Abstract
Introduction
Independent measurement of the levels of both the estrogen receptors, ERα and ERβ, in breast cancer could improve prediction of benefit from endocrine therapies. While ERα levels can be measured by positron emission tomography (PET) using 16α-[18F]fluoroestradiol (FES), no effective agent for imaging ERβ by PET has yet been reported.
Methods
We have prepared the fluorine-18 labeled form of 8β-(2-fluoroethyl)estradiol(8BFEE2), an analog of an ERβ-selective steroidal estrogen, 8β-vinylestradiol; efficient incorporation of fluorine-18 was achieved, but required very vigorous conditions. We have examined the biodistribution of this compound, as well as ofBr-041, an analog of a known non-steroidal ERβ-selective ligand (ERB-041), labeled with bromine-76. Studies were done in immature female rodents, with various pharmacological and endocrine perturbations to assess ERβ selectivity of uptake.
Results
Little evidence of ERβ-mediated uptake was observedwith either [18F]8BFEE2 or [76Br]Br-041. Attempts to increase the ERβ content of target tissues were not effective and failed to improve biodistribution selectivity.
Conclusions
Because on an absolute level, ERβ levels are low in all target tissues, these studies have highlighted the need to develop improved in vivo models for evaluating ERβ-selective radiopharmaceuticals for use in PET imaging. Genetically engineered breast cancer cells that are being developed to express either ERα or ERβ in a regulated manner, grown as xenografts in immune-compromised mice, could prove useful for future studies to develop ER subtype-selective radiopharmaceuticals.
Keywords: Estrogen receptor beta, estradiol, 8β-vinylestradiol, ERB-041, breast cancer
Introduction
Estrogen acting through the estrogen receptor (ER) functions as a major regulator of gene expression, and is associated with the stimulation of cell proliferation in female reproductive tissues and in certain hormone-regulated cancers. The estrogen receptor was cloned in 1985, and despite many intensive searches, no other ER gene was found until 1996, when two laboratories identified a second ER, which was termed ERβ, the original gene subsequently being called ERα. The amino acid sequence and the tissue distribution of ERβ are quite different from ERα. While the DNA binding domains are nearly identical, the N- and C-termini and the hinge domain are very different, and the ligand binding domains have only 59% amino acid identity. Also, while ERα often predominates (as in the uterus, kidney, liver and heart), both ERβ and ERα are found together in many tissues (breast, epididymis, thyroid, adrenal, and bone). ERβ is present at high levels in some cells (granulosa cells of ovary, testis, prostate, gastrointestinal tract, lung, and brain). With these differences in structure and tissue distribution, ERβ quickly became a popular target for the development of selective estrogens with potential for menopausal hormone replacement, regulation of fertility and other applications.
The functions of ERα and ERβ have been studied extensively in cells and in ERα- and ERβ-knockout mice, and by using ER subtype-selective ligands, many of which have been provided to the research community through our work, as well as through research programs at pharmaceutical laboratories, Wyeth, Merck, Lilly, and Schering. As a transcription factor, ERβ generally has lower activity than ERα, and when both are present in cells, ERβ overall appears to have a moderating effect on the activity of ERα. In terms of estrogen-driven proliferation, ERα and ERβ have been characterized as being in a “yin-yang relationship”, with ERα playing the role of a proliferative “accelerator” (the yang) and ERβ that of a proliferative “brake” on ERα action (the yin).
Normal human breast contains both ERβ and ERα, but ERβ levels decline in cancerous breast tissue with the progressive development of increased tumor grade and malignant character,whereas ERα levels are higher in cancerous vs. normal breast. Low ERβ protein levels correlate with poorer prognosis. Notably, however, ERβ protein levels do not correlate at all well with ERβ mRNA levels; also, the accuracy of ERβ immunoassays is compromised by several splice variant forms of ERβ, namely ERβ2, ERβcx, and ERβΔ5, that are detected by ERβ antibodies but do not bind ligand. Only the long form of ERβ, now termed ERβ1, binds estrogens, and it is the level of this ligand-binding form of ERβ that correlates best with a favorable outcome on tamoxifen therapy. In fact, recent work suggests that high ERβ1 levels are predictive of the success of endocrine therapy, even beyond that of ERα, and that ERβ might also account for the small fraction of breast cancer patientsthat respond to tamoxifen, despite being ERα negative (based on the results of standard immunoassays that detect only ERα). Therefore, the independent measurement of the levels of the active, ligand-binding forms of both ERα and ERβ proteins in breast tumors by positron-emission tomography (PET) using ER subtype-specific ligands might lead to a significant improvement in the selection of patients for targeted endocrine therapy.Our investigation in the area of ERβ-selective PET probes is built upon our prior work on the development of ERβ-selective ligands, as well as on that by others, and we have been guided by an evolving understanding of the ERβpharmacophore. Nevertheless, we find that there are substantial challenges in the development of radiopharmaceuticals suitable for imaging ERβ by PET.
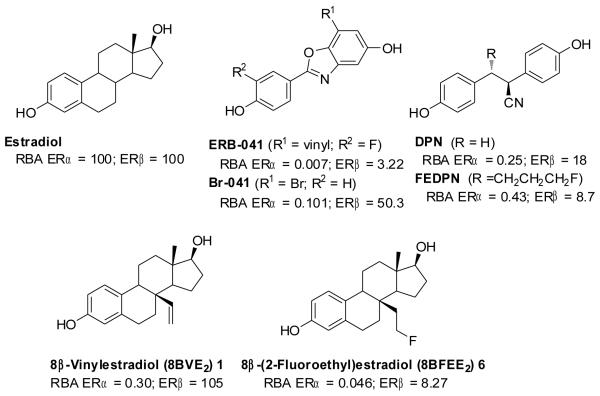
In prior work, we investigated the binding, fluorine-18 labeling, and biodistribution properties of the first ligand designed as an ERβ PET imaging agent, a non-steroidal ERβ-selective ligand, FEDPN, an analog of DPN, that ultimately proved to have limited potential for PET imaging of ERβ (Figure 1). We also previously investigated methods suitable for labeling, with bromine-76, a second non-steroidal ERβ ligand, Br-041, an analog of ERB-041, prepared by Wyeth. Here, we describe the synthesis, receptor binding and radiolabeling of 8β-(2-fluoroethyl)estradiol (8BFEE2)([18F]6), an analog of a structurally novel steroidal estrogen, 8β-vinyl estradiol (8BVE 2), recently reported by the Schering Company as an ERβ-selective ligand (Figure 1). We also report the biodistribution of both [18F]8BFEE2 and [76Br]Br-041(whose synthesis has been previously published), and we compare the two ERβ-selective ligands as PET imaging agents and discuss approaches that might facilitate future efforts to develop ERα- and ERβ-selective PET imaging agents.
Figure 1.
Structure of estradiol, and steroidal and non-steroidal ligands for the estrogen receptors ERα and ERβ.
Results
Synthesis of 8β-(2-Fluoroethyl)estradiol (8BFEE2, 6)
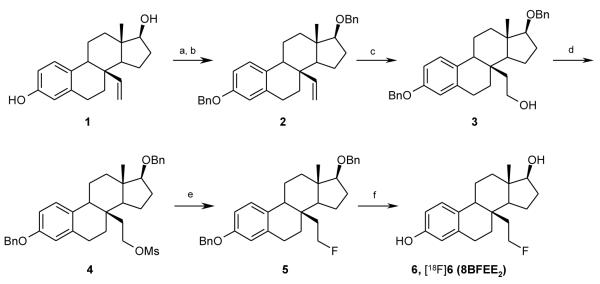
8β-Vinylestradiol (1) was first reacted with potassium carbonate, tetrabutylammonium iodide (TBAI), and benzylbromide in acetone, followed by benzylation at the 17β-hydroxyl group with sodium hydride, TBAI, and benzylbromide to give the 3,17β-bis-benzylether 2 (Scheme 1). Olefin2 was hydroborated and afterwards oxidized with hydrogenperoxide to give the bis-benzylatedtriol3. This material was reacted with methansulfonyl chloride in the presence of triethylamine to give mesylated precursor compound 4 in high yield (91%). To effect the fluorination reaction, we first tried classical methods, such as tetrabutylammonium fluoride (TBAF) or CsF in CH3CN or protic solvents in 130 °C for 2h. However, we obtained neither the fluorinated estradiol nor other by-products, recovering only the mesylate precursor in quantitative yield, thus indicating that it is stable but also quite unreactive and might require harsh conditions to achieve fluoride substitution.
Scheme 1.
aReaction conditions and reagents: (a) K2CO3, BnBr, TBAI, acetone, refl., 68%; (b) NaH, BnBr, TBAI, DMF, −10 °C – RT; (c) BH3•SMe2 in THF, 55 °C, then NaOH/H2O2, 0 °C, 44%; (d) MsCl, TEA, CH2Cl2, 0 °C-RT, 2 h, 91%; (e) n-Bu4NF•3H2O, 150 °C, 30 min, 77%; (f) Pd/C, H2, rt, 16 h, 90%. Radiolabeling conditions: (e) n-Bu4N[18F]F, TBAOMs, 180 °C, 30 min; (f) TMSI, CH3CN, 130 °C, 5 min.
We investigated more vigorous conditions, trying to effect fluoride displacement in neat TBAF, which without solvent behaves as a molten salt that is stable to quite high temperatures. Treatment of precursor 4 with TBAF at 150 °C for 30 min gave the fluorine-substituted estradiol 5 in high yield (77%). The final fluoroethylestradiol product 6 was obtained by cleavage of the two benzyl protecting groups by hydrogenolysis over palladium on charcoal in high yield (90%).After these transformation and purification, no material corresponding to the elimination product, 8β-vinyl-estradiol (1), was observed by 1H-NMR and HPLC analysis.
