Abstract
Background
Schizophrenia is associated with perceptual and physiological auditory processing impairments that may result from primary auditory cortex excitatory and inhibitory circuit pathology. High-frequency oscillations are important for auditory function and are often reported to be disrupted in schizophrenia. These oscillations may, in part, depend on upregulation of gamma-aminobutyric acid synthesis by glutamate decarboxylase 65 (GAD65) in response to high interneuron firing rates. It is not known whether levels of GAD65 protein or GAD65-expressing boutons are altered in schizophrenia.
Methods
We studied two cohorts of subjects with schizophrenia and matched control subjects, comprising 27 pairs of subjects. Relative fluorescence intensity, density, volume, and number of GAD65-immunoreactive boutons in primary auditory cortex were measured using quantitative confocal microscopy and stereologic sampling methods. Bouton fluorescence intensities were used to compare the relative expression of GAD65 protein within boutons between diagnostic groups. Additionally, we assessed the correlation between previously measured dendritic spine densities and GAD65-immunoreactive bouton fluorescence intensities.
Results
GAD65-immunoreactive bouton fluorescence intensity was reduced by 40% in subjects with schizophrenia and was correlated with previously measured reduced spine density. The reduction was greater in subjects who were not living independently at time of death. In contrast, GAD65-immunoreactive bouton density and number were not altered in deep layer 3 of primary auditory cortex of subjects with schizophrenia.
Conclusions
Decreased expression of GAD65 protein within inhibitory boutons could contribute to auditory impairments in schizophrenia. The correlated reductions in dendritic spines and GAD65 protein suggest a relationship between inhibitory and excitatory synapse pathology in primary auditory cortex.
Keywords: GAD65, postmortem human tissue, primary auditory cortex, quantitative microscopy, schizophrenia, stereology
Individuals with schizophrenia exhibit basic auditory processing deficits (1) that contribute to debilitating negative and cognitive symptoms. One such deficit is impaired tone frequency discrimination (2,3), assessed either behaviorally or as reduction in the mismatch negativity (MMN) response of the auditory event-related potential (3–5). The inability to properly discriminate between different frequencies may make phoneme identification difficult, translating to impaired speech comprehension in subjects with schizophrenia (6,7). Evidence suggests that features of schizophrenia stemming from disrupted tone processing have a significant impact on patients’ quality of life, as tone-matching performance is severely impaired in subjects who require long-term residential care (8). Correlated with tone-matching deficits, subjects with schizophrenia show reduced ability to use pitch-based acoustic cues to recognize vocally expressed emotion (2,9). Consequently, inability to recognize emotional components of speech, a negative symptom of schizophrenia (10), contributes to dysfunction in social interactions (11). Thus, basic impairments in tone frequency discrimination likely impair higher-order functions downstream of auditory stimulus processing, contributing to some of the signs and symptoms of schizophrenia.
Studies in animals suggest that the ability to discriminate frequency depends on auditory cortex function (12,13). The primary auditory cortex is located on Heschl’s gyrus, found within the Sylvian fissure on the superior temporal gyrus (STG). Reduction of STG gray matter volume is one of the most consistently reported gray matter volume change findings in schizophrenia subjects (14) and those who are genetically at risk (15). Specifically, findings include reductions of gray matter volume in Heschl’s gyrus in both cross-sectional analyses of subjects with schizophrenia (16–18) and longitudinal studies of high-risk individuals (19).
One measure of auditory neurophysiology known to depend on the integrity of the primary auditory cortex is the auditory steady state response (aSSR) (20–22). Many studies have found the aSSR to be abnormal in patients with schizophrenia (23–27). Steady state responses are generated in response to temporally modulated stimuli, are based on the synchronized activity of large populations of neurons, and represent the ability of neural circuits to oscillate at different frequencies (28). Subjects with schizophrenia exhibit abnormal aSSR entrainment to tones and white noise bursts modulated at gamma-range frequencies (30–80 Hz) (23,29). Individuals with schizophrenia also demonstrate reduced power of induced gamma-range oscillatory activity in response to an unmodulated pure tone (24). Altered high-frequency oscillatory activity may reflect a physiological impairment of auditory cortex circuitry that contributes to reduced ability to discriminate the features of auditory stimuli (30).
Inhibitory γ-aminobutyric acid (GABA)-ergic interneurons contribute to the generation of neural oscillatory activity through the production of rhythmic inhibitory postsynaptic potentials in excitatory neurons, inducing synchronization of their firing (31,32). It has been shown that rapid adjustments to levels of inhibition are required for controlling changes in oscillation frequency (33). This necessity of rapid adjustments to levels of inhibition suggests that modulation of the amount of GABA synthesized for release might be crucial for the ability of interneurons to mediate oscillatory activity. The GABA-producing activity of the 65 kDa isoform of glutamate decarboxylase (GAD65) is rapidly upregulated via binding to its cofactor pyridoxal-5’-phosphate under conditions of increased neural activity (34,35). Mice lacking GAD65 demonstrate reduced GABA release during sustained activation of inhibitory neurons (36). Glutamate decarboxylase 65 may therefore be particularly important for rapidly modulating GABA synthesis to maintain gamma-range oscillatory activity in the cortex during conditions of sustained high interneuron firing rates (37). Thus, impaired gamma-range oscillatory activity in auditory cortex in schizophrenia could indicate that GAD65-mediated GABA synthesis in inhibitory boutons is impaired in such a way as to be unable to keep up with the necessary high firing rates.
In the present study, we asked whether the relative level of GAD65 protein is reduced in the auditory cortex of subjects with schizophrenia. As auditory cortex circuitry is thought to participate in the generation of both aSSRs and the MMN component of the auditory event-related potential (20–22,38,39) and MMN reflects activity of the supragranular cortical layers (4,40), we wanted to determine whether GAD65 protein was reduced in this subregion of auditory cortex, specifically at its site of action: the bouton. To address this, we used quantitative fluorescence microscopy to assess levels of GAD65 protein within deep cortical layer 3 inhibitory boutons. We found that whereas the number and density of GAD65-expressing inhibitory boutons in deep layer 3 of primary auditory cortex were unaltered, these boutons contained less GAD65 protein, suggesting that the amount of GABA available for release when auditory cortex interneurons are firing at high rates may be reduced in subjects with schizophrenia.
Methods and Materials
Subjects and Animals
We studied two cohorts (Table 1 and Table S1 in Supplement 1) of subjects diagnosed with schizophrenia or schizoaffective disorder and matched control subjects included in our previous studies (41–44). We also studied a cohort of four male macaque monkeys (Macaca fascicularis) chronically exposed to haloperidol decanoate and four control macaques matched for sex and weight (43). See Supplemental Methods in Supplement 1 for further description.
Table 1.
