Abstract
Introduction
We previously reported a unique hypothalamic-pituitary-thyroid (HPT) axis profile in women with a menstrually related mood disorder (MRMD) who also had a history of sexual abuse (SA). In the present study, we sought to extend that work by examining the association of a SA history with HPT-axis disturbance in both MRMD and non-MRMD women.
Methods
Fifty-seven women met prospective criteria for MRMD (23 with a SA history) and 52 women were non-MRMD (18 with a SA history). Thyroid stimulating hormone (TSH), T4, (total and free) and T3 (total and free) were evaluated in serum together with thyroid hormone ratios reflecting T4 to T3 conversion.
Results
MRMD women, compared with non-MRMD women, had elevated T3/T4 ratios (ps≤0.01; reflecting increased conversion of T4 to T3) and lower free and total T4 concentrations (ps=0.01). Higher T3/T4 ratios and lower T4 concentrations predicted more severe premenstrual symptomatology in all women. A SA history, irrespective of MRMD status, was associated with elevated TSH concentrations (p=0.03). However, in MRMD women, a SA history was associated with elevated T3 concentrations (p=0.049), whereas in non-MRMD women, a SA history was associated with decreased T3 concentrations (p=0.02).
Conclusions
A MRMD and a SA history are associated with independent as well as interactive effects on the HPT-axis. The evidence that a MRMD moderates the influence of SA on T3 concentrations contributes to a growing body of work suggesting that a SA history may identify a distinct subgroup of women with MRMD.
Keywords: Menstrually related mood disorders, sexual abuse, thyroid hormones
Introduction
Alterations in the hypothalamus-pituitary-thyroid (HPT) axis are well described in mood disorders, particularly in stress related disorders (1–3). Briefly, activation of the HPT-axis involves hypothalamic release of thyrotropin-releasing hormone (TRH) that stimulates the pituitary release of thyroid stimulating hormone (TSH) that in turn activates the thyroidal production and release of the thyroid hormones, including thyroxin (T4) and tri-iodothyronine (T3), the more biologically potent thyroid hormone. The primary secretory product of the thyroid gland is T4, whereas the majority of T3 comes from extra-thyroidal conversion from T4 via deiodination (1,2).
While the HPT-axis is not responsive to minor stress, it is responsive to severe traumatic stress (4). A number of studies in men with combat related post-traumatic stress disorder (PTSD) have documented elevated T3 concentrations, together with an increase in the T3/T4 ratio, an index of increased peripheral conversion of T4 to T3 (5–7). The clinical relevance of this HPT-axis pattern in combat-related PTSD is suggested by the correlation of elevated T3 concentrations with more severe PTSD symptoms (6,8).
Sexual abuse (SA) represents another traumatic event with estimated lifetime prevalence of up to 22% in the general female population (9). Friedman and colleagues (2005) showed that women with PTSD due to childhood SA had similar HPT-axis profiles as men with combat-related PTSD with evidence for increased peripheral T4 to T3 conversion together with elevated T3 concentrations. Again, there was a positive correlation of PTSD symptoms with T3 concentrations and with the T3/T4 ratio (10).
In addition to PTSD, it has been consistently shown that SA histories are more prevalent in menstrually related mood disorders (MRMDs) (11–13). Up to 30% of childbearing age women experience some form a MRMD, and reported prevalence rates of the more severe form of a MRMD, premenstrual dysphoric disorder, range from 1.3% (after controlling for co-morbid psychopathology) (14) to 7% (15,16). MRMDs are characterized by significant emotional symptoms, physical symptoms and functional impairment that are isolated to the luteal phase of the menstrual cycle (17). Early research on the HPT-axis function in MRMD was inconclusive (18–21). However, more recent research suggests that a SA history may identify a subgroup of MRMD women for whom the HPT-axis is clinically relevant.
Work from our group has shown that MRMD women with a SA history exhibit higher serum T3 concentrations, a greater T3/T4 ratio, and TSH concentrations 75% greater when compared with both non-abused MRMD women and non-abused non-MRMD controls, a pattern remarkably similar to that reported in combat and SA related PTSD (22). These results suggested that MRMD women with a SA history have enhanced central drive of the HPT-axis and increased peripheral T4 to T3 conversion. Additionally, in MRMD women, we found that greater T3 concentrations were associated with more severe premenstrual symptoms (22) similar to findings in PTSD (6,8,10). However, our interpretation of those results was limited by the absence of a group of non-MRMD women with a SA history.
Consequently, in a larger sample than previously studied, we sought to evaluate whether the HPT-axis profile that we previously observed in MRMD women with SA histories (22) is unique to MRMD women with SA, and therefore may be of pathophysiological relevance, or would be evident in all women with SA histories. The conceptual framework guiding this investigation is based on the ample evidence that either very severe traumatic events or traumatic events experienced during critical developmental periods set lifetime trajectories for abnormalities in neuroendocrine systems, which can consequently contribute to poor mental and somatic health outcomes (23–25). Moreover, because MRMD women show unique long-term cardiovascular and adrenergic sequalea associated with an abuse history that is not seen in non-MRMD women with similar histories (13,26), we hypothesized that a history of SA would be associated with greater TSH and T3 concentrations and a greater T4 to T3 conversion in MRMD women only. We also sought to evaluate the clinical relevance of any HPT-axis dysregulation by examining the relationship of HPT-axis measures and premenstrual symptoms. We hypothesized that increased T4 to T3 conversion indices would be associated with more severe premenstrual symptoms.
