Abstract
BACKGROUND
Small-for-size syndrome (SFSS) may occur when graft volume is less than 45% of the standard liver volume, and it manifests as retarded growth and failure of the grafts and an increased mortality. However, its pathogenesis is poorly understood, and few effective interventions have been attempted.
AIMS
The present study aims to delineate the critical role of oxidant stress in SFSS and protective effects of a superoxide dismutase (SOD) mimetic, MnTBAP, on graft function, growth and survival in the recipient rats.
METHODS
Small size graft liver transplantation (SSGLT) was performed to determine the survival, graft injury and growth. MnTBAP was administered in SSGLT recipients (SSGLT+MnTBAP).
RESULTS
Serum ALT levels were sustained higher in SSGLT recipients, which were correlated with an increased apoptotic cell count and hepatocellular necrosis in liver sections. Malondialdehyde content, gene expression of TNF-α and IL-1β and DNA binding activity of NF-κB in the grafts were increased significantly in SSGLT recipients compared to sham-operated controls. Both phosphorylated p38 MAPK and nuclear c-jun were increased in SSGLT. All these changes were strikingly reversed by the administration of MnTBAP, with an increase in serum SOD activity. Moreover, in situ bromo-deoxyuridine incorporation demonstrated that graft regeneration in SSGLT+MnTBAP group was much profound than in the SSGLT group. Finally, the survival of recipients with MnTBAP treatments was significantly improved.
CONCLUSIONS
Enhanced oxidant stress with activation of the p38-c-Jun-NF-κB signaling pathway contributes to SFS-associated graft failure, retarded graft growth and poor survival. MnTBAP effectively reversed the pathologic changes in SFS-associated graft failure.
Keywords: Living donor liver transplantation, Small-for-size syndrome, Oxidant stress, SOD mimetics
INTRODUCTION
Liver transplantation is the only established treatment for liver failure as a result of acute toxicity, hepatitis or end-stage liver disease. More than 25,000 patients are on the waiting list in the US, and only 6,341 received transplants in 2011 with a waiting duration up to 6 years (www.unos.org). T o cope with the severe shortage of donor livers, living donor liver transplantation (LDLT) emerges as an alternative method to shorten the waiting duration and improve the donor liver quality (1). However, the associated ethnical concerns, surgical complexity, and complications in both donors and recipients still limit its wide application. In fact, the number of LDLT performed in the US has declined in recent 8 years (www.unos.org). One of the complications associated with LDLT is small-for-size syndrome (SFSS), which manifests as malfunction, loss or retarded growth of the graft, and is partially caused by excessive portal blood flow and obstructed hepatic venous outflow (2). Both changes may lead to disordered hemodynamics of the portal circulation (2). However, the transient increase in portal circulation could not explain the quick loss and delayed growth of the small size grafts; and the molecular mechanisms underlying the injury and delayed growth in SFSS, which happens when the ratios of graft volume over standard liver volume is less than 45%, are poorly understood.
MnTBAP is a synthetic non-peptidyl mimetic of superoxide dismutase (SOD) with a low molecular weight. It overcomes the extreme short half-life of scavenging activity of the enzyme, and may have a wider clinical application than natural SOD (3, 4). MnTBAP is also nonimmunogenic, and may cross the plasma membrane to neutralize reactive oxygen species (ROS) intracellularly. However, no study is available which investigates whether MnTBAP improves the graft function, survival and growth in small size graft liver transplantation (SSGLT), which is a valuable animal model of LDLT. Our previous studies have documented that significant oxidant stress is one of pathogenic mechanisms in ischemia/reperfusion (I/R)-induced graft injury in SSGLT recipient rats (5). We have employed the SSGLT model to investigate the protective effects of a preischemic maneuver against subsequent I/R-induced injury (5), and miRNA regulation of graft growth in rats (6). In this study, MnTBAP was administrated in the SSG recipients to determine its protective effects on graft function, growth and survival of small-for-size graft recipient rats in order to explore the critical role of oxidant stress in the SFSS development.
