Abstract
During acute Lyme disease, bacteria can disseminate to the central nervous system (CNS) leading to the development of meningitis and other neurologic symptoms. Here we have analyzed pooled cerebrospinal fluid (CSF) allowing a deep view into the proteome for patients diagnosed with early-disseminated Lyme disease and CSF inflammation. Additionally, we analyzed individual patient samples and quantified differences in protein abundance employing label-free quantitative mass spectrometry based methods. We identified 108 proteins that differ significantly in abundance in patients with acute Lyme disease from controls. Comparison between infected patients and control subjects revealed differences in proteins in the CSF associated with cell death localized to brain synapses and others that likely originate from brain parenchyma.
Keywords: Proteomics, mass spectrometry, Lyme disease, cerebrospinal fluid, Lyme neuroborreliosis
INTRODUCTION
Lyme Disease, caused by Borrelia burgdorferi sensu stricto, is the most common tick-borne disease in the United States 1. Vector-associated bacterial transmission from animal to human occurs as a consequence of an infected arthropod, the Ixodes tick, biting and taking a blood meal from a human host. Bacterial transmission occurs via tick salivary secretions after sustained attachment of an infected tick 2. Exposure to any of four or more distinct Borrelia burgdorferi ss genotypes may lead to a disseminated infection 3, 4 and dissemination to the CNS results in neurologic symptoms and clinical findings 2, 5 that commonly include Lyme meningitis, often accompanied by cranial neuritis. Approximately 10–15% of untreated patients will develop neurologic Lyme disease 6.
Cerebrospinal fluid (CSF) is routinely sampled to identify the presence of and monitor indicators of ongoing pathological processes in the CNS. Amongst other tests, clinicians measure total protein concentration, although until recently little has been known regarding the specific protein composition, or the proteome, of CSF in normal or disease states 7, 8. The intimate contact between the CSF and the brain parenchyma provides the rationale for proteome analysis of CSF as a means for gaining insight into neurological disease processes. Mass spectrometry (MS) and gel-based proteome analyses of CSF have been successfully applied to the characterization of normal subjects 7 and neurological diseases including Alzheimer’s disease 9, multiple sclerosis 10, Parkinson’s disease 11, chronic fatigue syndrome and neurologic post treatment Lyme disease Syndrome 8(reviewed in 12).
Presently, there is a lack of knowledge of the broad spectrum of changes in proteins bathing the CNS following early-disseminated Lyme disease. Here we characterize the CSF proteome for patients with acute Lyme neuroborreliosis as compared to the CSF proteome of control patients without evidence of CNS inflammation.
MATERIALS AND METHODS
Patient and control sample description
CSF samples with elevated WBC counts (ie, >5 WBCs/mm3) obtained from patients who underwent lumbar puncture for evaluation of neurologic symptoms associated with Lyme disease between 2006 and 2009 at either Massachusetts General Hospital or Maine Medical Center were provided for this study (see Table 1). All protected health information was removed prior to use, and only fully de-identified CSF aliquots were submitted for proteomic analysis. In this study we analyzed CSF from patients with Lyme disease (n=26) and control CSF samples (n=19). The mean age of Lyme disease patients was 27 (range 4–67), and mean age of controls 44 (3–83) (Supplemental table S1). Seventeen cases were male and 9 were female; among the control group 11 were male and 8 were female. Based upon CDC guidelines (2011), 22 cases met criteria for confirmed Lyme disease. The four other cases were diagnosed as Lyme disease on the basis of supportive clinical and laboratory data, but completion of two tiered laboratory testing was not documented. Recent or concurrent erythema migrans rashes were documented in 8 cases, and cranial neuritis, almost entirely VII th nerve palsies, was noted in 14 (see Table 1). One case did not meet our criteria for CSF pleocytosis, but had clinical signs of meningismus. All patients had positive serology for antibodies to Lyme disease and four (of six tested) had positive CSF/serum index for antibodies to B. burgdorferi consistent with intrathecal antibody synthesis. Western blots were interpreted according to CDC criteria13.
Table 1.
Clinical and laboratory characteristics of Lyme disease patients.
| Pt | Erythema | Cranial | Positive Serology
|
CSF WBC Differential
|
Total | Bb Index CSF/Serum
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Migrans | Neuritis | Screen | Western Blot1 | Total | % Polys | % Lymphs | % Monohistiocytes | Protein | Glucose | Done | Positive |
| 1 | no | yes | ELISA | n/a2 | 120 | 0 | 87 | 13 | 94 | 49.5 | yes | neg |
| 2 | yes | no | ELISA | IgG | 69 | 9 | 76 | 15 | 57 | 47 | yes | pos |
| 3 | no | no | ELISA | n/a | 6.5 | 22 | 78 | 0 | 35 | 74 | yes | neg |
| 4 | no | no | ELISA | IgG | 689 | 8 | 57 | 32 | 185 | 34 | yes | pos |
| 5 | no | yes | ELISA | IgG | 238 | 0 | 88 | 4 | 95 | 48 | yes | pos |
| 6 | no | no | n/a | IgM | 2 | 3 | 75 | 22 | 19 | 79 | n/a | n/a |
| 7 | no | no | ELISA | IgG | 495 | 0 | 91 | 0 | 140 | 72 | n/a | n/a |
| 8 | no | no | ELISA | IgG | 59 | 59 | 100 | 0 | 47 | 58 | yes | pos |
| 9 | no | no | ELISA | n/a | 21 | 12 | 78 | 8 | 19 | 71 | n/a | n/a |
| 10 | yes | no | ELISA | n/a | 30 | 0 | 85 | 15 | 67 | 114 | n/a | n/a |
| 11 | yes | no | ELISA | IgM | 544 | 6 | 85 | 9 | 173 | 29 | n/a | n/a |
| 12 | yes | yes | ELISA | IgM | 316 | 1 | 88 | 11 | 52 | 47 | n/a | n/a |
| 13 | yes | yes | ELISA | IgG,IgM | 13 | 0 | 79 | 21 | 24 | 55 | n/a | n/a |
| 14 | yes | yes | ELISA | IgM | 37 | 0 | 91 | 9 | 78 | 52 | n/a | n/a |