Methods
Procedure and subjects
The study protocol was approved by the University of North Carolina at Chapel Hill Committee on the Protection of the Rights of Human Subjects.
Women were recruited via newspaper, radio or posted advertisements targeting women with severe premenstrual symptoms (MRMD women) or women without premenstrual symptoms (non-MRMD women). In order to obtain roughly equal proportions of women with a history of SA in the two diagnostic groups it was necessary to selectively recruit non-MRMD women with abuse histories. After confirming MRMD status (below), women were invited for a diagnostic visit, during which they were assessed by a trained interviewer for medical histories, for current and past Axis I psychiatric disorders according to the Diagnostic and Statistical Manual of Mental Disorders 4th edition-Text revision (DSM-IV-TR) (27) using the MINI structured psychiatric interview (28) and for abuse histories using a validated structured interview (29). Also, during the same visit blood samples were drawn for evaluation of HPT-axis hormones concentrations. Women were included in the study if they were in good health, without current chronic medical conditions, including thyroid disorders, or current DSM-IV Axis I psychiatric disorders, including PTSD. None of the subjects was taking prescription medication including oral contraceptives, thyroid medications or psychotropic medications.
A total of 109 women were in our final sample: (a) MRMD with SA histories (n=23; 21%), (b) MRMD without SA histories (n=34; 31%), (c) non-MRMD with SA histories (n=18; 17%) and (d) non-MRMD without SA histories (n=34; 31%). Although the prevalence of SA histories in the subgroup of non-MRMD women was higher than the general population (9) due to targeted recruitment of non-MRMD women with an abuse history, the proportional distribution of women with a SA in the MRMD and non-MRMD groups was still not absolutely equivalent due to the well-documented association between MRMD and abuse histories (11–13).
MRMD diagnosis
MRMD and non-MRMD status was confirmed prospectively using the Daily record of severity of problems (DRSP) (30) that all women completed daily for two to three menstrual cycles. Forms were mailed back weekly in order to discourage retrospective reporting. The DRSP consists of 21 items and allows for the quantification of the severity of physical, emotional and behavioral symptoms of MRMD using a 6-point scale (1 absent, 2 minimal, 3 mild, 4 moderate, 5 severe and 6 extreme). To classify participants as having MRMD, each met the following criteria: (1) at least a 30% change in emotional symptom severity between the seven luteal phase days preceding menses compared with follicular phase days 4–10; (2) a rating of emotional symptoms as moderate, severe or extreme on at least two of the seven premenstrual days; (3) remission of symptoms shortly after the onset of menses followed by a clear symptom free period (≥ 6 consecutive days) during the early-to-mid follicular phase and (4) criteria 1–3 met in at least two menstrual cycles (30,31). Non-MRMD women met the following criteria: (1) no more than minimal emotional symptoms occurring on fewer than three days during the premenstrual week; (2) less than a 30% change in emotional symptom severity from the luteal to the follicular phase; and (3) these criteria met in at least two menstrual cycles.
Assessment of SA histories
A history of SA was verified using a validated structured interview (29), and included the following experiences in which force or threat of harm was used: (a) sexual touching with hands, mouth, or objects; (b) making the subject touch the perpetrator with hands, mouth, or objects; and (c) making the subject have vaginal or anal intercourse. If the subject was younger than 13 years of age at the time of first episode of SA, force or threat of harm was not required if implied by the age differential between perpetrator and victim. This measure of SA history has been associated with multiple measures of poor mental and physical health status in several studies (29,32). In addition, with this instrument, SA history is ascertained by interview using behaviorally specific questions after rapport with patients has been established. This type of methodology has been associated with more reliable and valid responses compared with other abuse history assessments (23).
Assessment of psychiatric histories
The MINI structured diagnostic interview (28) was used to evaluate all women for current and past psychiatric disorders. All diagnoses were based on a consensus diagnostic session with a clinical psychologist. Since lifetime histories of depressive disorders are prevalent in women with MRMD (33), one year in full remission was required for depressive disorders, while for other Axis I disorders three years in remission was required. However, two women who technically met criteria for current anxiety disorders were allowed to participate in the study. One of these women had current Agoraphobia, but her fear of crowds was not associated with Panic attacks. The other woman met criteria for MRMD and for generalized anxiety disorder but her anxiety was restricted in nature and was exacerbated during the premenstrual phase suggesting that her anxiety symptoms were a feature of her MRMD.
Hypothalamic-pituitary-thyroid axis Variables and Assays
Serum concentrations of TSH, total T4, free T4, total T3, free T3, and thyroid binding globulin (TBG) were evaluated. The majority of circulating thyroid hormones are bound to TBG and only a small fraction is unbound (or free) and biologically active. Hence, serum concentrations of T3 and T4 are measured as both free, which includes only unbound hormone, and total, which includes both bound and free hormone, fractions. Thyroid hormones ratios that reflect peripheral conversion of T4 to T3 and thyroid hormones binding were also calculated. The free T3/free T4ratio and total T3/free T4 ratio reflect peripheral conversion of T4 to T3, with higher ratios indicating more conversion. The free T3/total T3 ratio and free T4/total T4 ratio reflect thyroid hormone binding to serum proteins, with lower ratios indicating more binding.
Blood samples for the evaluation of HPT-axis hormones were drawn at a single time-point between 7:00 AM and 9:30 AM (median time of 9:00 AM) without respect to menstrual cycle phase. All women were required to have had at least 6 hours of sleep the night before the venipuncture. Blood was collected into serum separator tubes. Blood samples were centrifuged and serum was frozen at −40 °C. The samples from all patients for each parameter were analyzed in a single batch. Concentrations of thyroid hormones were measured by radioimmunoassay procedures using commercial kitspurchased from Becton-Dickinson. The intra-assay and inter-assay coefficients of variation were, respectively, 6% and 8% for total T4, 5% and 8% for free T4, 5% and 7% for total T3, 4%and 7% for free T3, and 7% and 9% for TBG.