RESULTS
Small-for-size-associated graft injury and its attenuation by MnTBAP
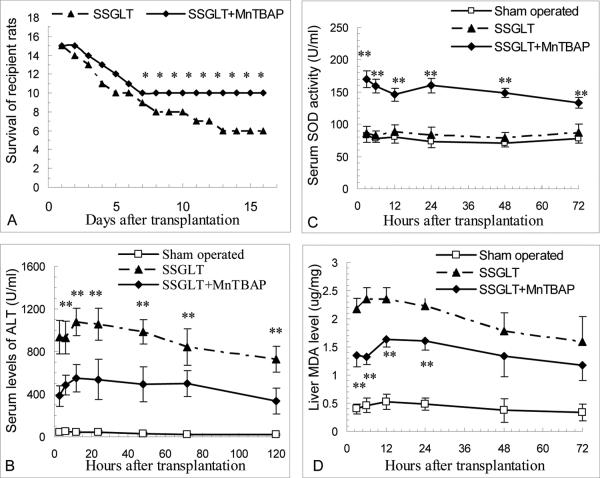
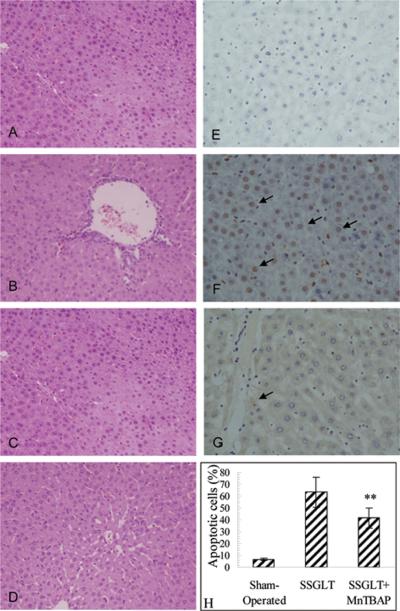
Graft injury and failure with increased mortality is often seen in small-for-size (SFS) liver transplantation with less 45% graft volume in rats (5). Recipient rats with small size liver transplantation at 30% of graft volume underwent severe graft damage with elevated serum levels of ALT activity (Fig. 1B), increased apoptotic cell counts (Fig. 2E&F) compared to the sham-operated animals, and focal to massive cell death in graft histology over one week after transplantation (Fig. 2A,B,C). The graft failure led to a poor survival rate (40%, 6/15) during the first two weeks after transplantation (Fig. 1A). However, with MnTBAP pretreatment in the donor, and the subsequent treatment in recipients after transplantation, serum ALT levels were much lower than in SSGLT recipients without MnTBAP treatment, so were the apoptotic cell counts (65.5±12.7 vs. 41.5±8.6%, p<0.01) 3 days after transplantation (Fig. 2G, H). The improvement in the graft damage was evidenced by H-E staining (Fig. 2D). All these contributed to an improved survival rate (66.7%, 10/15) in recipient rats with MnTBAP treatments (p<0.05) (Fig. 1A). Thus, it is evident that the graft failure and poor survival in 30% SSGLT recipient rats were strikingly improved by a series of MnTBAP treatments.
FIGURE 1.
Animal survival, serum SOD activity, injury and MDA content of small-for-size hepatic graft. A. Survival of SSGLT recipient rats with MnTBAP treatment. MnTBAP was given once daily after the transplantation until sacrifice. Improved survival was seen in those with MnTBAP treatment 8 days after the treatment. * p<0.05 compared to the SSGLT group (n=15). B. Serum ALT levels at different time points after SSGLT. Data were presented as mean±SD, n=6 in each group. *, p<0.05; **, p<0.01 compared to the SSGLT group. C. Increased serum SOD activity in SSGLT recipient rats with MnTBAP treatment was shown. Serum SOD activity was determined at indicated time points after the transplantation. n=6. ** p<0.01 compared to SSGLT. D. Decreased liver MDA levels in SSGLT recipient rats with MnTBAP treatment. Liver MDA was determined at indicated time points spectrophotometrically to represent oxidant stress. n=6 in each group. ** p<0.01 compared to SSGLT alone.
FIGURE 2.
Representative micrographs of liver histology after SSGLT with or without MnTBAP treatment. A–D: Hematoxylin and eosin (H–E) staining for small size liver graft sections at different time points after transplantation: A. 24 hrs after transplantation, B. 3 days after transplantation, C. 5 days after transplantation. D. 3 days after transplantation with MnTBAP treatment. E–G. Representative micrographs of in situ TUNEL staining of apoptotic cells in small size grafts. Each section was examined for 10 high-power fields, and both apoptotic and all cells were counted, and apoptotic cell count was expressed as follows: Apoptotic cell count (%) = (Apoptotic cells/total cell count) × 100. H. E. Sham-operated control. F. Small size graft at 30% volume 72 hours after transplantation. G. Small size graft at 30% volume from recipient rats with MnTBAP treatment. 400×. Average apoptotic cell count (%) in each group 3 days after transplantation. ** p<0.01 compared to SSGLT. n=6 in each group.