Summary of Subject Characteristics for Cohorts 1 and 2
| Cohort 1 |
Cohort 2 |
Total |
||||
|---|---|---|---|---|---|---|
| Control | Schizophrenia | Control | Schizophrenia | Control | Schizophrenia | |
| n | 15 | 15 | 12 | 12 | 27 | 27 |
| Mean Age (SD) | 46.8 (8.3) | 47.6 (5.5) | 45.1 (12.9) | 47.3 (13.4) | 46.0 (10.4) | 47.4 (9.6) |
| Range | 27–64 | 38–63 | 19–65 | 25–71 | 19–65 | 25–71 |
| Sex (F/M) | 6/9 | 6/9 | 4/8 | 4/8 | 10/17 | 10/17 |
| Handedness (R/L/A/U) | 10/4/0/1 | 7/3/1/4 | 11/1/0/0 | 6/2/1/3 | 21/5/0/1 | 13/5/2/7 |
| PMI (SD) | 13.9 (5.5) | 15.9 (6.6) | 18.0 (6.6) | 17.9 (8.8) | 15.7 (6.3) | 16.8 (7.6) |
| Storage Time, Months (SD) | 168 (29) | 167 (26) | 111 (27) | 102 (30) | 142 (40) | 138 (43) |
| Illness Duration, Years (SD) | 24.9 (5.6) | 22.1 (14.6) | 23.7 (10.6) | |||
| Range | 14–34 | 3–50 | 3–50 | |||
| Suicide, n (%) | 3 (20%) | 2 (17%) | 5 (19%) | |||
| Schizoaffective, n (%) | 3 (20%) | 4 (33%) | 7 (26%) | |||
| Living Independently ATOD, n (%) | 7 (47%) | 2 (17%) | 9 (33%) | |||
| Alcohol/Substance Abuse ATOD, n (%) | 9 (60%) | 7 (58%) | 16 (59%) | |||
| History of Cannabis Use, n (%) | 3 (20%) | 5 (42%) | 8 (30%) | |||
| Antipsychotic ATOD, n (%) | 13 (87%) | 11 (92%) | 24 (89%) | |||
| Benzodiazepine ATOD, n (%) | 1 (7%) | 5 (33%) | 1 (8%) | 1 (4%) | 6 (22%) | |
| Anticonvulsant ATOD, n (%) | 3 (20%) | 4 (33%) | 7 (26%) | |||
| Antidepressant ATOD, n (%) | 5 (33%) | 4 (33%) | 9 (33%) | |||
Each subject in cohorts 1 and 2 was previously matched to a normal comparison subject based on sex and as closely as possible for age and postmortem interval and group matched for handedness. There were no diagnostic group differences in age [t(52) = .517, p = .608] or postmortem interval [t(52) = .584, p = .561] or in the distribution of handedness between diagnostic groups (χ12 = 1.46, p = .314). Mean storage time did not differ between diagnostic groups [cohort 1: t(28) = .040, p = .968; cohort 2: t(22) = .596, p = .557].
A, ambidextrous; ATOD, at time of death; F, female; L, left-handed; M, male; PMI, postmortem interval; R, right-handed; U, unknown.
Immunohistochemistry
Auditory cortex containing tissue sections from matched pairs were processed together in immunohistochemistry runs. Glutamate decarboxylase 65 was detected using a mouse anti-GAD65 primary antibody (MAB351; Millipore, Billerica, Massachusetts), the specificity of which was assessed by Western blot and immunohistochemistry (see Supplemental Methods and Figure S1 in Supplement 1).
Quantification of GAD65-Immunoreactive Puncta
GAD65-immunoreactive (IR) boutons within deep cortical layer 3 of primary auditory cortex were quantified in this study using confocal microscopy. Stereologic sampling was conducted as shown in Figure 1. Sections were coded so that the experimenter was blind to diagnostic or drug exposure group, and sections were organized into sets so that sections from paired subjects were imaged during the same imaging session. Images were collected and processed as described in the Supplemental Methods in Supplement 1.
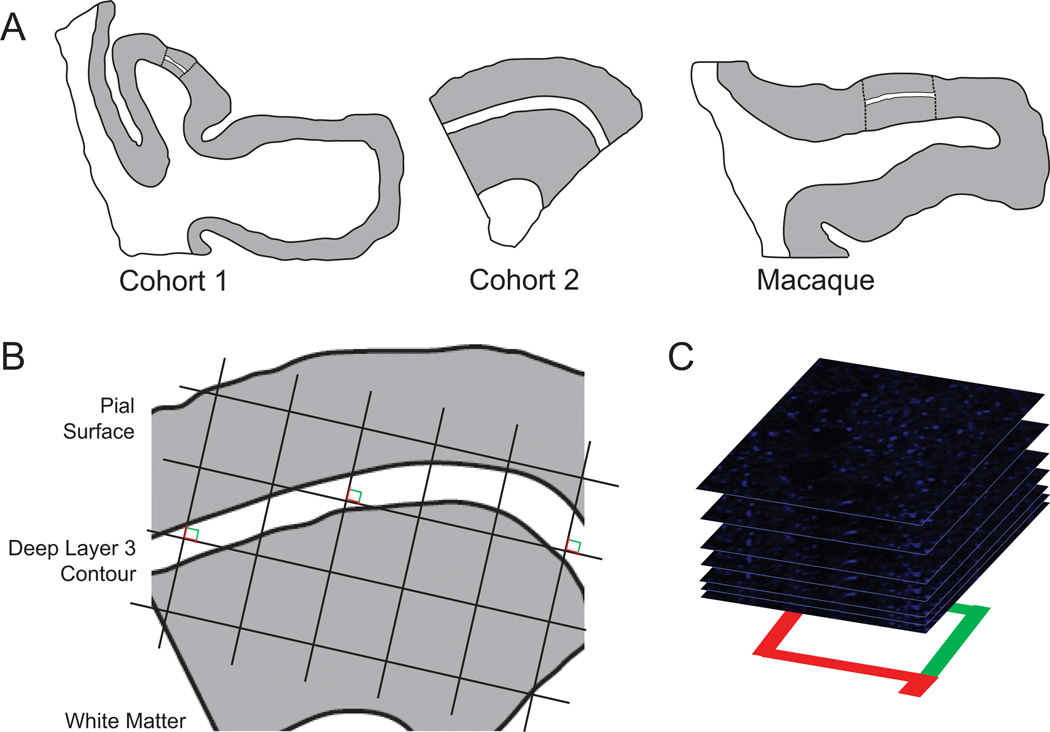
Figure 1.
Sampling of glutamate decarboxylase 65-immunoreactive boutons in primary auditory cortex deep layer 3. (A) Illustration of delineation of primary auditory cortex deep layer 3 on sections containing auditory cortex for human and antipsychotic exposed macaque cohorts. (Left) Cohort 1: Previously, every tenth section with a random start was selected from superior temporal gyrus blocks and processed for Nissl staining, and primary auditory cortex (Brodmann area 41) was identified using cytoarchitectonic criteria (42). Three Nissl-stained sections in which primary auditory cortex was cut perpendicular to the pial surface were selected for each subject, and sections adjacent to or nearby these sections were chosen for immunohistochemistry. (Middle) Cohort 2: Four primary auditory cortex blocks per subject were selected using a systematic uniformly random sampling scheme (90), designed to sample four primary auditory cortex blocks from each subject with equal probability. The central section of each block was stained for Nissl substance and used to delineate the cortical layer boundaries. From each selected block, one section adjacent to or nearby the center Nissl section was used in the present study. (Right) Antipsychotic-exposed macaques: Left hemisphere coronal superior temporal gyrus sections were generated similar to cohort 1 sections. Cytoarchitectonic criteria were used to identify primary auditory cortex, and three primary auditory cortex containing sections from each animal were selected for immunohistochemistry (43,44). For each human and monkey subject, the borders of layers 2/3 and 3/4 were identified on each of the adjacent Nissl-stained sections to determine the total layer 3 area for each subject. A contour outline (white) of the deepest one third of layer 3 was drawn in Stereo Investigator (MicroBright-Field Inc., Colchester, Vermont). The contours were aligned with the glutamate decarboxylase 65-labeled tissue sections using pial surface fiduciaries traced from the Nissl-stained sections. (B) A sampling grid was generated in Stereo Investigator to generate 12 to 14 sampling sites for each human subject and approximately 20 sites for each nonhuman primate subject. The grid size was determined based on the total deep layer 3 area across all tissue sections for a subject and the desired number of sampling sites per subject (12 for cohort 1 and 14 for cohort 2). The grid was then randomly rotated over the contour, and a sampling site was marked at every intersection between the grid and the deep layer 3 contour (shown as counting frames for the purpose of illustration; see Supplemental Methods in Supplement 1 for description of the associated point rule used in this study). (C) At each sampling site, a 10-µm thick stack of 46 image planes, each separated by .22 µm, was collected using spinning disk confocal microscopy.
See Supplemental Methods in Supplement 1 for additional details of stereologic calculations and approach to statistical analyses.