Estrogen Receptor Binding Affinity and Selectivity of 8β-(2-Fluroroethyl)estradiol (6)
The binding affinity of 8BFFE2 (6) for human ERα and ERβ was determined by a competitive radiometric binding assay with [3H]estradiol as a radio tracer. The binding affinity from competitive binding assay are expressed as relative binding affinity (RBA) values, with estradiol as standard set to RBA =100. Estradiol binds to ERα with Kd of 0.2 nM and ERβ with Kd of 0.5 nM. RBA values are given in Table 1.
Table 1.
Relative Binding Affinity (RBA) of 8β-Fluroroethyl Estradiol for ERα and ERβ.a
| ERα | ERβ | β/α ratio | |
|---|---|---|---|
| 1 (8BVE2) b | 0.30 | 105 | 350 |
| 6 (8BFEE2) | 0.046 ± 0.005 | 8.27 ± 0.98 | 179 |
| DPN | 0.25 ± 0.15 | 18.0 ± 2.0 | 72 |
| FEDPN | 0.43 ± 0.09 | 8.74 ± 1.87 | 20 |
| ERB-041 | 0.007 ± 0.001 | 3.22 ± 0.91 | 460 |
| Br-041 | 0.101 ± 0.02 | 50.3 ± 3.4 | 498 |
| estradiol | [100] | [100] | [1] |
Relative binding affinity (RBA) values of ERα and ERβ in this study were determined in a competitive radiometric binding assay, Estradiol = 100%. Values are expressed as percentages relative to the affinity of the indicated tritium-labeled estradiol as a radio tracer.
The binding affinity values for 1(8BVE2) are comparable to those reported previously for this compound .
The parent ERβ-selective ligand 8β-vinylestradiol (8BVE2) has an excellent affinity for ERβ and a poor affinity for ERα, giving it a β/α selectivity ratio of 350. The fluoroethyl analog (8BFEE2) had about a 10-fold lower affinity on both receptors, giving it a β/α selectivity ratio that is not much less than that of the parent compound. The lower ERβ binding affinity of 8BFEE2, however, was disappointing.
For comparison, we have shown in Table 1 the binding affinities of two other ERβ-selective ligands, DPN and ERB-041, as well as their analogs for radiolabeling, FEDPN and Br-041. The ERβ binding affinity of 8BFEE2is comparable to that of FEDPN. In prior work, we had prepared Br-041 in bromine-76 labeled form. In our hands, it has an ERβ selectivity equivalent to that of the parent compound, ERB-041, but a 16-fold higher affinity for ERβ.
Synthesis of 8β-[18F]Fluoroethylestradiol [18F]4
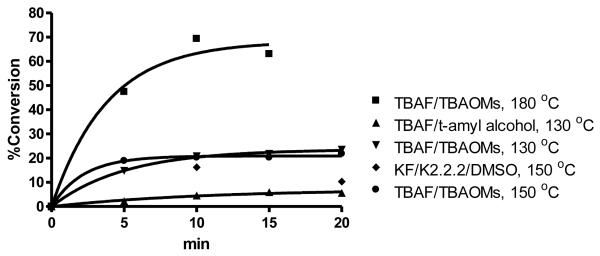
Initially, for radiolabeling, we followed the reaction conditionsof 150 °C for 30 min,as we had done in the preparation of authentic unlabeled compound 6. However, the yield of F-18 incorporation with the limited amount of n-Bu4N[18F]F was poor, below 20%. Even the use of TBAOMs as a molten salt solvent under these conditions did not improve the yield substantially. We then examined a number of other conditions typically used for fluorine-18 radiolabeling(Figure 2). The use of Kyptofix with K2CO3 at 130 °C gave only a low yield (13% at 20 min), and the use of the tertiary alcohol protic solvent system, which in many cases is effective in reducing side product formation through elimination or hydrolysis, gave even a lower yields (6%) .
Figure 2.
Optimization of conditions for radiofluorination
Because of the low reactivity but inherent stability of the precursor 4 that we had noted earlier, we simply used higher reaction temperature conditions with n-Bu4N[18F]F and TBAOMs as solvent. While yields of the [18F]5 were ca. 25% at 130 and 150 °C, by increasing the reaction temperature to 180 °C, we obtained the radiolabeled product in good yield (63%) within 15 min.
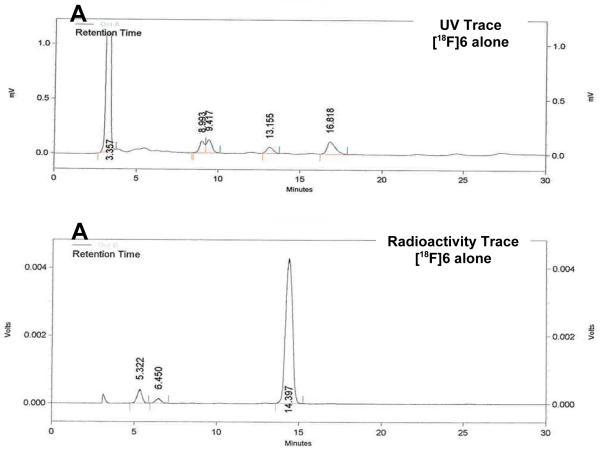
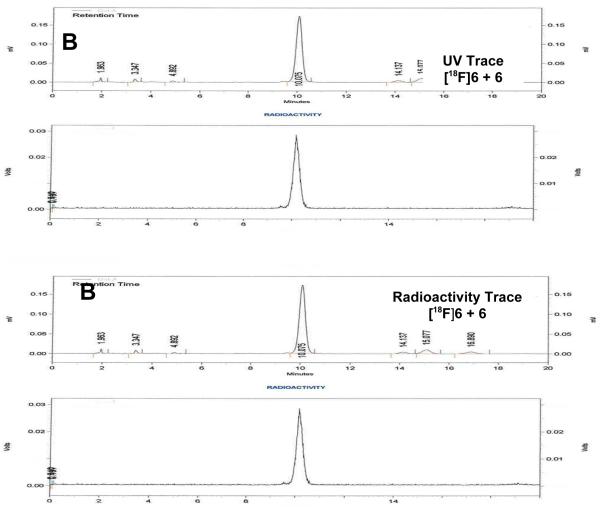
Based on these optimized reaction conditions, 8β-(2-[18F]fluoroethyl)estradiol ([18F]6) could be prepared by treatment of the methanesulfonate precursor 4 with n-Bu4N[18F]F in n-BuNOMs at 180 °C for 30 min to provide the F-18 labeled intermediate, followed by adding trimethylsilyl iodide (TMSI) at 130 °C for 5 min to remove the benzyl groups. The total radiochemical yield of F-18 labeled 8β-fluoroethylestradiol [18F]6 was 47% (decay corrected); the overall synthesis and purification time was approximately 90 min from end of bombardment (EOB), and the radiochemical purity of [18F]6 was shown to be over 99% by reversed phase HPLC, with essentially no coeluting impurities (Figure 3A). When a sample of authentic 6 was co-injected, [18F]6coeluted with the authentic material (Figure 3B). The effective specific activity of [18F]6 was 5.29 GBq/μmol (143 mCi/μmol, n = 4), which is somewhat lower than typical, but still sufficient for in vivo biodistribution studies.Again, no material corresponding to the elimination product, 8β-vinyl-estradiol (1), was observed to coelute with [18F]6by HPLC analysis.
Figure 3.
UV and Radiometric HPLC Profiles of [18F]6. HPLC conditions: (A) Radioactive [18F]6 alone: Altima silica Semi-Prep 250 mm × 10 mm, 10 μm (5% IPA/CH2CI2)/Hexane 40%:60%, 5.0 mL/min 275 nm, Rt = 14-15 min; (B) Radioactive [18F]6 co-injected with standard 6: Econosil, C18, 10 μm, 250 × 4.6 mm, CH3CN/water 50:50, 1.0 mL/min, 275 nm. Upper traces are UV, lower traces are radioactivity.
Tissue Distribution Studies with 8β-(2-[18F]Fluoroethyl)estradiol [18F]6 in Immature Female Sprague-Dawley Rats
In preliminary studies, we evaluated the tissue distribution of 8β-[18F]fluoroethylestradiol [18F]6 in female C57BL/6 mice using the same experimental design as described below for immature female rats. In this study (data not shown), the tissue distribution showed elevated lung uptake, suggesting that the ligand might have poor solubility; otherwise the distribution was unremarkable. We continued our studies with the more traditional animal model for the biodistribution of estrogens, immature female Sprague-Dawleyrats, paying greater attention to ensuring complete solubility of the agent.
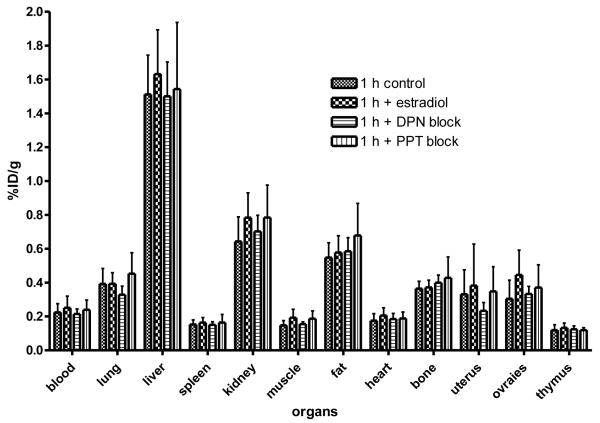
[18F]6, purified by HPLC, was reconstituted in 10% EtOH/saline. To further ensure the solubility of [18F]6 in these experiments in rats, we added 5% β-cyclodextrin. This resulting solution was injected (i.e., tail vein) at a radiotracer dose of 13 μCi/rat. The biodistribution was determined at 1 h postinjection as percent injected dose/gram (%ID/g) (Table 2, Figure 4). To investigate whether uptake was mediated by high-affinity binding to either ERα or ERβ, three sets of rats were coinjected with the [18F]6 and 20 μg of estradiol (E2, would block both ERα and ERβ), 200 μg of DPN (a selective ligand to block only ERβ) or 200μg of PPT (a selective ligand to block only ERα).
Table 2.
