Abstract
Background
Heart failure (HF) is the leading cause of hospitalization for Medicare beneficiaries. Nearly half of all HF patients have diastolic HF or HF with preserved ejection fraction (HF-PEF). Because these patients were excluded from major randomized clinical trials of neurohormonal blockade in HF there is little evidence about their role in HF-PEF.
Methods
The aims of the American Recovery & Reinvestment Act-funded National Heart, Lung, and Blood Institute-sponsored “Neurohormonal Blockade and Outcomes in Diastolic Heart Failure” are to study the long-term effects of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and aldosterone antagonists in four separate propensity-matched populations of HF-PEF patients in the OPTIMIZE-HF (Organized Program to Initiate Life-Saving Treatment in Hospitalized Patients with Heart Failure) registry. Of the 48,612 OPTIMIZE-HF hospitalizations occurring during 2003–2004 in 259 U.S. hospitals, 20,839 were due to HF-PEF (EF ≥40%). For mortality and hospitalization we used Medicare national claims data through December 31, 2008.
Results
Using a two-step (hospital-level and hospitalization-level) probabilistic linking approach, we assembled a cohort of 11,997 HF-PEF patients from 238 OPTIMIZE-HF hospitals. These patients had a mean age of 75 years, mean EF of 55%, were 62% women, 15% African American, and were comparable with community-based HF-PEF cohorts in key baseline characteristics.
Conclusions
The assembled Medicare-linked OPTIMIZE-HF cohort of Medicare beneficiaries with HF-PEF with long-term outcomes data will provide unique opportunities to study clinical effectiveness of various neurohormonal antagonists with outcomes in HF-PEF using propensity-matched designs that allow outcome-blinded assembly of balanced cohorts, a key feature of randomized clinical trials.
Keywords: Diastolic heart failure, neurohormonal antagonists, OPTIMIZE-HF, Medicare
1. Introduction
Heart failure (HF) is the leading cause of hospitalization for Medicare beneficiaries and is responsible for >1 million hospitalizations [1, 2]. Therapy with neurohormonal antagonists improve outcomes in systolic HF. Nearly half of the estimated 6 million HF patients in the United States (U.S.) have diastolic HF or HF with preserved ejection fraction (HF-PEF). Despite similar neurohormonal profile and prognosis as that of systolic HF [3, 4], HF-PEF patients were often excluded from major randomized clinical trials (RCTs) in HF and there is little evidence to guide therapy for these patients. When RCTs are impractical or unethical, propensity-matched studies can be used to derive evidence to guide therapy. Propensity scores could be used to design non-RCT studies while remaining blinded to study outcomes, a key feature of RCTs [5–9].
The purpose of the American Recovery & Reinvestment Act-funded National Heart, Lung, and Blood Institute-sponsored study “Neurohormonal Blockade and Outcomes in Diastolic Heart Failure” (R01-HL097047) is to estimate clinical effects of neurohormonal antagonists on long-term outcomes. This will be achieved by conducting four separate propensity-matched studies of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers and aldosterone antagonists in HF-PEF patients in the OPTIMIZE-HF (Organized Program to Initiate Life-Saving Treatment in Hospitalized Patients with Heart Failure) registry [4]. Because OPTIMIZE-HF did not collect data on unique patient or hospital identifiers or long-term outcomes, it was linked to Centers for Medicare and Medicaid Services (CMS) Medicare claims data using a complex probabilistic linking approach [10]. In the current article, we present the rationale and design of the study, the linking process, and compare the baseline characteristics of linked HF-PEF patients with those from RCTs and epidemiological studies of HF-PEF.
1. Methods
2.1. OPTIMIZE-HF
OPTIMIZE-HF is one of the largest HF registries in the U.S., the detail of which have been previously described [4]. OPTIMIZE-HF included extensive data from 48,612 HF hospitalizations occurring in 259 hospitals in 48 states during 2003–2004. Of the 48,612 hospitalizations, 20,839 were due to HF-PEF. GlaxoSmithKline (GSK) sponsored OPTIMIZE-HF but played no role in the design and conduct of the current study. A copy of OPTIMIZE-HF data was obtained from the GSK under a data use agreement (DUA) signed between the GSK and the University of Alabama at Birmingham (UAB). The study was approved by the UAB Institutional Review Board.
2.2. Medicare data
Medicare is the largest health insurance program in the U.S. that provides health care services to older Americans, over 97% of whom are eligible. The Medicare Provider Analysis and Review (MedPAR) File contains data on hospitalizations including dates of admission and discharge for fee-for-service Medicare beneficiaries and the Beneficiary Summary File contains demographic and enrollment information including dates of birth and death. All unique patient identifiers in both Medicare files were replaced with unique encrypted beneficiary identifiers (BeneID). Under a DUA signed between CMS and UAB, we obtained 100% MedPAR File and 100% Beneficiary Summary File between January 1, 2002 and December 31, 2008.