Results
GAD65-IR Bouton Intensity, Density, and Volume
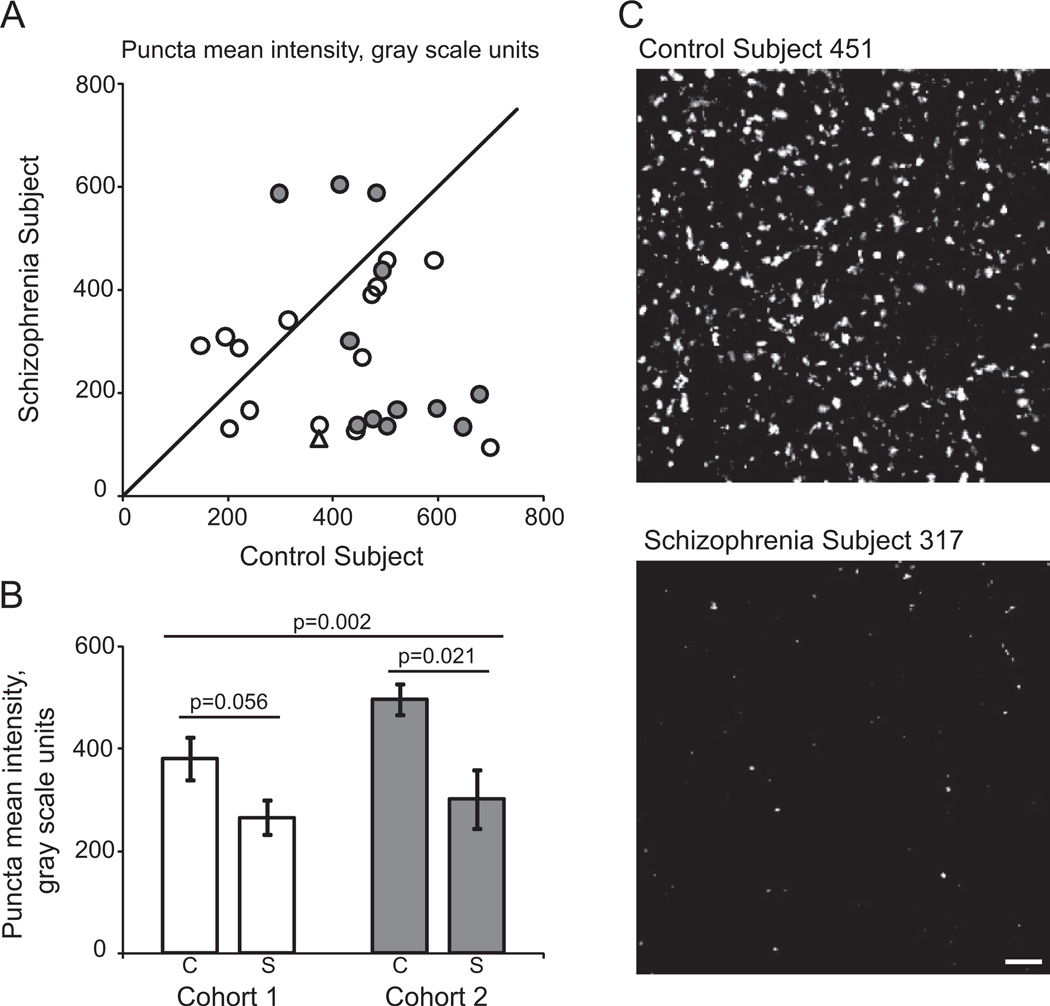
The fluorescence intensity of GAD65-IR puncta in deep layer 3 of the primary auditory cortex of subjects with schizophrenia was significantly decreased in both the primary [F(1,25.2) = 12.52, p = .002] and secondary statistical models [F(1, 46.5) = 15.74, p = .0002; Figure 2]. Estimated mean (95% confidence interval) fluorescence intensities derived from the primary model were 5.94 (5.73, 6.15) and 5.42 (5.21, 5.64) gray scale units on the natural logarithm scale in normal control subjects and subjects with schizophrenia, respectively, reflecting a 40.5% reduction in relative fluorescence intensity in subjects with schizophrenia.
Figure 2.
Within-bouton level of glutamate decarboxylase 65 protein is reduced in subjects with schizophrenia. (A) Mean puncta fluorescence intensity for each schizophrenia-control subject pair in cohort 1 (open circles and triangle) and cohort 2 (filled circles). Reference line represents schizophrenia = control subject values, where points below the line indicate a pair where control subject > schizophrenia and points above the line indicate schizophrenia > control subject. (B) Diagnostic group mean puncta fluorescence intensity for control (C) and schizophrenia (S) subjects in cohorts 1 (open bars) and 2 (gray bars). Error bars are ± SEM. (C) Representative projection image of glutamate decarboxylase 65-immunoreactive boutons from control subject 451 and schizophrenia subject 317 (cohort 1, pair 9, represented by open triangle in [A]). Scale bar represents 10 µm. Images are displayed across equivalent gray scale ranges.
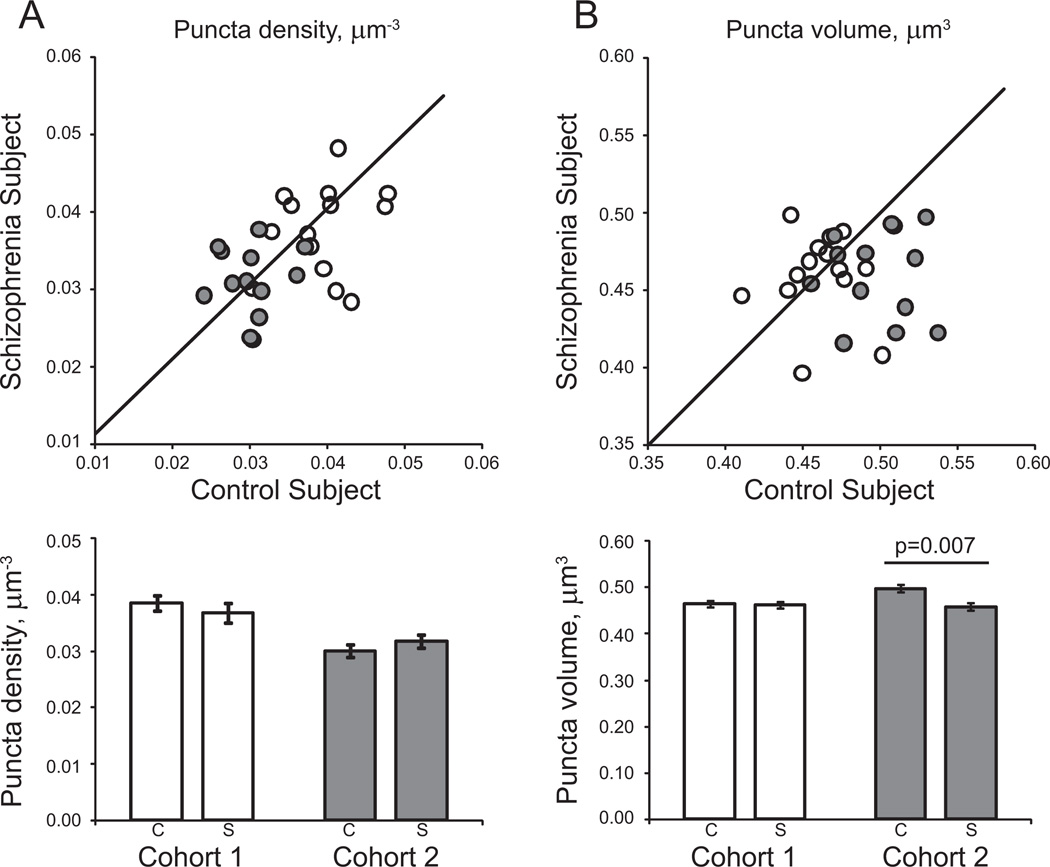
The density of GAD65-IR puncta was not significantly altered in subjects with schizophrenia compared with control subjects, in either the primary [F(1,24) = .84, p = .369] or secondary [F(1,46.8) = .22, p = .641] statistical models (Figure 3A).
