Abstract
Alzheimer’s disease (AD) is characterized by progressive, age-dependent degeneration of neurons in the central nervous system. A large body of evidence indicates that neurons affected in AD follow a dying-back pattern of degeneration, where abnormalities in synaptic function and axonal connectivity long precede somatic cell death. Mechanisms underlying dying-back degeneration of neurons in AD remain elusive but several have been proposed, including deficits in fast axonal transport (FAT). Accordingly, genetic evidence linked alterations in FAT to dying-back degeneration of neurons, and FAT defects have been widely documented in various AD models. In light of these findings, we discuss experimental evidence linking several AD-related pathogenic polypeptides to aberrant activation of signaling pathways involved in the phosphoregulation of microtubule-based motor proteins. While each pathway appears to affect FAT in a unique manner, in the context of AD, many of these pathways might work synergistically to compromise the delivery of molecular components critical for the maintenance and function of synapses and axons. Therapeutic approaches aimed at preventing FAT deficits by normalizing the activity of specific protein kinases may help prevent degeneration of vulnerable neurons in AD.
Keywords: Alzheimer’s disease, axonal transport, synapse, kinase, phosphatase, amyloid, presenilin, tau, kinesin, dynein, signaling, amyloid, ApoE, GSK3, CK2
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease representing the most prevalent form of dementia worldwide. The vast majority of AD patients have the sporadic, late onset form of the disease (sAD). The gradual loss of cognitive function that characterizes AD involves progressive dysfunction and degeneration of specific neuronal populations within the central nervous system (Holtzman, et al., 2011). The major histopathological hallmarks of AD include extracellular plaques composed of amyloid-β (Aβ) peptides and intracellular fibrillar tau aggregates known as neurofibrillary tangles (NFTs) (Holtzman, et al., 2011). Rare, familial forms of AD (fAD) are caused by mutations in amyloid precursor protein (APP) and presenilins 1 and 2 (PS1, PS2) (Bekris, et al., 2010), polypeptides functionally related to Aβ production (O’Brien and Wong, 2011). In addition, the identification of tau mutations as causative of various human disorders (collectively termed tauopathies) has directly linked abnormalities in tau to neurodegeneration (Goedert and Jakes, 2005). Similar clinical characteristics between fAD and sAD suggested the existence of common pathogenic mechanisms (Bossy-Wetzel, et al., 2004) fueling the development of animal AD models based on expression of fAD-related mutant polypeptides (Ashe and Zahs, 2010). Although molecular pathways linking fAD-associated forms of APP and PS, as well as mutant tau to neurodegeneration are not fully elucidated, an analysis of the early pathogenic events in these animal models and in brains of SAD patients reveals potential mechanisms.
Neurons affected in AD follow a “dying-back” pattern of degeneration
Synaptic dysfunction during early sAD stages is thought to underlie the memory and cognitive deficits characteristic of this disease (Bell and Claudio Cuello, 2006). Accordingly, neuronal populations affected in AD feature abnormalities in synapse morphology and marked reductions in the total number of synapses (DeKosky and Scheff, 1990, Masliah, et al., 1991a, Masliah, et al., 1991b). Moreover, Aβ peptides reportedly inhibit synaptic function in a variety of experimental systems [reviewed in (Shankar and Walsh, 2009, Tampellini and Gouras, 2010)].
Studies of the neuropathology in sAD brains mapped the earliest signs of neurodegeneration to medial temporal lobe structures, including the transentorhinal and entorhinal cortices, the subiculum and the hippocampus (Braak, et al., 2006). Interestingly, numerous studies demonstrate that neuropil threads, which are dystrophic neuronal processes (i.e. axons and dendrites) containing tau inclusions, often precede the deposition of detectable levels of NFTs in neuronal cell bodies within affected brain regions (Ghoshal, et al., 2002, Su, et al., 1997, Vana, et al., 2011). Moreover, dystrophic axons displaying aberrant accumulation of membrane-bounded organelles (MBOs) and cytoskeletal abnormalities are present in AD brains (Dessi, et al., 1997, Praprotnik, et al., 1996). In many cases, these dystrophic axons were observed before detectable deposition of classical tau and amyloid pathologies (Kowall and Kosik, 1987). This evidence, and the significant reduction in myelination observed within brain areas affected in AD (Ihara, et al., 2010), suggests that axonal dysfunction and degeneration represents an important component of AD pathogenesis.
Consistent with results from the post-mortem studies described above, several independent reports recently documented alterations in white matter of patients affected by mild cognitive impairment (MCI) in vivo. MCI is thought to represent a symptomatic pre-dementia stage of AD, because it involves cognitive deficits beyond those seen with normal aging, yet not severe enough to meet clinical criteria of dementia (Petersen, et al., 2001). Significantly, both quantitative high-resolution magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) studies in MCI patients documented axonal degeneration in the parahippocampal gyrus, an anatomically discrete brain area that includes the perforant pathway (Kalus, et al., 2006, Rogalski, et al., 2009, Stoub, et al., 2006). The perforant pathway represents an important axonal tract that connects neurons in the entorhinal cortex (one of the earliest brain areas affected in AD) to the dentate gyrus and other areas of the hippocampus (Witter, 2007). Abnormalities detected using MRI and DTI appear consistent with axonal degeneration, and by extension, loss of synaptic connectivity within the medial temporal lobe regions and other cortical regions (Stebbins and Murphy, 2009). Early signs of white matter degeneration also occur within the frontal, temporal, and parietal cortices, corpus callosum, and cholinergic system of MCI patients (Bozzali, et al., 2011, Huang and Auchus, 2007, Medina, et al., 2006, Swartz, et al., 2003). Importantly, these decrements in white matter integrity correlated well with deficits in cognitive and executive functions in many of these studies (Huang and Auchus, 2007, Kalus, et al., 2006, Rogalski, et al., 2009, Stoub, et al., 2006, Swartz, et al., 2003).
The development of transgenic fAD animal models has facilitated the study of early pathogenic events in the course of disease (Coleman and Perry, 2002, Price, et al., 2000). Currently, no model accurately recapitulates all aspects of AD (Ashe and Zahs, 2010, Gilley, et al., 2011a). Still, fAD models illuminated dysfunctional signaling pathways linked to specific fAD variants. Behavioral, electrophysiological, and pathological studies in fAD mouse models based on expression of pathogenic forms of APP, presenilin, and/or tau documented early abnormalities in synaptic function, and signs of axonal pathology (i.e. axonal swellings and spheroids) prior to overt cell death (Bell and Claudio Cuello, 2006, Gilley, et al., 2011a). Transgenic expression of fAD-related mutant APPV717A (PDAPP line) (Games, et al., 1995) and other fAD-related APP mutants (i.e. APPV717F and APPK670N, M671L) (Mucke, et al., 2000) leads to significant loss of synapses that precedes Aβ plaque formation. Similarly, in the widely studied 3XTg-AD mouse model (harboring mutant PS1M146V, APPSWE, and tauP301L), synaptic dysfunction and axonal pathology takes place prior to the deposition of amyloid plaques and tau aggregation (Cai, et al., 2011, Oddo, et al., 2003). Studies using the 5xFAD (harboring APPK670N, M671L,I716V,V717I; PS1M146L,L286V) mouse model (Oakley, et al., 2006) documented axonal swellings and spheroids at 3 months of age, before signs of memory deficits are detected at 6 months of age (Jawhar, et al., 2011). Deficits in synaptic transmission in APPswe/PS1M146L (Zhang, et al., 2005) and APPswe/PS 1ΔE9 mice (Goto, et al., 2008, Machova, et al., 2008) indicate that fAD-related pathogenic proteins cause functional impairments in synaptic function. In vivo imaging studies using DTI in the Tg2576 and PDAPP mouse models extended these observations, showing that alterations in white matter integrity coincide with deposition of amyloid-β pathology (Song, et al., 2004).
Taken together, both experimental and pathological evidence indicate that neuronal populations affected in AD follow a dying-back pattern of neuronal degeneration, where substantial reductions in synaptic function and axonal connectivity long precede neuronal cell death. Fitting the multi-factorial complexity of an age-related neurodegenerative disease, numerous pathogenic pathways are proposed to contribute to axonal degeneration in AD (Coleman, 2011). These include impaired axonal transport, cytoskeleton abnormalities, increased oxidative stress, imbalances in calcium signaling, mitochondrial dysfunction, inflammation-related neuronal damage, impaired protein degradation machinery, and a compromised blood-brain-barrier, among others. A detailed review of all these mechanisms is well beyond the scope of this review, but this material is discussed elsewhere (Bamburg and Bloom, 2009, Morawe, et al., 2012, Ye, et al., 2011, Yu, et al., 2009, Zlokovic, 2011). Given the association of dying-back degeneration to axonal transport abnormalities (see below), pathogenic mechanisms featuring such abnormalities represent the main topic of this review.