Biodistribution of 8β-(2-[18F]Fluoroethyl)estradiol [18F]6in Immature Female Sprague Dawley Rats
| percentage injected dose/gram ±SDa (n = 5) |
||||
|---|---|---|---|---|
| 1 h | 1 h (E2 blocked)b |
1 h (DPN blocked)c |
1 h (PPT blocked)d |
|
| blood | 0.22 ± 0.05 | 0.25 ± 0.07 | 0.22 ± 0.03 | 0.24 ± 0.06 |
| lung | 0.39 ± 0.09 | 0.39 ± 0.06 | 0.33 ± 0.05 | 0.45 ± 0.12 |
| liver | 1.52 ± 0.23 | 1.63 ± 0.26 | 1.50 ± 0.20 | 1.54 ± 0.39 |
| spleen | 0.15 ± 0.03 | 0.16 ± 0.03 | 0.15 ± 0.02 | 0.16 ± 0.05 |
| kidney | 0.64 ± 0.14 | 0.78 ± 0.15 | 0.70 ± 0.10 | 0.78 ± 0.19 |
| muscle | 0.15 ± 0.03 | 0.19 ± 0.05 | 0.15 ± 0.01 | 0.19 ± 0.05 |
| fat | 0.55 ± 0.09 | 0.58 ± 0.10 | 0.58 ± 0.08 | 0.68 ± 0.19 |
| heart | 0.18 ± 0.04 | 0.21 ± 0.04 | 0.18 ± 0.03 | 0.19 ± 0.04 |
| bone | 0.36 ± 0.04 | 0.37 ± 0.04 | 0.40 ± 0.04 | 0.43 ± 0.12 |
| uterus | 0.33 ± 0.14 | 0.38 ± 0.25 | 0.23 ± 0.05 | 0.35 ± 0.15 |
| ovaries | 0.30 ± 0.11 | 0.44 ± 0.15 | 0.33 ± 0.04 | 0.37 ± 0.13 |
| thymus | 0.12 ± 0.03 | 0.13 ± 0.03 | 0.12 ± 0.02 | 0.12 ± 0.02 |
SD is standard deviation. Rats were injected via the tail vein with 13 μCi of [18F]6.
Dose includes estradiol (20 μg).
Dose includes DPN (200 μg).
Dose includes PPT (200 μg).
Figure 4.
Biodistribution of 8β-(2-[18F]Fluoroethyl)estradiol [18F]6in Immature Female Sprague Dawley Rats. The organ most rich in ERα is the uterus, and the organs most rich in ERβ are the ovaries. Uptake of [18F]6 in these organs was low and was not effectively blocked by estradiol (would block uptake by both ERα and ERβ), by DPN (an ERβ-selective ligand that would block uptake by ERβ) and PPT (an ERα-selective ligand that would block uptake by ERα).
ERβ is rich in the granulosa cells of the ovary, and in normal female animals the ovary is considered a principal target tissue for ERβ. Uptake of [18F]6 in the ovary was as high as in the uterus, which has only low levels of ERβ, and was higher than that in non-target tissues, muscle, heart, thymus, and spleen.Uptake infat was somewhat higher, and kidney and liver even higher. However, [18F]6 uptake in the ovary was not notably high, nor was it substantially blocked by any of the three agents, estradiol, DPN or PPT; thus, ovarian uptake does not appear to be ERβ mediated. Uptake of [18F]6 in the lung was as high as in the ovaries, and while lung is a tissue that contains more ERβ than ERα, this uptake was again not blocked by any unlabeled ligand. The somewhat elevated uptake in liver and kidney is typical for lipophilic agents such as steroids that are eliminated by both hepatobiliary and renal routes.
Tissue Distribution Studies with 8β-(2-[18F]Fluoroethyl)estradiol [18F]6 in Immature Female Sprague-Dawley Rats with Hormonal Manipulations
While the ovary is considered to be an ERβ target tissue, ERβ is found principally in granulosa cells of the follicle, which in immature animals form only a small percentage of ovarian mass. In addition, because the ovaries are the major estrogen biosynthetic tissue, ER in the ovary might be saturated by endogenous production of estradiol, although this is not likely to be a problem in these immature animals. In an effort to improve the characteristics of the ovary as an ERβ target through pharmaceutical and hormonal manipulation, immature female Sprague-Dawley rats were treated with Letrozole, an aromatase inhibitor, to lower estradiol production (10 μg per day for three days), and then with pregnant mare serum gonadotropin (PMSG) at a dose (5 IU) that is well known to induce massive folliculogenesis and expansion of the granulosa cell population after 48 hours .
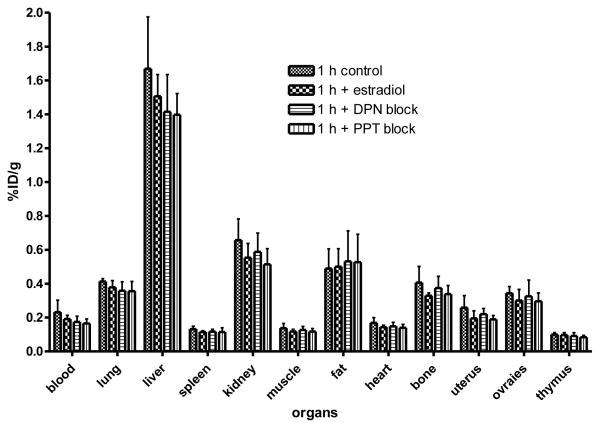
Animals hormonally prepared in the above manner, were injected with [18F]6 at 18 μCi/animal, and biodistribution was determined at 1 h post injection and expressed as percent injected dose/gram (Figure 5, Table 3). As before, some animals were blocked with 20 μg of estradiol or with 200 μg of DPN or PPT. Despite these hormonal manipulations, the biodistribution of [18F]6 was not significantly different from that of untreated immature female rats (Table 3, Figure 4) (See Discussion).
Figure 5.
Biodistribution of 8β-(2-[18F]Fluoroethyl)estradiol [18F]6in Immature Female Sprague Dawley Rats with Letrozole and PMSG treatments.The organ most rich in ERα is the uterus, and the organs most rich in ERβ are the ovaries. Uptake of [18F]6 in these organs was low and was not effectively blocked by estradiol (would block uptake by both ERα and ERβ), by DPN (an ERβ-selective ligand that would block uptake by ERβ) and PPT (an ERα-selective ligand that would block uptake by ERα).
Table 3.
Biodistribution of 8β-(2-[18F]Fluoroethyl)estradiol [18F]6in Immature Female Sprague Dawley Rats with Letrozole and PMSG treatments.
| percentage injected dose/gram ±SDa (n = 5) |
||||
|---|---|---|---|---|
| 1 h | 1 h (E2 blocked)b |
1 h (DPN blocked)c |
1 h (PPT blocked)d |
|
| blood | 0.23 ± 0.07 | 0.19 ± 0.02 | 0.17 ± 0.03 | 0.16 ± 0.03 |
| lung | 0.41 ± 0.02 | 0.38 ± 0.04 | 0.36 ± 0.05 | 0.36 ± 0.06 |
| liver | 1.67 ± 0.31 | 1.51 ± 0.13 | 1.41 ± 0.22 | 1.40 ± 0.12 |
| spleen | 0.13 ± 0.16 | 0.12 ± 0.07 | 0.12 ± 0.01 | 0.12 ± 0.02 |
| kidney | 0.66 ± 0.12 | 0.55 ± 0.08 | 0.59 ± 0.11 | 0.51 ± 0.09 |
| muscle | 0.14 ± 0.03 | 0.12 ± 0.01 | 0.13 ± 0.02 | 0.12 ± 0.02 |
| fat | 0.49 ± 0.12 | 0.50 ± 0.11 | 0.53 ± 0.18 | 0.53 ± 0.16 |
| heart | 0.17 ± 0.03 | 0.14 ± 0.01 | 0.15 ± 0.02 | 0.14 ± 0.02 |
| bone | 0.40 ± 0.10 | 0.33 ± 0.02 | 0.37 ± 0.07 | 0.34 ± 0.05 |
| uterus | 0.26 ± 0.07 | 0.20 ± 0.04 | 0.22 ± 0.03 | 0.19 ± 0.02 |
| ovaries | 0.34 ± 0.04 | 0.30 ± 0.06 | 0.32 ± 0.10 | 0.30 ± 0.05 |
| thymus | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.02 | 0.08 ± 0.01 |
SD is standard deviation. Rats were injected via the tail vein with 18 μCi of [18F]6.
Dose includes estradiol (20 μg).
Dose includes DPN (200 μg).
Dose includes PPT (200 μg).
Tissue Distribution Studies with Another ERβ-Selective Estrogen, Br-041, in Immature Female Mice
In earlier work, we developed the radiochemistry methods to label Br-041, a non-steroidal ligand having high affinity and selectivity for ERβ, with bromine-76. This compound is a close analog of ERB-041, which has been quite extensively studied as an ERβ-selective ligand. Both ERB-041 and Br-041 (Figure 1) were prepared by the Wyeth company. The radiobromination of Br-041 required considerable investigation of the nature of the active bromination species, minimization of chemically reactive interfering substances, etc., but eventually an effective protocol for the preparation of 76Br-041 was developed.
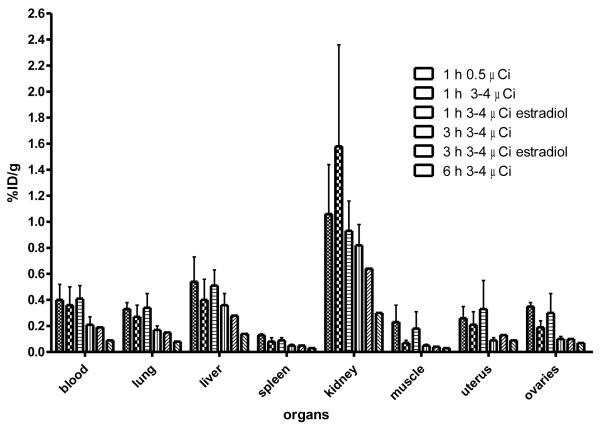
As part of this current study, we investigated the biodistribution of 76Br-041 in immature mice using the same protocol as that used for [18F]6 (Table 2, Figure 4). The results, given in Table 4 and Figure 6, again show no attributes expected for uptake mediated by ERβ. Uptake in all tissues studied at 1 and 3 hours was relatively low, and there was no evidence of blocking uptake in any tissue by estradiol; uptake at later times is very low. Distributions of a low and a high dose of 76Br-041 at 1 hour are essentially the same, indicating that effective specific activity is not limiting target tissue uptake. (See Discussion for further considerations).