| 15 | no | no | ELISA | IgG,IgM | 56 | 0 | 91 | 9 | 86 | 56 | n/a | n/a |
| 16 | no | yes | ELISA | IgM | 146 | 2 | 81 | 17 | 71 | 77 | n/a | n/a |
| 17 | yes | no | ELISA | IgG,IgM | 180 | 1 | 88 | 11 | 135 | 50 | n/a | n/a |
| 18 | yes | no | ELISA | IgG | 11 | 55 | 34 | 11 | 22 | 62 | n/a | n/a |
| 19 | no | yes | ELISA | IgM | 10 | 10 | 78 | 12 | 30 | 61 | n/a | n/a |
| 20 | no | yes | ELISA | IgM | 66 | 3 | 68 | 29 | 25 | 49 | n/a | n/a |
| 21 | no | yes | ELISA | IgM | 164 | 1 | 84 | 15 | 55 | 54 | n/a | n/a |
| 22 | no | yes | ELISA | IgM | 72 | 3 | 84 | 13 | 53 | 52 | n/a | n/a |
| 23 | no | yes | ELISA | IgG,IgM | 339 | 11 | 83 | 6 | 75 | 51 | n/a | n/a |
| 24 | no | yes | ELISA | IgM | 964 | 3 | 93 | 4 | 199 | 38 | n/a | n/a |
| 25 | no | yes | ELISA | IgG | 135 | 2 | 89 | 9 | 204 | 83 | n/a | n/a |
| 26 | no | yes | ELISA | IgM | 323 | 8 | 74 | 18 | 145 | 49 | n/a | n/a |
CDC criteria
data not available (test not done)
Our comparator control group consisted of CSF from patients who had lumbar punctures performed for a variety of clinical indications. Fever was present in 11/19 control patients. A variety of other diagnoses (postoperative fever, alcohol withdrawal, pneumonia, pyelonephritis) was made in 6 of the 11 cases. Of the 5 remaining cases presenting with fever and headache, 2 had completely negative Lyme blood and/or CSF diagnostic studies and one had a positive serum IgM immunoblot, negative IgG immunoblot, and negative CSF capture EIA studies and was not felt to have central nervous system Lyme disease. Two patients were diagnosed with non-meningitic viral syndromes based on their clinical, laboratory, and epidemiologic features and resolution without therapy for neuroborreliosis.
CSF samples used in this study were transported to the microbiology laboratory, clarified by centrifugation, and the supernatants stored at −70 °C prior to shipping overnight on dry ice to the Pacific Northwest National Laboratory, and stored at −80 °C prior to preparation and analysis.
The study protocol was approved by the institutional review boards of the Massachusetts General Hospital, Maine Medical Center, and Pacific Northwest National Laboratory. The use of excess cerebrospinal fluid, a clinical specimen collected solely for non-research purposes, falls within the guidelines for expedited review promulgated by the Office for Human Research Protections, Department of Health and Human Services following the passage of HIPAA.
BORRELIA culture conditions
The Bb strain B31 from the American Type Culture Collection (ATCC®) was obtained and expanded by incubation in Barbour-Stoenner-Kelly (BSK) media with antibiotics for 7–10 days at 34 °C until log phase was reached. A Petroff-Hausser hemocytometer under dark-field microscopy was used to determine when log phase was reached (70–80% confluency, 70–80% viability, ≈107 spirochetes/mL). The cells were expanded, washed by centrifugation, and killed by adding solid urea to each sample to a final concentration of 8 M. Control samples were Hanks added to CSF (1:3.28) with 8 M urea.
Proteomic methods
Our characterization of the CSF proteome was performed in a manner similar to that previously reported 7, 8. Briefly, pooled CSF samples were subjected to trypsin proteolysis and then fractionated by strong cation exchange (SCX) chromatography. The resultant fractionated peptides were identified, following reversed phase liquid chromatography (LC) separation, by tandem mass spectrometry (MS/MS) for creation of the accurate mass and time tag database. CSF samples from individual patients were analyzed by high resolution LC-MS employing the accurate mass and time tag label free quantification approach 7, 8.
Protein digestion
CSF peptide samples were prepared as previously described 8. Briefly, CSF proteins were denatured with 8 M urea and disulfide bonds were reduced with 5 mM dithiothreitol at 37 °C for 60 min. Proteins were then digested with porcine Trypsin (Promega, Madison, WI) over night at 37 °C. Tryptic peptide mixtures were cleaned up by solid phase extraction, with a 1-mL SPE C18 column (Discovery DSC-18, Supelco, Bellefonte, PA) as described previously 14. Final peptide concentrations were determined by BCA assay (Pierce). All tryptic digests were snap frozen in liquid nitrogen and stored at −80 °C until further processing and analysis. Ammonium bicarbonate and acetonitrile were from Fisher Scientific (Fair Lawn, NJ). Sequencing grade, modified trypsin was from Promega (Madison, WI). Bicinchoninic acid (BCA) assay reagents and standards were from Pierce (Rockford, IL). All other reagents were from Sigma-Aldrich (St.Louis, MO). Water was purified using a Barnstead Nanopure Infinity water purification system (Dubuque, IA).
Off-line strong cation exchange (SCX) fractionation
Tryptic peptide mixtures from pooled CSF samples from either 12 control samples or 9 patient samples were fractionated by strong cation exchange (SCX) chromatography to reduce sample complexity. 460 Pg of tryptic peptides from pooled patient samples or 700 μg from control pooled samples were suspended in a final volume of 900 μL in 10 mM ammonium formate, pH 3.0, 25% acetonitrile and fractionated by SCX chromatography as described previously 14. A total of 35 SCX fractions were collected for the patient pooled sample and 25 fractions from the pooled control sample and lyophilized prior to high resolution, gradient, reversed phase capillary LC-MS/MS analysis.
Reversed phase capillary LC-MS/MS and LC-MS analysis
Peptide fractions were separated by high resolution, reversed phase capillary liquid chromatography as previously described using an automated four-column capillary liquid chromatography system 15 in-line with mass spectrometers modified with electrodynamic ion funnels developed and assembled in-house 16. SCX fractions were analyzed using an LTQ mass spectrometer and label free quantification of CSF was performed as previously described 8.