Data analysis
First, we compared the four subgroups of women with respect to demographic characteristics (age and ethnicity), sexual abuse histories (age at first SA and years since last SA), psychiatric histories (depressive disorders, anxiety disorders, PTSD and substance and/or alcohol abuse and/or dependence) and premenstrual symptom severity. A 2 (SA history) × 2 (MRMD status) analysis of variance was used to compare continuous data and Pearson’s Chi-squared test was used to compare categorical data. Significant interactions were followed by post-hoc analyses using the Scheffe test for continuous data and Pearson’s Chi-squared test or Fisher’s exact t-test, as appropriate, for categorical data.
Second, we examined individual and interactive effects of current MRMD status and SA histories on concentrations and ratios of thyroid hormones. For each of the six thyroid hormones and four ratios we performed univariate general linear model analyses with each thyroid measure as the dependent variable and with MRMD status (MRMD or non-MRMD) and SA history (SA history or no SA history) as between-subjects factors. Where significant interactions emerged, they were followed with post-hoc analyses using the independent-samples t-test.
Finally, using Spearman’s rank order correlation, we evaluated the association between premenstrual symptom severity and HPT-axis measures. Average premenstrual symptom severity from the first menstrual cycle was analyzed in order to avoid the confound that repeated symptom monitoring over several months may have on symptoms (34). Core MRMD premenstrual symptoms included: depression (“felt depressed, sad, “down” or “blue”, “felt hopeless” and “felt worthless or guilty”), anxiety symptom (“felt anxious, “keyed up” or “on edge””), mood lability (“had mood swings” and “was more sensitive to rejection or my feelings were easily hurt”) and anger (“felt angry, irritable” and “had conflicts or problems with people”). Luteal phase symptoms were averaged over the six days preceding menses.
Data were analyzed with the PASW 18.0 for Windows (Chicago, Illinois). All continuous data are expressed as means ± standard deviations, all categorical data as number and percent.
Results
Demographics and Psychiatric Histories
There were no differences between the four subgroups in age, ethnicity, age at first SA experience, time since last SA experience, or prevalence of past anxiety disorders (Table 1). Histories of depressive disorders, PTSD and substance and/or alcohol abuse or dependence were more prevalent in non-MRMD women with SA histories relative to non-MRMD women with no SA history (X2 = 14.44, p < 0.001, Fisher’s exact test p < 0.001 and p = 0.005, respectively). Histories of alcohol and/or substance abuse or dependence were also more prevalent in MRMD women with SA histories relative to MRMD women with no SA histories (X2 = 13.07, p < 0.001). As expected, total premenstrual symptom severity and each of the core symptom severities was higher in MRDM women when compared to non-MRMD women irrespective of SA histories (ps < 0.001; data not shown).
Table 1.
Demographic characteristics and psychiatric histories of all women (n=109) stratified by MRMD status and SA history; mean ± SD, n (%).
| MRMD, n=57 | Non-MRMD, n=52 | |||
|---|---|---|---|---|
| SA, n=23 | No SA, n=34 | SA, n=18 | No SA, n=34 | |
| Age, years | 32.43 ± 6.27 | 35.94 ± 8.37 | 35.78 ± 7.13 | 33.47 ± 7.97 |
| Race, white, n(%) | 15 (65) | 24 (71) | 12 (67) | 21 (62) |
| Age at first SA, years | 10.17 ± 5.69 | - | 13.00 ± 7.27 | - |
| Time after last SA, years | 19.52 ± 8.10 | - | 18.61 ± 8.89 | - |
| Depression history, n(%) A | 11 (48) | 11 (32) | 12 (67) | 5 (15) |
| Anxiety history, n(%) | 3 (13) | 9 (27) | 4 (22) | 2 (6) |
| PTSD history, n(%) A | 2 (9) | 2 (6) | 9 (50) | 0 |
| Alcohol and/or substance abuse and/or dependence history, n(%) A,B | 11 (48) | 2 (6) | 6 (33) | 1 (3) |
MRMD - menstrually related mood disorders; SA – sexual abuse; PTSD – post-traumatic stress disorder.
non-MRMD women: SA > no SA, p < .05
MRMD women: SA > no SA, P < .05
HPT-Axis Measures
Table 2 summarizes results of HPT-axis measures stratified by MRMD status and by SA histories. All women with a SA history, irrespective of MRMD status, had higher TSH concentrations (F [108, 3] = 4.88; p = 0.03; Figure 1) and a lower free T3/total T3 ratio (F [108, 3] = 3.92; p = 0.05), the latter suggesting increased binding of T3 in women with SA histories.
Table 2.