Table 4.
Biodistribution of [76Br]Br-ERB-041in Immature Female Mice
| percentage injected dose/gram ±SDa (n = 3) |
||||||
|---|---|---|---|---|---|---|
| 1 h (low dose)b |
1 h (high dose)b |
1 h (high dose, E2blocked)b,c |
3 h (high dose)b |
3 h (high dose, E2blocked)b,c |
6 h (high dose) b |
|
| blood | 0.40 ± 0.12 | 0.36 ± 0.14 | 0.41 ± 0.10 | 0.21 ± 0.06 | 0.19 ± 0.08 | 0.09 ± 0.01 |
| lung | 0.33 ± 0.05 | 0.27 ± 0.09 | 0.34 ± 0.11 | 0.17 ± 0.03 | 0.15 ± 0.05 | 0.08 ± 0.01 |
| liver | 0.54 ± 0.19 | 0.40 ± 0.16 | 0.51 ± 0.12 | 0.36 ± 0.09 | 0.28 ± 0.10 | 0.14 ± 0.02 |
| spleen | 0.13 ± 0.01 | 0.08 ± 0.03 | 0.09 ± 0.02 | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.03 ± 0.00 |
| kidney | 1.06 ± 0.38 | 1.58 ± 0.78 | 0.93 ± 0.23 | 0.82 ± 0.16 | 0.64 ± 0.25 | 0.30 ± 0.12 |
| muscle | 0.23 ± 0.13 | 0.07 ± 0.02 | 0.18 ± 0.13 | 0.05 ± 0.01 | 0.04 ± 0.02 | 0.03 ± 0.01 |
| uterus | 0.26 ± 0.09 | 0.21 ± 0.10 | 0.33 ± 0.22 | 0.09 ± 0.02 | 0.13 ± 0.02 | 0.09 ± 0.03 |
| ovaries | 0.35 ± 0.03 | 0.19 ± 0.05 | 0.30 ± 0.15 | 0.10 ± 0.02 | 0.10 ± 0.04 | 0.07 ± 0.02 |
SD is standard deviation.;
Mice were injected via the tail vein with 0.5 μCi (low dose) or 3-4 μCi (highdose).;
Dose includes estradiol (E2, 20 μg).
Figure 6.
Biodistribution of [76Br]Br-ERB-041in Immature Female Mice.The organ most rich in ERα is the uterus, and the organs most rich in ERβ are the ovaries. Uptake of [76Br]Br-ERB-041in these organs was low and was not at all blocked by estradiol (would block uptake by both ERα and ERβ).
Discussion
Interest and Challenges in Imaging Estrogen Receptor β
Our efforts to develop a PET agent for imaging ERβ are driven by the general interest in measuring ER levels in breast cancer, and possibly other cancers as well. Measurement of ERα, considered to be the pro-proliferative ER subtype, can be done effectively using the well-established PET imaging agent, 16α-[18F]fluoroestradiolFES, a steroidal ligand having a distinct binding preference for ERα. The generally higher levels of ERα also help to enforce the ERα specificity of FES PET images, and in several studies, FES-PET images in breast cancer were shown to have predictive value for response to endocrine therapies. ERβ, the anti-proliferative ER subtype, is also found in breast tissue, with ERβ levels being highest in normal breast and in breast cancer, but declining with the progressive development of malignant character, a change that mirrors the general increase in ERα levels. Therefore, independent measurement of both ERα and ERβ levels in breast tumors might provide a more definitive way of predicting disease outcome and response to endocrine therapies. ERβ may also play a role in defining the stage of other cancers, such as those of the prostate and lung.
While immunohistochemical (IHC) assays have become the standard method for measuring ERα levels, immunological assays of ERβ by IHC is complicated by the existence of varied isoforms of ERβwhose functional activities are often different and whose presence is detected to different degrees by various antibodies. Thus, measurement of ERβ activity, that is, in the form of its capacity to bind a ligand rather than its antigenic behavior, might provide a more functionally relevant measure of ERβ in tumors. It might be particularly beneficial if this could be done by PET imaging, which is non-invasive and can be done repeatedly to monitor disease progression or response to therapies.
Determination of ERβ levels by PET, however, presents challenges that are both inherent and operational. The inherent challenge is that, overall, ERβ levels are less to much less than those of ERα; this factor demands ligands of exceptionally high affinity and high specificity to both observe ERβ by PET and to distinguish it from ERα. The operational challenge is that in normal experimental animals, there is no tissue that is rich in ERβand thus able to serve as a strong positive control target tissue for the uptake of ERβ PET imaging agents; for ERα PET imaging agents, the uterus nicely fulfills this role by being highly enriched in ERα.
Current Status of Development of PET Imaging Agents for Estrogen Receptor β
Thus far, we have examined three different ERβ-selective ligands for their potential as PET imaging agents for ERβ (Figure 1). FEDPN is an analog of DPN, one of the first ERβ-selective ligands to be described. There was no obvious site at which DPN could be conveniently labeled with fluorine-18 by currently available radiofluorination methodology; so, we examined analogs into which fluorine could be introduced by standard nucleophilic substitution reactions . Attachment of a fluoroethyl group, which afforded this option, had the consequence of lowering somewhat both the ERβ affinity and selectivity from that of the parent compound, and in extensive studies we did with both normal rats and mice, and ERα and ERβ knockout mice, we obtained only hints that the tissue uptake of FEDPN was being mediated by ERβ.
In this study, we have studied two additional agents as PET imaging agents for ERβ. 8β-Vinylestradiol (8BVE2), which has both high affinity and selectivity for ERβ, is unique among ERβ selective ligands by having a steroidal structure. While there are several sites at which fluorine-18 might be introduced into 8BVE2, we did not elect to use the 16α position, the site where FES is radiolabeled, because this substitution enhances affinity for ERα. Thus, we chose to replace the 8β-vinyl group with a 2-fluoroethyl substituent. While this substitution preserved ERβ binding selectivity, it did reduce binding affinity to some extent, so that 8BFEE2 bound to ERβ with an affinity similar to that of FEDPN. Although it required rather harsh conditions to achieve radiofluorination, we were able to obtain [18F]8BFEE2 in very good radiochemical yield. Again, our extensive investigations of the biodistribution of this agent in mice and rats failed to provide unambiguous evidence for tissue uptake mediated by ERβ.
Our third investigation centered on Br-041, a close analog of ERB-041, the ERβ-selective ligand that underwent intensive preclinical investigation by the Wyeth Company and even began clinical trials. Although Br-041 was described in the initial papers on the medicinal chemical development of ERB-041, it was not selected for development because its ERβ selectivity was somewhat less than that of ERB-041. Notably, however, its measured (Table 1) and reported affinity for ERβ is actually greater than that of ERB-041. While ERB-041 contains a fluorine substituent and could in principle be labeled by isotopic substitution, this would require preparation of an ortho-[18F]fluorophenol, which generally entails a multistep process for labeling with [18F]fluoride ion, the isotope precursor required to achieve the high specific activity needed for receptor imaging.
We were attracted to Br-041 because it appeared possible for it to be radiolabeled by isotopic substitution at the site of bromine substitution using electrophilic bromination methods on a tin precursor. While considerable optimization was required to accomplish this, we eventually succeeded and published this methodology some time ago. In the studies presented here, we found, as we did with the other two agents, that the biodistribution of Br-041 did not provide evidence of ERβ-mediated uptake, despite its very favorable binding affinity profile (Table 1).
Future Prospects for the Development of Estrogen Receptor β PET Imaging Agents
The major operational challenge that we have encountered in the development of PET agents for imaging ERβ is the lack of a suitable target tissue that can serve as a positive control site for assessing ERβ-mediated uptake in biodistribution or microPET imaging studies. In this respect, the use of ERα or ERβ knockout animals is not useful, because although ERβ can be genetically deleted, if there is no selective, ERβ-mediated uptake in any target tissue, the deletion of ERβ will not result in any observable reduction in uptake. Deletion of ERα, by contrast, has a marked effect on the uptake of an ERα-selective imaging agent, such as FES, by the ERα-rich uterus, as we demonstrated in a previous study.
Some tissues, such as ovary, prostate, lung, and certain brain regions, are considered to be “rich in ERβ”. Measurements of the ERs, however, are now typically done at the mRNA level or by Western blot immunoassay of the protein, without absolute quantification of the ligand binding capacity of the ERβ. Thus, in practice, “rich” means “much more ERβ than ERα”, rather than a high absolute level of ERβ. By contrast, target tissue concentrations of ERα were established before the ERs were sequenced, and of necessity, receptor levels were determined by radioligand binding assays from which high absolute levels of ERα could be established directly .
Because ERβ is rich only in the granulosa cell component of ovarian follicles, which constitutes just a small percent by weight of the ovaries of immature rats, we attempted to enrich the granulosa cell complement of the ovaries by inducing folliculogenesis with a 2-day treatment with PMSG. This was done after suppression of ovarian steroidogenesis with Letrozole, to lower possible endogenous production of estradiol ; it is known that the expansion of the granulosa cell complement of the ovary by PMSG is not blocked by Letrozole treatment. In the one case where we examined it, this hormonal treatment failed to improve the level or selectivity of the uptake of 8BFEE2 by the ovary. Notably, we subsequently found (M. Jeyakumar, unpublished) and learned through further analysis of the literature that while PMSG treatment greatly expands the granulosa cell content of the ovary, it does not increase the level of ERβ and can actually decrease it ! Thus, other approaches to the development of ERβ-positive control target tissues for characterizing the potential of ERβ-selective ligands for PET imaging of this ER subtype are needed.