Figure 3.
Glutamate decarboxylase 65-immunoreactive puncta density and volume in deep layer 3 of primary auditory cortex of subjects with schizophrenia. (A) (Top) Mean puncta density for each schizophrenia-control subject pair in cohort 1 (open circles) and cohort 2 (filled circles). Reference line represents schizophrenia = control subject values, where points below the line indicate a pair where control subject > schizophrenia and points above the line indicate schizophrenia > control subject. (Bottom) Diagnostic group mean puncta density for control (C) and schizophrenia (S) subjects in cohorts 1 (open bars) and 2 (gray bars). (B) (Top) Mean puncta volume for each schizophrenia-control subject pair in cohort 1 (open circles) and cohort 2 (filled circles). Reference line represents schizophrenia = control subject values, where points below the line indicate a pair where control subject > schizophrenia and points above the line indicate schizophrenia > control subject. (Bottom) Diagnostic group mean puncta volume for control (C) and schizophrenia (S) subjects in cohorts 1 (open bars) and 2 (gray bars). Error bars are ± SEM.
The reduction of GAD65-IR puncta volume in subjects with schizophrenia relative to normal control subjects was nearly statistically significant in the primary model [F (1,26.6) = 3.75, p = .063] and was statistically significant in the secondary model [F (1,49.6) = 4.80, p = .033; Figure 3B]. This effect was largely driven by the cohort 2 subject pairs in both the primary [F (1,10.7) = 11.24, p = .007] and secondary [F (1,17.8) = 10.72, p = .004] statistical models. Estimated mean (95% confidence interval) volumes derived from the primary model were .499 (.484, .515) and .465 (.449, .481) µm3 for control subjects and subjects with schizophrenia in cohort 2, respectively, reflecting a 6.8% reduction in subjects with schizophrenia. Because we suspected that the volume decrease in cohort 2 might be driven by the significant decrease in fluorescence intensity, we repeated the primary and secondary multivariate analysis of covariance models for volume with mean intensity as a covariate, using both cohorts. There was a highly significant effect of intensity [primary model: F (1,655) = 114.16, p < .001; secondary model: F (1,669) = 102.78, p < .001] but the effect of diagnosis on bouton volume was still significant [primary model: F (1,25.1) = 8.39, p = .008; secondary model: F (1,44.8) = 11.16, p = .002].
Independent Living Status
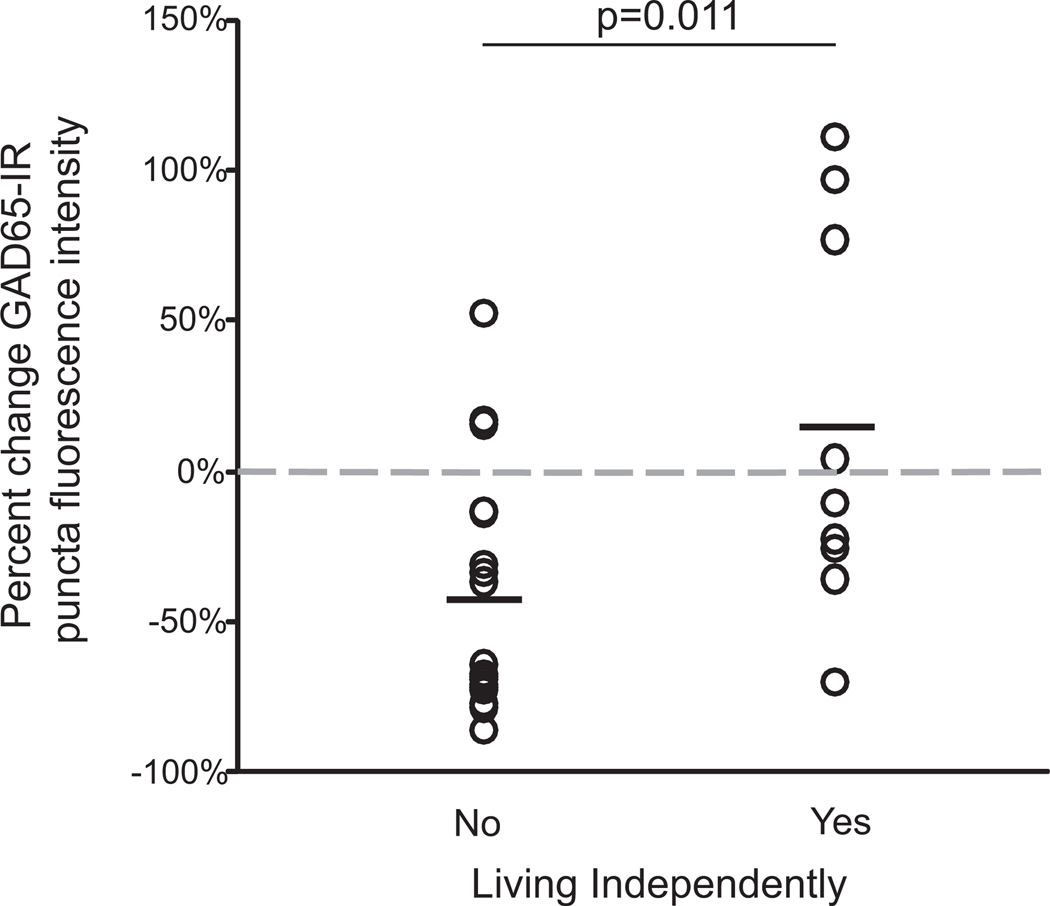
We found that the percent reduction in mean GAD65-IR bouton fluorescence intensity relative to matched control subjects was significantly greater for schizophrenia subjects who were not living independently at time of death than for those who were [t (24) = 2.77, p = .011; Figure 4]. In contrast, there was no significant relationship between independent living status and the percent changes in GAD65-IR bouton density or volume relative to control subjects.
Figure 4.
Relationship between independent living status of schizophrenia subjects and the reduction in mean glutamate decarboxylase 65-immunoreactive (GAD65-IR) bouton fluorescence intensity. The percent change in GAD65-IR bouton fluorescence intensity in subjects with schizophrenia is significantly greater in pairs where the schizophrenia subject was not living independently at time of death [t(24) = 2.77, p = .011]. Horizontal lines indicate mean pairwise percent change relative to control subjects. Gray dashed line indicates zero percent change. There was no significant relationship between independent living status and percent change in GAD65-IR bouton density [t(24) = .09, p = .925] or volume [t(24) = 1.86, p = .075] relative to control subjects (data not shown).
Clinical Factors
We tested for associations between GAD65-IR mean bouton density, fluorescence intensity, or volume and a number of clinical factors. We identified a significantly greater percent increase in GAD65-IR bouton density in subjects who were taking benzodiazepines at time of death (see Supplemental Results and Figure S2 in Supplement 1 for details).