Data analysis
LTQ raw data was extracted using Extract_MSn (version 3.0) and analyzed with the SEQUEST algorithm (V27 revision 12) searching the MS/MS data against the human IPI database (Version 3.39) and sequences from the B. burgdorferi B31 protein database that contained 1737 protein entries. Database search parameters and FDR analysis was performed as previously described 8. Analysis of quantification of the differences in protein abundance between control and LM patient CSF samples was performed as previously described 8 and visualized using in-house software DAnTE 17. Briefly, peptide intensities from the LC-MS analyses were log2 transformed. Peptide abundances were then “rolled up” to the unique protein level employing the Z-rollup method (based on trends observed at the peptide level) implemented in DAnTE. ANOVA and clustering analyses were also performed using DAnTE. Functional enrichment and pathway analysis was performed with Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com). Right-tailed Fisher’s exact test was used to calculate a p-value determining the probability that each biological function and/or disease assigned to our data set is due to chance alone. Annotated tissue expression profile for CSF proteins was retrieved from Ensemble-Biomart (www.Ensembl.org)
RESULTS
We performed an in-depth analysis of the CSF proteome, followed by a quantitative analysis of differences in protein abundance occurring in the CSF of 26 patients with Lyme disease and evidence of CNS dissemination and from 19 control subjects without Lyme disease. For the deepest view of the CSF proteome, we first analyzed an unbiased subset of pooled patient and control samples by LC-MS/MS following fractionation by strong cation exchange (SCX) chromatography. We then analyzed a second set of individual patient and control samples that lacked sufficient sample volume for fractionation, leveraging the accurate mass and time (AMT) tag database initially created from the analysis of the pooled sample sets. This allowed for high throughput label-free quantitative analysis of the differences in CSF protein abundance profiles between patients with disseminated Lyme disease with neurologic symptoms and control subjects.
Analysis of pooled CSF samples for human proteins
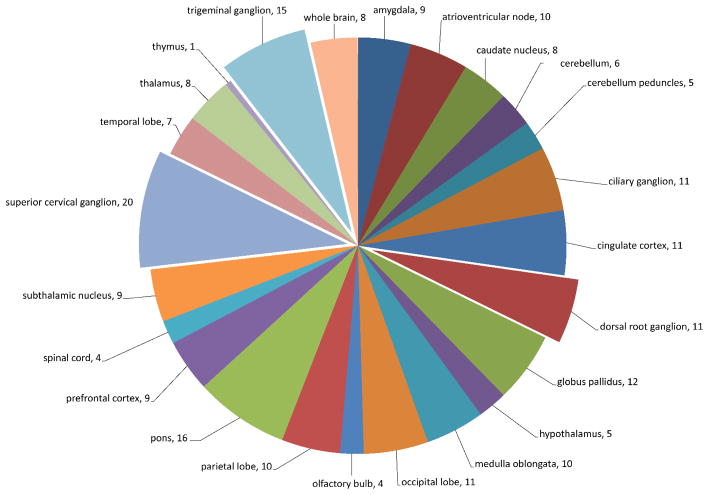
In-depth analysis of highly fractionated pooled patient (n=9) and control (n=12) samples resulted in the identification of 5625 peptides covering 1104 proteins in the Lyme patient pooled samples and 4778 peptides corresponding to 906 proteins in the control pooled samples. There were 1458 non-redundant (unique) proteins confidently identified in total, with 552 proteins being identified in both Lyme disease and control samples. A total of 552 proteins were uniquely identified in the Lyme pooled sample and 354 proteins were uniquely identified in control subjects (Figure 1 and Supplementary Table S2). Approximately 60% of the proteins identified CSF samples were unique to the CSF proteome and not present in the plasma proteome 14 (Supplementary Table S2). Collectively 49% of the CSF proteome of Lyme and control subjects were annotated as being expressed in CNS associated tissues (Supplementary Table S2 and Figure 2). A comparison between proteins identified in previous studies of brain postsynaptic density (PSD) tissue 18, plasma 14, and CSF revealed 111 proteins common to all three proteomes and 102 proteins (7%) were identified in the CSF and brain tissue, but not in blood plasma (Supplementary Table S2 and Figure 3). There were approximately 2 times more plasma proteins uniquely identified in the CSF of Lyme subjects compared to control, consistent with the likelihood of reduced blood-brain barrier integrity.
Figure 1.
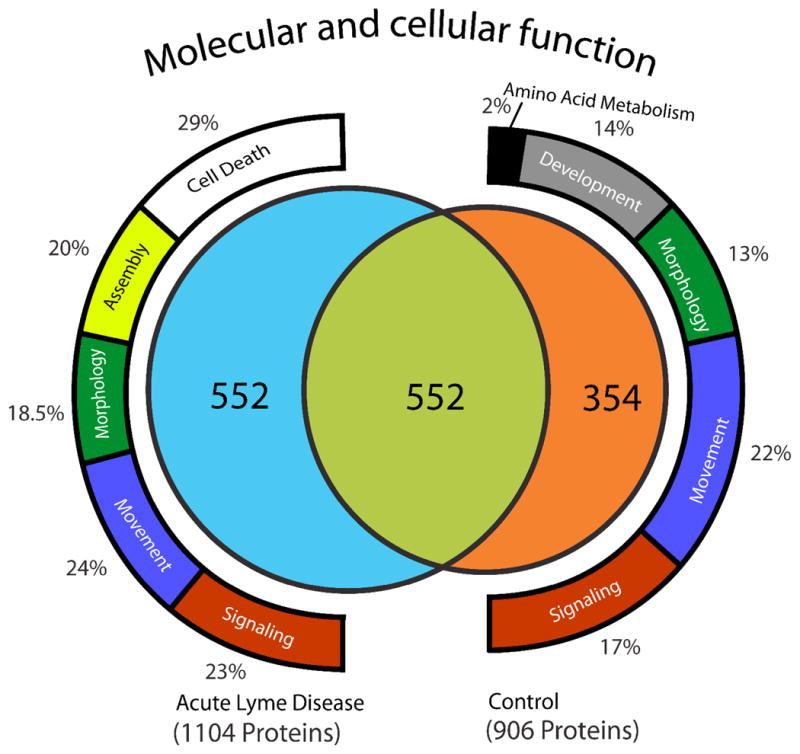
Cerebrospinal fluid proteome of pooled samples from subjects with acute Lyme disease and non-infected control subjects. Pooled CSF from Lyme disease and control samples were digested, fractionated offline by strong cation exchange, and analyzed by reversed phase LC-MS/MS. We identified peptides attributable to a total of 1458 proteins considering all sample fractions, 906 in the pooled control sample and 1104 in the pooled acute Lyme disease sample. There were 552 proteins common to both samples, 552 were uniquely identified in the acute Lyme disease pooled sample and 354 were uniquely identified in the control pooled sample. Functional analysis for proteins unique to either control or Lyme subject pooled samples was performed employing Ingenuity Pathway tools (www.ingenuity.com) revealing the presence of proteins associated with cell death in the acute Lyme disease pooled samples. In contrast proteins relating to development and amino acid metabolism were singularly identified in pooled control samples.