An intriguing and potentially powerful approach that is just emerging is the development of ER-negative human breast cancer cell lines that are engineered to express either ERα or ERβ under the control of a tetracycline-regulated promoter. The expression of the ERs in a regulated manner is important, because expression of ERα and particularly ERβ in ER-negative cells is known to suppress their growth. If these human breast cancer cells engineered for the regulated expression of ERα or ERβ could be grown as robust xenografts in athymic or other immunocompromised mice, then the biodistribution of both radiolabeled ERα and ERβ-selective ligands could be studied directly. Mediation of this uptake by the ER subtypes could be verified by comparing uptake before and after induction of ER expression by tetracycline, and any selective uptake by a xenograft could be challenged with blockage by estradiol (for both ERs), PPT (for ERα only) or DPN (for ERβ only). As good versions of these new, genetically engineered xenograft systems become available, we intend to use them to further examine the development of PET imaging agents for ERβ.
Conclusion
We have prepared in fluorine-18 labeled form 8BFEE26, an analog of an ERβ-selective steroidal estrogen, and we have examined the biodistribution in immature female rodents of this compound, as well as Br-041, an analog of a non-steroidal ERβ-selective ligand (ERB-041), labeled with bromine-76. Little evidence of ERβ-mediated uptake was observed with either radiopharmaceutical, and our attempts to increase the ERβ content of target tissues by hormonal manipulation did not result in improved biodistribution. These studies have highlighted the need for improved in vivo models for developing ERβ-selective radiopharmaceuticals for use as PET imaging agents to measure ERβ levels in breast tumors. Genetically engineered breast cancer cells that are being developed to express either ERα or ERβ in a regulated manner, grown as xenografts in immune-compromised mice, could prove useful in future efforts to develop ER subtype-selective radiopharmaceuticals.
Experimental Section
General Methods and Materials
All chemical was commercially purchased from Sigma-Aldrich Chemical Company. Flash column chromatography was performed with silica gel (Merck, 230-400 mesh ASTM). Analytical thin layer chromatography (TLC) was performed with Merck silica gel F-254 glass-backed plates. Visualization on TLC was monitored by UV light. 1H and 13C NMR spectra were obtained on Varian Unity 500 (500 MHz) and are reported in parts per million downfield from internal tetramethylsilane. Mass spectra were obtained on 70 eV using the micro-mass 70-VSE mass spectrometer.High performance liquid chromatography (HPLC) was carried out on a Thermo Co. system with a semi-preparative column. H218O was purchased from Rotem Industries. The screw cap test tubes used for fluoride incorporation were purchased from Fisher Scientific (Pyrex no. 9825). [18F]Fluoride ion was produced at Washington University by 18O(p,n)18F reaction through irradiation of 95% enriched [18O]water, using either the JSW BC16/8 cyclotron (The Japan Steel Works Ltd.) or the CS15 cyclotron (The Cyclotron Corp.). Radiochemical purification of [18F]6 utilized a reversed-phase semi-preparative HPLC column (Altima silica Semi-Prep 250 mm × 10 mm, 10 μm (5% IPA/CH2CI2) = 40%:60% Hexane 5.0 mL/min 254 nm). For quality control, the radiochemical purity of [18F]6 was determined by analytical HPLC (Econosil, C18, 10 μm, 250 × 4.6 mm, CH3CN/water = 50:50, 1.0 mL/min, 275 nm). TLC plates were analyzed using a Bioscan, Inc., System 200 imaging scanner. Radioactivity was determined with a dose calibrator. Radiochemical yields are decay-corrected to the beginning of synthesis time.
3,17β-Bis(benzyloxy)-8β-vinyl-estra-1,3,5(10)-triene (2)
8β-Vinyl-estra-1,3,5(10)-triene-3,17β-diol (1) (1.00 g, 3.4 mmol) and (1.85 g,13.4 mmol) potassium carbonate in acetone (20 mL) were stirred at 55 °C for 1 h. After cooling to room temperature acetone (20 ml), benzylbromide(4.0 mL, 33.5 mmol) and tetrabutylammoniumiodide (0.05 g, 0.1 mmol)were added, and the resulting mixture then refluxed for 2 h. The solution was filtered, the filtrate evaporated and the crude material purified by column chromatography on silica gel (n-hexane/ethyl acetate,1:1) to obtain 3-benzyloxy-8β-vinyl-estra-1,3,5(10)-trien-17β-ol(0.89 g, 68%). 1H NMR (300 MHz, CDCl3) δ 0.80 (s, 3 H) 1.20-1.85 (m, 7 H) 1.97 (dt, 1 H) 2.04-2.20 (m, 2 H) 2.23-2.32 (m, 1 H) 2.37-2.46 (m, 1 H) 2.69-2.93 (m, 2 H) 3.62-3.69 (m, 1 H) 4.88 (dd, 1 H) 5.02 (s, 2 H) 5.05 (dd, 1 H) 5.57 (dd, 1 H) 6.68 (d, 1 H) 6.77 (dd, 1 H) 7.18 (d, 1 H) 7.29-7.45 (m, 5 H).
At −10 °C, a solution of 0.88 g (2.3 mmol) 3-benzyloxy-8β-vinyl-estra-1,3,5(10)-trien-17β-ol in dimethylformamide (DMF,10 mL) was added dropwise to sodium hydride (mineral oil dispersion, 60%. 0.91 g, 2.3 mmol)) in DMF (10 mL)and stirred for 1 h. At the same temperature tetrabutylammonium iodide (0.35 g, 1.0 mmol) and benzylbromide (2.7 mL, 22.6 mol) were added, and the mixture then warmed up to room temperature. A sodium hydroxide solution (0.1 N, 20 mL) and water (80 mL)were added before repeated extraction with dichloromethane. The combined organic layers were washed with brine and dried with sodium sulphate. The crude was filtered over a short column of silica gel (ethyl acetate/n-hexane, 1:1) to obtain 3,17β-bis(benzyloxy)-8β-vinyl-estra-1,3,5(10)-triene(2)as raw material (1.60 g, >100%).1H NMR (300 MHz, CDCl3) δ 0.89 (s, 3 H) 1.24-1.87 (m, 7 H) 1.98-2.26 (m, 4 H) 2.40 (dd, 1 H) 2.66-2.75 (m, 1 H) 2.80-2.89 (m, 1 H) 3.39-3.45 (m, 1 H) 4.57 (s, 2 H) 4.87 (dd, 1 H) 5.01 (s, 2 H) 5.05 (dd, 1 H) 5.57 (dd, 1 H) 6.67 (d, 1 H) 6.76 (dd, 1 H) 7.16-7.45 (m, 11 H).
2-[3,17β-Bis(benzyloxy)-estra-1,3,5(10)-trien-8β-yl]-ethanol (3)
To a solution of unpurified 3,17β-bis(benzyloxy)-8β-vinyl-estra-1,3,5(10)-triene (2) (0.76 g,1.1 mmol, calculated on pure material) in THF (10 mL)was added slowly) of borane-dimethyl sulfide complex (2 M in THF, 3.0 ml, 6.0 mmol) and heated to 55 °C for 1 h. After 16 h at room temperature the solution was cooled to 0 °C, then sodium hydroxide solution (2 N, 20 mL)was added, followed by the addition of hydrogen peroxide (30%, 20 mL). The mixture was extracted several times with ethyl acetate, and the combined organic layers were washed with saturated aqueous sodium thiosulfate solution and dried with sodium sulphate. The crude material was purified by column chromatography on silica gel (n-hexane/ethyl acetate, 3:2) to obtain 0.24 g (44%, calculated on pure material) of colorless 2-[3,17β-bis(benzyloxy)-estra-1,3,5(10)-trien-8β-yl]-ethanol (3). 1H NMR (500 MHz, CDCl3) δ1.11 (s, 3H), 1.23-1.30 (m, 1H), 1.30-1.46 (m, 3H), 1.53-1.60 (m, 2H), 1.64-1.74 (m, 2H), 1.84 (ddd, J= 14.0, 11.2, 5.9 Hz, 1H), 1.96-2.05 (m, 2H), 2.11-2.18 (m, 2H), 2.31 (dd, J= 12.4, 2.8 Hz, 1H), 2.77-2.83 (m, 2H), 3.39 (t, J= 8.4 Hz, 1H), 3.44 (td, J= 10.5, 4.7 Hz, 1H), 3.76 (td, J= 10.6, 5.9 Hz, 1H), 4.51-4.61 (m, 2 H), 5.03 (s, 2H), 6.73 (d, J= 2.6 Hz, 1H), 6.76 (dd, J= 8.4, 2.8 Hz, 1H), 7.11 (d, J= 8.4 Hz, 1H), 7.27-7.40 (m, 8H), 7.41-7.45 (m, 2 H).
2-[3,17β-Bis(benzyloxy)-estra-1,3,5(10)-trien-8β-yl]-ethyl methanesulfonate (4)
To a solution of 2-hydroxyethylestradiol (3) (50 mg, 100.7 μmol) in CH2Cl2 (10 mL) was added triethylamine (42.1 μL, 302 μmol) at room temperature with subsequent cooling to 0 °C. Methanesulfonyl chloride (11.7 μL, 151 μmol) was added slowly to the reaction mixture at 0 °C and stirring continued for 2 h. Water (10mL) was added to the reaction mixture, which was then extracted with CH2Cl2 (5 mL × 3). The combined organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography, 30% EtOAc/hexane, to give mesylated compound 4 (53 mg, 91%) as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 1.08 (s, 3H), 1.24-1.43 (m, 2H), 1.44-1.61 (m, 2H), 1.62-1.75 (m, 2H), 1.91-2.08 (m, 3H), 2.11-2.20 (m, 2H), 2.34 (dd, J= 12.2, 2.6 Hz, 1H), 2.73-2.88 (m, 5H), 3.10 (qd, J= 7.3, 4.8 Hz, 2H), 3.39 (t, J= 8.4 Hz, 1H), 3.92 (ddd, J= 11.3, 9.5, 4.7 Hz, 1H), 4.28 (ddd, J= 11.1, 9.6, 6.1 Hz, 1H), 4.52-4.59 (m, 2H), 5.03 (s, 2H), 6.75 (d, J= 2.6 Hz, 1H), 6.78 (dd, J= 8.6, 2.8 Hz, 1H), 7.11 (d, J= 8.2 Hz, 1H), 7.27-7.36 (m, 6H), 7.36-7.41 (m, 2H), 7.41-7.45 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 208.7, 157.0, 139.0, 137.4, 137.2, 131.4, 128.5, 128.3, 127.9, 127.45, 127.35, 127.26, 125.5, 114.8, 112,1, 88.4, 71.5, 69.9, 68.4, 65.4, 55.5, 48.8, 45.8, 43.0, 38.8, 38.3, 37.3, 32.6, 31.4, 28.7, 27.4, 25.7, 21.5, 21.0, 17.7, 13.1, 8.6; MS (FAB) m/z 574.2 (M)+, 479.2, 389.2, 249.2, 165.2, 104.7 (100). HRMS (FAB) calcd for C35H42O5S 574.2753, found 574.2753.