Correlation Between Dendritic Spine Density and GAD65-IR Bouton Fluorescence Intensity and Density in Cohort 1 Subjects
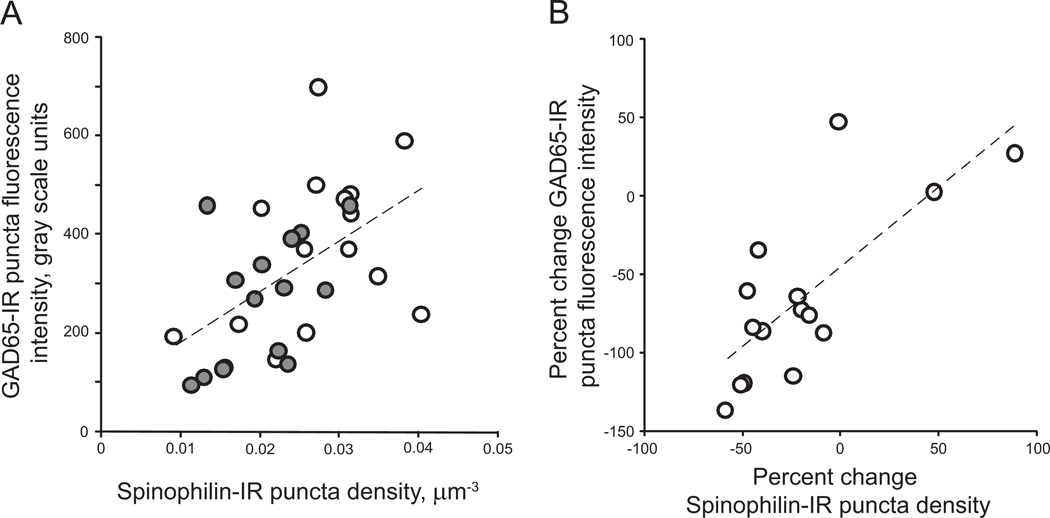
Given the importance of balanced excitatory and inhibitory neurotransmission in neural processing of auditory stimuli (45,46) and in modulating neural oscillations (33), we hypothesized that there are concurrent reductions in inhibitory and excitatory components of primary auditory cortex circuitry. We examined whether GAD65-IR bouton mean intensity was correlated with previously measured spinophilin-IR puncta density in deep layer 3 of primary auditory cortex for cohort 1 subjects (44). We found that GAD65-IR bouton fluorescence intensity was significantly correlated with dendritic spine density in this cohort of subjects (r = .525, p = .003; Figure 5A). The strength of the correlation was observed to be greater for schizophrenia subjects compared with control subjects (control: r = .379, p = .162; schizophrenia: r = .505, p = .055). In addition, we found a significant correlation between the percent changes in GAD65-IR puncta fluorescence intensity and dendritic spine density in schizophrenia subjects (r = .745, p = .001; Figure 5B). Similarly, we observed a correlation between GAD65-IR bouton density and spinophilin-IR spine density (r = .457, p = .01; Figure S3 in Supplement 1). The strength of this correlation was also greater for schizophrenia subjects than control subjects (control: r = .351, p = .2; schizophrenia: r = .583, p = .02). Finally, the percent reductions in GAD65-IR boutons and spinophilin-IR spine densities in schizophrenia subjects relative to control subjects were significantly correlated (r = .718, p = .003; Figure S3 in Supplement 1).
Figure 5.
Correlations between mean and percent change in glutamate decarboxylase 65-immunoreactive (GAD65-IR) puncta fluorescence intensity and spine density in primary auditory cortex. (A) Mean GAD65-IR puncta fluorescence plotted as a function of spinophilin-immunoreactive (IR) puncta density for each subject in cohort 1. Open circles = control subject, filled circles = schizophrenia subject. Dashed line represents the regression line. (Pearson r = .525, p = .003). (B) The percent change in GAD65-IR puncta mean fluorescence intensity plotted as a function of the percent change in spinophilin-IR puncta density for pairs in cohort 1 (schizophrenia subjects relative to matched control subjects). Dashed line represents the regression line. (Pearson r = .745, p = .001).
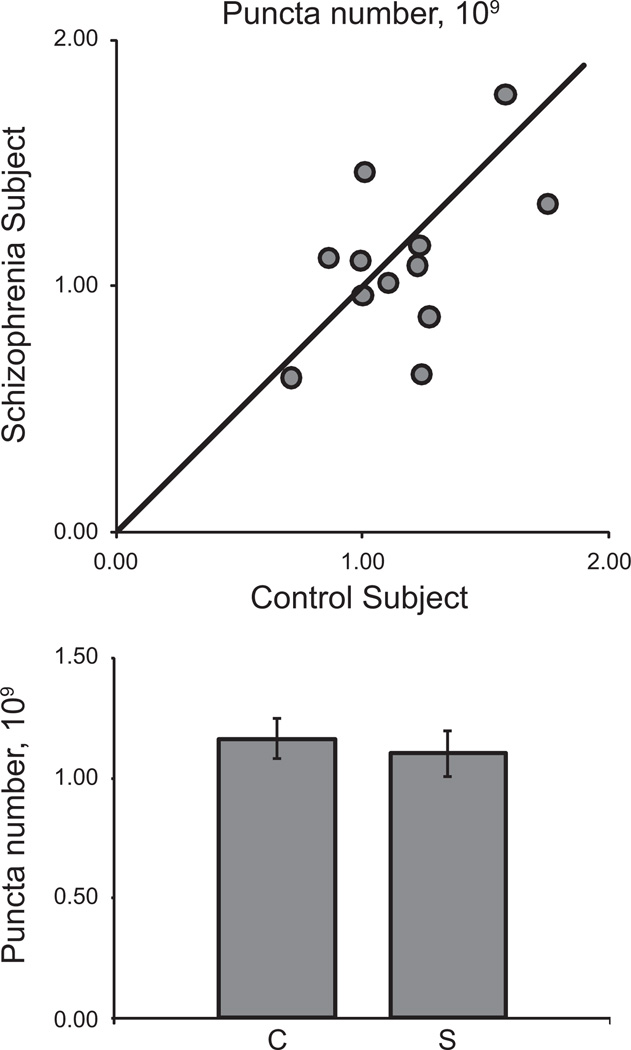
Estimation of GAD65-IR Bouton Number in Cohort 2 Subjects
We were able to estimate the total number of GAD65-IR puncta in primary auditory cortex deep layer 3 for the 12 subject pairs of cohort 2 and found that the absolute numbers of GAD65-IR boutons were not altered in schizophrenia in either the primary [F(1,10) = .644, p = .441] or secondary [F(1,18) = .065, p = .802] analysis of covariance (ANCOVA) models (Figure 6). Because of the differences in tissue processing methods, we were not able to estimate bouton number in subject pairs from cohort 1.
Figure 6.
Glutamate decarboxylase 65-immunoreactive puncta number in deep layer 3 of primary auditory cortex of subjects with schizophrenia. (Top) Mean puncta number for each schizophrenia-control subject pair in cohort 2. Reference line represents schizophrenia = control subject values, where points below the line indicate a pair where control subject > schizophrenia and points above the line indicate schizophrenia > control subject. (Bottom) Diagnostic group mean puncta number for control (C) and schizophrenia subjects (S). Error bars are ± SEM.
Antipsychotic Exposed Monkeys
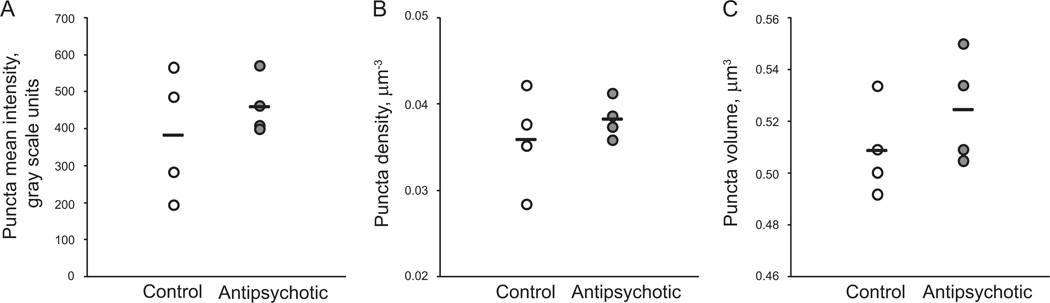
We observed no effect of chronic haloperidol exposure on relative fluorescence intensity [F(1,3) = 1.72; p = .281], density [F(1,18.6) = .51; p = .483], or volume [F(1,2.88) = 2.82; p = .195] of GAD65-IR puncta in deep layer 3 of primary auditory cortex (Figure 7).
Figure 7.
Chronic antipsychotic exposure does not alter glutamate decarboxylase 65-immunoreactive puncta mean intensity (A), density (B), or volume (C) in adult male macaques. Horizontal lines indicate group means.
