.Shimoyama M, Szeto HH, Schiller PW, Tagaito Y, Tokairin H, Eun CM, Shimoyama N. Differential analgesic effects of a mu-opioid peptide, [Dmt1]DALDA, and morphine. Pharmacol. 2009;83:33–37. doi: 10.1159/000167878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Wu G, Wu LJ. Neuronal and microglial mechanisms of neuropathic pain. Molecular Brain. 2011;4:31. doi: 10.1186/1756-6606-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leventhal L, Smith V, Hornby G, Andree TH, Brandt MR, Rogers KE. Differential and synergistic effects of selective norepinephrine and serotonin reuptake inhibitors in rodent models of pain. J Pharmacol Exp Ther. 2007;320:1178–1185. doi: 10.1124/jpet.106.109728. [DOI] [PubMed] [Google Scholar]
- 7.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 9.Kim HY, Chung JM, Chung K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: The effect of mitochondrial electron transport complex inhibitors. Neurosci Lett. 2008;447:87–91. doi: 10.1016/j.neulet.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 11.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Wang X, Partilla JS, Bishop-Mathis K, Benaderet TS, Dersch CM, Simpson DS, Prisinzano TE, Rothman RB. Differential effects of opioid agonists on G protein expression in CHO cells expressing cloned human opioid receptors. Brain Res Bull. 2008;77:49–54. doi: 10.1016/j.brainresbull.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spetea M, Bohotin CR, Asim MF, Stübegger K, Schmidhammer H. In vitro and in vivo pharmacological profile of the 5-benzyl analogue of 14-methoxymetopon, a novel mu opioid analgesic with reduced propensity to alter motor function. Eur J Pharm Sci. 2010;41:125–135. doi: 10.1016/j.ejps.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neilan CL, Janvey AJ, Bolan E, Berezowska I, Nguyen TM, Schiller PW, Pasternak GW. Characterization of the binding of [3H][Dmt1]H-Dmt-D-Arg-Phe-Lys-NH2, a highly potent opioid peptide. J Pharmacol Exp Ther. 2003;306:430–436. doi: 10.1124/jpet.103.049742. [DOI] [PubMed] [Google Scholar]
- 15.Zhao GM, Qian X, Schiller PW, Szeto HH. Comparison of [Dmt1]DALDA and DAMGO in binding and G protein activation at mu, delta, and kappa opioid receptors. J Pharmacol Exp Ther. 2003;307:947–54. doi: 10.1124/jpet.103.054775. [DOI] [PubMed] [Google Scholar]
- 16.Yaksh TL, Reddy SV. Studies in the primate on the analgetic effects associated with intrathecal actions of opiates, alpha-adrenergic agonists and baclofen. Anesthesiology. 1981;54:451–467. doi: 10.1097/00000542-198106000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Loomis CW, Jhamandas K, Milne B, Cervenko F. Monoamine and opioid interactions in spinal analgesia and tolerance. Pharmacol Biochem Behav. 1987;26:445–451. doi: 10.1016/0091-3057(87)90146-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl-hydroperoxide in neuronal cell lines. Biochem Pharmacol. 2005;70:1796–1806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Fidanboylu M, Griffiths LA, Flatters SJ. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS One. 2011;6:e25212. doi: 10.1371/journal.pone.0025212. [DOI] [PMC free article] [PubMed] [Google Scholar]