Axonal Transport: A critical cellular process underlying axonal and synaptic function
The ubiquitous tissue expression of fAD-associated gene products contrasts sharply with the selective vulnerability of neurons observed in AD suggesting that one or more cellular features render neuronal cells increasingly vulnerable. Unlike any other cell type, neurons feature unusually long dendrites and axons, cytoplasmic projections that facilitate the reception, processing and transmission of chemical information via synaptic contacts with other neurons. Axonal length vary from the relatively short distances required to communicate with interneurons to the remarkably long distances observed in projection neurons that connect anatomically remote areas of the nervous system (Mattson and Magnus, 2006). Neuronal cells display a unique reliance on axonal transport mechanisms because molecular constituents required for appropriate function and maintenance of synapses and axons are synthesized and packaged in MBOs in the neuronal cell body. Therefore, axonal transport represents a vital cellular process for the maintenance of proper axonal connectivity, synaptic function, and ultimately neuron survival (Morfini, et al., 2001a, Morfini, et al., 2009a).
Axonal transport is categorized into two major rate classes, termed slow and fast axonal transport (FAT), respectively [reviewed in (Morfini, et al., 2011)]. Slow transport involves the movement of cytoskeletal structures and cytosolic proteins at rates of 0.1–6 mm/day. FAT on the other hand, involves rapid movement of MBOs at rates of 50–400 mm/day. The intrinsic polarity of microtubules within axons imparts directionality to transport, in that anterograde FAT is plus-end directed (from cell body to terminals) and retrograde FAT is minus-end directed (from terminals to cell body).
The kinesin protein superfamily has numerous members [reviewed in (Hirokawa, et al., 2010)]. From these, conventional kinesin (i.e. kinesin-1) represents the most abundant and best-characterized anterograde-directed motor protein expressed in mature neurons (Wagner, et al., 1989). A wide variety of MBOs including mitochondria, synaptic vesicle precursors, axolemmal constituents, and secretory products are transported by conventional kinesin [reviewed in (Morfini, et al., 2011)]. Conventional kinesin exists as a heterotetramer composed of two kinesin heavy chains (KHCs or kinesin 1s) and two kinesin light chains (KLCs). KHCs are responsible for binding to microtubules and ATP hydrolysis, providing the mechanochemical activity that powers movement of MBO cargoes by the conventional kinesin holoenzyme. On the other hand, KLCs play a major role in binding conventional kinesin to specific MBO cargoes (Stenoien and Brady, 1997). Multiple KHC and KLC isoforms exist in mammalian neurons, which interact in various combinations to facilitate the targeting of biochemically heterogeneous forms of conventional kinesin to specific MBO cargoes (DeBoer, et al., 2008). Retrograde FAT of MBOs containing trophic factors, degradation products and lysosomes from the axonal compartment to the neuronal cell body is dependent upon cytoplasmic dynein (cDyn), a multisubunit complex consisting of two dynein heavy chain and various additional protein subunits that either regulate DHC activity or target DHC to specific MBO cargoes (Susalka and Pfister, 2000).
Neurons are highly vulnerable to functional alterations in microtubule-based motor proteins responsible for the execution of FAT. Genetic evidence supports this contention, as reductions in conventional kinesin and cDyn function resulting from mutations in selected motor subunits are sufficient to promote degeneration of specific neuronal populations (Morfini, et al., 2009a, Pfister, et al., 2006, Roy, et al., 2005). For example, autosomal dominant loss of function mutations in kinesin-1A result in hereditary spastic paraplegia (Reid, et al., 2002), a disease featuring well-documented dying-back degeneration of upper motor neurons (Deluca, et al., 2004). Similarly, mutations in several cDyn subunits, including dynein heavy chain and dynactin result in dying-back degeneration of sensory and/or motor neurons (Eschbach and Dupuis, 2011). Relevant to this review, pathological studies across a wide spectrum of neurodegenerative diseases indicate that neurons featuring deficits in FAT undergo dying-back degeneration (Chevalier-Larsen, et al., 2008, Dupuis, et al., 2009, Lai, et al., 2007, Puls, et al., 2005). Taken together, these observations clearly indicate that reductions in FAT alone suffice to induce dying-back degeneration of neurons.
Axonal transport defects in AD
Recently, the term “dysferopathy” (from the Greek word “fero”: to carry, transport) was coined by our group to describe neurodegenerative diseases featuring alterations in FAT and dying-back degeneration of neurons (Morfini, et al., 2007b, Morfini, et al., 2009a). Significantly, a large body of evidence demonstrated alterations in FAT in animal models of AD and tauopathies (Gotz, et al., 2006), prompting us to posit that AD could be described, at least in part, as a dysferopathy (Morfini, et al., 2009a). Accordingly, Drosophila models that express fAD-related forms of Aβ (Zhao, et al., 2010), APP (Gunawardena and Goldstein, 2001, Rusu, et al., 2007), and human tau (Mudher, et al., 2004) all exhibit FAT deficits. Similarly, transgenic mouse lines harboring mutant PS1 (Lazarov, et al., 2007, Pigino, et al., 2003), APP/PS1 (Chen, et al., 2011, Wirths, et al., 2007), APP/PS1/tau (Cai, et al., 2011, Desai, et al., 2009), human apolipoprotein E4 (Tesseur, et al., 2000), and mutant tau (Gilley, et al., 2011b, Yoshiyama, et al., 2007, Zhang, et al., 2004) all show evidence of FAT impairments, as well as synaptic and axonal degeneration. For instance, axonal transport of mitochondria, specific Trk receptors, APP, synaptophysin, and syntaxin are reduced in mice expressing mutant PS1 (Lazarov, et al., 2007, Pigino, et al., 2003). Manganese enhanced MRI studies in mutant APP transgenic mice (Smith, et al., 2007) and APP/PSI/tau triple transgenic mice (Kim, et al., 2011) supported these observations by demonstrating reductions in FAT prior to deposition of neuropathological inclusions in vivo. Interestingly, environmental enrichment in APPswe/PS1ΔE9 mice promoted a reduction in tau hyperphosphorylation and concomitantly increased the expression levels of conventional kinesin subunits, suggesting that induction of brain plasticity modulates the toxic pathways elicited by pathogenic forms of APP and PS1 (Hu, et al., 2010). Collectively, these independent observations provide strong evidence that FAT deficits represent a common feature among the diverse pathologies associated with the expression of AD-related polypeptides.
What mechanisms underlie axonal transport defects in AD?
The precise mechanisms underlying FAT deficits in AD are a subject of intense investigation by numerous research groups, and multiple pathways are likely involved (Table 1). For instance, some reports suggested that increasing microtubule stability reduces tau-mediated FAT deficits (Zhang, et al., 2012). Studies by the Mandlekow’s group led to the proposal that tau protein reduces FAT by physically interfering with the binding of conventional kinesin to microtubules (Ebneth, et al., 1998, Mandelkow, et al., 2003). However, physical blockade of conventional kinesin by tau was not supported by other studies (Morfini, et al., 2007a, Yuan, et al., 2008). Tau also was proposed to block microtubule binding of conventional kinesin and to revert CDyn directionality (Dixit, et al., 2008), but other reports contradict this possibility (LaPointe, et al., 2009, McVicker, et al., 2011). Recent studies indicate that amyloid-β may inhibit FAT through multiple mechanisms including activation of N-methyl-D-aspartate receptors and activation of specific kinases (see below) (Decker, et al., 2010, Tang, et al., 2012). Unrelated to tau and amyloid-β, recent work suggests that lysosomal dysfunction in AD has a deleterious effect on FAT (Boland, et al., 2008, Lee, et al., 2011). While all of these pathogenic pathways warrant further investigation and discussion, this review focuses on pathways featuring abnormal activation of kinases and phosphatases, a major AD hallmark (Chung, 2009).
Table 1.