Mean concentration of HPT-axis measures in four subgroups of women; mean ± SD.
| MRMD, n=57 | Non-MRMD, n=52 | |||
|---|---|---|---|---|
| SA, n=23 | No SA, n=34 | SA, n=18 | No SA, n=34 | |
| TSH, μU/mL A | 1.71±0.85 | 1.45±0.66 | 2.10 ±1.15 | 1.63±0.74 |
| Free T4, ng/dL B | 0.92±0.18 | 0.90±0.19 | 0.99±0.23 | 1.06±0.20 |
| Total T4, μg/dL B | 7.13±1.08 | 6.91±1.11 | 7.73±1.37 | 7.49±1.21 |
| Free T3, ng/dL C | 2.43±0.68 | 2.31±0.45 | 2.04±0.50 | 2.41±0.50 |
| Total T3, ng/dL C | 141.85±29.57 | 132.35±17.54 | 131.01±21.07 | 138.64±21.19 |
| TBG μg/mL | 19.82±3.88 | 18.79±2.82 | 21.08±4.37 | 20.14±3.68 |
| Free T3 to free T4 ratio D | 2.70±0.75 | 2.61±0.44 | 2.09±0.47 | 2.31±0.44 |
| Total T3 to free T4 ratio D | 158.88±42.41 | 151.17±31.43 | 136.99±32.28 | 135.43±30.48 |
| Free T3 to total T3 ratio E | 0.017±0.003 | 0.018±0.003 | 0.016±0.003 | 0.017±0.003 |
| Free T4 to total T4 ratio | 0.129±0.02 | 0.132±0.027 | 0.132±0.037 | 0.144±0.034 |
TSH = thyroid stimulating hormone; T4 = thyroxin; T3 = tri-iodothyronine; TBG = thyroid binding globulin; MRMD = menstrually related mood disorders; SA = sexual abuse.
SA > no SA, p < .05
MRMD < non-MRMD, p <.05
MRMD x SA histories interaction, p < .05
MRMD > non-MRMD, p < .05
SA < no SA, p < .05
Figure 1.
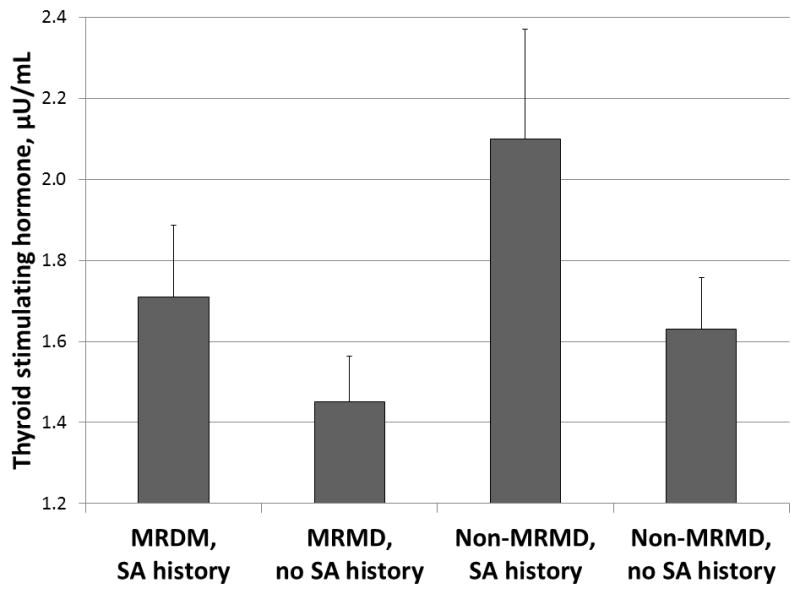
Concentrations of thyroid stimulating hormone (± SEM) stratified by MRMD status and SA histories.
Abbreviations: MRMD = menstrually related mood disorders; SA = sexual abuse; SEM = standard error of mean.
SA histories > no SA histories: F (3, 108) = 4.88, p=0.03.
On the other hand, MRMD women, irrespective of abuse histories, had lower free T4 (F [108, 3] = 8.28; p = 0.01) and total T4 concentrations (F [108, 3] = 6.30; p = 0.01), and higher free T3/free T4 ratio (F [108, 3] = 18.39; p < 0.001) and total T3/free T4 ratios (F [108, 3] = 7.82; p = 0.01; Figure 2), relative to non-MRMD women. This HPT-axis pattern suggests an increase in peripheral conversion of T4 to T3 in MRMD women.
Figure 2.
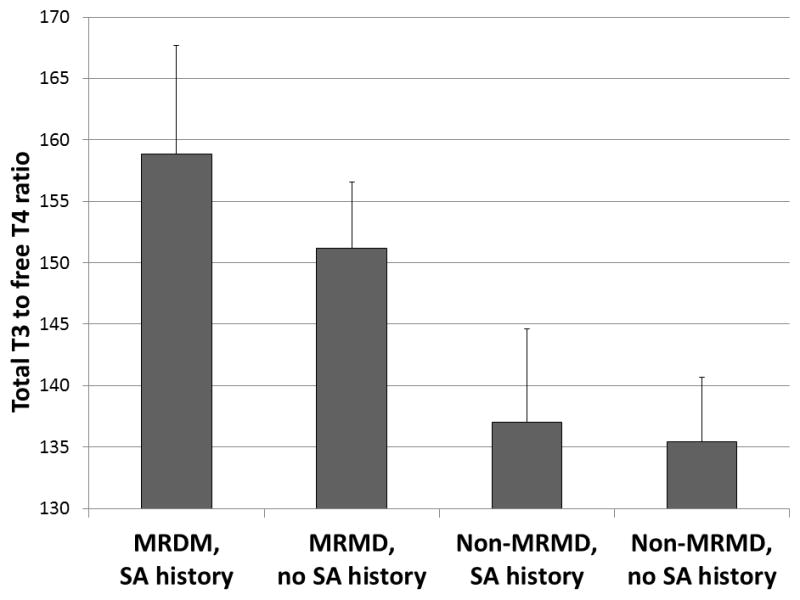
Total T3/free T4 ratio (± SEM) stratified by MRMD status and SA histories.
