Abstract
We have developed a general procedure for covalently attaching complementary restriction fragments to RNA molecules and visualizing the fragment in the electron microscope after denaturation. This is accomplished by creating psoralen monoadducts on the restriction fragment by irradiation at 390 nm before hybridization to the RNA and subsequently converting the monoadducts to RNA . DNA crosslinks by irradiation at 360 nm. Using this procedure, we have determined the location of a crosslink placed in Escherichia coli 165 rRNA by a previous irradiation. A consistent assignment is obtained by marking the crosslinked RNA with restriction fragments complementary to the 5' end or the 3' end of the 165 rRNA molecule.
Full text
PDF
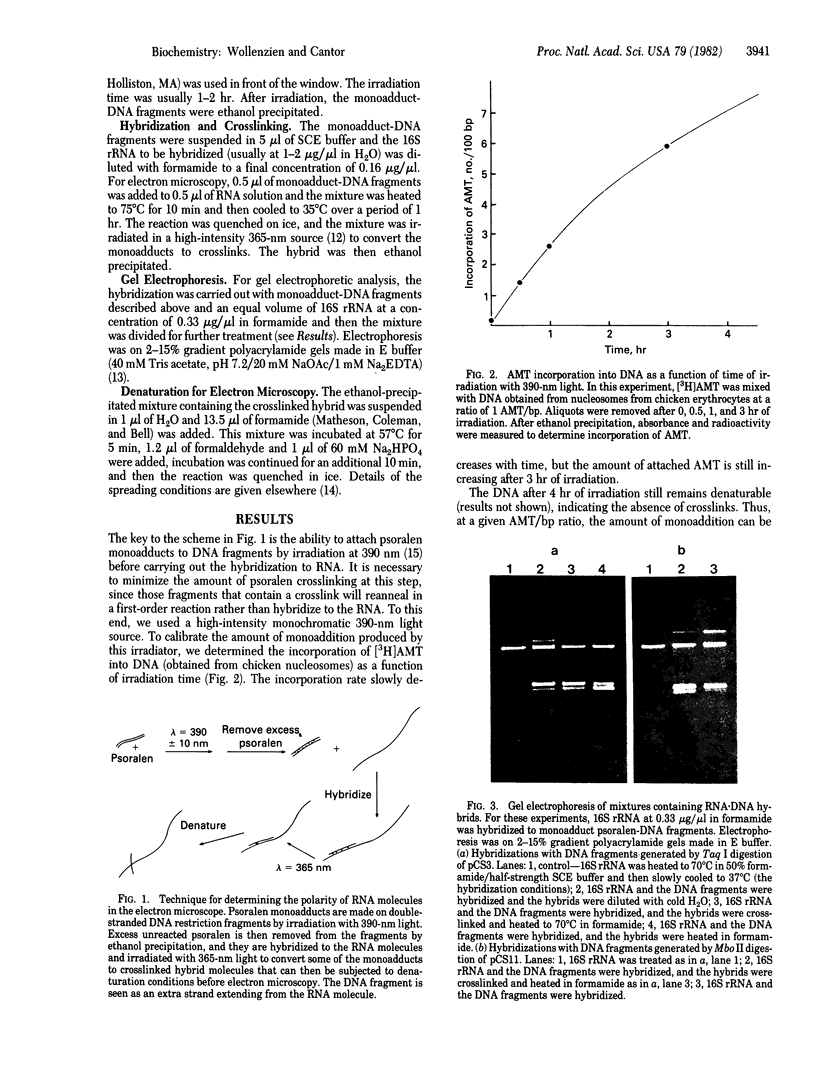
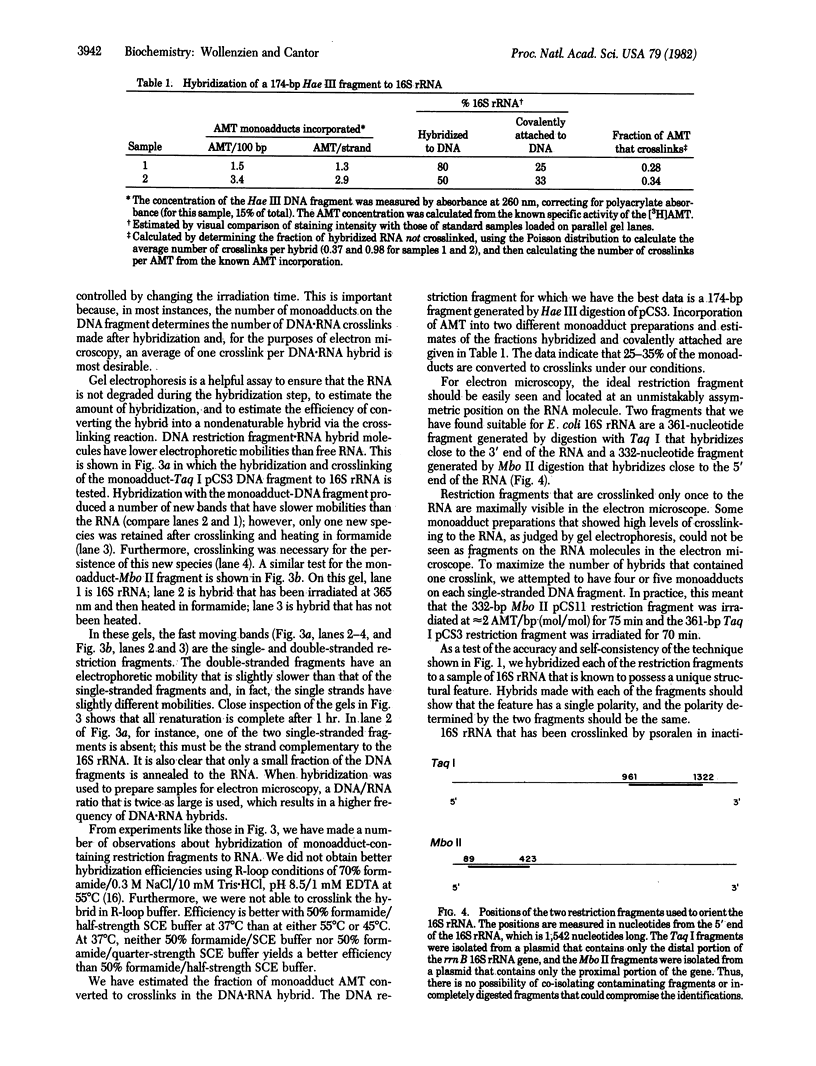
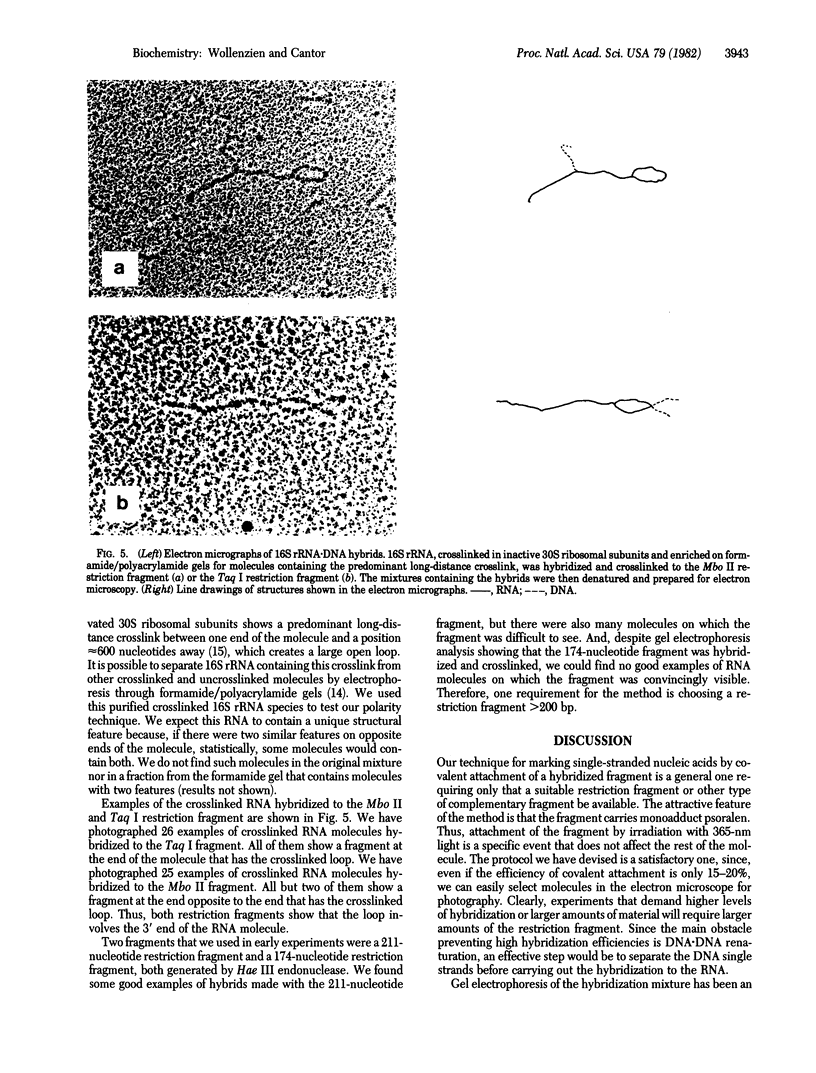

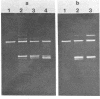
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amils R., Matthews E. A., Cantor C. R. Reconstitution of 50 S ribosomal subunits from Escherichia coli. Methods Enzymol. 1979;59:449–461. doi: 10.1016/0076-6879(79)59107-1. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequencing by partial ribosubstitution. J Mol Biol. 1978 Feb 15;119(1):83–99. doi: 10.1016/0022-2836(78)90271-1. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Angerer L. M., Yen P. H., Hershey N. D., Davidson N. Electron microscopic visualization of tRNA genes with ferritin-avidin: biotin labels. Nucleic Acids Res. 1978 Feb;5(2):363–384. doi: 10.1093/nar/5.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P. K., Cantor C. R. Photochemical production of psoralen - DNA monoadducts capable of subsequent photocrosslinking. Nucleic Acids Res. 1978 Oct;5(10):3619–3633. doi: 10.1093/nar/5.10.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. A method for the purification and reconcentration of nucleic acids eluted or extracted from polyacrylamide gels. Anal Biochem. 1973 Sep;55(1):317–320. doi: 10.1016/0003-2697(73)90322-9. [DOI] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Jacobson A. B., Spahr P. F. Studies on the secondary structure of single-stranded RNA from the bacteriophage MS2. II Analysis of the RNase IV cleavage products. J Mol Biol. 1977 Sep 25;115(3):279–294. doi: 10.1016/0022-2836(77)90155-3. [DOI] [PubMed] [Google Scholar]
- Peterlin B. M., Sullivan M., Westphal H., Maizel J. V., Jr Secondary structure map of psoralen-crosslinked adenovirus DNA studied by electron microscopy. Virology. 1978 May 15;86(2):391–397. doi: 10.1016/0042-6822(78)90079-x. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Psoralen-crosslinked secondary structure map of single-stranded virus DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2649–2653. doi: 10.1073/pnas.73.8.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodja A., Davidson N. Gene mapping and gene enrichment by the avidin-biotin interaction: use of cytochrome-c as a polyamine bridge. Nucleic Acids Res. 1978 Feb;5(2):385–401. doi: 10.1093/nar/5.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammana P., Cantor C. R., Wollenzien P. L., Hearst J. E. Crosslinking studies on the organization of the 16 S ribosomal RNA within the 30 S Escherichia coli ribosomal subunit. J Mol Biol. 1979 Nov 25;135(1):271–283. doi: 10.1016/0022-2836(79)90352-8. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- Wollenzien P. L., Hearst J. E., Squires C., Squires C. Determining the polarity of the map of crosslinked interactions in Escherichia coli 16 S ribosomal RNA. J Mol Biol. 1979 Nov 25;135(1):285–291. doi: 10.1016/0022-2836(79)90353-x. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N. A technique for mapping transfer RNA genes by electron microscopy of hybrids of ferritin-labeled transfer RNA and DNA: the phi-80hpsu+3-system. J Mol Biol. 1973 Jun 25;78(1):1–21. doi: 10.1016/0022-2836(73)90424-5. [DOI] [PubMed] [Google Scholar]