Figure 2.
Distribution pattern for CNS proteins identified in the CSF proteome of Lyme disease and control subject samples.
Figure 3.
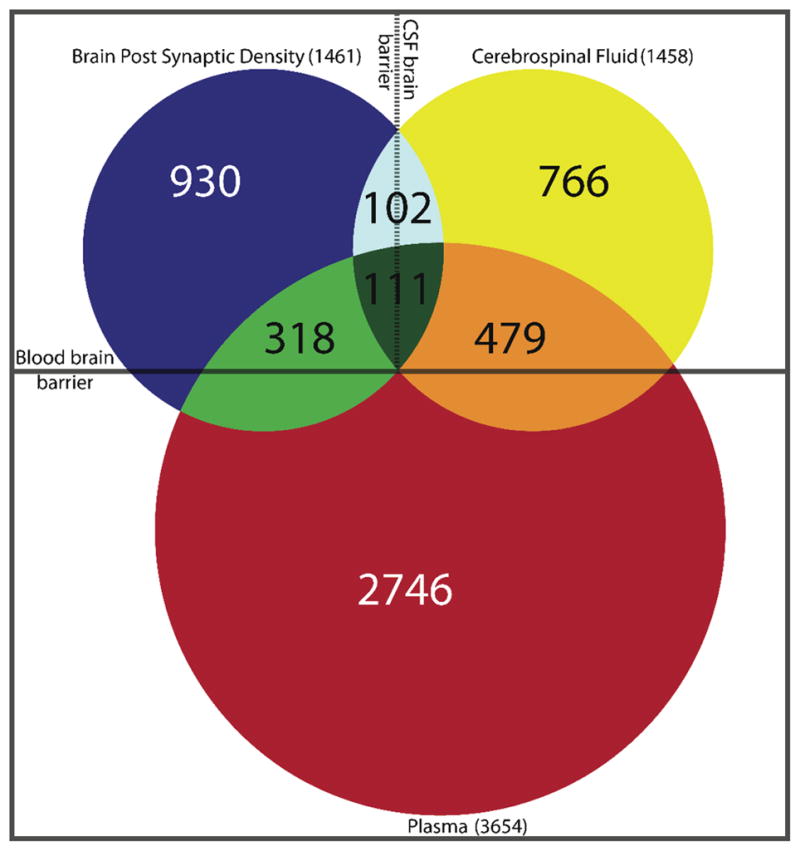
Venn diagram with CNS compartmentalization. Comparison between the CSF proteome of combined normal subjects and acute Lyme disease patients (yellow circle) with previously reported plasma proteome (red circle) and brain postsynaptic density proteome (blue circle).
Pathway analysis for all proteins identified in the pooled CSF samples (employing Ingenuity Pathway Analysis, www.ingenuity.com) led to the identification of enriched canonical signaling associated with acute phase signaling response, complement cascade, coagulation, and both intrinsic and extrinsic prothrombin activation pathways (Supplementary Table S2), consistent with a pro-inflammatory response. Analysis of enrichment of protein function identified in only one of the two pooled samples (disease or control) or common to both was performed (Figure 1). Notable was the presence of 116 proteins (29% of the unique proteins) associated with cell death for the Lyme patients; this functional category was not enriched in the proteins uniquely identified in the control sample or in the proteins common to both pooled samples. These results are consistent with previous reports showing that Borrelia induces inflammatory processes resulting in apoptosis of neuronal cells 19, 20.
Analysis of pooled CSF samples for bacterial proteins
In addition to searching for peptides derived from human proteins, the LC-MS/MS data sets were searched for peptides derived from Borrelia. We were unable to detect any peptides from B. burgdorferi.
Determination of detection limit of bacterial peptides
We spiked a range of B. burgdorferi (104, 105, 106, and 107 organisms per mL of CSF) into control CSF to determine our nominal level of detection for bacterial proteins in unfractionated CSF. We detected bacterial derived proteins, such as the antigenic outer surface lipoproteins OspA and OspB among others, in CSF spiked with 105 or greater Bb organisms per mL.
Analysis of non-fractionated individual CSF samples
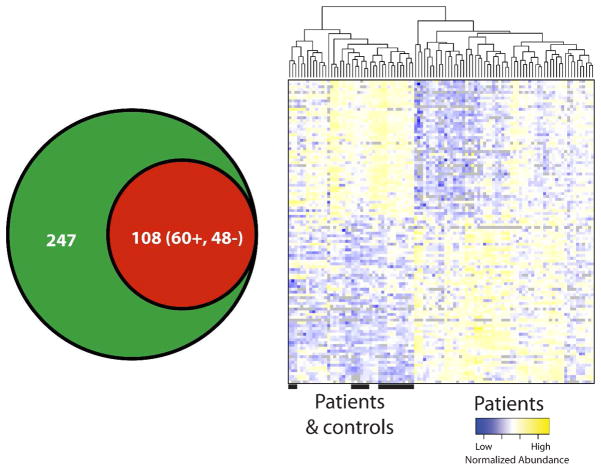
Analysis of non-fractionated CSF from a second set of individual Lyme patient samples and control subjects employing a label-free quantitative MS-based approach 21 was performed. Analysis of CSF from 26 Lyme patients and 7 control subjects resulted in the identification of 247 unique proteins (2 or more peptides identified for all proteins) across all samples (Supplementary Table S3).