Mechanisms proposed to underlie axonal transport deficits in AD and molecular components involved.
| Proposed Mechanism | Effector | Effect on FAT | Target Motor or Cargo | Motor Subunit modified | Effect on motor function | References |
|---|---|---|---|---|---|---|
| Lysosome Impairment | Unknown | Inhibits aFAT and rFAT | Endo-lysosomes | n.a. | n.a. | Lee et al., 2011 |
| Steric Interference | Tau | Inhibits FAT | Vesicles, Mitochondria and Neurofilaments | n.a. | Physically interferes with cKinesin attachment to MTs | Mandelkow et al., 2003 and Stamer et al., 2002 |
| NMDA Receptor Activity | Amyloid-β via GSK3β | Inhibits aFAT and rFAT | Vesicles and Mitochondria | n.a | n.a. | Decker et al., 2010 |
| Motor protein regulation | Tau | Inhibits aFAT and rFAT | cKinesin and cDyn | n.a. | Reduces cKinesin-microtubule interaction. Reverses cDyn direction |
Dixit et al., 2008 |
| cJun N-terminal kinase 3 (JNK3) | Inhibits aFAT | cKinesin | KHC | Reduces MT binding | Morfini et al., 2009b | |
| Inhibits rFAT | cDyn | n.a | Unknown | Morfini et al., unpublished | ||
| p38α MAPK | Inhibits aFAT | cKinesin | KHC | Reduces MT binding |
Bosco et al., 2010 Morfini et al., unpublished |
|
| Amyloid-β via CK2 | Inhibits aFAT | cKinesin | KLC | Dissociates cargoes | Pigino et al, 2009 | |
| Inhibits rFAT | cDyn | Unknown | Unknown | Pigino et al, 2009 | ||
| Amyloid-β, Tau, and PS1 via GSK3β | Inhibits aFAT | cKinesin Vesicles and Mitochondria | KLC | Inhibits cKinesin binding to cargoes Reduces mitochondrial transport |
Morfini et al., 2002b & 2004; Pigino et al., 2003; Decker et al. 2010; Rui et al., 2006 | |
| Protein kinase Cδ (PKCδ) | Increases rFAT | cDyn | DIC | Unknown |
Morfini et al., 2007b Morfini et al, unpublished |
Abbreviations: cKinesin: conventional kinesin. cDyn: cytoplasmic dynein. KHC: kinesin heavy chain. KLC: kinesin light chain. DIC: dynein intermediate chain. aFAT and rFAT: anterograde and retrograde fast axonal transport, respectively. n.a.: not analyzed
Phosphotransferases Regulate Fast Axonal Transport
Basic neuronal functions depend upon the exquisitely regulated delivery of specific MBOs to numerous specialized axonal compartments. For example, saltatory conduction relies on the localized delivery of MBOs containing sodium channels at the nodes of Ranvier. Similarly, effective neurotransmission requires the sustained supply of synaptic vesicle precursors to hundreds of “en passant” and terminal synapses in CNS neurons (Morfini, et al., 2001b). The required specificity of local cargo delivery to the appropriate location strongly suggests the existence of regulatory mechanisms for FAT, fueling experiments aimed at illuminating a molecular basis for FAT regulation.
Multiple mechanisms have been proposed to regulate FAT in vivo. Some are based on regulation of motor protein activities by autoinhibition (Verhey and Hammond, 2009), phosphorylation (Dillman and Pfister, 1994, Morfini, et al., 2009a), calcium-dependent interactions with MBO-associated binding partners (Wang and Schwarz, 2009), or recruitment of specific adaptor proteins (Kardon and Vale, 2009). Other mechanisms proposed are based on the modification of microtubule tracks, including acetylation, tyrosination and polyglutamination of tubulin, among others (Hammond, et al., 2010). Dysfunction in any or all these regulatory mechanisms may be important to AD pathogenesis. Because alterations in kinase-based signaling pathways are widely recognized in AD (Chung, 2009), the remainder of this review focuses on the regulation of motor proteins by phosphorylation.
Do abnormalities in the phosphoregulation of motor proteins contribute to the FAT deficits in AD?
As discussed above, genetic and pathological evidence clearly indicate that deficits in FAT suffice to cause dying-back degeneration of neurons. While mutations in molecular motor subunits are not known in AD patients, alterations in regulatory mechanisms for FAT might underlie the abnormalities in FAT characteristic of AD (Morfini, et al., 2009a). At present, a large body of experimental evidence indicates that phosphorylation of motor proteins represents a major mechanism for the regulation of FAT (Table 1). Significantly, abnormal patterns of protein phosphorylation (i.e., tau and neurofilaments) and increased activation of protein kinases represent major AD hallmarks (Chung, 2009). Moreover, several kinases abnormally activated in AD affect FAT, including glycogen synthase kinase 3 (GSK3), cyclin-dependent kinase 5 (Cdk5), and casein kinase 2 (CK2), among others (Morfini, et al., 2009a). Taken together, these observations provide a rationale for the hypothesis that alterations in the activity of numerous kinases underlie the FAT defects observed in AD. Such abnormalities in kinase activity may also be associated with other aspects of AD pathology, including altered synaptic function and gene expression.
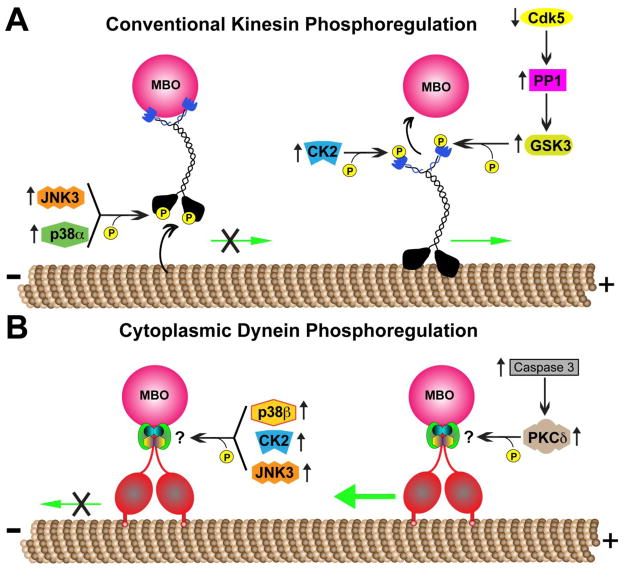
A variety of protein kinases regulate specific functional activities of conventional kinesin (Table 1 and Fig. 1a). For example, cJun amino-terminal kinase 3 (JNK3) was recently identified as a mitogen activated protein kinase (MAPK) that phosphorylates serine 176 within the motor domain of KHC. This phosphorylation event impairs the binding of conventional kinesin to microtubules and its translocation along axons in vivo (Morfini, et al., 2009b). Another MAPK associated with reduction in conventional kinesin motility is p38α (Bosco, et al., 2010), which also phosphorylates KHCs directly causing reductions in microtubule binding (Morfini et al, unpublished). The protein kinases CK2 and GSK3 regulate the binding of conventional kinesin to MBO cargoes through a mechanism involving phosphorylation of KLCs (Morfini, et al., 2002, Pigino, et al., 2003). Interestingly, some evidence suggesting these kinases may act in concert to promote the regulation of MBO cargo delivery. For example, CK2 phosphorylates one or more “priming sites” on KLCs that in render these subunits phosphorylatable by GSK3 (Morfini, et al., 2002).
Fig. 1.
Phosphoregulation of motor protein complexes that mediate fast axonal transport (FAT). (A) Biochemically heterogeneous forms of conventional kinesins are regulated through numerous kinases that phosphorylate specific motor protein subunits and regulate specific functional activities. For example, the microtubule-binding activity of kinesin heavy chains (KHCs) is inhibited by c-Jun N-terminal kinase 3 (JNK3) and p38-mediated phosphorylation. In contrast, both glycogen synthase kinase 3 (GSK3) and casein kinase 2 (CK2) phosphorylate kinesin light chains (KLCs), promoting dissociation of bound membrane bound organelle (MBO) cargoes. Cyclin-dependent kinase 5 (Cdk5) and protein phosphatase 1 (PP1) indirectly promote MBO cargo dissociation through mechanisms involving GSK3 activation. (B) Similarly, some kinases promote reductions in cytoplasmic dynein (cDyn)-mediated retrograde FAT; however, the precise mechanisms mediating their effects remain unclear. Interestingly, protein kinase C delta (PKCδ) increases retrograde FAT; but again, the underlying mechanisms are unknown. In the context of Alzheimer’s disease and other neurodegenerative diseases, these observations provide a molecular link between abnormal kinase/phosphatase signaling, deficits in FAT, and dying-back degeneration of neurons. Green arrows indicate movement of motor complexes; an “X” indicates inhibition of this movement; the larger green arrow indicates enhanced movement.
Consistent with the complex protein networks that characterize phosphorylation-based signaling mechanisms, additional kinases and phosphatases indirectly regulate conventional kinesin function. These include Cdk5 and protein phosphatase 1 (PP1), which together delineate a pathway regulating activation of GSK3 within the axonal compartment (Morfini, et al., 2004). Indeed, localized inhibition of Cdk5 promotes, through an unknown mechanism, increased activation of PP1. In turn, PP1 dephosphorylates an inhibitory phosphate group within GSK3 (serine 9), leading to its activation and phosphorylation of KLCs, among other substrates (Morfini, et al., 2004, Plattner, et al., 2006).
Phosphorylation-dependent regulatory mechanisms for conventional kinesin-based FAT are better characterized than those regulating cDyn-based FAT. However, experimental evidence from studies in the isolated squid axoplasm preparation illuminated specific protein kinases that regulate retrograde FAT (Table 1 and Fig. 1b). For example, the non-conventional protein kinase C (PKC) isoform, PKCδ, stimulates an increase in retrograde FAT (Morfini, et al., 2007b), likely through a mechanism involving direct phosphorylation of specific cDyn subunits (Morfini et al., unpublished). Axonal PKCδ can be activated through a variety of different pathways, including proteolytic cleavage by caspase 3, and caspase 3 can enhance retrograde FAT (Morfini, et al., 2007b). In contrast, the protein kinases JNK3 (Morfini, et al., 2009b), CK2 (Pigino, et al., 2009) and p38β (Morfini et al., in preparation) inhibit retrograde FAT in isolated squid axoplasm. However, the molecular mechanisms by which these kinases inhibit CDyn-based motility have not yet been defined.