Oxidant stress is responsible for SFS-associated graft damage
In order to determine whether oxidant stress is a crucial factor causing severe damage in SSGLT, malondialdehyde (MDA) content in SSG was assayed over time after transplantation. As shown in Fig. 1D, MDA content was markedly increased 3 hours and sustained during the first 3 days after transplantation. In contrast, MnTBAP treatment significantly lowered MDA levels in the graft tissues over time. As a proof of the effectiveness of MnTBAP treatment, elevated serum SOD activity was observed in recipient rats during the first 3 days compared to the sham-operated or SSGLT recipient rats (Fig. 1C). These data indicate that elevated oxidant stress may be one of critical factors responsible for the marked damage in the small size grafts after transplantation, and that the dismutation of superoxide anions or other anti-oxidant effects by MnTBAP appears to be the prime mechanism for its protection as indicated in a long-term animal study with a fatty liver model (7).
TNF-α and IL-1β are the inflammatory mediators for SFS-associated graft injury
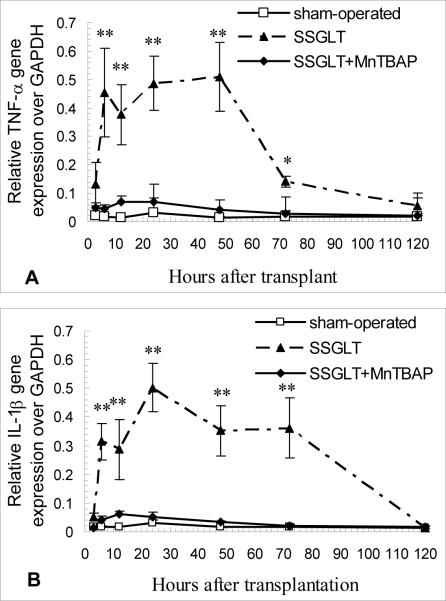
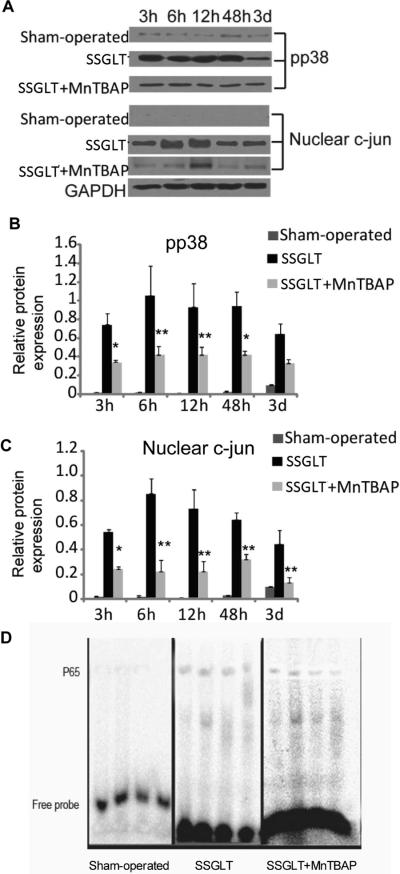
Enhanced oxidant stress in the SFS grafts results in up-regulation of inflammatory cytokines, such as TNF-α and IL-1β, which in turn attract inflammatory infiltration, and activate intracellular signaling pathways. As shown in Fig. 3, gene expression of both TNF-α and IL-1β cytokines was markedly up-regulated as early as 3 hours after the transplant, and sustained over 3 days. The downstream signaling molecule, such as p38 MAPK, was phosphorylated as evidenced by the fact that phosphorylated p38 MAPK content in the cytoplasm was increased significantly (Fig. 4A, B). C-jun is a transcription factor that binds to the enhancer heptamer motif, and re-rallies the activating signaling to further activate genes involved in stress and inflammatory responses. It is also evident that c-jun in the nuclear fraction of small size grafts was strikingly increased, indicating that the nuclear translocation of this transcription factor was enhanced over time after transplantation (Fig. 4A,C). Moreover, NF-κB is a transcription factor responsible for a strong inflammatory response to oxidant stress, and was activated as documented by enhanced DNA binding activity of its p65 subunit in the nuclear extract as shown in Fig. 4D. In contrast, enhanced expression of TNF-α and IL-1β genes and the activation of p38 MAPK, c-jun and NF-κB in the small size grafts were all abrogated markedly by the treatment with SOD mimetic, MnTBAP (Fig. 3, 4). These data indicate that antioxidant treatment not only minimized oxidant stress, but also reduced the inflammatory cytokine release, and abolished intracellular signaling molecules that are key inflammatory regulators, such as NF-κB (8).
FIGURE 3.