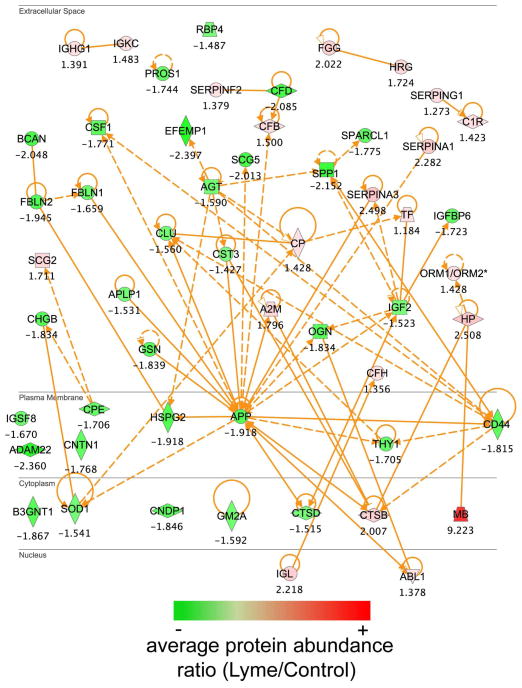
Comparison of quantified protein abundances between Lyme patient and control samples employing a one way analysis of variance (ANOVA) resulted in 108 proteins being identified as significantly different in abundance (p ≤0.05), where 48 were decreased and 60 increased in Lyme compared to control samples (Figure 4A). Unsupervised hierarchical clustering of the 108 proteins revealed that there were identifiable disease-associated differences in the CSF proteome that allowed for the partial separation of disease and control samples (Figure 4B). Samples organized into two main clusters, one cluster being composed of patients (right, cluster 1) and the second cluster (left, cluster 2) being a mixture of negative control samples and 7 patient samples. Annotation of the protein-tissue expression profile, employing Ensembl-Biomart (www.ensembl.org/biomart), was performed (Supplementary Table S4 and Figure 2). The greatest numbers of significantly different proteins (20/31 annotated proteins) were annotated as being expressed in the superior cervical ganglion and trigeminal ganglion. Additionally, eleven proteins (gene symbols – CD59, CNTN1,CSF1, FBLN1, FCGBP,HRG, LYNX1, NCAM1, NEGR1, PZP, THY1) expressed in the dorsal root ganglion differed in abundance in Lyme patient CSF samples. These findings were consistent with a previous report of dorsal root ganglion inflammatory lesions in non-human primate studies of neuroborreliosis 20. Network analysis of proteins that were found to be significantly different by ANOVA (p ≤ 0.05) in abundance between Lyme patient samples and control subjects and known functional and disease related activities, shown in Figure 5, highlights the quantitative differences identified in the CSF proteome. Notably, there is a reduction in the amyloid precursor protein (gene symbol, APP) in patient samples and this protein is at a highly connected node, participating as a member of a large subset of proteins that have altered abundance levels in diseased versus control state (Figure 5). Many of the proteins that displayed differential abundance are associated with neurological disorders.
Figure 4.
Analysis of individual patient samples led to the quantification of 247 proteins. An analysis of variance of the protein abundances revealed 108 proteins as being differentially abundant (p value < 0.05) (A) where 60 were increased and 48 were decreased in abundance relative to control. Unsupervised cluster analysis of the subject samples shows that the quantified protein abundances (rows in matrix) allowed for partial segregation of patient and control samples (columns) (B).
Figure 5.
Network analysis of proteins found to be significantly altered in abundance in the CSF proteome by ANOVA (p value <0.05) following analysis of individual subject samples. Network analysis was performed employing ingenuity pathway tools (www.ingenuity.com) on proteins that were annotated in the Ingenuity database (101 of the 108 proteins). Illustrated are proteins, colored by ratio (Lyme/Control) of average protein abundances, with known functional and disease associated relationships that differed in abundance levels in the CSF.
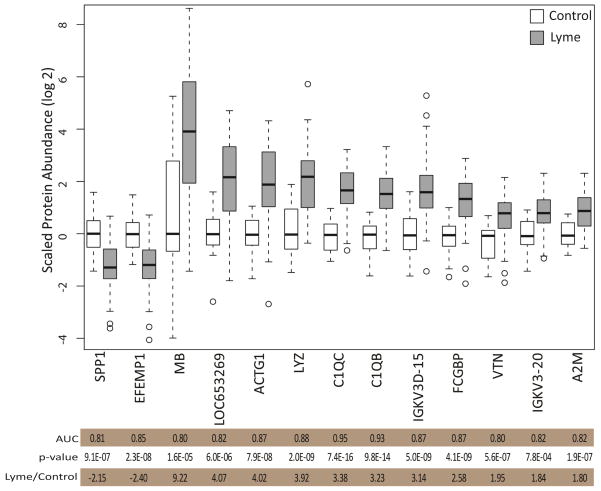
The descriptive and discriminating power of the differences in protein abundance can be quantified employing a receiver operating characteristic (ROC) curve analysis by calculating the area under the ROC curve (AUC). The discriminating power of each of the 247 proteins was calculated. Selection of proteins that were significantly different in abundance by ANOVA (p values ≤ 0.05) and that had an AUC ≥ 0.8 resulted in identification of a panel of 13 proteins (Figure 6).
Figure 6.
Proteins with greatest discriminating power for Lyme disease. ROC curve analysis of all proteins identified and quantified in the CSF led to the identification of 13 proteins that had good discriminating power with areas under the curve (AUC) greater than 0.8. Shown are the scaled protein abundance values of the 13 proteins that show the greatest discriminating power in the ROC curve analysis.
We compared the identified proteins that significantly differed in relative abundance in the Lyme CSF proteome with those previously reported significantly changed for subjects with chronic neurological diseases for which there are data (Parkinson’s Disease 11, multiple sclerosis 12, Alzheimer’s disease 9, and late (post-treatment) neurological Lyme disease 8 (Supplementary Table S5). There were 53 proteins that uniquely differed in Lyme disease (not identified as changing in any of the chronic diseases above), and of these proteins, 37 were previously identified in the plasma proteome 14.
DISCUSSION
Here we present, for the first time, a detailed analysis of the proteome of the CSF of patients with acute Lyme neuroborreliosis. We found that the proteome reflects increased immune activation, with components of the complement cascade in particular being increased in abundance.
Proteins in the CSF originate from plasma proteins crossing the blood-cerebrospinal fluid barrier as well as proteins from the brain parenchyma and associated tissues crossing the brain-cerebrospinal fluid barrier 22, 23. CSF functions as a sink for degradation products resulting from both normal and pathologic processes occurring in the brain parenchyma and meninges. Accordingly, differences in CSF proteome composition in patients with Lyme disease presumably reflect increased innate immune activation with a cellular response, proteins from brain tissue itself, and plasma proteins from damage to the blood-brain barrier 24.