Misregulation of Phosphotransferase-based pathways in AD
Mechanisms underlying FAT impairments in sAD are not well understood and multiple possibilities exist (see above and Table 1). However, experimental evidence from various fAD animal models illuminated specific kinase-based pathways linking fAD-related proteins to alterations in FAT. In AD, aberrant patterns of protein phosphorylation and misregulation of phosphotransferase activities have long been recognized. Moreover, the presence of abnormally phosphorylated proteins (e.g. tau) represents a major AD hallmark. One implication of these observations is that aberrant activation of multiple, apparently interconnected phosphotransferase-based pathways might lead to abnormalities in FAT and dying-back degeneration in AD. A growing body of evidence appears consistent with this hypothesis, because AD-related pathogenic factors including Aβ, PS1, ApoE and tau affect the activities of specific phosphotransferases and promote alterations in FAT through different mechanisms [reviewed in (Morfini, et al., 2009a)] (Fig. 2).
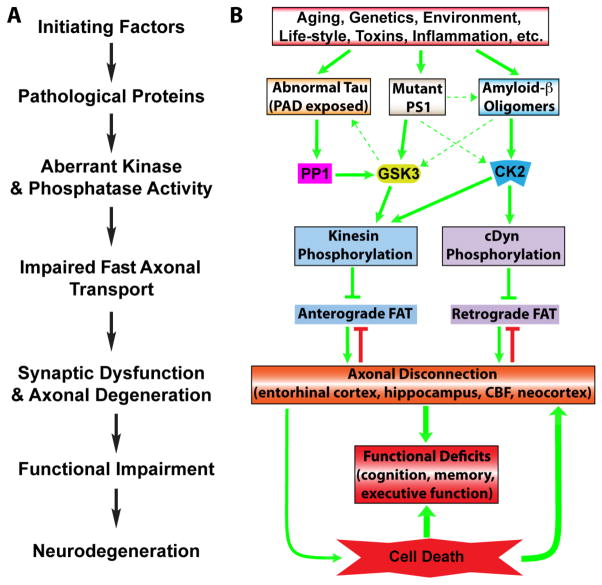
Fig. 2.
Signaling pathways linking alterations in phosphotransferases to axonal transport defects, axonal degeneration, and cell death in AD. (A) A general schematic for the proposed role of phosphotransferases and axonal dysfunction in the pathogenesis of numerous neurodegenerative diseases characterized by a dying-back pattern of degeneration. (B) Plugging Alzheimer’s disease (AD)-specific pathologies into the schematic clearly suggests that there are many parallel and potentially interacting pathways that begin with abnormalities in known disease-associated factors (i.e. abnormal tau protein, mutant PS1, amyloid-β oligomers), and through aberrant activation of kinase and/or phosphatases, lead to impaired axonal transport, neuroanatomical disconnection of disease-related brain structures, functional deficits and eventually neurodegeneration (later in disease). Indeed, relatively subtle abnormalities in fast axonal transport, synaptic function and connectivity among neurons likely underlie the initial clinical symptoms of AD and as the disease progresses these pathways are further activated ultimately causing neurodegeneration and further functional impairment. Dashed arrows indicate secondary interconnections among toxic pathways that might contribute to pathological progression; green arrows indicate facilitating mechanisms, blunt-ended arrows indicate inhibitory mechanisms.
Amyloid-β and Phosphotransferase Misregulation
Since the identification of Aβ as the major constituent of senile plaques in AD brains, Aβ has been considered a central player in AD pathogenesis (Benilova et al., 2012). The discovery of fAD-related mutations in APP, the precursor protein from which Aβ is generated, further supported this idea (O’Brien and Wong, 2011). Aβ peptides are first generated from proteolytic cleavage of APP within the plasma membrane by secretase proteases, later undergoing aggregation and conformational changes that lead to the formation of oligomeric (oAβ) and fibrillar (fAβ) species (Thinakaran and Koo, 2008). Forerunners in the list of potential mechanisms by which aggregated Aβ species, particularly oAβ, promote neuronal degeneration involve the induction of synaptic dysfunction (Ferreira and Klein, 2011, Moreno, et al., 2009) and promotion of FAT impairments through abnormal activation of kinases (Pigino, et al., 2009).
Studies in cell culture (Hiruma, et al., 2003, Wang, et al., 2010) and animal models (Kasa, et al., 2000, Smith, et al., 2007) recently linked various disease-relevant forms of extracellular and intracellular Aβ to deficits in FAT triggered by various pathogenic pathways. For instance, Rui and colleagues showed that Aβ peptides and fibrils inhibit mitochondrial transport in cultured neurons via a GSK3-dependent mechanism (Rui, et al., 2006). Similarly, treating cultured neurons with oAβ inhibits FAT of dense core vesicles and mitochondria by a mechanism involving N-methyl-D-aspartate receptor-dependent and GSK3 activation (Decker, et al., 2010, Tang, et al., 2012). Independently, Pigino and co-workers showed that intracellular oAβ inhibits anterograde FAT though a mechanism involving CK2 activation, phosphorylation of KLCs, and dissociation of MBO cargoes from conventional kinesin (Pigino, et al., 2009). Interestingly, oAβ also inhibited retrograde FAT, but the exact mechanisms underlying the reductions in CDyn-based motility were not addressed in these studies. Of note, these effects of oAβ on FAT were observed in isolated axons, suggesting axon-autonomous effects of oAβ that are consistent with dying-back degeneration in AD (Pigino, et al., 2009).
Presenilin-1, Apolipoprotein E and Phosphotransferase Misregulation
Subsets of fAD cases result from mutations in PS1 and PS2. Specifically, there are over 170 PS1 mutations and 14 PS2 mutations, with most mutations altering APP processing in ways that increase the production of toxic Aβ species (Thinakaran and Koo, 2008). PS are the catalytic core of the aspartyl protease γ-secretase that cleaves APP to yield Aβ (O’Brien and Wong, 2011). The role of presenilins in Aβ generation led to the hypothesis that these proteins play an important role in AD pathogenesis.
Several lines of evidence link fAD-related mutant PS1 to GSK3 and to changes in FAT. First, the cytoplasmic domain of PS1 binds to GSK3 and this interaction is altered by fAD-related mutations (Takashima, et al., 1998, Tesco and Tanzi, 2000). Second, in mammalian cell culture models, mutant PS1 activates GSK3 and inhibits anterograde FAT. Indeed, genetic deletion of PS1 or expression of mutant PS1 polypeptides all resulted in elevated GSK3 activity, increased tau and KLC phosphorylation, and reduced binding of conventional kinesin to MBO cargoes (Pigino, et al., 2003). Additionally, two transgenic mouse lines harboring different PS1 mutants showed impairment in anterograde FAT of APP and neurotrophin receptors in the sciatic nerve (Lazarov, et al., 2007). These impairments were correlated with increased phosphorylation of GSK3 substrates (i.e. neurofilaments and tau), and functional motor deficits (Lazarov, et al., 2007). Finally, pathogenic forms of PS1 could affect FAT through alterations in Aβ production, which can elevate CK2 kinase activity (Pigino et al., 2009). Thus, another important etiological factor for fAD is clearly linked to FAT dysfunction by directly affecting the activity of phosphotransferases known to regulate conventional kinesin function.
Three apolipoprotein E (ApoE) gene alleles exist in humans: ApoE2, ApoE3, and ApoE4. Significantly, genetic studies linked the apoE4 allele to increased risk of developing sAD (Hyman, et al., 1996). Interestingly, transgenic mice featuring neuronal expression of human apoE4 display significant axonal degeneration (Tesseur, et al., 2000). Moreover, abnormal accumulation of MBOs in affected axons further suggested inhibition of FAT (Tesseur, et al., 2000). Relevant to this review, apolipoprotein receptor activation promotes GSK3 inactivation through a mechanism involving PI3 kinase and Akt activation (Beffert, et al., 2002). Moreover, ApoE appears to modulate the activities of these kinases in an isoform-specific manner (Laffont, et al., 2002). Thus, a major risk factor for sAD may be linked to increased GSK3 activation and inhibition of FAT.