Discussion
We found that GAD65-IR bouton fluorescence intensity was decreased within deep layer 3 in 27 subjects with schizophrenia relative to matched control subjects. Because the intensity data that we collect using our microscopy and image processing methods are linearly related to underlying object fluorophore content (47), we interpret the decrease in bouton fluorescence intensity as a decrease in within-bouton GAD65 protein content. However, caution must be used in interpretation of the magnitude of protein reduction, as the stoichiometry of the binding of this antibody to GAD65 protein in tissue is not known for certain to be linear. The reductions in GAD65-IR bouton fluorescence and decreased spine density were correlated within subjects in the 15 subject pairs for whom both measures were available. Finally, we report that the reductions in GAD65-IR bouton fluorescence intensity were larger in those subjects who were not living independently at time of death.
GAD65 Protein Studies
The few prior studies that have examined GAD65 protein levels in the cortex of subjects with schizophrenia report mixed results. Assessments of protein by Western blot suggest that GAD65 protein level is unchanged in prefrontal and occipital cortices in subjects with schizophrenia (48,49), and one study in temporal cortex reported a nonsignificant 27% decrease in GAD65 compared with control subjects (50). Differences between these reports of GAD65 protein levels and our own could be due to cohort-specific differences in subject characteristics such as age and postmortem interval but most likely can be attributed to the different methods used. We may have observed a larger reduction in GAD65 protein expression because in contrast to the Western blot studies where GAD65 protein from a less specific region was analyzed, we measured GAD65 protein at its site of action within boutons. For example, in a recent study using a similar approach to assess glutamate decarboxylase 67 (GAD67) protein, a larger deficit was found within boutons than within total gray matter when both were assessed in the same subjects (51). A potential biological explanation for the discrepancy between total gray matter and within-bouton levels of GAD65 and GAD67 protein is that transport of GAD65/GAD67 protein from the soma to the axon bouton is impaired in subjects with schizophrenia. This could result in relatively normal levels of protein detected unless within-bouton protein levels are examined exclusively. Future immunohistochemistry studies could test this possibility by quantifying GAD65 fluorescence within the somata of inhibitory neurons. Another possible source of discrepancy is that different cortical regions were examined, and reduction of GAD65 protein may not occur universally but may be more pronounced in auditory cortex. Future studies will be necessary to evaluate whether reduced GAD65 protein within inhibitory boutons is specific to deep layer 3 or is a feature across cortical layers in primary auditory cortex and other regions.
GAD65-IR Bouton Density, Number, and Volume
We found that the density of GAD65-IR inhibitory boutons was unaltered in deep layer 3 of primary auditory cortex, despite significantly reduced bouton fluorescence intensity. Our finding that GAD65-IR bouton density is unchanged in schizophrenia agrees with a previous report that GAD65-IR puncta density is unaltered in the anterior cingulate and prefrontal cortices (52). A potential difficulty in interpreting a lack of change in bouton density between groups is that gray matter volume, particularly in the STG, is consistently reported to be reduced in schizophrenia (14–19). Therefore, even though we identified no change in bouton density in schizophrenia, it is possible that the number of boutons is reduced, and an accompanying reduction in regional gray matter volume masks this effect when bouton density is calculated—this is known as the reference trap (53). The stereological techniques described in Dorph-Petersen et al. (41) that we used to generate the tissue sections for cohort 2 allowed us to systematically randomly sample the entire primary auditory cortex. Our previous study showed no significant reduction in auditory cortex layer 3 gray matter volume in schizophrenia (41). Similarly, using unbiased stereological principles of sampling, we estimated the number of GAD65-IR boutons in auditory cortex deep layer 3 for each subject and found that the absolute numbers of GAD65-IR boutons were not altered in schizophrenia.
We identified a reduction in mean bouton volume in subjects with schizophrenia, which was significant within one cohort (cohort 2) of schizophrenia subjects relative to matched control subjects. Given the difference between control and schizophrenia subject GAD65-IR bouton fluorescence intensity in cohort 2 pairs, we tested whether reduced intensity mediated the reduction in volume. Instead, we found that after correcting for bouton fluorescence intensity, the association of reduced GAD65-IR bouton volume with schizophrenia was strengthened. This evidence suggests that the reduction in GAD65-IR bouton volume is not fully attributable to reduced GAD65 fluorescence intensity and may be an effect of schizophrenia. If so, reduced bouton volume may further contribute to inhibitory bouton impairment and lead to reduced inhibitory neurotransmission, as bouton size is positively correlated with the number of active zones (54,55). However, caution must be used in the interpretation of this finding, as reduced bouton volume in schizophrenia subjects was only observed in one of our two cohorts studied.
Clinical Factors
We assessed the association between GAD65-IR bouton alterations and a number of clinical factors, including whether the subject had a diagnosis of schizoaffective disorder or schizophrenia, died by suicide, was living independently at time of death, and had a history of cannabis use or alcohol or other substance abuse. We found that the percent reduction in GAD65-IR bouton fluorescence intensity was greater in those subjects who were not living independently at time of death. One study has shown that tone-matching ability is particularly impaired in schizophrenia subjects who require long-term residential care (8), and reductions in auditory MMN amplitude are correlated with impairments in daily function in patients (56). Social perception and emotion responsivity are independently correlated with functional outcome in schizophrenia patients (57). Taken together, these findings indicate that reductions in GAD65 protein levels within inhibitory boutons, auditory processing, and social and emotional cognition are all associated with poor functional outcome in schizophrenia.
A number of our subjects were taking antipsychotics, benzodiazepines, antidepressants, or anticonvulsant medications at time of death. For a discussion of the association of these medications with GAD65-IR bouton characteristics, see Supplemental Discussion in Supplement 1.
Implications for Regulation of the Synthesis of GABA by GAD65 in Schizophrenia
Glutamate decarboxylase 65 exists predominantly in the inactive apoenzyme form and readily associates with synaptic vesicle membranes (58), localizing it to the presynaptic bouton where it is available to synthesize GABA for release. Association of inactive GAD65 with its cofactor pyridoxal-5’-phosphate to form the active holoenzyme form of GAD65 is promoted by increased metabolism during periods of synaptic activity (reviewed in [35]). Glutamate decarboxylase 65 thus serves as a molecular switch, allowing GABA synthesis to be upregulated during conditions of high GABA demand, i.e., during periods of high interneuron firing rates (36). Impairments in inhibitory transmission during increased neuronal activity have been demonstrated in GAD65 knockout mice, which despite having no difference in baseline levels of GABA (59), exhibit impaired neural plasticity (60) and behavioral deficits (61). Our finding of reduced GAD65 protein within inhibitory boutons in schizophrenia therefore suggests that upregulation of GABA production would be impaired when interneurons fire at high rates, leading to depletion of GABA and depression of inhibitory synapses under these conditions.