Limited data are available on the characteristics of the CSF proteome in meningitis due to different bacterial or viral pathogens. A recent proteome analysis of CSF in patients with pneumococcal meningitis 25 highlighted an array of proteins up-regulated in a disease with a massive inflammatory reaction characterized with a predominantly neutrophil response. We identified many differentially abundant proteins in the Lyme CSF samples that play central roles in innate immunity. These data were consistent with a prior report of increased levels of the marker of innate immune activation, neopterin, in CSF of patients with neurologic Lyme disease 26. We observed decreased levels of amyloid precursor protein (APP) in LM patient samples compared with normal subjects consistent with previous reports by Mattsson et al. 27. Amyloid metabolism is sensitive and is markedly affected by inflammation associated with innate immune activation, as has been reported for several neurodegenerative diseases 28. Reduction in APP abundance was also observed as a function of patient disease severity for viral encephalitis in the case of HIV infection 29. Kallikrein 6 (KLK6) is present at reduced levels in Lyme samples and is thought to play a role in removal of α-synuclein and prevent its polymerization, where accumulation is associated with Lewy body formations and Parkinson’s disease 30. Additionally it has been shown to generate amyloid fragments from amyloid precursor protein in cell culture, and may in part contribute to the reduced levels quantified in the CSF of Lyme patients 31.
The small chemokine CXCL13 has been proposed for use as a biomarker of neurologic Lyme disease 32. CXCL13 is a B lymphocyte chemo-attractant and ligand for the G protein-coupled receptor CXCR5. Increased levels in the CSF are associated with increased numbers of B lymphocytes in the CSF 32. CXCL13 has been shown to be released by monocytes following exposure to the outer surface proteins from Borrelia and recognition by the toll like receptor 2 (TLR2).33 We did not identify CXCL13 in our broad coverage of the CSF proteome, and CXCL13 was not identified in previous analyses of human plasma or normal CSF proteomes 7, 14. It is possible that it is below the limit of detection by the methods used 34, 35 and a targeted proteomics approach applying LC-SRM-MS and synthetic, stable isotopically labeled peptides may eventually lead to detection of CXCL13 in patient blood plasma in future studies 36. In contrast, we did identify proteins such as CD44, which is also associated with TLR2 signaling and plays a role in the presentation of lipoproteins (such as OspC from Bb 37) to the TLR2 signaling complex. CD44, decreased in abundance in the CSF proteome for Lyme patients (Figure 5), has been associated with inflammation (reviewed in 38), and has also been reported to play a role as a negative regulator of TLR mediated inflammation 39.
We identified 13 proteins out of 247 possible discriminating proteins with favorable receiver operating characteristic curves. This group of proteins constitutes a potential candidate protein profile for discriminating Lyme from control samples. Several proteins in the panel were associated with the complement cascade: C1QB, C1QC, and VTN. C1QB and C1QC are positive effector molecules for innate immunity, while VTN is a negative effector 40. Complement activation and increased levels of C1q and C3a in CSF as a consequence of CNS involvement in Lyme disease have been previously reported41. Receiver operating characteristic analysis revealed that complement component C1QC shows the greatest discriminating power for all proteins with an AUC of 0.95. Osteopontin (SPP1) was reduced in Lyme patient samples and had an AUC of 0.81. Osteopontin has been shown to act as a cytokine, enhancing the production of interferon gamma and IL-12 42. Pathway analysis showed that these proteins are linked to the centrally located and highly connected amyloid precursor protein (Figure 5).
Of particular interest were proteins identified and known to be present in brain tissue. We detected 20 proteins differing in abundance in patients with Lyme that were present in the postsynaptic density (PSD) tissue. These proteins directly or indirectly participate in signal transduction events that underlie the processes of learning and memory 18. The presence of these proteins in the CSF may reflect restructuring of the synaptic arbor as a consequence of toxic processes such as inflammation coincident with infection.
Comparison of the data set with a recent report describing the CSF proteome of patients with post-treatment Lyme disease8 revealed 11 proteins in common that differed from controls in abundance: C1QB, C1QC, IGFBP6, SERPINA3, GSN, CD44, CNTN1, CNDP1, AGT, OMG, and ORM2. Several of these proteins are brain-derived and participate in axonal maintenance and growth regulation. The relationship in pathophysiology, if any, between acute meningitis in Lyme disease and late neurological post-treatment Lyme disease is not clear.
We failed to detect evidence of bacterial peptides in any of our samples. Bacterial load in the CSF is low even when there is clinical evidence of dissemination, with CSF often being both culture and PCR negative 43. Ex vivo spiking experiments required a level of 105 organisms per mL for detection.
There are several possible limitations to our study. Formal and complete Lyme disease testing was not available in all control patients, since in many patients epidemiologic and clinical features were not consistent with Lyme disease. CSF pleocytosis is considered to be an important finding in patients with acute Lyme meningitis 44, 45, so the absence of CSF pleocytosis in all of our control patients supports their inclusion for this analysis. The absence of pleocytosis also makes the presence of other acute meningitic processes unlikely. In addition, we are unable to resolve if the findings that we report are specific to meningitis caused by Borrelia burgdorferi as compared to other infectious agents that might cause a lymphocytic meningitis. Future studies that compare the proteome in Lyme disease with the proteome in the CSF of other acute infections (i.e., viral or mycobacterial meningitis) that present with a similar, predominantly lymphocytic or monocytic, cellular response will be necessary to determine how much of the response reported here is specific to infection with Borrelia.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Center for Research Resources (5P41RR018522-10) and the National Institute of General Medical Sciences (8 P41 GM103493-10) from the National Institutes of Health to RDS), and by NIH grant AI059010 and grant 87200 from the Shriners Hospital for Crippled Children to HSW. The analytical work was performed in the Environmental Molecular Sciences Laboratory, U. S. Department of Energy Office of Biological and Environmental Research national scientific user facility located at Pacific Northwest National Laboratory in Richland, Washington. Pacific Northwest National Laboratory is operated by Battelle for the U.S. Department of Energy under Contract No. DE-AC05-76RLO 1830. Additional support for clinical sample acquisition was provided by the Maine Medical Center Neuroscience Institute. The authors acknowledge the assistance of Drs. Mark Eggena and Cheryl Liechty in provision of Penobscot Bay Medical Center, Rockland Maine in the provision of cerebrospinal fluid samples.