Tau and Phosphotransferase Misregulation
Historically, tau was considered a microtubule-associated protein that binds to and stabilizes microtubules (Wang and Liu, 2008). However, a more diverse set of biological functions has been attributed to tau in recent years, including a role as a signaling molecule (Kanaan, et al., 2011a, Kanaan, et al., 2011b, Morris, et al., 2011). In AD, tau undergoes increased phosphorylation and aggregation, leading to hyperphosphorylated and aggregated forms of tau found in NFTs, neuropil threads and neuritic plaques. Animal models based on mutant tau expression recapitulate some aspects of tau aggregation and several abnormal modifications of tau seen in AD (i.e. phosphorylation, truncation, etc.), thus offering novels insights into AD pathogenesis. A large number of studies documented behavioral deficits, synaptic and axonal abnormalities (e.g. swellings and spheroids), reductions in FAT of specific MBOs, and neurodegeneration in transgenic mouse models expressing human wild-type tau (Probst, et al., 2000), mutant P301L tau (Gilley, et al., 2011b, Lin, et al., 2005), mutant P301S tau (Yoshiyama, et al., 2007), mutant R406W tau (Zhang, et al., 2004), and mutant G272V/P301S tau (Leroy, et al., 2007) transgenic mouse models.
Until recently, molecular mechanisms linking disease-associated modifications of tau (i.e. mutations, aggregation, and phosphorylation) to FAT deficits remained unknown. However, recent work identified a signaling cascade by which pathological forms of tau activate axonal PP1. Active PP1 in turn activates GSK3 via dephosphorylation at serine 9, ultimately increasing KLC phosphorylation and dissociation of conventional kinesin from MBO cargoes (LaPointe, et al., 2009). Further studies identified a phosphatase-activating domain (PAD, aa 2–18) within the extreme N-terminus of tau that, when abnormally exposed, promotes activation of the PP1-GSK3 signaling cascade, leading to inhibition of conventional kinesin-based anterograde FAT (Kanaan, et al., 2011b).
Identification of PAD as a potential signaling motif within tau provided a basis for the hypothesis that disease-associated modifications of tau promote increased PAD exposure and inhibition of anterograde FAT. Indeed, PAD contains a putative PP1 binding site (aa 5–8) that is likely responsible for activating this cascade upon PAD exposure (Liao, et al., 1998). Numerous disease-associated forms of tau exist in which PAD is expected to be exposed, including tau aggregates, short N-terminal isoforms, AT8 phosphorylated tau and mutant tau. Consistent with model, these forms of tau cause significant deficits in anterograde FAT (Kanaan, et al., 2011b, LaPointe, et al., 2009). Interestingly, some of these toxic forms of tau did not require aggregation, suggesting that pre-filamentous forms of tau with PAD exposed can elicit this effect (Kanaan, et al., 2011b). Accordingly, recent experiments suggested that oligomeric forms of tau might represent a major toxic conformation of aggregated tau leading to FAT inhibition (Patterson, et al., 2011a, Patterson, et al., 2011b).
Using a novel PAD-specific antibody, immunohistochemical studies showed that increased PAD exposure represents an early molecular event that precedes formation of NFTs in sAD (Kanaan, et al., 2011b, Patterson, et al., 2011a). Curiously, tau abnormalities are pervasive in AD and some neurons appear to survive for decades with tau pathology, suggesting that some protective mechanisms that prevent the toxic effects associated with sustained PAD exposure exist in neurons. Indeed, phosphorylation of tyrosine 18, which is located within PAD, was identified as a modification that prevents PAD-mediated activation of the PP1-GSK3 cascade and FAT inhibition (Kanaan, et al., 2011a). In AD brains, a subset of neurons containing PAD-exposed tau were also positive for tyrosine 18 phosphorylated tau, suggesting that tyrosine 18 phosphorylation by non-receptor tyrosine kinases may help mitigate the toxic effects of PAD exposure in human neurons. Collectively, these data suggest that early pathological changes in tau protein lead to increased PAD exposure, which induces aberrant activation of a PP1-GSK3 cascade, phosphorylation of KLCs and inhibition of conventional kinesin-based anterograde FAT (Kanaan, et al., 2011a, Kanaan, et al., 2011b).
Interactions Between Pathogenic Factors
Studies in isolated squid axoplasm, a unique experimental system for the study of axon-specific events, demonstrate that pathogenic forms of tau and Aβ can both inhibit FAT in an axon-autonomous manner, but through independent mechanisms. However, an arguably more appropriate view in the context of AD complexity is that these factors act in overlapping and potentially synergistic ways (Fig. 2). In the case of tau, the relationship between GSK3 activation, PAD exposure and FAT inhibition may exist as a feed-forward cycle. In this scenario, one or more postulated upstream initiating events (i.e. aging, genetics, environment, toxins, life-style, diet, neuroinflammation, Aβ42 oligomers etc.) promote modifications in tau that result in increased PAD exposure, leading to activation of the PP1-GSK3 cascade that inhibits anterograde FAT. Abnormally activated GSK3 may subsequently phosphorylate other protein substrates, including tau, further increasing PAD exposure and/or inducing aggregation. The suggestion that Aβ-related signaling represents one of the important upstream events that precipitate changes in tau conformation associated with increased PAD exposure is particularly attractive. Indeed, Aβ exposure in cultured neurons and in animal models induces activation of several kinases, including CK2 and GSK3, that can phosphorylate tau and potentially affect the self-aggregation of tau (Avila, et al., 2006, Hernandez, et al., 2010). Moreover, both cell culture (Rapoport, et al., 2002) and animal models (Roberson, et al., 2007) of Aβ toxicity require tau for toxicity, including Aβ-induced FAT deficits (Vossel, et al., 2010).
Additional evidence supports the notion that convergent pathways independently affect FAT in AD. For example, Peethumnongsin and colleagues demonstrated an interesting link between PS1 and tau in the context of FAT impairments (Peethumnongsin, et al., 2010). A double transgenic mouse line was generated containing a conditional knockout of PS1 and expressing wild-type tau proteins. In these animals, axonopathy and FAT impairments were accelerated when compared to either single transgenic line alone, suggesting an additive interaction between PS1 dysfunction and tau. Moreover, the double transgenic line exhibited reduced neurotrophin signaling, impaired learning and memory, and increased cortical neurodegeneration. Perhaps the convergence of tau and PS1 abnormalities lies at the level of GSK3 activation since both of them inhibit anterograde FAT via signaling cascades involving GSK3-mediated phosphorylation of KLCs. Lastly, the activity of other kinases with known effects on FAT (i.e. JNK, Cdk5 and p38) are increased in sAD (Pei, et al., 2001, Zhu, et al., 2001) and fAD animal models (Otth, et al., 2003, Rossner, et al., 2001). The complexity and potential overlap in pathological cascades associated with FAT impairments in AD support the notion that AD exhibits characteristics of a dysferopathy induced, at least in part, by misregulation of phosphotransferases (Fig. 2). However, further studies are required to establish the precise contribution of these events to AD pathology in vivo.
Phosphotransferases and Pathogenic Proteins: Which are Viable Therapeutic Targets?
The most important insight that can be gleaned from the strong link between misregulation of phosphotransferases, FAT deficits and synaptic degeneration in AD is the identification of potential therapeutic targets. In this regard, the observations discussed here provide a novel conceptual framework for devising novel therapeutic treatments in AD. If the FAT deficits that characterize this disease indeed result from alterations in the activity of phosphotransferases, these could be corrected using pharmacological approaches. Identifying specific phosphotransferase activities relevant to FAT deficits, synaptic dysfunction and axonal degeneration in AD would represent a critical step. After this, specific inhibitors could be developed, or already known agents could be used to restore these enzymatic activities to their normal levels, without compromising their basic cellular functions. One would expect that preventing decrements in FAT would have a positive outcome for patients. Moreover, if FAT deficits are treated early enough, the loss of neuronal connectivity that accompanies AD could be prevented.
Alternatively, therapeutic interventions aimed at the pathological conformations that initiate the toxic cascades may be effective. Since oAβ appears to be the primary toxic form of amyloid, approaches that either dissolve or sequester oAβ, or ones that block the ability of oAβ to activate CK2 may prevent the toxic cascades leading to decrements in FAT. Similarly, blocking the ability of mutant PS1 to activate GSK3 may be therapeutic. Lastly, therapies based on blocking the effects of aberrantly exposed PAD in tau represent a viable approach. Regardless of the approach, the most critical advancement that is required prior to development of effective therapies is a reliable, reproducible, accurate and specific diagnostic method for identifying early alterations in phosphotransferase activity and FAT efficiency in AD.
Conclusions
Studies continue to demonstrate a strong link between phosphotransferase misregulation, FAT deficits and neurodegeneration in AD, as well as other neurodegenerative diseases. The unique burden of maintaining FAT and well-orchestrated delivery of specific cargoes to select subdomains of neurons makes them particularly susceptible to perturbations in FAT. This notion supports the long recognized, yet unexplained, fact that adult-onset neurodegenerative diseases typically feature increased vulnerability of projection neurons within specific brain regions, while interneurons are typically less affected or even spared (Mattson and Magnus, 2006). Furthermore, deficits in FAT in AD are likely not an all-or-nothing phenomena, but instead might occur in localized regions (e.g. within discrete axonal subdomains) early in disease development. Such localized deficits might allow neurons to survive for a long time before manifesting signs of dysfunction and death. One can envision a degenerative cascade in which FAT impairments would first affect the regions most reliant on proper transport function – the synapse. After a currently undefined threshold is reached, synaptic deficits would promote axonal degeneration. Eventually, the axonal degeneration leads to overt neurodegeneration as the trophic signaling and other target-derived components are no longer available. As discussed here, the traditional pathological factors (e.g. tau, Aβ, PS1, ApoE) appear to play a direct role in precipitating FAT impairments through their actions on phosphotransferase-based pathways. Not surprisingly, there are multiple pathways that seem to converge, and in some cases may act synergistically, on FAT (Fig. 2).