Abbreviations: MRMD = menstrually related mood disorders; T4 = thyroxin; T3 = tri-iodothyronine; SA = sexual abuse; SEM = standard error of mean.
MRMD > non-MRMD: F (3, 108) = 7.82, p=0.01.
There was an MRMD x SA interaction for free T3 concentrations (F [108, 3] = 5.42; p=0.02; Table 2) and a trend for total T3 concentrations (F [108, 3] = 3.77; p = 0.055), reflecting the directionally different effect that a SA history had on T3 concentrations in MRMD versus non-MRMD women. Post-hoc analyses revealed that MRMD women with SA histories had elevated free T3 concentrations compared with non-MRMD women with SA histories (t = −2.03, p = 0.049), while non-MRMD women with SA histories had lower free T3 concentrations than non-MRMD women without SA histories (t = −2.53, p = 0.02). Although same pattern was evident for total T3 concentrations, individual paired comparisons were not statistically significant.
Relationship of thyroid hormones and luteal phase symptoms
Because many women have some premenstrual symptomatology, albeit not MRMD, and because we have previously documented that a history of abuse is associated with greater premenstrual symptom severity in both MRMD and non-MRMD women (29), we examined the relationship of HPT-axis measures to premenstrual symptoms in the full sample of women. Lower free and total T4 concentrations, and higher free T3/free T4 and total T3/free T4 ratios (all ps <0.05; Table 3) were associated with greater premenstrual anxiety, mood lability and anger symptom severity and total symptom severity. A higher free T3/free T4 ratio was also correlated with greater premenstrual depressive symptom severity (p<0.001).
Table 3.
The association between premenstrual symptoms and thyroid hormones in all women; Spearman’s rho (p).
| Premenstrual symptoms: | Free T4, ng/dL | Total T4, μg/dL | Free T3 to free T4 ratio | Total T3 to free T4 ratio |
|---|---|---|---|---|
| Depression | −0.18 (0.07) | −0.067 (0.49) | 0.410 (<0.001) | 0.12 (0.23) |
| Anxiety | −0.20 (0.04) | −0.191 (0.05) | 0.409 (<0.001) | 0.27 (0.01) |
| Mood lability | −0.25 (0.01) | −0.196 (0.04) | 0.354 (<0.001) | 0.24 (0.01) |
| Anger | −0.22 (0.02) | −0.206 (0.03) | 0.292 (0.002) | 0.26 (0.01) |
| Total symptom severity | −0.24 (0.01) | −0.21 (0.03) | 0.37 (<0.001) | 0.25 (0.01) |
T4 = thyroxin; T3 = tri-iodothyronine.
In bold p≤0.05
Discussion
The results of this study suggest that MRMD status and SA histories are associated with independent, as well as interactive effects on the HPT-axis. For example, greater free T3/free T4 and total T3/free T4 ratios together with lower T4 serum concentrations in MRMD women when compared to non-MRMD women suggest increased peripheral conversion of T4 to T3 in MRMD. Whether the increased peripheral T4 to T3 conversion also reflects greater central conversion in MRMD we cannot say. However, peripheral thyroid hormones readily cross into brain via energy-dependent transport mechanisms (1,2). Thus, it would be unusual for central events to go unchanged when the brain receives a distinctly different proportion of T3 to T4. Furthermore, we found that the low T4 concentrations and elevated T3/T4 ratios were associated with increased premenstrual symptom severity in the entire sample. Additional clinical relevance of increased T4 to T3 conversion that we found in MRMD women (22) and that Wang with colleagues have reported in PTSD (6,8) comes from the evidence that propranolol, a centrally acting beta-adrenergic receptor blocker, is widely used in treatment of thyrotoxicosis and is effective in reducing T3 concentrations (35). Animal studies have shown that propranolol decreases the expression of type II deiodinase, the enzyme involved in the conversion of T4 to T3 in central nervous system, consequentially leading to decreased conversion of T4 to T3 (1,2,36). Propranolol has been shown to be effective in reducing symptom severity in women with severe premenstrual syndrome (37), and several small studies have shown that propranolol is effective in both the treatment and prevention of PTSD (38,39). Taken together, these findings suggest that increased T4 to T3 conversion in both periphery and brain might be pathophysiologically relevant in MRMD.
It must be noted, however, that in the present study not all thyroid hormones were supportive of increased T4 to T3 conversion in MRMD women since T3 concentrations were not elevated in MRMD women as a whole. Greater T4 to T3 conversion together with elevated T3 concentrations have been previously reported in men with combat related PTSD (5–8) and in women with PTSD due to childhood SA (10). It is important to emphasize that our participants did not have current PTSD. In the absence of PTSD, it is possible that in women with MRMD, certain compensatory mechanisms might be responsible for keeping T3, which is the most potent thyroid hormone, within the normal range. For example, lower free T4, which we documented in MRMD, would be expected, via feedback regulation at the pituitary, to increase TSH that would consequently lead to an augmented T4 production by the thyroid gland. However, TSH levels were not significantly elevated in response to lower free T4 concentrations in MRMD women as a whole when compared to non-MRMD women, suggesting possible alterations in the normal feedback regulation of the HPT-axis that may represent a protective mechanism preventing overt stimulation with T3.