Footnotes
Conflict of interest statement
Thomas E. Angel- no conflict, Jon M. Jacobs- no conflict, Robert P. Smith- no conflict, Mark S. Pasternack- no conflict, Susan Elias- no conflict, Marina A. Gritsenko- no conflict, Anil Shukla- no conflict, Edward C. Gilmore- no conflict, Carol McCarthy- no conflict, David G. Camp II- no conflict, Richard D. Smith- no conflict, H. Shaw Warren- no conflict
References
- 1.Casjens SR, Fraser-Liggett CM, Mongodin EF, Qiu WG, Dunn JJ, Luft BJ, Schutzer SE. Whole genome sequence of an unusual Borrelia burgdorferi sensu lato isolate. Journal of bacteriology. 2011;193(6):1489–90. doi: 10.1128/JB.01521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216(4552):1317–9. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 3.Alghaferi MY, Anderson JM, Park J, Auwaerter PG, Aucott JN, Norris DE, Dumler JS. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: Lack of correlation with invasive and noninvasive genotypes. Journal of Clinical Microbiology. 2005;43(4):1879–1884. doi: 10.1128/JCM.43.4.1879-1884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Wormser GP, Schriefer ME, Luft BJ. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67(7):3518–24. doi: 10.1128/iai.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hildenbrand P, Craven DE, Jones R, Nemeskal P. Lyme Neuroborreliosis: Manifestations of a Rapidly Emerging Zoonosis. American Journal of Neuroradiology. 2009;30(6):1079–1087. doi: 10.3174/ajnr.A1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halperin JJ. Nervous system lyme disease: diagnosis and treatment. Rev Neurol Dis. 2009;6(1):4–12. [PubMed] [Google Scholar]
- 7.Schutzer SE, Liu T, Natelson BH, Angel TE, Schepmoes AA, Purvine SO, Hixson KK, Lipton MS, Camp DG, Coyle PK, Smith RD, Bergquist J. Establishing the proteome of normal human cerebrospinal fluid. PLoS One. 2010;5(6):e10980. doi: 10.1371/journal.pone.0010980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schutzer SE, Angel TE, Liu T, Schepmoes AA, Clauss TR, Adkins JN, Camp DG, Holland BK, Bergquist J, Coyle PK, Smith RD, Fallon BA, Natelson BH. Distinct cerebrospinal fluid proteomes differentiate post-treatment lyme disease from chronic fatigue syndrome. Plos One. 2011;6(2):e17287. doi: 10.1371/journal.pone.0017287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maarouf CL, Andacht TM, Kokjohn TA, Castano EM, Sue LI, Beach TG, Roher AE. Proteomic analysis of Alzheimer’s disease cerebrospinal fluid from neuropathologically diagnosed subjects. Curr Alzheimer Res. 2009;6(4):399–406. doi: 10.2174/156720509788929318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoop MP, Dekker LJ, Titulaer MK, Lamers RJ, Burgers PC, Sillevis Smitt PA, van Gool AJ, Luider TM, Hintzen RQ. Quantitative matrix-assisted laser desorption ionization-fourier transform ion cyclotron resonance (MALDI-FT-ICR) peptide profiling and identification of multiple-sclerosis-related proteins. J Proteome Res. 2009;8(3):1404–14. doi: 10.1021/pr8010155. [DOI] [PubMed] [Google Scholar]
- 11.Constantinescu R, Andreasson U, Li S, Podust VN, Mattsson N, Anckarsater R, Anckarsater H, Rosengren L, Holmberg B, Blennow K, Wikkelso C, Ruetschi U, Zetterberg H. Proteomic profiling of cerebrospinal fluid in parkinsonian disorders. Parkinsonism Relat Disord. 2010;16(8):545–9. doi: 10.1016/j.parkreldis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Kroksveen AC, Opsahl JA, Aye TT, Ulvik RJ, Berven FS. Proteomics of human cerebrospinal fluid: Discovery and verification of biomarker candidates in neurodegenerative diseases using quantitative proteomics. J Proteomics. 2011;74(4):371–88. doi: 10.1016/j.jprot.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Lyme disease--United States 1995. MMWR Morb Mortal Wkly Rep. 1996;45(23):481–4. [PubMed] [Google Scholar]
- 14.Liu T, Qian WJ, Gritsenko MA, Xiao WZ, Moldawer LL, Kaushal A, Monroe ME, Varnum SM, Moore RJ, Purvine SO, Maier RV, Davis RW, Tompkins RG, Camp DG, Smith RD, Injury IHR. High dynamic range characterization of the trauma patient plasma proteome. Molecular & Cellular Proteomics. 2006;5(10):1899–1913. doi: 10.1074/mcp.M600068-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livesay EA, Tang K, Taylor BK, Buschbach MA, Hopkins DF, LaMarche BL, Zhao R, Shen Y, Orton DJ, Moore RJ, Kelly RT, Udseth HR, Smith RD. Fully automated four-column capillary LC-MS system for maximizing throughput in proteomic analyses. Analytical Chemistry. 2008;80(1):294–302. doi: 10.1021/ac701727r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaffer SA, Prior DC, Anderson GA, Udseth HR, Smith RD. An ion funnel interface for improved ion focusing and sensitivity using electrospray ionization mass spectrometry. Analytical chemistry. 1998;70(19):4111–9. doi: 10.1021/ac9802170. [DOI] [PubMed] [Google Scholar]
- 17.Polpitiya AD, Qian WJ, Jaitly N, Petyuk VA, Adkins JN, Camp DG, 2nd, Anderson GA, Smith RD. DAnTE: a statistical tool for quantitative analysis of -omics data. Bioinformatics. 2008;24(13):1556–8. doi: 10.1093/bioinformatics/btn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayes A, van de Lagemaat LN, Collins MO, Croning MD, Whittle IR, Choudhary JS, Grant SG. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat Neurosci. 2011;14(1):19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers TA, Kaushal D, Philipp MT. Microglia are mediators of Borrelia burgdorferi-induced apoptosis in SH-SY5Y neuronal cells. PLoS Pathog. 2009;5(11):e1000659. doi: 10.1371/journal.ppat.1000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramesh G, Borda JT, Gill A, Ribka EP, Morici LA, Mottram P, Martin DS, Jacobs MB, Didier PJ, Philipp MT. Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. J Neuroinflammation. 2009;6:23. doi: 10.1186/1742-2094-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmer JS, Monroe ME, Qian WJ, Smith RD. Advances in proteomics data analysis and display using an accurate mass and time tag approach. Mass Spectrom Rev. 2006;25(3):450–82. doi: 10.1002/mas.