3,17β-Bis(benzyloxy)-8β-(2-fluoroethyl)-estra-1,3,5(10)-triene (5)
The mixture of mesylated compound 4 (9 mg, 15.7 μmol) and tetrabutylaminium fluoride trihydrate (500 mg) was heated at 150 °C (using oil bath) for 30 min. The reaction mixture was cooled to room temperature, diluted with water (5 mL) and extracted with EtOAc (2 mL × 3). The combined organic layer was dried over Na2SO4 and evaporated under reduced pressure. The residue was purified by flash column chromatography, 20% EtOAc/hexane to furnish fluorinated compound 5 (6 mg, 77%) as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 1.08 (s, 3H), 1.24-1.35 (m, 2H), 1.37-1.47 (m, 2H), 1.49-1.61 (m, 2H), 1.62-1.73 (m, 2H), 1.87-1.97 (m, 1H), 1.98-2.04 (m, 2H), 2.10-2.19 (m, 2H), 2.32 (d, J= 12.2 Hz, 1H), 2.72-2.86 (m, 2H), 3.39 (t, J= 8.2 Hz, 1H), 4.17-4.33 (m, 1H), 4.40-4.61 (m, 3H), 5.03 (s, 2H), 6.73 (d, J= 2.6 Hz, 1H), 6.77 (dd, J= 8.5, 2.7 Hz, 1H), 7.11 (d, J= 8.4 Hz, 1H), 7.27-7.36 (m, 6H), 7.36-7.41 (m, 2H), 7.41-7.45 (m, 2H);13C NMR (125 MHz, CDCl3) δ 208.8, 139.1, 137.6, 131.6, 128.6, 128.3, 127.9, 127.5, 127.4, 127.3, 125.5, 114.8, 112.0, 88.6, 82.6 (d, J = 161 Hz, 1C), 71.6, 70.0, 55.7, 53.9, 49.0, 39.0, 33.0, 29.5 (d, J = 19.2 Hz, 1C), 29.2, 27.5, 25.8, 21.2, 21.0, 20.8, 14.1, 13.3; MS (CI) m/z 499 (M+H)+, 479, 451, 391, 287, 181, 119, 91 (100). HRMS calcd for C34H39FO2 499.3006, found 499.3012.
8β-(2-Fluoroethyl)estra-1,3,5(10)-trien-3,17β-diol (6)
The suspension of fluorinated compound 5 (5 mg, 10.027 μmol) and Pd/C (10 mg) in MeOH(10 mL) were stirred under H2 atmosphere at room temperature for 16 h. The reaction mixture was filtrated through Celite, and the filtrate was concentrated by rotary evaporation. The residue was purified by flash column chromatography, 40% EtOAc/hexane to obtain debenzylated compound 6 (3.7 mg, 90%). 1H NMR (500 MHz, CDCl3) δ 0.92 (s, 3H), 1.12-1.25 (m, 2H), 1.31-1.55 (m, 5H), 1.58 (d, J= 17.0 Hz, 1H) 1.61-1.68 (m, 1H), 1.76-1.97 (m, 3H), 1.99-2.06 (m, 1H), 2.07-2.13 (m, 1H), 2.25 (d, J= 12.4 Hz, 1H), 2.63-2.79 (m, 2H), 3.51-3.70 (m, 1H), 4.10-4.26 (m, 1H), 4.31-4.47 (m, 1H), 4.49 (br. s., 1H), 6.51 (d, J= 2.8 Hz, 1H), 6.55 (dd, J= 8.4, 2.8 Hz, 1H), 7.00 (d, J= 8.4 Hz, 1H); MS (CI) m/z 319 (M+H)+, 299, 281, 73, 59. HRMS calcd for C20H27 FO2319.2073, found 319.2073.
General Radiochemical Synthesis of 8β-(2-[18F]Fluoroethyl)estradiol(6)
Fluorine-18 radionuclide was produced by the 18O(p,n)18F reaction on an enriched water target. Oxygen-18 water containing the F-18 anion was transferred to a reaction vessel containing tetrabutylammonium bicarbonate TBAHCO3 (40% in water, 2μL), and CH3CN was added. The water in reaction vessel was removed by azeotropic distillation with CH3CN at 110 °C under a gentle stream of N2. The reaction vessel was cooled to room temperature, and the 8β-(2-methanesulfonyloxyethyl)estradiolsubstrate (4) (2 mg, 3.480 μmol) was added to reaction vessel, followed by addition of n-Bu4NOMs (500 mg). The capped reaction vessel was heated at 160 °C for 30 min and cooled down to room temperature. TMSI (50μL, 351μmol) was added, followed by heating 130 °C for 5 min. The reaction mixture was completely dried at 110 °C under a gentle stream of N2, and the residue was dissolved with 45% of (5% IPA/ CH2Cl2) and 55%of hexane. This resulting solution was purified by normal phase semi-preparative HPLC (5%, IPA/CH2Cl2):hexane = 45:55, 4 mL/min) to obtain the desired product [18F]6, which eluted at 18-21 min (47% decay-corrected radiochemical yield from end of synthesis, in a total synthesis time of approximately 90 min). The identity of the collected material was confirmed by co-injection with authentic compound 6 by reversed phase analytical HPLC (CH3CN:water = 50:50, 1 mL/min). The eluted fractions containing [18F]6 were concentrated under a gentle stream of N2, and the was residue dissolved with 10% EtOH/saline or 10% EtOH/saline (containing 5% β-cyclodextrin) (1.5 mL) for biodistribution studies. The radiochemical purity as determined reverse phase HPLC was greater than 99% in all cases, and effective specific activitywas 5.29 GBq/μmol (143 mCi/μmol, n = 4) .
Estrogen Receptor Binding Affinity Assay
Relative binding affinities were determined by a competitive radiometric binding assay, as previously described, with purified full-length human ERα and ERβ (PanVera/InVitrogen, Carlsbad, CA). Incubations were for 18–24 h at 0 °C, the receptor–ligand complexes were then adsorbed onto hydroxyapatite (BioRad, Hercules, CA), and unbound ligand was washed away. The binding affinities are expressed as relative binding affinity (RBA) values, with the RBA of estradiol set to 100. The values given are the average ±range or SD of two or more independent determinations. Estradiol binds to ERα with a Kd of 0.2 nM and to ERβ with a Kd of 0.5 nM.
Tissue Distribution Studies
In the following experiments, animals were handled in accordance with the Guidelines for the Care and Use of Research Animals established by the Animal Studies Committee at the Washington University School of Medicine. A complete description of the animal handling procedure, including animal care, anesthesia and monitoring,can be found in Sharp et al. . After tracer administration, the animals were allowed to wake up and maintain normal husbandry until euthanasia by cervical dislocation.
For the biodistribution studies of [18F]6 (Table 2, Figure 4), immature female Sprague-Dawley rats (21 days, n = 5 per each blocking agent point) were used. [18F]6 was concentrated under N2 gas, reconstituted in ethanol, and diluted with isotonic saline-5% β-cyclodextrin to obtain 10% ethanol/saline solution. Anesthetized rats under 1.5-2% isoflurane/O2 were injected via tail vein with 13 μCi (100 μL of 10%EtOH/saline) of F-18 labeled radio tracer. One set of rats was also coinjected with 20 μg of estradiol (E2) to block uptake mediated by the estrogen receptor, and two other sets of animals were coinjected with 200 μg (100 μL of 15% EtOH/saline) of DPN or PPT for blocking ERβ or ERα, respectively. At 1 h post-injection, each set of rats was sacrificed. Blood, lung, liver, spleen, left kidney, abdominal muscle, fat from flank area, heart, bone (tibia/fibula), uterus, ovaries, and thymus were removed and placed in a vial for weighing and counted on the Beckmann Gamma counter along with a standard dilution of the injectate. Uptake was calculated as percent injected dose (%ID) per gram.
For the biodistribution study of [18F]6 in Letrozole- and PMSG-treated animals (Table 3, Figure 5), immature female Sprague-Dawley rats (21 days, n = 5 per each blocking agent point) were treated with 10 μg Letrozole in 200 μL saline IV daily for 3 days, with an injection of 5 IU PMSG in 200 μL saline IV on the second day; the biodistribution study was done 48 hours after the PMSG dose, using the same protocol as described above with a dose of 18 μCi of [18F]6.
Biodistribution studies with [76Br]Br-041 used material prepared as described previously, and followed the same protocol used for the biodistribution of [18F]6 but was conducted in female C57BL/6 mice (18-22 g, 6.5 weeks old, n= 4-5 per each of blocking agent point). The injectate (0.5 or 3-4 μCi/animal) was prepared in 10% ethanol/saline without β-cyclodextrin.
Acknowledgment
We are grateful for support of this research from the National Institutes of Health (PHS R01 CA025836).