Despite recent advances in our understanding of the role for phosphotransferase misregulation in FAT impairments, much remains to be done. Age-related neurodegenerative diseases such as AD are complex, multifaceted diseases that are likely built upon a foundation of aging-related changes as well as other disease-specific mechanisms. Indeed, the mechanisms of FAT impairments discussed above are likely not the only factors at play. Unknown factors affecting FAT and/or phosphotransferases will no doubt come to light with continued research, as well as mechanisms that are not centered around FAT. Regardless, once axons start degenerating, neurons loose their functional connections and are headed down a one-way street in the wrong direction (Brady and Morfini, 2010). Thus, FAT represents a critical target in studies aimed at understanding disease mechanisms in AD. Developing therapeutic strategies that effectively help prevent impairments in FAT and loss of neuronal connectivity in AD should help rescue affected neurons from reaching the “point of no return”.
Acknowledgments
This paper is dedicated to the loving memory of Mario Felipe Morfini (GM). This work was supported by NIH T32 AG020506-07 and Alzheimer’s Association NIRG-10-174461 (NMK); NIH NS23868 (STB); NIH AG09466 (LIB); Alzheimer’s Association NIRGD-11-206379 (to GP); Brain Research Foundation grants (OL and GM); NIH/NIA AG033570 and NIA 1RC1AG036208-01 ARRA (OL); and NIH NS066942A and ALS/CVS Therapy Alliance grants (GM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashe KH, Zahs KR. Probing the biology of Alzheimer’s disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J, Santa-Maria I, Perez M, Hernandez F, Moreno F. Tau phosphorylation, aggregation, and cell toxicity. J Biomed Biotechnol. 2006;2006:74539. doi: 10.1155/JBB/2006/74539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, Bloom GS. Cytoskeletal pathologies of Alzheimer disease. Cell Motil Cytoskeleton. 2009;66:635–649. doi: 10.1002/cm.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Yu CE, Bird TD, Tsuang DW. Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol. 2010;23:213–227. doi: 10.1177/0891988710383571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KF, Claudio Cuello A. Altered synaptic function in Alzheimer’s disease. Eur J Pharmacol. 2006;545:11–21. doi: 10.1016/j.ejphar.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28:6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, Goolsby H, Fontaine BA, Lemay N, McKenna-Yasek D, Frosch MP, Agar JN, Julien JP, Brady ST, Brown RH., Jr Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004;10(Suppl):S2–9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Padovani A, Caltagirone C, Borroni B. Regional grey matter loss and brain disconnection across Alzheimer disease evolution. Curr Med Chem. 2011;18:2452–2458. doi: 10.2174/092986711795843263. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S, Morfini G. A perspective on neuronal cell death signaling and neurodegeneration. Mol Neurobiol. 2010;42:25–31. doi: 10.1007/s12035-010-8128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Zhang XM, Macklin LN, Cai H, Luo XG, Oddo S, Laferla FM, Struble RG, Rose GM, Patrylo PR, Yan XX. BACE1 Elevation is Involved in Amyloid Plaque Development in the Triple Transgenic Model of Alzheimer’s Disease: Differential Abeta Antibody Labeling of Early-Onset Axon Terminal Pathology. Neurotox Res. 2011 doi: 10.1007/s12640-011-9256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Epelbaum S, Delatour B. Fiber Tracts Anomalies in APPxPS1 Transgenic Mice Modeling Alzheimer’s Disease. J Aging Res. 2011;2011:281274. doi: 10.4061/2011/281274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Larsen ES, Wallace KE, Pennise CR, Holzbaur EL. Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150Glued subunit of dynactin. Hum Mol Genet. 2008;17:1946–1955. doi: 10.1093/hmg/ddn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH. Aberrant phosphorylation in the pathogenesis of Alzheimer’s disease. BMB Rep. 2009;42:467–474. doi: 10.5483/bmbrep.2009.42.8.467. [DOI] [PubMed] [Google Scholar]
- Coleman M. Molecular signaling how do axons die? Adv Genet. 2011;73:185–217. doi: 10.1016/B978-0-12-380860-8.00005-7. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- DeBoer SR, You Y, Szodorai A, Kaminska A, Pigino G, Nwabuisi E, Wang B, Estrada-Hernandez T, Kins S, Brady ST, Morfini G. Conventional kinesin holoenzymes are composed of heavy and light chain homodimers. Biochemistry. 2008;47:4535–4543. doi: 10.1021/bi702445j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker H, Jurgensen S, Adrover MF, Brito-Moreira J, Bomfim TR, Klein WL, Epstein AL, De Felice FG, Jerusalinsky D, Ferreira ST. N-methyl-D-aspartate receptors are required for synaptic targeting of Alzheimer’s toxic amyloid-beta peptide oligomers. J Neurochem. 2010;115:1520–1529. doi: 10.1111/j.1471-4159.2010.07058.x. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Deluca GC, Ebers GC, Esiri MM. The extent of axonal loss in the long tracts in hereditary spastic paraplegia. Neuropathol Appl Neurobiol. 2004;30:576–584. doi: 10.1111/j.1365-2990.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- Desai MK, Sudol KL, Janelsins MC, Mastrangelo MA, Frazer ME, Bowers WJ. Triple-transgenic Alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57:54–65. doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessi F, Colle MA, Hauw JJ, Duyckaerts C. Accumulation of SNAP-25 immunoreactive material in axons of Alzheimer’s disease. Neuroreport. 1997;8:3685–3689. doi: 10.1097/00001756-199712010-00006. [DOI] [PubMed] [Google Scholar]
- Dillman JF, 3rd, Pfister KK. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, Fergani A, Braunstein KE, Eschbach J, Holl N, Rene F, Gonzalez De Aguilar JL, Zoerner B, Schwalenstocker B, Ludolph AC, Loeffler JP. Mice with a mutation in the dynein heavy chain 1 gene display sensory neuropathy but lack motor neuron disease. Exp Neurol. 2009;215:146–152. doi: 10.1016/j.expneurol.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschbach J, Dupuis L. Cytoplasmic dynein in neurodegeneration. Pharmacol Ther. 2011;130:348–363. doi: 10.1016/j.pharmthera.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Ferreira ST, Klein WL. The Abeta oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol Learn Mem. 2011 doi: 10.1016/j.nlm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Ghoshal N, Garcia-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, Binder LI. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol. 2002;177:475–493. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- Gilley J, Adalbert R, Coleman MP. Modelling early responses to neurodegenerative mutations in mice. Biochem Soc Trans. 2011a;39:933–938. doi: 10.1042/BST0390933. [DOI] [PubMed] [Google Scholar]
- Gilley J, Seereeram A, Ando K, Mosely S, Andrews S, Kerschensteiner M, Misgeld T, Brion JP, Anderton B, Hanger DP, Coleman MP. Age-dependent axonal transport and locomotor changes and tau hypophosphorylation in a “P301L” tau knockin mouse. Neurobiol Aging. 2011b doi: 10.1016/j.neurobiolaging.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta. 2005;1739:240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Goto Y, Niidome T, Hongo H, Akaike A, Kihara T, Sugimoto H. Impaired muscarinic regulation of excitatory synaptic transmission in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Eur J Pharmacol. 2008;583:84–91. doi: 10.1016/j.ejphar.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Gotz J, Ittner LM, Kins S. Do axonal defects in tau and amyloid precursor protein transgenic animals model axonopathy in Alzheimer’s disease? J Neurochem. 2006;98:993–1006. doi: 10.1111/j.1471-4159.2006.03955.x. [DOI] [PubMed] [Google Scholar]
- Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/s0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21:572–583. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Gomez de Barreda E, Fuster-Matanzo A, Lucas JJ, Avila J. GSK3: a possible link between beta amyloid peptide and tau protein. Exp Neurol. 2010;223:322–325. doi: 10.1016/j.expneurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Hiruma H, Katakura T, Takahashi S, Ichikawa T, Kawakami T. Glutamate and amyloid beta-protein rapidly inhibit fast axonal transport in cultured rat hippocampal neurons by different mechanisms. J Neurosci. 2003;23:8967–8977. doi: 10.1523/JNEUROSCI.23-26-08967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YS, Xu P, Pigino G, Brady ST, Larson J, Lazarov O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer’s disease-linked APPswe/PS1DeltaE9 mice. FASEB J. 2010;24:1667–1681. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Auchus AP. Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:259–264. doi: 10.1196/annals.1379.021. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Gomez-Isla T, West H, Briggs M, Chung H, Growdon JH, Rebeck GW. Clinical and neuropathological correlates of apolipoprotein E genotype in Alzheimer’s disease. Window on molecular epidemiology. Ann N Y Acad Sci. 1996;777:158–165. doi: 10.1111/j.1749-6632.1996.tb34414.x. [DOI] [PubMed] [Google Scholar]