Up-regulated expression of TNF-α and IL-1β genes in the mediation of small size graft injury. Increased TNF-α (A) and IL-1β (B) gene expression was demonstrated at an early phase (2–3 days) after SSGLT, and MnTBAP significantly attenuated the enhanced TNF-α and IL-1β gene expression as demonstrated by real time RT-PCR analysis in the graft tissue. The GAPDH gene was used as a house-keeping control. n=6, *, p<0.05, **, p<0.01 compared to the SSGLT group. The sequences of the forward and reverse primers are: 5′-CGA TTT GCC ATT TCA TAC CAG-3′ and 5′-AGT ACT TGG GCA GGT TGAC-3′ for TNF-α (amplicon size: 180 bp); 5′-TGT GGA TCC CAA ACA ATA CCC-3′ and 5′-TAT GTC CCG ACC ATT GC-3′ for IL-1β (amplicon size: 175 bp); 5′-TGT GCA GTG CCA GCC TCG TCT-3′ and 5′-TTG CCG TGG GTA GAG TCA TAC-3′ for GAPDH (amplicon size: 190 bp).
FIGURE 4.
Western blot analysis of activation of p38 MAPK and c-jun signaling pathway in small size graft tissue. A. Phosphorylated p38 MAPK and nuclear c-Jun levels in small size graft tissue at various time points were determined by Western blot analysis using GAPDH as a loading control (shown are the representative images). Please note that the activation of p38 MAPK was observed during the first 48 hours, whereas, nuclear c-jun was enhanced during the first 12 hours after the transplantation. The activation of both p38 MAPK and c-jun was attenuated by the treatment with MnTBAP. B&C. Densitometrical analysis of the Western blot images of p38 MAPK and c-jun was performed with image-pro plus software (n=6). * p < 0.05, ** p < 0.01 compared to the SSGLT group. D. Electrophoretic mobility shift assays (EMSA) of NF-κB in small size graft tissue after transplantation. The biotin 3'-end-labeled, double-stranded specific DNA probe is : 5'-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3' (the bold face indicates NF-κB-binding sites). EMSA image of NF-κB p65 subunit in Sham-operated, SSGLT and SSGLT + MnTBAP groups (n=4). Early activation of NF-κB was demonstrated in the graft tissue of SSGLT recipient rats compared to Sham-operated group, and decreased DNA binding activity was shown in SSGLT + MnTBAP group in comparison to SSGLT.
Improved graft growth contributing to a better survival in SSGLT recipients
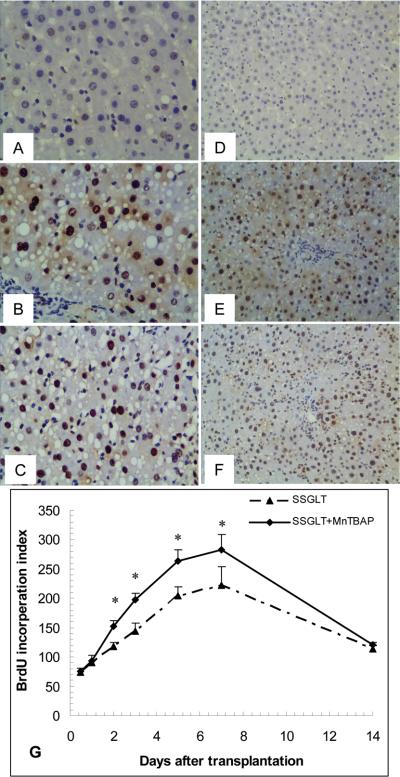
One critical issue in small size graft transplantation is whether the graft will grow after transplantation. The growth rate and capacity of the small size grafts affect the overall graft function and animal survival (6). We employed an in situ 5-bromo-20-deoxyuridine (BrdU) incorporation assay to determine the graft regeneration after transplantation. As shown in Fig. 5, BrdU-positive cells in small size grafts were increased about 3-fold at day 7 in SSGLT without MnTBAP treatment compared to that at 12 hours after transplantation, and then the BrdU-positive cell count started to decline. In contrast, BrdU-positive cell count was significantly higher than in those without MnTBAP treatment from Day 2 to Day 7, which clearly demonstrates that graft growth in the recipient rats with MnTBAP treatments was more profound than in those without the treatment, and that the improved graft growth positively affected the animal survival as shown in Fig. 1.
FIGURE 5.
Graft regeneration assessed by in situ bromodeoxyuridine (BrdU) incorporation. The BrdU-positive cell counts were significantly increased in MnTBAP-treated rats compared to those without MnTBAP treatments after transplantation. Immunohistochemical staining for BrdU-positive cells in liver tissue from the control (A, D), SSGLT (B, E), and SSGLT with MnTBAP treatment (C, F) groups 48h after transplantation. Original magnification 400× (A, B, C) and 200× (D, E, F). The BrdU-positive cells were counted in each section for 10 high-power fields. * p < 0.05 compared to the SSGLT group at the same time point, n=5 at each time point of the two groups.