Implications for Specific Inhibitory Neuron Populations
Evidence suggests that GAD65 is the predominant glutamate decarboxylase (GAD) isoform expressed in the cannabinoid receptor type 1 (CB1)-IR boutons of cholecystokinin (CCK)-expressing interneurons, whereas GAD65 and GAD67 are co-expressed in the boutons of parvalbumin (PV)-IR basket cells and GAD67 is the predominant isoform in the axon cartridges of chandelier neurons (62). Therefore, reduced GAD65 protein expression in CCK boutons is likely to greatly impair the function of these interneurons. Activating CB1 receptors decreases GABA release from CCK-IR neurons and disrupts auditory sensory gating, as well as hippocampal and cortical theta and gamma range oscillatory activity in rats (63). Further, reduced event-related gamma activity is observed in chronic cannabis users (64), paralleling electrophysiological disruptions observed in schizophrenia (4,23–27,29). Decreased GAD65 protein within CCK boutons would reduce GABA available for release from these neurons, which would have a similar effect to that of CB1 receptor agonists, suggesting that such an alteration could, at least in part, be responsible for the disrupted oscillatory activity and auditory evoked potentials associated with schizophrenia. Reduced GAD65 protein could also impair PV-IR basket cell function. Parvalbumin-IR basket cells exhibit high firing rates (reviewed in [65]), a condition under which GAD65 is thought to be necessary for maintaining adequate levels of GABA needed for release (34,36). These fast-spiking interneurons are important for modulating the responses of primary auditory cortex pyramidal neurons to auditory stimuli (46) and for the induction of gamma oscillations (66,67), which are abnormal in schizophrenia. In contrast, chandelier cells, the terminals of which express predominantly GAD67 (62), would probably be less affected by reduced GAD65 protein than PV-IR and CCK-IR interneurons. Taken together, impairment of GABA release as the result of decreased GAD65 protein in CCK-IR and PV-IR boutons may contribute to some features of abnormal auditory cortex function and auditory processing in schizophrenia. Future studies determining whether reduced GAD65 expression is common to all GABAergic boutons in schizophrenia or specific to a certain population of interneurons will provide more information as to how reduced within-bouton expression of GAD65 protein impacts inhibitory neuron circuitry in schizophrenia.
Impaired GAD65-Mediated GABA Synthesis Could Disrupt Auditory Processing
Impaired GABAergic neurotransmission resulting from loss of GAD65 may impair the processing of stimuli in auditory cortex. Altering inhibition impairs the frequency tuning and response properties of auditory cortex neurons (68–70). An auditory stimulus elicits both excitation and inhibition in auditory cortex, which shape the postsynaptic response to the stimulus. Inhibition that roughly balances excitation contributes to the frequency selectivity of pyramidal neurons by scaling down the level of excitation in response to noncharacteristic frequencies (45) and by narrowing the tuning curve of the postsynaptic response through a lateral inhibition mechanism (46). Reduced inhibition caused by reduced GAD65 in schizophrenia may broaden frequency tuning of auditory cortex neurons and thus impair stimulus discrimination.
Reduced GABA available for release resulting from loss of GAD65 protein could also contribute to alterations in gamma-range aSSRs to modulated tones and noise measured in subjects with schizophrenia (24,29). Rapid adjustments in levels of inhibition balance large fluctuations in excitation during gamma oscillations and regulate the oscillation frequency (33,71). If insufficient GABA is available for release during this process due to reduced GAD65 protein, this could lead to the reductions of gamma band power and phase synchrony in schizophrenia (24). Interestingly, in human visual cortex, GABA concentration is directly proportional to the frequency of gamma oscillations and is inversely related to stimulus discrimination threshold (72). If the same is true for auditory cortex, reduced GABA synthesis resulting from decreased GAD65 protein may contribute to disruptions in gamma oscillations and auditory discrimination impairments in schizophrenia. However, the relationship between steady state GABA levels and GAD65 protein remains to be determined, and studies of GABA levels in human cortex report conflicting findings (73–75).
Reduced GAD65 Protein Levels May Compensate for Reduced Excitatory Activity
We observed a correlation between GAD65-IR bouton fluorescence intensity and dendritic spine density reductions, a finding that is consistent with other studies that have demonstrated that GAD expression and spine density are often co-regulated in cerebral cortex. Additionally, we found that dendritic spine density and percent reduction were significantly correlated with GAD65-IR bouton density and percent reductions in schizophrenia subjects. Reduced dendritic spine density occurs in multiple cortical regions in schizophrenia (76), and reduced expression of GABAergic markers is also conserved across cortical regions (77–79). Further, correlation between expression levels of excitatory and inhibitory synapse markers in the prefrontal cortex appears to be stronger in schizophrenia subjects than in control subjects (80). We also observed stronger correlations between spine density and both within-bouton GAD65 protein and GAD65-IR bouton density in schizophrenia subjects compared with control subjects. This raises the possibility that reduced inhibition and reduced spine density are related pathologies in schizophrenia.
Several lines of evidence indicate that reducing or disrupting GAD activity can have stability-promoting effects on dendritic spines. Monocular deprivation results in elimination of spines in layer 2/3 of visual cortex, but when GAD65 is disrupted, monocular deprivation-induced loss of spines does not occur (81). Further, GAD65 knockout mice are reported to have elevated spine density (81), and spine density increases when cultured neurons are exposed to the GAD inhibitor mercaptopropionic acid (82). Conversely, studies using a variety of methods have demonstrated that reducing neuronal activity or sensory input decreases expression of GAD (83–86). Therefore, our finding of correlated reductions in within-bouton GAD65 protein and spine density would be unlikely to indicate that reduced GAD65 expression causes spine loss in schizophrenia. Instead, reduced excitatory drive from the loss of dendritic spines may lead to a compensatory reduction in GAD65 protein expression. This could be evaluated by determining whether a reduction in within-bouton GAD65 expression occurs subsequent to accelerated loss of spines, e.g., during normal adolescent spine pruning (87,88), or in animal models of adolescentonset spine reduction (89).
Conclusions
In summary, our study is the first to find that GAD65 protein levels are reduced within GAD65-expressing boutons of primary auditory cortex in subjects with schizophrenia. This reduction may impair inhibitory transmission during auditory processing, causing impaired oscillatory activity and disrupting stimulus discrimination. Understanding the cell types in which GAD65 expression is reduced in schizophrenia will be important for the future design of therapies directed at this pathological feature. Similarly, studies that investigate the temporal relationship between GAD65 protein and excessive dendritic spine loss will be important to determine how to best target these features for prevention.
Supplementary Material
Acknowledgments
This work was supported by Grants MH071533 (RAS), MH084053 (DAL), and MH085108 (KNF).
We thank Dr. C. Sue Johnston for assistance with the clinical data; Ruth Henteleff, Jennifer Ciuchta, and the research staff of the Translational Neuroscience Program for technical assistance; and Dr. Gregg Homanics for providing the glutamate decarboxylase 65 knockout tissue.
Footnotes
These results were presented, in part, at the Annual Meetings of the Society for Neuroscience, Nov 18, 2008, Washington, DC; Oct 19, 2009, Chicago, Illinois; and Nov 15, 2010, San Diego, California.
ARS is a statistical consultant to Johnson & Johnson Pharmaceutical Research and Development. DAL currently receives investigator-initiated research support from the Bristol-Myers Squibb Foundation, Bristol-Myers Squibb, Curridium Ltd, and Pfizer and in 2007 to 2010 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, BioLine RX, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, Neurogen, and SK Life Science. CEM, KMD, KNF, JKA-A, K-AD-P, and RAS have no biomedical financial interests or potential conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
Supplementary material cited in this article is available online.