- Ihara M, Polvikoski TM, Hall R, Slade JY, Perry RH, Oakley AE, Englund E, O’Brien JT, Ince PG, Kalaria RN. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer’s disease, and dementia with Lewy bodies. Acta Neuropathol. 2010;119:579–589. doi: 10.1007/s00401-009-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawhar S, Wirths O, Schilling S, Graubner S, Demuth HU, Bayer TA. Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate A{beta} formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice. J Biol Chem. 2011;286:4454–4460. doi: 10.1074/jbc.M110.185819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus P, Slotboom J, Gallinat J, Mahlberg R, Cattapan-Ludewig K, Wiest R, Nyffeler T, Buri C, Federspiel A, Kunz D, Schroth G, Kiefer C. Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia. Neuroimage. 2006;30:713–720. doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Kanaan NM, Morfini G, Pigino G, Lapointe NE, Andreadis A, Song Y, Leitman E, Binder LI, Brady ST. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol Aging. 2011a doi: 10.1016/j.neurobiolaging.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Morfini GA, LaPointe NE, Pigino GF, Patterson KR, Song Y, Andreadis A, Fu Y, Brady ST, Binder LI. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci. 2011b;31:9858–9868. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasa P, Papp H, Kovacs I, Forgon M, Penke B, Yamaguchi H. Human amyloid-beta1–42 applied in vivo inhibits the fast axonal transport of proteins in the sciatic nerve of rat. Neurosci Lett. 2000;278:117–119. doi: 10.1016/s0304-3940(99)00863-0. [DOI] [PubMed] [Google Scholar]
- Kim J, Choi IY, Michaelis ML, Lee P. Quantitative in vivo measurement of early axonal transport deficits in a triple transgenic mouse model of Alzheimer’s disease using manganese-enhanced MRI. Neuroimage. 2011;56:1286–1292. doi: 10.1016/j.neuroimage.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall NW, Kosik KS. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer’s disease. Ann Neurol. 1987;22:639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- Laffont I, Takahashi M, Shibukawa Y, Honke K, Shuvaev VV, Siest G, Visvikis S, Taniguchi N. Apolipoprotein E activates Akt pathway in neuro-2a in an isoform-specific manner. Biochem Biophys Res Commun. 2002;292:83–87. doi: 10.1006/bbrc.2002.6586. [DOI] [PubMed] [Google Scholar]
- Lai C, Lin X, Chandran J, Shim H, Yang WJ, Cai H. The G59S mutation in p150(glued) causes dysfunction of dynactin in mice. J Neurosci. 2007;27:13982–13990. doi: 10.1523/JNEUROSCI.4226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, Brady ST. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J Neurosci Res. 2009;87:440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Morfini GA, Pigino G, Gadadhar A, Chen X, Robinson J, Ho H, Brady ST, Sisodia SS. Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer’s disease-linked mutant presenilin 1. J Neurosci. 2007;27:7011–7020. doi: 10.1523/JNEUROSCI.4272-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neurosci. 2011;31:7817–7830. doi: 10.1523/JNEUROSCI.6412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy K, Bretteville A, Schindowski K, Gilissen E, Authelet M, De Decker R, Yilmaz Z, Buee L, Brion JP. Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice. Am J Pathol. 2007;171:976–992. doi: 10.2353/ajpath.2007.070345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Li Y, Brautigan DL, Gundersen GG. Protein phosphatase 1 is targeted to microtubules by the microtubule-associated protein Tau. J Biol Chem. 1998;273:21901–21908. doi: 10.1074/jbc.273.34.21901. [DOI] [PubMed] [Google Scholar]
- Lin WL, Zehr C, Lewis J, Hutton M, Yen SH, Dickson DW. Progressive white matter pathology in the spinal cord of transgenic mice expressing mutant (P301L) human tau. J Neurocytol. 2005;34:397–410. doi: 10.1007/s11068-006-8726-0. [DOI] [PubMed] [Google Scholar]
- Machova E, Jakubik J, Michal P, Oksman M, Iivonen H, Tanila H, Dolezal V. Impairment of muscarinic transmission in transgenic APPswe/PS1dE9 mice. Neurobiol Aging. 2008;29:368–378. doi: 10.1016/j.neurobiolaging.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Albright T, Mallory M, Terry RD. Immunoelectron microscopic study of synaptic pathology in Alzheimer’s disease. Acta Neuropathol. 1991a;81:428–433. doi: 10.1007/BF00293464. [DOI] [PubMed] [Google Scholar]
- Masliah E, Terry RD, Alford M, DeTeresa R, Hansen LA. Cortical and subcortical patterns of synaptophysin like immunoreactivity in Alzheimer’s disease. Am J Pathol. 1991b;138:235–246. [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVicker DP, Chrin LR, Berger CL. The nucleotide-binding state of microtubules modulates kinesin processivity and the ability of Tau to inhibit kinesin-mediated transport. J Biol Chem. 2011;286:42873–42880. doi: 10.1074/jbc.M111.292987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Morawe T, Hiebel C, Kern A, Behl C. Protein Homeostasis, Aging and Alzheimer’s Disease. Mol Neurobiol. 2012 doi: 10.1007/s12035-012-8246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno H, Yu E, Pigino G, Hernandez AI, Kim N, Moreira JE, Sugimori M, Llinas RR. Synaptic transmission block by presynaptic injection of oligomeric amyloid beta. Proc Natl Acad Sci U S A. 2009;106:5901–5906. doi: 10.1073/pnas.0900944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Burns M, Stenoien DL, Brady S. Axonal Transport. In: Brady S, Siegel G, Albers RW, Price D, editors. Basic Neurochemistry. Elsevier Academic Press; Boston, MA: 2011. pp. 146–164. [Google Scholar]
- Morfini G, Pigino G, Mizuno N, Kikkawa M, Brady ST. Tau binding to microtubules does not directly affect microtubule-based vesicle motility. J Neurosci Res. 2007a;85:2620–2630. doi: 10.1002/jnr.21154. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Opalach K, Serulle Y, Moreira JE, Sugimori M, Llinas RR, Brady ST. 1-Methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc Natl Acad Sci U S A. 2007b;104:2442–2447. doi: 10.1073/pnas.0611231104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Brown H, Pant HC, Pigino G, DeBoer S, Beffert U, Brady ST. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Richards B, Brady ST. Regulation of kinesin: implications for neuronal development. Dev Neurosci. 2001a;23:364–376. doi: 10.1159/000048720. [DOI] [PubMed] [Google Scholar]
- Morfini G, Tsai MY, Szebenyi G, Brady ST. Approaches to study interactions between kinesin motors and membranes. Methods Mol Biol. 2001b;164:147–162. doi: 10.1385/1-59259-069-1:147. [DOI] [PubMed] [Google Scholar]
- Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH, Jr, Brown H, Tiwari A, Hayward L, Edgar J, Nave KA, Garberrn J, Atagi Y, Song Y, Pigino G, Brady ST. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009a;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET, Bagnato C, Han D, Huang CF, Banker G, Pigino G, Brady ST. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci. 2009b;12:864–871. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudher A, Shepherd D, Newman TA, Mildren P, Jukes JP, Squire A, Mears A, Drummond JA, Berg S, MacKay D, Asuni AA, Bhat R, Lovestone S. GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol Psychiatry. 2004;9:522–530. doi: 10.1038/sj.mp.4001483. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Otth C, Mendoza-Naranjo A, Mujica L, Zambrano A, Concha II, Maccioni RB. Modulation of the JNK and p38 pathways by cdk5 protein kinase in a transgenic mouse model of Alzheimer’s disease. Neuroreport. 2003;14:2403–2409. doi: 10.1097/00001756-200312190-00023. [DOI] [PubMed] [Google Scholar]
- Patterson KR, Remmers C, Fu Y, Brooker S, Kanaan NM, Vana L, Ward S, Reyes JF, Philibert K, Glucksman MJ, Binder LI. Characterization of prefibrillar Tau oligomers in vitro and in Alzheimer disease. J Biol Chem. 2011a;286:23063–23076. doi: 10.1074/jbc.M111.237974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson KR, Ward SM, Combs B, Voss K, Kanaan NM, Morfini G, Brady ST, Gamblin TC, Binder LI. Heat shock protein 70 prevents both tau aggregation and the inhibitory effects of preexisting tau aggregates on fast axonal transport. Biochemistry. 2011b;50:10300–10310. doi: 10.1021/bi2009147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peethumnongsin E, Yang L, Kallhoff-Munoz V, Hu L, Takashima A, Pautler RG, Zheng H. Convergence of presenilin- and tau-mediated pathways on axonal trafficking and neuronal function. J Neurosci. 2010;30:13409–13418. doi: 10.1523/JNEUROSCI.1964-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer’s disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis. 2001;3:41–48. doi: 10.3233/jad-2001-3107. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Pfister KK, Shah PR, Hummerich H, Russ A, Cotton J, Annuar AA, King SM, Fisher EM. Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet. 2006;2:e1. doi: 10.1371/journal.pgen.0020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci U S A. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem. 2006;281:25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- Praprotnik D, Smith MA, Richey PL, Vinters HV, Perry G. Filament heterogeneity within the dystrophic neurites of senile plaques suggests blockage of fast axonal transport in Alzheimer’s disease. Acta Neuropathol. 1996;91:226–235. doi: 10.1007/s004010050420. [DOI] [PubMed] [Google Scholar]