In addition to the HPT-axis profile in all MRMD women noted above, we extended our previous results (22) and partially supported our a priori hypotheses by showing that a history of SA identifies a unique subgroup of MRMD women with elevated T3 concentrations when compared to non-MRMD women with a SA history. This is consistent with other prior work as well, demonstrating a different impact of an abuse history on cardiovascular and neuroendocrine reactivity to mental stress and beta-adrenergic receptor responsivity in women with MRMD versus women without MRMD (13,26,40). There are a number of sociodemographic factors, such as time elapsed after last traumatic experience and/or age at the time of first trauma that might influence HPT-axis measures and the relationship of thyroid hormone concentrations to symptoms, and could account for the differential effect of SA on T3 concentrations in the two diagnostic groups (41,42). In our study, however, the MRMD and non-MRMD women were similar in age at first SA experience and time elapsed after last SA episode suggesting that these factors are unlikely to contribute to the SA-related differences in T3 concentrations in the two diagnostic groups.
Another possibility for the diagnosis related differences in the association that SA had on T3 concentrations may relate to coping mechanisms. It has been suggested that different adaptive responses to traumatic stress may influence whether the HPT-axis is activated or suppressed (3,6). For example, in combat situations involving “fight or flight”, mobilization of the HPT-axis is necessary for survival whereas in war prisoners, serious threat is associated with powerlessness and therefore a “shutting down” or immobilization of the HPT-axis might be a more adaptive strategy for survival (3,6). Although in the current study coping strategies were not evaluated, we can speculate that the non-MRMD women might employ avoidant coping strategies following SA, as a recent review indicates (43), and hence an ‘immobilization’ response leading to lower T3 concentrations. In contrast, the elevated total T3 seen in the women with MRMD and a SA history may be part of an arousal response because, in addition to the evidence for increased conversion of T4 to T3 that we found in all MRMD women, the thyroid gland itself can, under emergency situations, meet the body’s metabolic needs by direct secretion of T3 (44). Although normally, the dynamics of the HPT axis are such that negative feedback mechanisms would restore values to baseline levels, the possibility exists that increased free and total T3 concentrations could be sustained in the face of severe stress (45). At the same time, because T3 can easily enter the brain and promote noradrenergic neurotransmission (46), increased T3 may perpetuate further arousal. This is consistent with our prior work showing that MRMD women with an abuse history have greater norepinephrine reactivity to stress relative to non-abused MRMD women, while an abuse history was not associated with differences in norepinephrine reactivity in non-MRMD women (13). Thus, the persistent elevation of total T3 seen in the women with MRMD and a history of SA (and also in men with combat-related PTSD) may represent an adaptation gone awry if the thyroid system continues to prepare the organism for a severe stressor that no longer exists but somehow remains encoded in the brain (45).
Finally, a SA history was associated with an increased TSH concentration, irrespective of MRMD status, suggesting increased central HPT-axis drive associated with SA. Thus, our hypothesis that only MRMD women would show an effect of SA on HPT-axis measures was not supported. We also observed a trend for increased free T3 binding in women with SA histories possibly suggesting a compensatory mechanism from overt stimulation that increased central drive of HPT-axis might cause. Findings of elevated TSH concentrations in women with a SA history may help to explain the previous consistent findings for greater variability in TSH concentrations in MRMD women when compared to non-MRMD women (19–21). Since it is well established that SA histories are more prevalent among MRMD women when compared to non-MRMD women (12,13), greater TSH variability in MRMD women reported previously might be explained by a higher proportion of women with a SA history in the MRMD group. Our findings for elevated TSH associated with a SA history is consistent with both our previous study showing that MRMD women with a SA history exhibited 75% higher TSH concentrations when compared with non-abused MRMD women and with non-abused non-MRMD women (22), and is also consistent with a study in combat veterans with PTSD (6).
The major limitation of our study is that HPT-axis hormones were evaluated at a single time point. A single assessment of the HPT-axis measures might not give a valid picture of the dynamic function of the axis. Thus, in general, for all dynamic endocrine testing pharmacologic challenge and/or repeated measured are preferred. Lack of control for menstrual cycle phase might also be considered another limitation. We and others have previously shown that concentrations of HPT-axis hormones are different in the luteal versus follicular phases of the menstrual cycle (13,19). However, MRMD and SA-related differences in HPT-axis hormone concentrations remained consistent in both cycle phases (13, 19). On the other hand, the major strengths of this study included prospective assessment for MRMD and the use of well-validated instruments for assessment of psychiatric disorders and abuse histories.
In conclusion, our results suggest that both MRMD and SA histories may be associated with unique HPT-axis disturbances, while also suggesting that a MRMD moderates the influence of SA on free and total T3 concentrations, the most biologically active thyroid hormone. Moreover, our results indicate that the unique HPT-axis abnormalities seen in the MRMD sample (increased T4 to T3 conversion coupled with low free T4) is related to more severe premenstrual symptomatology, underscoring the pathophysiological relevance of this profile in MRMD. Finally, SA histories may be associated with increased central drive of the HPT-axis as suggested by elevated TSH concentrations. Since polymorphisms in the transporter genes that actively transport thyroid hormones through the blood-brain barrier are related to different mental-health sequalea (1,2), future studies exploring the association of such genetic polymorphisms with MRMD symptoms, and the moderating influence of an abuse history on that association, may be indicated. One remarkable feature of these findings is that, on average, it had been nearly twenty years since women had experienced sexual abuse, suggesting long-term, persistent disturbance in HPT-axis function following severe traumatic stress, as others have shown following combat exposure (5–8). Lifetime prevalence of SA is approximately 22% in the general female population (9) and is associated with increased risk for psychiatric and somatic disorders (23,47,48). Our findings add to existing evidence and suggest that dysregulation of endocrine function can persists for years after traumatic experiences and may be an important underlying mechanism contributing to increased risk for poor health in SA victims. Healthcare providers should maintain a high level of suspicion for subclinical thyroid dysfunction and consider screening for HPT-axis dysfunction in women with a MRMD diagnosis and/or a SA history.