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regeniter A, Kuhle J, Mehling M, Moller H, Wurster U, Freidank H, Siede WH. A modern approach to CSF analysis: pathophysiology, clinical application, proof of concept and laboratory reporting. Clin Neurol Neurosurg. 2009;111(4):313–8. doi: 10.1016/j.clineuro.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Ramesh G, Borda JT, Dufour J, Kaushal D, Ramamoorthy R, Lackner AA, Philipp MT. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am J Pathol. 2008;173(5):1415–27. doi: 10.2353/ajpath.2008.080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goonetilleke UR, Scarborough M, Ward SA, Gordon SB. Proteomic analysis of cerebrospinal fluid in pneumococcal meningitis reveals potential biomarkers associated with survival. J Infect Dis. 2010;202(4):542–50. doi: 10.1086/654819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasse T, Murr C, Meyersbach P, Schmutzhard E, Wachter H, Fuchs D. Neopterin Production and Tryptophan Degradation in Acute Lyme Neuroborreliosis Versus Late Lyme Encephalopathy. European Journal of Clinical Chemistry and Clinical Biochemistry. 1994;32(9):685–689. doi: 10.1515/cclm.1994.32.9.685. [DOI] [PubMed] [Google Scholar]
- 27.Mattsson N, Bremell D, Anckarsater R, Blennow K, Anckarsater H, Zetterberg H, Hagberg L. Neuroinflammation in Lyme neuroborreliosis affects amyloid metabolism. BMC Neurol. 2010;10:51. doi: 10.1186/1471-2377-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gisslen M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, Spudich S, Hagberg L, Rosengren L, Price RW, Zetterberg H. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angel TE, Jacobs JM, Spudich SS, Gritsenko MA, Fuchs D, Liegler T, Zetterberg H, Camp DG, 2nd, Price RW, Smith RD. The cerebrospinal fluid proteome in HIV infection: change associated with disease severity. Clin Proteomics. 2012;9(1):3. doi: 10.1186/1559-0275-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwata A, Maruyama M, Akagi T, Hashikawa T, Kanazawa I, Tsuji S, Nukina N. Alpha-synuclein degradation by serine protease neurosin: implication for pathogenesis of synucleinopathies. Human Molecular Genetics. 2003;12(20):2625–2635. doi: 10.1093/hmg/ddg283. [DOI] [PubMed] [Google Scholar]
- 31.Little SP, Dixon EP, Norris F, Buckley W, Becker GW, Johnson M, Dobbins JR, Wyrick T, Miller JR, MacKellar W, Hepburn D, Corvalan J, McClure D, Liu X, Stephenson D, Clemens J, Johnstone EM. Zyme, a novel and potentially amyloidogenic enzyme cDNA isolated from Alzheimer’s disease brain. The Journal of biological chemistry. 1997;272(40):25135–42. doi: 10.1074/jbc.272.40.25135. [DOI] [PubMed] [Google Scholar]
- 32.Rupprecht T, Koedel U, Angele B, Kastenbauer S, Wilske B, Pfister HW. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Journal of Neurology. 2005;252:34–35. doi: 10.1212/01.wnl.0000171349.06645.79. [DOI] [PubMed] [Google Scholar]
- 33.Rupprecht T, Kirschning C, Popp B, Kastenbauer S, Fingerle V, Pfister HW, Koedel U. Borrelia garinii induces CXCL13 production in human monocytes through TLR2. Journal of Neurology. 2007;254:30–30. doi: 10.1128/IAI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marra CM, Tantalo LC, Sahi SK, Maxwell CL, Lukehart SA. CXCL13 as a Cerebrospinal Fluid Marker for Neurosyphilis in HIV-Infected Patients With Syphilis. Sexually Transmitted Diseases. 2010;37(5):283–287. doi: 10.1097/OLQ.0b013e3181d877a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian WJ, Kaleta DT, Petritis BO, Jiang HL, Liu T, Zhang X, Mottaz HM, Varnum SM, Camp DG, Huang L, Fang XM, Zhang WW, Smith RD. Enhanced Detection of Low Abundance Human Plasma Proteins Using a Tandem IgY12-SuperMix Immunoaffinity Separation Strategy. Molecular & Cellular Proteomics. 2008;7(10):1963–1973. doi: 10.1074/mcp.M800008-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T, Hossain M, Schepmoes AA, Fillmore TL, Sokoll LJ, Kronewitter SR, Izmirlian G, Shi T, Qian W-J, Leach RJ, Thompson IM, Chan DW, Smith RD, Kagan J, Srivastava S, Rodland KD, Camp DG., Ii Analysis of serum total and free PSA using immunoaffinity depletion coupled to SRM: correlation with clinical immunoassay tests. Journal of Proteomics. (0) doi: 10.1016/j.jprot.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an Outer Surface Protein on Borrelia-Burgdorferi during Tick Feeding. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(7):2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends in Molecular Medicine. 2001;7(5):213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- 39.Kawana H, Karaki H, Higashi M, Miyazaki M, Hilberg F, Kitagawa M, Harigaya K. CD44 suppresses TLR-mediated inflammation. Journal of Immunology. 2008;180(6):4235–4245. doi: 10.4049/jimmunol.180.6.4235. [DOI] [PubMed] [Google Scholar]
- 40.Singh B, Su YC, Riesbeck K. Vitronectin in bacterial pathogenesis: a host protein used in complement escape and cellular invasion. Molecular Microbiology. 2010;78(3):545–560. doi: 10.1111/j.1365-2958.2010.07373.x. [DOI] [PubMed] [Google Scholar]
- 41.Henningsson AJ, Ernerudh J, Sandholm K, Carlsson SA, Granlund H, Jansson C, Nyman D, Forsberg P, Nilsson Ekdahl K. Complement activation in Lyme neuroborreliosis--increased levels of C1q and C3a in cerebrospinal fluid indicate complement activation in the CNS. Journal of neuroimmunology. 2007;183(1–2):200–7. doi: 10.1016/j.jneuroim.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–4. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 43.Marques AR. Lyme Disease: A Review. Current Allergy and Asthma Reports. 2010;10(1):13–20. doi: 10.1007/s11882-009-0077-3. [DOI] [PubMed] [Google Scholar]
- 44.Djukic M, Schmidt-Samoa C, Lange P, Spreer A, Neubieser K, Eiffert H, Nau R, Schmidt H. Cerebrospinal fluid findings in adults with acute Lyme neuroborreliosis. Journal of Neurology. 2012;259(4):630–6. doi: 10.1007/s00415-011-6221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tumani H, Nolker G, Reiber H. Relevance of cerebrospinal fluid variables for early diagnosis of neuroborreliosis. Neurology. 1995;45(9):1663–70. doi: 10.1212/wnl.45.9.1663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.