References
- 1.McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J. Event-related potentials in schizophrenia: Their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophr Res. 1991;4:209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- 2.Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- 3.Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111:1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- 4.Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG, Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: Intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res. 1994;667:192–200. doi: 10.1016/0006-8993(94)91496-6. [DOI] [PubMed] [Google Scholar]
- 5.Naatanen R, Kahkonen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: A review. Int J Neuropsychopharmacol. 2009;12:125–135. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- 6.Javitt DC. When doors of perception close: Bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasai K, Nakagome K, Itoh K, Koshida I, Hata A, Iwanami A, et al. Impaired cortical network for preattentive detection of change in speech sounds in schizophrenia: A high-resolution event-related potential study. Am J Psychiatry. 2002;159:546–553. doi: 10.1176/appi.ajp.159.4.546. [DOI] [PubMed] [Google Scholar]
- 8.Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia. Imprecision or distractibility? Arch Gen Psychiatry. 2000;57:1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- 9.Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biol Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Shea TL, Sergejew AA, Burnham D, Jones C, Rossell SL, Copolov DL, Egan GF. Emotional prosodic processing in auditory hallucinations. Schizophr Res. 2007;90:214–220. doi: 10.1016/j.schres.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Leitman DI, Ziwich R, Pasternak R, Javitt DC. Theory of Mind (ToM) and counterfactuality deficits in schizophrenia: Misperception or misinterpretation? Psychol Med. 2006;36:1075–1083. doi: 10.1017/S0033291706007653. [DOI] [PubMed] [Google Scholar]
- 12.Heffner HE, Heffner RS. Hearing loss in Japanese macaques following bilateral auditory cortex lesions. J Neurophysiol. 1986;55:256–271. doi: 10.1152/jn.1986.55.2.256. [DOI] [PubMed] [Google Scholar]
- 13.Rybalko N, Suta D, Nwabueze-Ogbo F, Syka J. Effect of auditory cortex lesions on the discrimination of frequency-modulated tones in rats. Eur J Neurosci. 2006;23:1614–1622. doi: 10.1111/j.1460-9568.2006.04688.x. [DOI] [PubMed] [Google Scholar]
- 14.McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J Psychiatry. 2004;161:1121–1124. doi: 10.1176/appi.ajp.161.6.1121. [DOI] [PubMed] [Google Scholar]
- 16.Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: A magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 20.Jeschke M, Lenz D, Budinger E, Herrmann CS, Ohl FW. Gamma oscillations in gerbil auditory cortex during a target-discrimination task reflect matches with short-term memory. Brain Res. 2008;1220:70–80. doi: 10.1016/j.brainres.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 21.Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Penuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22:10501–10506. doi: 10.1523/JNEUROSCI.22-23-10501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. Int J Audiol. 2003;42:177–219. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- 23.Brenner CA, Sporns O, Lysaker PH, O’Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O’Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, O’Donnell BF. Steady state responses: Electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35:1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamm JP, Gilmore CS, Picchetti NA, Sponheim SR, Clementz BA. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biol Psychiatry. 2011;69:989–996. doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenz D, Jeschke M, Schadow J, Naue N, Ohl FW, Herrmann CS. Human EEG very high frequency oscillations reflect the number of matches with a template in auditory short-term memory. Brain Res. 2008;1220:81–92. doi: 10.1016/j.brainres.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 31.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 32.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 33.Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62:566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J Neurochem. 2006;97:385–396. doi: 10.1111/j.1471-4159.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- 35.Martin DL, Martin SB, Wu SJ, Espina N. Regulatory properties of brain glutamate decarboxylase (GAD): The apoenzyme of GAD is present principally as the smaller of two molecular forms of GAD in brain. J Neurosci. 1991;11:2725–2731. doi: 10.1523/JNEUROSCI.11-09-02725.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci U S A. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaur S, Rose HJ, Lazar R, Liang K, Metherate R. Spectral integration in primary auditory cortex: Laminar processing of afferent input, in vivo and in vitro. Neuroscience. 2005;134:1033–1045. doi: 10.1016/j.neuroscience.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 39.Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat Neurosci. 2007;10:1594–1600. doi: 10.1038/nn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: Implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorph-Petersen KA, Delevich KM, Marcsisin MJ, Zhang W, Sampson AR, Gundersen HJ, et al. Pyramidal neuron number in layer 3 of primary auditory cortex of subjects with schizophrenia. Brain Res. 2009;1285:42–57. doi: 10.1016/j.brainres.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA. Pyramidal cell size reduction in schizophrenia: Evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 44.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 46.Wu GK, Arbuckle R, Liu BH, Tao HW, Zhang LI. Lateral sharpening of cortical frequency tuning by approximately balanced inhibition. Neuron. 2008;58:132–143. doi: 10.1016/j.neuron.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fish KN, Sweet RA, Deo AJ, Lewis DA. An automated segmentation methodology for quantifying immunoreactive puncta number and fluorescence intensity in tissue sections. Brain Res. 2008;1240:62–72. doi: 10.1016/j.brainres.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. GAD67 and GAD65 mRNA and protein expression in cerebrocortical regions of elderly patients with schizophrenia. J Neurosci Res. 2004;76:581–592. doi: 10.1002/jnr.20122. [DOI] [PubMed] [Google Scholar]
- 49.Guidotti A, Auta J, Davis JM, Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: A postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 50.Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: Clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/s0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- 53.Braendgaard H, Gundersen HJ. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods. 1986;18:39–78. doi: 10.1016/0165-0270(86)90112-3. [DOI] [PubMed] [Google Scholar]
- 54.Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol. 2011;520:434–452. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz R, Cano R, Casanas JJ, Gaffield MA, Betz WJ, Tabares L. Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. J Neurosci. 2011;31:2000–2008. doi: 10.1523/JNEUROSCI.4663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rasser PE, Schall U, Todd J, Michie PT, Ward PB, Johnston P, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37:131–140. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathews JR, Barch DM. Emotion responsivity, social cognition, and functional outcome in schizophrenia. J Abnorm Psychol. 2010;119:50–59. doi: 10.1037/a0017861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang YC, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 60.Choi SY, Morales B, Lee HK, Kirkwood A. Absence of long-term depression in the visual cortex of glutamic acid decarboxylase-65 knock-out mice. J Neurosci. 2002;22:5271–5276. doi: 10.1523/JNEUROSCI.22-13-05271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kash SF, Tecott LH, Hodge C, Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fish KN, Sweet RA, Lewis DA. Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex. 2011;21:2450–2460. doi: 10.1093/cercor/bhr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol Psychiatry. 2008;63:1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Edwards CR, Skosnik PD, Steinmetz AB, O’Donnell BF, Hetrick WP. Sensory gating impairments in heavy cannabis users are associated with altered neural oscillations. Behav Neurosci. 2009;123:894–904. doi: 10.1037/a0016328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 66.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen QC, Jen PH. Bicuculline application affects discharge patterns, rate-intensity functions, and frequency tuning characteristics of bat auditory cortical neurons. Hear Res. 2000;150:161–174. doi: 10.1016/s0378-5955(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 2000;11:1137–1140. doi: 10.1097/00001756-200004070-00045. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- 71.Mann EO, Mody I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat Neurosci. 2010;13:205–212. doi: 10.1038/nn.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choe BY, Kim KT, Suh TS, Lee C, Paik IH, Bahk YW, et al. 1H magnetic resonance spectroscopy characterization of neuronal dysfunction in drug-naïve, chronic schizophrenia. Acad Radiol. 1994;1:211–216. doi: 10.1016/s1076-6332(05)80716-0. [DOI] [PubMed] [Google Scholar]
- 74.Stanley JA, Drost DJ, Williamson PC, Carr TJ. In vivo proton MRS study of glutamate and schizophrenia. In: Nasrallah HA, Pettegrew JW, editors. NMR Spectroscopy in Psychiatric Brain Disorders. Washington, DC: American Psychiatric Press; 1995. pp. 21–44. [Google Scholar]
- 75.Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. Gamma-aminobutyric acid concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: Advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 78.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. AmJ Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 80.Fung SJ, Webster MJ, Weickert CS. Expression of VGluT1 and VGAT mRNAs in human dorsolateral prefrontal cortex during development and in schizophrenia. Brain Res. 2011;1388:22–31. doi: 10.1016/j.brainres.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 82.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hendry SH, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1:701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- 84.Patz S, Wirth MJ, Gorba T, Klostermann O, Wahle P. Neuronal activity and neurotrophic factors regulate GAD-65/67 mRNA and protein expression in organotypic cultures of rat visual cortex. Eur J Neurosci. 2003;18:1–12. doi: 10.1046/j.1460-9568.2003.02702.x. [DOI] [PubMed] [Google Scholar]
- 85.Welker E, Soriano E, Van der LH. Plasticity in the barrel cortex of the adult mouse: Effects of peripheral deprivation on GAD-immunoreactivity. Exp Brain Res. 1989;74:441–452. doi: 10.1007/BF00247346. [DOI] [PubMed] [Google Scholar]
- 86.Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- 87.Huttenlocher PR. Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 88.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 89.Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gundersen HJ. The smooth fractionator. J Microsc. 2002;207:191–210. doi: 10.1046/j.1365-2818.2002.01054.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.