DISCUSSION
The scarcity of donor livers drives to use small, split or margin grafts for transplantation in orthotopic or LDLT, which often leads to the onset of SFSS (9), presenting as malfunction and retarded growth of the grafts with significant morbidity and mortality (10). Although the causes of SFSS may be multi-factorial in a clinical setting, including lower graft volume over standard liver volume (GV/SLV) (<30–40%), more than 30% steatosis, prolonged ischemia/reperfusion time in graft-related factors, insufficient graft volume is the key factor leading to the pathophysiologic changes, such as portal hyperperfusion with impaired venous outflow and hepatic artery flow, in turn causing sinusoidal congestion and endothelial dysfunction, as well as parenchymal cell death (9, 10). Surgical procedures, for example, porto-systemic shunt and splenectomy, were implemented for reducing hyperperfusion in preventing the occurrence of SFSS (10, 11). Therefore, these studies support the hypothesis that an increase in portal flow with increased portal pressure (see the supplemental data) may be one of crucial factors contributing to the development of SFSS. On the other hand, it was found that portal hyperperfusion in small size grafts at 29% graft volume is the major cause and stimulus for the graft growth in pig recipients (2). Thus, it appears that disordered hemodynamics in the portal circulation plays a significant role in SFSS development although there is no close correlation between portal pressure and flow in the liver transplantation setting (12). Moreover, the portal hyperperfusion hypothesis could not explain all changes in SSFS, such as significant apoptosis and/or necrosis, retarded growth, etc. There is no study available so far to reveal the underlying molecular mechanisms of retarded graft growth and to delineate the critical role of oxidant stress in the mediation of graft dysfunction and delayed growth. Studies aiming at exploring the molecular mechanisms are hampered by the surgical difficulties in establishing rodent models of SSGLT.
In the present study we employed a rat model of SSLGT at 30% graft volume to investigate the critical role of oxidant stress and down-stream signaling mechanisms in the SFSS development. Our data have demonstrated that marked graft injury occurred with highly elevated serum ALT levels, increased apoptotic cell counts and focal to massive cell death in histology during the first 5 days after the transplantation. The survival rate in the recipients was approximately 40% over two weeks. These changes were in parallel with an increase in liver MDA content, which is the classic indicator of enhanced lipid peroxidation, increased expression of inflammatory cytokines, TNF-α and IL-1β, activation of p38 MAPK and c-jun, as well as NF-κB. The enhanced TNF-α and IL-1β expression in the SSG caused the activation of down-stream signaling molecules, such as p38 MAPK and c-jun activation. C-jun is the substrate of JNK, which binds to and phosphorylates c-jun at serines 63 and 73 located within its transactivation domain under stressful and inflammatory stimuli, and triggers the translocation of c-jun into the nuclei to activate the genes involved in stress and inflammation (13). JNK plays an important role in the stress response and is activated by ROS and cytokines, such as TNF-α (14), both of which were obviously increased in the small size grafts (5, 15), contributing to pronounced apoptosis in the graft histology as shown by TUNEL staining in the present study and our previous studies (16). At the same time, the activation of p38 MAPK and JNK elicits the translocation of NF-κB 65 subunit into the nuclei to turn on stress or inflammatory genes, and leads to the initiation and perpetuation of the inflammatory responses (8). In contrast, the treatment with MnTBAP partially reversed all these changes, resulted in much lower serum ALT levels, reduced apoptotic cell counts and improved histology, increased serum SOD activity and lower liver MDA contents, as well as a much improved survival rate in the recipients. All of these data demonstrate that oxidant stress plays a pivotal role in the SFSS development, and that TNF-α/IL-1β initiated-activation of the p-38 MAPK/JNK/c-jun-NF-κB pathway is attributable for the oxidant stress-elicited signaling mechanisms during the onset of SSFS. The treatment with MnTBAP or other SOD mimetics proves to be effective in ameliorating oxidant stress in vitro (17) and radiation-induced oxidant stress in rats (18). To our best knowledge, up to date we are the first group in using this potent antioxidant in improving graft function and survival in SSGLT recipients.