Acknowledgments
Grant support: NIH: R01 MH051246 and UL 1RR025747, and The Foundation of Hope for the Research and Treatment of Mental Illness.
Acronyms
- DRSP
Daily record of severity of problems
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders 4th edition-Text revision
- HPT
hypothalamus-pituitary-thyroid
- MRMD
menstrually related mood disorder
- PTSD
post-traumatic stress disorder
- SA
sexual abuse
- T4
thyroxin
- T3
tri-iodothyronine
- TBG
thyroid binding globulin
- TRH
thyrotropin-releasing hormone
- TSH
thyroid stimulating hormone
Footnotes
Potential conflicts of interest: None.
References
- 1.Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20:1101–14. doi: 10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 2.Bunevicius R. Thyroid disorders in mental patients. Curr Opin Psychiatry. 2009;22:391–5. doi: 10.1097/YCO.0b013e328329e1ae. [DOI] [PubMed] [Google Scholar]
- 3.Wang S. Traumatic stress and thyroid function. Child Abuse Negl. 2006;30:585–8. doi: 10.1016/j.chiabu.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Mason JW. A review of psychoendocrine research on the pituitary thyroid system. Psychosom Med. 1968;30:666–81. doi: 10.1097/00006842-196809000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Mason J, Southwick S, Yehuda R, Wang S, Riney S, Bremner D, Johnson D, Lubin H, Blake D, Zhou G, Gusman F, Charney D. Elevation of serum free triiodothyronine, total triiodothyronine, thyroxine-binding globulin, and total thyroxine levels in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1994;51:629–41. doi: 10.1001/archpsyc.1994.03950080041006. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Mason J. Elevations of serum T3 levels and their association with symptoms in World War II veterans with combat-related posttraumatic stress disorder: replication of findings in Vietnam combat veterans. Psychosom Med. 1999;61:131–8. doi: 10.1097/00006842-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kozarić-Kovacić D, Karlović D, Kocijan-Hercigonja D. Elevation of serum total triiodothironine and free triiodothironine in Croatian veterans with combat-related post-traumatic stress disorder. Mil Med. 2002;167:846–9. [PubMed] [Google Scholar]
- 8.Wang S, Mason J, Southwick S, Johnson D, Lubin H, Charney D. Relationships between thyroid hormones and symptoms in combat-related posttraumatic stress disorder. Psychosom Med. 1995;57:398–402. doi: 10.1097/00006842-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Plichta SB, Falik M. Prevalence of violence and its implications for women’s health. Womens Health Issues. 2001;11:244–58. doi: 10.1016/s1049-3867(01)00085-8. [DOI] [PubMed] [Google Scholar]
- 10.Friedman MJ, Wang S, Jalowiec JE, McHugo GJ, McDonagh-Coyle A. Thyroid hormone alterations among women with posttraumatic stress disorder due to childhood sexual abuse. Biol Psychiatry. 2005;57:1186–92. doi: 10.1016/j.biopsych.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Pilver CE, Levy BR, Libby DJ, Desai RA. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14:383–93. doi: 10.1007/s00737-011-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golding JM, Taylor DL, Menard L, King MJ. Prevalence of sexual abuse history in a sample of women seeking treatment for premenstrual syndrome. J Psychosom Obstet Gynaecol. 2000;21:69–80. doi: 10.3109/01674820009075612. [DOI] [PubMed] [Google Scholar]
- 13.Girdler SS, Sherwood A, Hinderliter AL, Leserman J, Costello NL, Straneva PA, Pedersen CA, Light KC. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. 2003;65:849–56. doi: 10.1097/01.psy.0000088593.38201.cd. [DOI] [PubMed] [Google Scholar]
- 14.Gehlert S, Song IH, Chang CH, Hartlage SA. The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychol Med. 2009;39(1):129–36. doi: 10.1017/S003329170800322X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biggs WS, Demuth RH. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2011;84:918–24. [PubMed] [Google Scholar]
- 16.Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28 (Suppl 3):1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham J, Yonkers KA, O’Brien S, Eriksson E. Update on research and treatment of premenstrual dysphoric disorder. Harv Rev Psychiatry. 2009;17:120–37. doi: 10.1080/10673220902891836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brayshaw ND, Brayshaw DD. Thyroid hypofunction in premenstrual syndrome. N Engl J Med. 1986;315:1486–7. doi: 10.1056/NEJM198612043152313. [DOI] [PubMed] [Google Scholar]
- 19.Girdler SS, Pedersen CA, Light KC. Thyroid axis function during the menstrual cycle in women with premenstrual syndrome. Psychoneuroendocrinology. 1995;20:395–403. doi: 10.1016/0306-4530(94)00068-9. [DOI] [PubMed] [Google Scholar]
- 20.Roy-Byrne PP, Rubinow DR, Hoban MC, Grover GN, Blank D. TSH and prolactin responses to TRH in patients with premenstrual syndrome. Am J Psychiatry. 1987;144:480–4. doi: 10.1176/ajp.144.4.480. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt PJ, Grover GN, Roy-Byrne PP, Rubinow DR. Thyroid function in women with premenstrual syndrome. J Clin Endocrinol Metab. 1993;76:671–4. doi: 10.1210/jcem.76.3.8445024. [DOI] [PubMed] [Google Scholar]