Abbreviations
- 8BFEE2
8β-(2-fluoroethyl)estradiol, an ERβ-selective ligand
- 8BVE2
8β-vinylestradiol, an ERβ-selective ligand
- Br-041
an analog of ERB-041, an ER β-selective ligand
- ERβ-selective ligand DPN
diarylpropionitrile, an ERβ-selective ligand: E2, estradiol
- ER
estrogen receptor
- ERB-041
an ERβ-selective ligand
- FES
16α-[18F]fluoroestradiol
- PMSG
pregnant mare serum gonadotropin
- PPT
propylpyrazoletriol, an ERα-selective ligand
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- [3].Harris HA. The unexpected science of estrogen receptor-beta selective agonists: a new class of anti-inflammatory agents? Nucl Recept Signal. 2006;4:e012. doi: 10.1621/nrs.04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu. Rev. Physiol. 2001;63:165–92. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- [5].Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of estrogen action. Physiol. Rev. 2001;81:1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- [6].Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr. Rev. 2005;26:465–78. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- [7].Minutolo F, Macchia M, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor beta ligands: recent advances and biomedical applications. Med. Res. Rev. 2011;31:364–442. doi: 10.1002/med.20186. [DOI] [PubMed] [Google Scholar]
- [8].Nilsson S, Koehler KF, Gustafsson JA. Development of subtype-selective oestrogen receptor-based therapeutics. Nat. Rev. Drug Discov. 2011;10:778–92. doi: 10.1038/nrd3551. [DOI] [PubMed] [Google Scholar]
- [9].Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol. Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- [10].Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu. Rev. Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- [11].Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143:4172–7. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- [12].Meyers MJ, Sun J, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor subtype-selective ligands: asymmetric synthesis and biological evaluation of cis- and trans-5,11-dialkyl-5,6,11, 12-tetrahydrochrysenes. J. Med. Chem. 1999;42:2456–68. doi: 10.1021/jm990101b. [DOI] [PubMed] [Google Scholar]
- [13].Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. 2001;44:4230–51. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- [14].Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J. Med. Chem. 2000;43:4934–47. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- [15].Stauffer SR, Huang YR, Aron ZD, Coletta CJ, Sun J, Katzenellenbogen BS, et al. Triarylpyrazoles with basic side chains: development of pyrazole-based estrogen receptor antagonists. Bioorg. Med. Chem. 2001;9:151–61. doi: 10.1016/s0968-0896(00)00226-1. [DOI] [PubMed] [Google Scholar]
- [16].Stauffer SR, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Acyclic amides as estrogen receptor ligands: synthesis, binding, activity and receptor interaction. Bioorg. Med. Chem. 2000;8:1293–316. doi: 10.1016/s0968-0896(00)00075-4. [DOI] [PubMed] [Google Scholar]
- [17].Fink BE, Mortensen DS, Stauffer SR, Aron ZD, Katzenellenbogen JA. Novel structural templates for estrogen-receptor ligands and prospects for combinatorial synthesis of estrogens. Chem. Biol. 1999;6:205–19. doi: 10.1016/S1074-5521(99)80037-4. [DOI] [PubMed] [Google Scholar]
- [18].Ghosh U, Ganessunker D, Sattigeri VJ, Carlson KE, Mortensen DJ, Katzenellenbogen BS, et al. Estrogenic diazenes: heterocyclic non-steroidal estrogens of unusual structure with selectivity for estrogen receptor subtypes. Bioorg. Med. Chem. 2003;11:629–57. doi: 10.1016/s0968-0896(02)00309-7. [DOI] [PubMed] [Google Scholar]
- [19].Mortensen DS, Rodriguez AL, Carlson KE, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Synthesis and biological evaluation of a novel series of furans: ligands selective for estrogen receptor alpha. J Med Chem. 2001;44:3838–48. doi: 10.1021/jm010211u. [DOI] [PubMed] [Google Scholar]
- [20].Mortensen DS, Rodriguez AL, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Furans with basic side chains: synthesis and biological evaluation of a novel series of antagonists with selectivity for the estrogen receptor alpha. Bioorg. Med. Chem. Lett. 2001;11:2521–4. doi: 10.1016/s0960-894x(01)00488-7. [DOI] [PubMed] [Google Scholar]
- [21].Compton DR, Sheng S, Carlson KE, Rebacz NA, Lee IY, Katzenellenbogen BS, et al. Pyrazolo[1,5-a]pyrimidines: estrogen receptor ligands possessing estrogen receptor beta antagonist activity. J. Med. Chem. 2004;47:5872–93. doi: 10.1021/jm049631k. [DOI] [PubMed] [Google Scholar]
- [22].De Angelis M, Stossi F, Carlson KA, Katzenellenbogen BS, Katzenellenbogen JA. Indazole estrogens: highly selective ligands for the estrogen receptor beta. J Med Chem. 2005;48:1132–44. doi: 10.1021/jm049223g. [DOI] [PubMed] [Google Scholar]
- [23].De Angelis M, Stossi F, Waibel M, Katzenellenbogen BS, Katzenellenbogen JA. Isocoumarins as estrogen receptor beta selective ligands: Isomers of isoflavone phytoestrogens and their metabolites. Bioorg. Med. Chem. 2005;13:6529–42. doi: 10.1016/j.bmc.2005.07.014. [DOI] [PubMed] [Google Scholar]
- [24].Zhou HB, Comninos JS, Stossi F, Katzenellenbogen BS, Katzenellenbogen JA. Synthesis and evaluation of estrogen receptor ligands with bridged oxabicyclic cores containing a diarylethylene motif: estrogen antagonists of unusual structure. J. Med. Chem. 2005;48:7261–74. doi: 10.1021/jm0506773. [DOI] [PubMed] [Google Scholar]
- [25].Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD, et al. Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-beta ligands. J. Med. Chem. 2004;47:5021–40. doi: 10.1021/jm049719y. [DOI] [PubMed] [Google Scholar]
- [26].Blizzard TA. Selective estrogen receptor modulator medicinal chemistry at Merck. Curr. Top. Med. Chem. 2008;8:792–812. doi: 10.2174/156802608784535066. A review. [DOI] [PubMed] [Google Scholar]
- [27].Richardson TI, Dodge JA, Wang Y, Durbin JD, Krishnan V, Norman BH. Benzopyrans as selective estrogen receptor beta agonists (SERBAs). Part 5: Combined A- and C-ring structure-activity relationship studies. Bioorg. Med. Chem. Lett. 2007;17:5563–6. doi: 10.1016/j.bmcl.2007.08.009. [DOI] [PubMed] [Google Scholar]
- [28].Fritzemeier KH, Hillisch A, Elger W, Kaufmann U, Kollenkirchen U, Kosemund D, et al. Biological effects of ERalpha- and ERbeta-selective estrogens. Ernst Schering Res Found Workshop. 2004:127–50. [PubMed] [Google Scholar]
- [29].Hegele-Hartung C, Siebel P, Peters O, Kosemund D, Muller G, Hillisch A, et al. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5129–34. doi: 10.1073/pnas.0306720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831–42. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- [31].Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol. Endocrinol. 2003;17:203–8. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- [32].Speirs V, Carder PJ, Lane S, Dodwell D, Lansdown MR, Hanby AM. Oestrogen receptor beta: what it means for patients with breast cancer. Lancet Oncol. 2004;5:174–81. doi: 10.1016/S1470-2045(04)01413-5. [DOI] [PubMed] [Google Scholar]
- [33].Shaaban AM, O’Neill PA, Davies MP, Sibson R, West CR, Smith PH, et al. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am. J. Surg. Pathol. 2003;27:1502–12. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- [34].Balfe P, McCann A, McGoldrick A, McAllister K, Kennedy M, Dervan P, et al. Estrogen receptor alpha and beta profiling in human breast cancer. Eur. J. Surg. Oncol. 2004;30:469–74. doi: 10.1016/j.ejso.2004.02.010. [DOI] [PubMed] [Google Scholar]
- [35].Nakopoulou L, Lazaris AC, Panayotopoulou EG, Giannopoulou I, Givalos N, Markaki S, et al. The favourable prognostic value of oestrogen receptor beta immunohistochemical expression in breast cancer. J. Clin. Pathol. 2004;57:523–8. doi: 10.1136/jcp.2003.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Omoto Y, Kobayashi S, Inoue S, Ogawa S, Toyama T, Yamashita H, et al. Evaluation of oestrogen receptor beta wild-type and variant protein expression, and relationship with clinicopathological factors in breast cancers. Eur. J. Cancer. 2002;38:380–6. doi: 10.1016/s0959-8049(01)00383-5. [DOI] [PubMed] [Google Scholar]
- [37].Palmieri C, Cheng GJ, Saji S, Zelada-Hedman M, Warri A, Weihua Z, et al. Estrogen receptor beta in breast cancer. Endocr Relat Cancer. 2002;9:1–13. doi: 10.1677/erc.0.0090001. [DOI] [PubMed] [Google Scholar]
- [38].Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–41. [PubMed] [Google Scholar]
- [39].Speirs V, Carder PJ, Lansdown MR. Oestrogen receptor beta: how should we measure this? Br. J. Cancer. 2002;87:687. doi: 10.1038/sj.bjc.6600534. discussion 8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Saji S, Hirose M, Toi M. Clinical significance of estrogen receptor beta in breast cancer. Cancer Chemother. Pharmacol. 2005;56(Suppl 1):21–6. doi: 10.1007/s00280-005-0107-3. [DOI] [PubMed] [Google Scholar]
- [41].Esslimani-Sahla M, Kramar A, Simony-Lafontaine J, Warner M, Gustafsson JA, Rochefort H. Increased estrogen receptor betacx expression during mammary carcinogenesis. Clin. Cancer Res. 2005;11:3170–4. doi: 10.1158/1078-0432.CCR-04-2298. [DOI] [PubMed] [Google Scholar]
- [42].Esslimani-Sahla M, Simony-Lafontaine J, Kramar A, Lavaill R, Mollevi C, Warner M, et al. Estrogen receptor beta (ER beta) level but not its ER beta cx variant helps to predict tamoxifen resistance in breast cancer. Clin. Cancer Res. 2004;10:5769–76. doi: 10.1158/1078-0432.CCR-04-0389. [DOI] [PubMed] [Google Scholar]