The growth of small size graft is critical for the overall function and well-being of the recipients in LDLT (6). One of the factors which give rise to SSFS is the delayed regeneration response after the implantation (19). The ischemia/reperfusion-induced graft damage, hyperperfusion-associated volume stress, poor quality of donor organs (with steatosis or extended cold-ischemia duration) may all contribute to the further damage and impaired growth of the small-size grafts in recipients (10). In the present study, we found that the treatment with MnTBAP markedly improved the growth of the small size grafts as evidenced with significantly increased BrdU in situ incorporation. The improved growth of the small size grafts in these recipients may be the results of reduced oxidant stress, minimized stress and inflammatory responses as well as injurious process in the grafts. However, it remains to be investigated whether MnTBAP acts on hepatocytes directly or through other molecular pathways, such as inhibiting oxidant stress and/or ameliorating the injury process, in promoting graft growth (6).
In conclusion, the results presented in this study demonstrate that enhanced oxidant stress with activation of the p38 MAPK-c-Jun-NF-κB signaling pathway contributes to small-for-size associated graft failure, retarded graft growth and poor survival. MnTBAP is an effective antioxidant, which strikingly reversed the pathologic changes of small-for-size-associated graft failure, and may have potential clinical application in a transplant setting.
MATERIALS AND METHODS
Animals and small size graft liver transplantation
Male Spraque-Dawley rats (weighing 220–250g) were purchased from Slack Shanghai Laboratory Animal Co., Ltd. (Shanghai, China), and fed standard pellet diet on a 12-hours light/dark cycle with free access to water and food. The animal experiment protocol was approved by the Institutional Ethical Committee of Animal Experimentation, and the experiments were performed strictly according to governmental and international guidelines. Animals were fasted overnight prior to surgical procedures (allowing a free access to water). All surgical procedures were performed under sterile conditions. Animals were divided into Sham-operated (n=6), SSGLT (n=36), and SSGLT + MnTBAP (n=36) groups. Additional 30 animals were used for in situ BrdU incorporation experiment. SSGLT was performed with “two-cuff” method as we previously described (5). The median lobe of the liver was retained to ensure that graft volume/standard liver volume (GV/SLV) was no higher than 30% (20). Rats were restrained in the supine position after administration of anesthetics (ketamine and xylazine) as we previously reported (15). MnTBAP with 99% purity, purchased from Alexis Biochemicals (San Diego, CA) was dissolved in normal saline (NS) and injected intraperitoneally at 10 mg/kg, 4 hours prior to transplant in donor rats and once daily till sacrifice in recipient rats. The SSGLT group received the same volume of normal saline injections each time when MnTBAP was given to rats in the SSGLT + MnTBAP group. After transplant surgery, rats were kept warm for one hour, and provided with 10% glucose in drinking water. In the Sham-operated group, the ligaments around the liver were separated. The abdomen was closed after the liver lobes were
turned upside down and returned back. Normally recipient rats did not receive any antibiotics unless confirmed infection occurred. Otherwise, gentamycin was injected intraperitoneally at 10 mg/kg, once daily for 3–5 days. Fifteen recipient rats from each group were used to observe the survival rate, and the remaining were sacrificed at specific time points to collect liver graft tissue for histologic examinations. Blood samples were collected for serum levels of ALT and SOD activity assays at time points as indicated in the result section.
Serum ALT
Serum ALT was assayed with a routine method using an auto-analyzer (Hitachi 7600-10, Hitachi High-Technologies Corporation, Japan).
Liver histology
Liver tissues were fixed in 10% neutralized formalin at 4°C, paraffin-embedded, sectioned at 4 μm in thickness, and stained with hematoxylin and eosin (H–E) using a standard method.
TUNEL assay
Frozen sections of small size grafts were fixed with 4% paraformaldehyde in PBS, and stained to determine apoptotic cells with an in situ cell death detection kit from Roche-Boehringer Mannheim (Indianapolis, IN) as we previously described (21).
Serum SOD activity and MDA content in the liver graft tissue
Serum SOD activity was determined with the SOD Assay Kit (Jiancheng Biotechnology, Nanjing, China). The SOD activity was expressed as units per milligrams of protein as we previously described (15). MDA content was determined spectrophotometrically by a commercially available kit from Jiancheng Biotechnology (Nanjing, China).
Western blot analysis for phosphorylation of p38 MAPK and c-jun
Total and nuclear protein was extracted from liver graft tissue, and protein concentration was determined as we described previously (22). Western blot analysis was performed according to the method we described previously (6). The primary antibodies against phosphorylated p38 (pp38) MAPK, c-jun (Abcam, Cambridge, MA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cell signaling Technology, Danvers, MA) were used.
Quantitative RT-PCR of TNF-α and IL-1β gene expression
Total RNA was extracted from snap-frozen liver graft tissue by TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription reactions and real-time PCR were run as we previously reported (23). The rat GAPDH was used as the house-keeping gene control. TNF-α, IL-1β and β-actin primers were designed using Primer 3 software, synthesized by Sangon Biotechnology Co. (Shanghai, China).