- Price DL, Wong PC, Markowska AL, Lee MK, Thinakaren G, Cleveland DW, Sisodia SS, Borchelt DR. The value of transgenic models for the study of neurodegenerative diseases. Ann N Y Acad Sci. 2000;920:179–191. doi: 10.1111/j.1749-6632.2000.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Probst A, Gotz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. 2000;99:469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- Puls I, Oh SJ, Sumner CJ, Wallace KE, Floeter MK, Mann EA, Kennedy WR, Wendelschafer-Crabb G, Vortmeyer A, Powers R, Finnegan K, Holzbaur EL, Fischbeck KH, Ludlow CL. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann Neurol. 2005;57:687–694. doi: 10.1002/ana.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta -amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid E, Kloos M, Ashley-Koch A, Hughes L, Bevan S, Svenson IK, Graham FL, Gaskell PC, Dearlove A, Pericak-Vance MA, Rubinsztein DC, Marchuk DA. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10) Am J Hum Genet. 2002;71:1189–1194. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Rogalski EJ, Murphy CM, deToledo-Morrell L, Shah RC, Moseley ME, Bammer R, Stebbins GT. Changes in parahippocampal white matter integrity in amnestic mild cognitive impairment: a diffusion tensor imaging study. Behav Neurol. 2009;21:51–61. doi: 10.3233/BEN-2009-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner S, Mehlhorn G, Schliebs R, Bigl V. Increased neuronal and glial expression of protein kinase C isoforms in neocortex of transgenic Tg2576 mice with amyloid pathology. Eur J Neurosci. 2001;13:269–278. [PubMed] [Google Scholar]
- Roy S, Zhang B, Lee VM, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu P, Jansen A, Soba P, Kirsch J, Lower A, Merdes G, Kuan YH, Jung A, Beyreuther K, Kjaerulff O, Kins S. Axonal accumulation of synaptic markers in APP transgenic Drosophila depends on the NPTY motif and is paralleled by defects in synaptic plasticity. Eur J Neurosci. 2007;25:1079–1086. doi: 10.1111/j.1460-9568.2007.05341.x. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Walsh DM. Alzheimer’s disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KD, Kallhoff V, Zheng H, Pautler RG. In vivo axonal transport rates decrease in a mouse model of Alzheimer’s disease. Neuroimage. 2007;35:1401–1408. doi: 10.1016/j.neuroimage.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Kim JH, Lin SJ, Brendza RP, Holtzman DM. Diffusion tensor imaging detects age-dependent white matter changes in a transgenic mouse model with amyloid deposition. Neurobiol Dis. 2004;15:640–647. doi: 10.1016/j.nbd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer’s disease and mild cognitive impairment. Behav Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenoien DL, Brady ST. Immunochemical analysis of kinesin light chain function. Mol Biol Cell. 1997;8:675–689. doi: 10.1091/mbc.8.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:10041–10045. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JH, Deng G, Cotman CW. Transneuronal degeneration in the spread of Alzheimer’s disease pathology: immunohistochemical evidence for the transmission of tau hyperphosphorylation. Neurobiol Dis. 1997;4:365–375. doi: 10.1006/nbdi.1997.0164. [DOI] [PubMed] [Google Scholar]
- Susalka SJ, Pfister KK. Cytoplasmic dynein subunit heterogeneity: implications for axonal transport. J Neurocytol. 2000;29:819–829. doi: 10.1023/a:1010995408343. [DOI] [PubMed] [Google Scholar]
- Swartz RH, Sahlas DJ, Black SE. Strategic involvement of cholinergic pathways and executive dysfunction: Does location of white matter signal hyperintensities matter? J Stroke Cerebrovasc Dis. 2003;12:29–36. doi: 10.1053/jscd.2003.5. [DOI] [PubMed] [Google Scholar]
- Takashima A, Murayama M, Murayama O, Kohno T, Honda T, Yasutake K, Nihonmatsu N, Mercken M, Yamaguchi H, Sugihara S, Wolozin B. Presenilin 1 associates with glycogen synthase kinase-3beta and its substrate tau. Proc Natl Acad Sci U S A. 1998;95:9637–9641. doi: 10.1073/pnas.95.16.9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Gouras GK. Synapses, synaptic activity and intraneuronal abeta in Alzheimer’s disease. Front Aging Neurosci. 2010:2. doi: 10.3389/fnagi.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Scott DA, Das U, Edland SD, Radomski K, Koo EH, Roy S. Early and Selective Impairments in Axonal Transport Kinetics of Synaptic Cargoes Induced by Soluble Amyloid beta-Protein Oligomers. Traffic. 2012 doi: 10.1111/j.1600-0854.2012.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G, Tanzi RE. GSK3 beta forms a tetrameric complex with endogenous PS1-CTF/NTF and beta-catenin. Effects of the D257/D385A and FAD-linked mutations. Ann N Y Acad Sci. 2000;920:227–232. doi: 10.1111/j.1749-6632.2000.tb06927.x. [DOI] [PubMed] [Google Scholar]
- Tesseur I, Van Dorpe J, Bruynseels K, Bronfman F, Sciot R, Van Lommel A, Van Leuven F. Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord. Am J Pathol. 2000;157:1495–1510. doi: 10.1016/S0002-9440(10)64788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vana L, Kanaan NM, Ugwu IC, Wuu J, Mufson EJ, Binder LI. Progression of tau pathology in cholinergic Basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. Am J Pathol. 2011;179:2533–2550. doi: 10.1016/j.ajpath.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Hammond JW. Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol. 2009;10:765–777. doi: 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MC, Pfister KK, Bloom GS, Brady ST. Copurification of kinesin polypeptides with microtubule-stimulated Mg-ATPase activity and kinetic analysis of enzymatic properties. Cell Motil Cytoskeleton. 1989;12:195–215. doi: 10.1002/cm.970120403. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85:148–175. doi: 10.1016/j.pneurobio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener Dis. 2010;7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Weis J, Kayed R, Saido TC, Bayer TA. Age-dependent axonal degeneration in an Alzheimer mouse model. Neurobiol Aging. 2007;28:1689–1699. doi: 10.1016/j.neurobiolaging.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Witter MP. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res. 2007;163:43–61. doi: 10.1016/S0079-6123(07)63003-9. [DOI] [PubMed] [Google Scholar]
- Ye X, Tai W, Zhang D. The early events of Alzheimer’s disease pathology: from mitochondrial dysfunction to BDNF axonal transport deficits. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Yu JT, Chang RC, Tan L. Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Prog Neurobiol. 2009;89:240–255. doi: 10.1016/j.pneurobio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Yuan A, Kumar A, Peterhoff C, Duff K, Nixon RA. Axonal transport rates in vivo are unaffected by tau deletion or overexpression in mice. J Neurosci. 2008;28:1682–1687. doi: 10.1523/JNEUROSCI.5242-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB, 3rd, Lee VM, Brunden KR. The microtubule-stabilizing agent, epothilone d, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Higuchi M, Yoshiyama Y, Ishihara T, Forman MS, Martinez D, Joyce S, Trojanowski JQ, Lee VM. Retarded axonal transport of R406W mutant tau in transgenic mice with a neurodegenerative tauopathy. J Neurosci. 2004;24:4657–4667. doi: 10.1523/JNEUROSCI.0797-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gong B, Liu S, Fa M, Ninan I, Staniszewski A, Arancio O. Synaptic fatigue is more pronounced in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:137–140. doi: 10.2174/1567205053585936. [DOI] [PubMed] [Google Scholar]
- Zhao XL, Wang WA, Tan JX, Huang JK, Zhang X, Zhang BZ, Wang YH, YangCheng HY, Zhu HL, Sun XJ, Huang FD. Expression of beta-amyloid induced age-dependent presynaptic and axonal changes in Drosophila. J Neurosci. 2010;30:1512–1522. doi: 10.1523/JNEUROSCI.3699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA. Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem. 2001;76:435–441. doi: 10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]