- [43].Girault I, Andrieu C, Tozlu S, Spyratos F, Bieche I, Lidereau R. Altered expression pattern of alternatively spliced estrogen receptor beta transcripts in breast carcinoma. Cancer Lett. 2004;215:101–12. doi: 10.1016/j.canlet.2004.05.006. [DOI] [PubMed] [Google Scholar]
- [44].Dimolea E, Tiniakos DG, Vassilaros S, Goutas N, Kittas C, Alexis MN. ERb1 contributes to breast cancer response to tamoxifen; International Congress on Hormonal Steroids and Hormones and Cancer 2006;Athens; Greece. September 2006; Abstract 120. [Google Scholar]
- [45].Seo JW, Comninos JS, Chi DY, Kim DW, Carlson KE, Katzenellenbogen JA. Fluorine-Substituted Cyclofenil Derivatives as Estrogen Receptor Ligands: Synthesis and Structure-Affinity Relationship Study of Potential PET Agents for Imaging Estrogen Receptors in Breast Cancer. J. Med. Chem. 2006 doi: 10.1021/jm0512037. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vijaykumar D, Al-Qahtani MH, Welch MJ, Katzenellenbogen JA. Synthesis and biological evaluation of a fluorine-18 labeled estrogen receptor-alpha selective ligand: [F-18] propyl pyrazole triol. Nucl. Med. Biol. 2003;30:397–404. doi: 10.1016/s0969-8051(02)00446-8. [DOI] [PubMed] [Google Scholar]
- [47].Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–9. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- [48].Yoo J, Dence CS, Sharp TL, Katzenellenbogen JA, Welch MJ. Synthesis of an estrogen receptor beta-selective radioligand: 5-[18F]fluoro-(2R,3S)-2,3-bis(4-hydroxyphenyl)pentanenitrile and comparison of in vivo distribution with 16alpha-[18F]fluoro-17beta-estradiol. J. Med. Chem. 2005;48:6366–78. doi: 10.1021/jm050121f. [DOI] [PubMed] [Google Scholar]
- [49].Zhou D, Zhou H, Jenks CC, Lewis JS, Katzenellenbogen JA, Welch MJ. Bromination from the macroscopic level to the tracer radiochemical level: (76)Br radiolabeling of aromatic compounds via electrophilic substitution. Bioconjugate Chem. 2009;20:808–16. doi: 10.1021/bc800313c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hillisch A, Peters O, Kosemund D, Muller G, Walter A, Schneider B, et al. Dissecting physiological roles of estrogen receptor alpha and beta with potent selective ligands from structure-based design. Mol. Endocrinol. 2004;18:1599–609. doi: 10.1210/me.2004-0050. [DOI] [PubMed] [Google Scholar]
- [51].Carlson KE, Choi I, Gee A, Katzenellenbogen BS, Katzenellenbogen JA. Altered ligand binding properties and enhanced stability of a constitutively active estrogen receptor: Evidence that an open pocket conformation is required for ligand interaction. Biochemistry. 1997;36:14897–905. doi: 10.1021/bi971746l. [DOI] [PubMed] [Google Scholar]
- [52].Kim DW, Jeong HJ, Lim ST, Sohn MH, Katzenellenbogen JA, Chi DY. Facile nucleophilic fluorination reactions using tert-alcohols as a reaction medium: significantly enhanced reactivity of alkali metal fluorides and improved selectivity. The Journal of organic chemistry. 2008;73:957–62. doi: 10.1021/jo7021229. [DOI] [PubMed] [Google Scholar]
- [53].Senderoff SG, McElvany KD, Carlson KE, Heiman DF, Katzenellenbogen JA, Welch MJ. Methodology for the synthesis and specific activity determination of 16 alpha-[77Br]-bromoestradiol-17 beta and 16 alpha-[77Br]-11 beta-methoxyestradiol-17 beta, two estrogen receptor-binding radiopharmaceuticals. The International journal of applied radiation and isotopes. 1982;33:545–51. doi: 10.1016/0020-708x(82)90010-2. [DOI] [PubMed] [Google Scholar]
- [54].Kiesewetter DO, Kilbourn MR, Landvatter SW, Heiman DF, Katzenellenbogen JA, Welch MJ. Preparation of four fluorine-18-labeled estrogens and their selective uptakes in target tissues of immature rats. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1984;25:1212–21. [PubMed] [Google Scholar]
- [55].Younes M, Honma N. Estrogen receptor beta. Archives of pathology & laboratory medicine. 2011;135:63–6. doi: 10.5858/2010-0448-RAR.1. [DOI] [PubMed] [Google Scholar]
- [56].Alexiadis M, Eriksson N, Jamieson S, Davis M, Drummond AE, Chu S, et al. Nuclear receptor profiling of ovarian granulosa cell tumors. Hormones & cancer. 2011;2:157–69. doi: 10.1007/s12672-011-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Byers M, Kuiper GG, Gustafsson JA, Park-Sarge OK. Estrogen receptor-beta mRNA expression in rat ovary: down-regulation by gonadotropins. Mol. Endocrinol. 1997;11:172–82. doi: 10.1210/mend.11.2.9887. [DOI] [PubMed] [Google Scholar]
- [58].Fatum M, Gyo Y, Diana P, Laufer N, Simon A. Is estradiol mandatory for an adequate follicular and embryo development? A mouse model using aromatase inhibitor (anastrozole) J. Assist. Reprod. Genet. 2006;23:407–12. doi: 10.1007/s10815-006-9089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, et al. Estrogen Receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol. Endocrinol. 2008;22:1032–43. doi: 10.1210/me.2007-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dehdashti F, Flanagan FL, Mortimer JE, Katzenellenbogen JA, Welch MJ, Siegel BA. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur. J. Nucl. Med. 1999;26:51–6. doi: 10.1007/s002590050359. [DOI] [PubMed] [Google Scholar]
- [61].Dehdashti F, Mortimer JE, Siegel BA, Griffeth LK, Bonasera TJ, Fusselman MJ, et al. Positron tomographic assessment of estrogen receptors in breast cancer: comparison with FDG-PET and in vitro receptor assays. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1995;36:1766–74. [PubMed] [Google Scholar]
- [62].Dehdashti F, Mortimer JE, Trinkaus K, Naughton MJ, Ellis M, Katzenellenbogen JA, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res. Treat. 2009;113:509–17. doi: 10.1007/s10549-008-9953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Linden HM, Kurland BF, Peterson LM, Schubert EK, Gralow JR, Specht JM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:4799–805. doi: 10.1158/1078-0432.CCR-10-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Linden HM, Stekhova SA, Link JM, Gralow JR, Livingston RB, Ellis GK, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:2793–9. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- [65].McGuire AH, Dehdashti F, Siegel BA, Lyss AP, Brodack JW, Mathias CJ, et al. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in metastatic breast carcinoma. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1991;32:1526–31. [PubMed] [Google Scholar]
- [66].Mortimer JE, Dehdashti F, Siegel BA, Katzenellenbogen JA, Fracasso P, Welch MJ. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: correlation with estrogen receptor status and response to systemic therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 1996;2:933–9. [PubMed] [Google Scholar]
- [67].Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19:2797–803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- [68].Asgari M, Morakabati A. Estrogen receptor beta expression in prostate adenocarcinoma. Diagnostic pathology. 2011;6:61. doi: 10.1186/1746-1596-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Archives of pathology & laboratory medicine. 2010;134:e48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- [70].Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. 2001;44:4230–51. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- [71].Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology. 1999;140:800–4. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]
- [72].Roman-Blas JA, Castaneda S, Cutolo M, Herrero-Beaumont G. Efficacy and safety of a selective estrogen receptor beta agonist, ERB-041, in patients with rheumatoid arthritis: a 12-week, randomized, placebo-controlled, phase II study. Arthritis care & research. 2010;62:1588–93. doi: 10.1002/acr.20275. [DOI] [PubMed] [Google Scholar]
- [73].Katzenellenbogen JA, Heiman DF, Carlson KE, Lloyd JE. In Vivo and In Vitro Steroid Receptor Assays in the Design of Estrogen Pharmaceuticals. In: Eckelman WC, editor. Receptor Binding Radiotracers. CRC Press; Boca Raton, LA: 1982. pp. 93–126. [Google Scholar]
- [74].Shanle EK, Hawse JR, Xu W. Generation of stable reporter breast cancer cell lines for the identification of ER subtype selective ligands. Biochem. Pharmacol. 2011;82:1940–9. doi: 10.1016/j.bcp.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tee MK, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, et al. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol. Biol. Cell. 2004;15:1262–72. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–8. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- [77].Stender JD, Frasor J, Komm B, Chang KC, Kraus WL, Katzenellenbogen BS. Estrogen-regulated gene networks in human breast cancer cells: involvement of E2F1 in the regulation of cell proliferation. Mol. Endocrinol. 2007;21:2112–23. doi: 10.1210/me.2006-0474. [DOI] [PubMed] [Google Scholar]
- [78].Jiang SY, Jordan VC. Growth regulation of estrogen receptor-negative breast cancer cells transfected with complementary DNAs for estrogen receptor. J. Natl. Cancer Inst. 1992;84:580–91. doi: 10.1093/jnci/84.8.580. [DOI] [PubMed] [Google Scholar]
- [79].Katzenellenbogen JA, Johnson HJ, Myers HN. Photoaffinity Labels for Estrogen Binding Proteins of Rat Uterus. Biochemistry. 1973;12:4085–92. doi: 10.1021/bi00745a010. [DOI] [PubMed] [Google Scholar]
- [80].Sharp TL, Dence CS, Engelbach JA, Herrero P, Gropler RJ, Welch MJ. Techniques necessary for multiple tracer quantitative small-animal imaging studies. Nucl. Med. Biol. 2005;32:875–84. doi: 10.1016/j.nucmedbio.2005.05.010. [DOI] [PubMed] [Google Scholar]