- 22.Girdler SS, Thompson KS, Light KC, Leserman J, Pedersen CA, Prange AJ., Jr Historical sexual abuse and current thyroid axis profiles in women with premenstrual dysphoric disorder. Psychosom Med. 2004;66:403–10. doi: 10.1097/01.psy.0000127690.38525.ab. [DOI] [PubMed] [Google Scholar]
- 23.Leserman J. Sexual abuse history: prevalence, health effects, mediators, and psychological treatment. Psychosom Med. 2005;67(6):906–15. doi: 10.1097/01.psy.0000188405.54425.20. [DOI] [PubMed] [Google Scholar]
- 24.Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav. 2003;79(3):471–8. doi: 10.1016/s0031-9384(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 25.Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: implications for the pathophysiology of depression and anxiety. Arch Womens Ment Health. 2003;6(1):15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- 26.Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26:201–13. doi: 10.1037/0278-6133.26.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. text rev. [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 29.Leserman J, Drossman DA, Li Z, Toomey TC, Nachman G, Glogau L. Sexual and physical abuse history in gastroenterology practice: how types of abuse impact health status. Psychosom Med. 1996;58:4–15. doi: 10.1097/00006842-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Endicott, Nee J, Harrison W. Daily record of severity of problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41–9. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- 31.Rubinow DR, Roy-Byrne PP, Hoban MC, Gold PW, Post RM. Prospective assessment of menstrually related mood disorders. Am J Psychiatry. 1984;141:684–686. doi: 10.1176/ajp.141.5.684. [DOI] [PubMed] [Google Scholar]
- 32.Girdler SS, Pedersen CA, Straneva PA, Leserman J, Stanwyck CL, Benjamin S, Light KC. Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Res. 1998;81(2):163–78. doi: 10.1016/s0165-1781(98)00074-2. [DOI] [PubMed] [Google Scholar]
- 33.Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- 34.Blake F, Salkovskis P, Gath D, Day A, Garrod A. Cognitive therapy for premenstrual syndrome: a controlled trial. J Psychosom Res. 1998;45:307–18. doi: 10.1016/s0022-3999(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson OR, Melander A, Tegler L. Effects and plasma levels of propranolol and metoprolol in hyperthyroid patients. Eur J Clin Pharmacol. 1980;18:315–20. doi: 10.1007/BF00561388. [DOI] [PubMed] [Google Scholar]
- 36.Yasuzawa-Amano S, Toyoda N, Maeda A, Kosaki A, Mori Y, Iwasaka T, Nishikawa M. Expression and regulation of type 2 iodothyronine deiodinase in rat aorta media. Endocrinology. 2004;145:5638–45. doi: 10.1210/en.2004-0632. [DOI] [PubMed] [Google Scholar]
- 37.Diegoli MS, da Fonseca AM, Diegoli CA, Pinotti JA. A double-blind trial of four medications to treat severe premenstrual syndrome. Int J Gynaecol Obstet. 1998;62:63–7. doi: 10.1016/s0020-7292(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 38.Shad MU, Suris AM, North CS. Novel combination strategy to optimize treatment for PTSD. Hum Psychopharmacol. doi: 10.1002/hup.1171. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 40.Bunevicius R, Hinderliter AL, Light KC, Leserman J, Pedersen CA, Girdler SS. Histories of sexual abuse are associated with differential effects of clonidine on autonomic function in women with premenstrual dysphoric disorder. Biol Psychol. 2005;69:281–96. doi: 10.1016/j.biopsycho.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Haviland MG, Sonne JL, Anderson DL, Nelson JC, Sheridan-Matney C, Nichols JG, Carlton EI, Murdoch WG. Thyroid hormone levels and psychological symptoms in sexually abused adolescent girls. Child Abuse Negl. 2006;30:589–98. doi: 10.1016/j.chiabu.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 42.De Bellis MD, Burke L, Trickett PK, Putnam FW. Antinuclear antibodies and thyroid function in sexually abused girls. J Trauma Stress. 1996;9:369–78. doi: 10.1007/BF02110669. [DOI] [PubMed] [Google Scholar]
- 43.Walsh K, Fortier MA, Dilillo D. Adult Coping with Childhood Sexual Abuse: A Theoretical and Empirical Review. Aggress Violent Behav. 2010;15:1–13. doi: 10.1016/j.avb.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollander CS, Mitsuma T, Shenkman L, Woolf P, Gershengorn MC. Thyrotropin-releasing hormone: evidence for thyroid response to intravenous injection in man. Science. 1972;175:209–10. doi: 10.1126/science.175.4018.209. [DOI] [PubMed] [Google Scholar]
- 45.Prange AJ., Jr Thyroid axis sustaining hypothesis of posttraumatic stress disorder. Psychosom Med. 1999;61:139–40. doi: 10.1097/00006842-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Mason GA, Bondy SC, Nemeroff CB, Walker CH, Prange AJ., Jr The effects of thyroid state on beta-adrenergic and serotonergic receptors in rat brain. Psychoneuroendocrinology. 1987;12:261–70. doi: 10.1016/0306-4530(87)90050-3. [DOI] [PubMed] [Google Scholar]
- 47.Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med. 2009;71(8):805–12. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- 48.Chen LP, Murad MH, Paras ML, Colbenson KM, Sattler AL, Goranson EN, Elamin MB, Seime RJ, Shinozaki G, Prokop LJ, Zirakzadeh A. Sexual abuse and lifetime diagnosis of psychiatric disorders: systematic review and meta-analysis. Mayo Clin Proc. 2010;85(7):618–29. doi: 10.4065/mcp.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]