Electrophoretic mobility shift assays (EMSA)
To determine NF-κB activation, we performed EMSA as we described previously (5, 24). Competition reaction mixtures contained a 100-fold molar excess of non-labeled double-stranded oligo deoxynucleotide.
In situ BrdU incorporation for determination of graft growth
In order to examine mitogenic response after liver transplantation, recipient rats in SSGLT or SSGLT + MnTBAP groups were intravenously injected with BrdU (50 mg/kg) at 12, 24, 48h and 3, 5, 7 and 14 days after the transplantation. They were sacrificed 2 hours after the injection, and the liver tissue was collected and snap-frozen. Frozen sections at 10 μm were prepared in a Cryostat and stained with antibodies against BrdU as previously described (25). Labeling index was determined by counting the number of BrdU-positive nuclei per 1000 nuclei in 10 randomly selected high-power fields under light microscope.
Statistical analysis
All data are presented as means ± standard deviation (SD) and analyzed with the SPSS statistic package. Comparisons of same parameters at corresponding time points between two groups were analyzed by unpaired student t test. Differences among more than two groups were analyzed with one-way analysis of variance with subsequent Newman–Keuls tests. The survival curves of recipient animals were estimated with the Kaplan-Meier method and analyzed by the log-rank test. p values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
Funding information: This study was supported in part by Wu Jie-Ping Medical Foundation (320.670010009) to S. Wang, the National Institutes of Health (DK069939) and a Technology Transfer fund from UC Davis Medical Center to J.W. YYC is a recipient of the China Scholarship Council Award.
Abbreviations used in this manuscript
- ALT
alanine aminotransferase
- BrdU
5-bromo-20-deoxyuridine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- LDLT
living donor liver transplantation
- IL-1β
interleukin-1β
- MAPK
mitogen-activated protein kinase
- MDA
malondialdehyde
- NF-κB
nuclear factor-kappa B
- MnTBAP
Mn(III)tetrakis(4-benzoic acid)porphyrin chloride
- PBS
phosphate buffered saline
- SFSS
small-for-size syndrome
- SOD
superoxide dismutase
- SSGLT
small size graft liver transplantation
- SSG
small size graft
- TNF-α
tumor necrosis factor-α
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling.
Footnotes
There is no financial or any other conflict of interest for all authors.
All authors declare no conflict of interest.
Contributions from each author: Yi-Yao Cui: Designed and performed most of the experiments, and was responsible for data collection and initial manuscript preparation
Jian-Ming Qian: Supervision and technical guidance for liver transplantation.
Ai-Hua Yao: Assisting liver transplant surgeries and animal care.
Xiao-Feng Qian: Histologic examination.
Zhen-Yu Ma: Partial data analysis.
Xiao-Min Zha: Assisting liver transplant surgeries and animal care.
Yi Zhao: Assisting liver transplant surgeries and measuring liver MDA concentration.
Ding Qiang: Assisting liver transplant surgeries and animal care.
Jia Zhao: Animal care and electrophoretic mobility shift assays.
Shui Wang: Overall supervision and funding support.
Jian Wu: Conceptual vision, data analysis, manuscript preparation and partial funding support.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Florman S, Miller CM. Live donor liver transplantation. Liver Transpl. 2006;12(4):499. doi: 10.1002/lt.20754. [DOI] [PubMed] [Google Scholar]
- 2.Fondevila C, Hessheimer AJ, Taura P, et al. Portal hyperperfusion: mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl. 2010;16(3):364. doi: 10.1002/lt.21989. [DOI] [PubMed] [Google Scholar]
- 3.Malassagne B, Ferret PJ, Hammoud R, et al. The superoxide dismutase mimetic MnTBAP prevents Fas-induced acute liver failure in the mouse. Gastroenterology. 2001;121(6):1451. doi: 10.1053/gast.2001.29590. [DOI] [PubMed] [Google Scholar]
- 4.Salvemini D, Wang ZQ, Zweier JL, et al. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286(5438):304. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 5.Qian JM, Zhang H, Wu XF, et al. Improvement of recipient survival after small size graft liver transplantation in rats with preischemic manipulation or administering antisense against nuclear factor-kappaB. Transplant Int. 2007;20(9):784. doi: 10.1111/j.1432-2277.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Murad M, Cui YY, et al. miRNA regulation of liver growth after 50% partial hepatectomy and small size grafts in rats. Transplantation. 2011;91(3):293. doi: 10.1097/TP.0b013e318204756c. [DOI] [PubMed] [Google Scholar]
- 7.Laurent A, Nicco C, Tran Van Nhieu J, et al. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology. 2004;39(5):1277. doi: 10.1002/hep.20177. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Venugopal SK, He S, et al. Ethanol induces apoptosis in hepatocytes by a pathway involving novel protein kinase C isoforms. Cellular Signal. 2007;19(11):2339. doi: 10.1016/j.cellsig.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez HD, Liu ZW, Cashman S, et al. Small for size syndrome following living donor and split liver transplantation. World J Gastrointest Surg. 2010;2(12):389. doi: 10.4240/wjgs.v2.i12.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikegami T, Shimada M, Imura S, et al. Current concept of small-for-size grafts in living donor liver transplantation. Surgery Today. 2008;38(11):971. doi: 10.1007/s00595-008-3771-1. [DOI] [PubMed] [Google Scholar]
- 11.Botha JF, Langnas AN, Campos BD, et al. Left lobe adult-to-adult living donor liver transplantation: small grafts and hemiportocaval shunts in the prevention of small-for-size syndrome. Liver Transpl. 2010;16(5):649. doi: 10.1002/lt.22043. [DOI] [PubMed] [Google Scholar]
- 12.Sainz-Barriga M, Scudeller L, Costa MG, et al. Lack of a correlation between portal vein flow and pressure: toward a shared interpretation of hemodynamic stress governing inflow modulation in liver transplantation. Liver Transplant. 2011;17(7):836. doi: 10.1002/lt.22295. [DOI] [PubMed] [Google Scholar]
- 13.Park SJ, Oh EJ, Yoo MA, Lee SH. Involvement of DNA-dependent protein kinase in regulation of stress-induced JNK activation. DNA Cell Biol. 2001;20(10):637. doi: 10.1089/104454901753340622. [DOI] [PubMed] [Google Scholar]
- 14.Win S, Than TA, Han D, et al. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286(40):35071. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He SQ, Zhang YH, Venugopal SK, et al. Delivery of antioxidative enzyme genes protects against ischemia/reperfusion-induced liver injury in mice. Liver Transpl. 2006;12(12):1869. doi: 10.1002/lt.21001. [DOI] [PubMed] [Google Scholar]
- 16.Liu PG, He SQ, Zhang YH, Wu J. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J Gastroenterol. 2008;14(18):2832. doi: 10.3748/wjg.14.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez MJ, Cederbaum AI. Antioxidant and pro-oxidant effects of a manganese porphyrin complex against CYP2E1-dependent toxicity. Free Radic Biol Med. 2002;33(1):111. doi: 10.1016/s0891-5849(02)00865-1. [DOI] [PubMed] [Google Scholar]
- 18.Abou-Seif MA, El-Naggar MM, El-Far M, et al. Amelioration of radiation-induced oxidative stress and biochemical alteration by SOD model compounds in pre-treated gamma-irradiated rats. Clin Chim Acta. 2003;337(1–2):23. doi: 10.1016/s0009-8981(03)00192-x. [DOI] [PubMed] [Google Scholar]
- 19.Haga J, Shimazu M, Wakabayashi G, et al. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transpl. 2008;14(12):1718. doi: 10.1002/lt.21622. [DOI] [PubMed] [Google Scholar]
- 20.Yao A, Li X, Pu L, et al. Impaired hepatic regeneration by ischemic preconditioning in a rat model of small-for-size liver transplantation. Transplant Immunol. 2007;18(1):37. doi: 10.1016/j.trim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Liu L, Yen RD, et al. Liposome-mediated extracellular superoxide dismutase gene delivery protects against acute liver injury in mice. Hepatology. 2004;40(1):195. doi: 10.1002/hep.20288. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Lingala S, Khoobyari S, et al. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol. 2011;55(10):838. doi: 10.1016/j.jhep.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wege H, Le HT, Chui MS, et al. Telomerase reconstitution immortalizes human fetal hepatocytes without disrupting their differentiation potential. Gastroenterology. 2003;124(2):432. doi: 10.1053/gast.2003.50064. [DOI] [PubMed] [Google Scholar]
- 24.Chen R, Qiu W, Liu Z, et al. Identification of JWA as a novel functional gene responsive to environmental oxidative stress induced by benzo[a]pyrene and hydrogen peroxide. Free Radic Biol Med. 2007;42(11):1704. doi: 10.1016/j.freeradbiomed.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Zern MA, Lizarzaburu ME, et al. Poly(cationic lipid)-mediated in vivo gene delivery to mouse liver. Gene Ther. 2003;10(2):180. doi: 10.1038/sj.gt.3301861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.