2.3. Probabilistic linking of OPTIMIZE-HF with Medicare data
We used a modified Duke probabilistic linking approach to link unique OPTIMIZE-HF patients to the Medicare data [10]. We excluded 11 Veterans Affairs (VA) hospitals as services provided in VA hospitals are not paid by the Medicare. Linking involved a two-step process: (1) hospital-level, and (2) hospitalization-level. The purpose of the hospital level linkage was to identify the 248 non-VA OPTIMIZE-HF hospitals in the CMS MedPAR data so that they could be used to link unique patients. We began by creating unique “4-variable key” in both OPTIMIZE-HF and Medicare data, using patients’ sex and dates of birth, admission, and discharge. For example, a hospitalization record of a female patient with date of birth of May 21, 1930, date of admission of December 24, 2003, date of discharge of January 08, 2004 was given a “4-variable key” of “May211930Dec242003Jan082004F”.
2.4. Hospital-level linking
We then used the “4-variable key” to identify and link matching hospitalization records in both datasets. If at least 5 hospitalizations from an OPTIMIZE-HF hospital could be found in any of the 2927 hospitals in the Medicare data, then that OPTIMIZE-HF hospital was assigned the corresponding Medicare provider number. For example, if hospital X in OPTIMIZE-HF had 29 hospitalizations (X1 to X29) and 5 of those (e.g., X1 to X5) occurred in Medicare provider number ABC1234 in, then hospital X was assigned the ABC1234. When an OPTIMIZE-HF hospital linked to more than one Medicare hospitals, we linked them to the Medicare hospital with the highest frequency of linked hospitalizations. We also collected Medicare provider numbers for 214 of the 259 OPTIMIZE-HF hospitals by manually checking their names and addresses in a CMS master file for U.S. hospitals. When <5 hospitalizations occurred in a Medicare hospital, we manually linked them based on Medicare provider numbers in both data.
2.5. Patient-level linking
After the hospital-level linking was completed, we added Medicare provider number to the “4-variable keys” to create a new “5-variable keys” for hospitalization-level linking. For example, the “5-variable key” for the hospitalization “May211930Dec242003Jan082004F” occurring in hospital ABC1234 would be “May211930Dec242003Jan082004FABC1234”. The hospitalization-level linking was restricted to OPTIMIZE-HF hospitalizations due to HF-PEF (EF ≥40%) and Medicare hospitalizations associated with a primary discharge diagnosis of HF (ICD-9-CM diagnosis coded of 428.x, 402.x1, 404.x1, or 404.x3). We began by requiring exact matches on all 5 variables. All linked hospitalizations were then removed, and linking process was re-run on the remaining hospitalizations using several relaxed criteria, but never relaxing the criteria for date of birth and hospital Medicare Provider Number (MPN) (Table 1).
Table 1.
Patient-level linking between HF-PEF patients in OPTIMIZE-HFand Medicare claims data
| Rules | Date of birth | Date of admission | Date of discharge | Sex | Medicare provider number | All patients
|
Patients ≥65 years
|
||
|---|---|---|---|---|---|---|---|---|---|
| Hospitalization records | Unique patients | Hospitalization records | Unique patients | ||||||
| 1 | • | • | • | • | • | 12,184 | 11,035 | 11,035 | 10,001 |
| 2 | • | • | • | x | • | 125 | 111 | 119 | 107 |
| 3 | • | •, +1 | • | • | • | 244 | 216 | 220 | 193 |
| 4 | • | •, −1 | • | • | • | 110 | 102 | 97 | 89 |
| 5 | • | • | •, +1 | • | • | 59 | 57 | 53 | 51 |
| 6 | • | • | •, −1 | • | • | 39 | 37 | 34 | 32 |
| 7 | • | •, +1 | • | x | • | <11 | <11 | <11 | <11 |
| 8 | • | •, −1 | • | x | • | <11 | <11 | <11 | <11 |
| 9 | • | • | •, +1 | x | • | <11 | <11 | <11 | <11 |
| 10 | • | • | •, −1 | x | • | <11 | <11 | <11 | <11 |
| 11 | • | • | x | • | • | 504 | 435 | 477 | 412 |
| Total | 13,270 | 11,997 | 12,040 | 10,889 | |||||
+1 = corresponding date + 1 day
−1 = corresponding date − 1 day
When an OPTIMIZE-HF hospitalization linked to multiple Medicare hospitalizations with exact matches on all 5 variables but different BeneID, that record was excluded as it could not be determined which one of the multiple Medicare hospitalization was truly related to the OPTIMIZE-HF patient. Finally, using unique BeneID from the Medicare data, we identified unique patients in the OPTIMIZE-HF data. When one OPTIMIZE-HF patient had more than one hospitalization, we kept the first hospitalization.
2.6. Comparison with other HF-PEF cohorts
We then compared the baseline characteristics of linked HF-PEF patients with those of the unlinked HF-PEF hospitalizations. Finally, we compared baseline characteristics and one-year mortality of the linked HF-PEF patients in the OPTIMIZE-HF with those from four RCTs of HF-PEF (I-PRESERVE, CHARM-Preserved, DIG Ancillary, and PEP-CHF) and three epidemiological datasets of HF-PEF (National HF Project, Mayo Clinic, Rochester, MN and EFFECT study, Canada) [11–17].
2.7. Study designs: Assembling balanced cohorts
We will use propensity scores to assemble matched cohorts in which patients receiving and not receiving a neurohormonal antagonist will be well-balanced in all measured baseline characteristics [18–20]. Propensity score of a patient to receive a drug such as ACE inhibitors will be that patient’s probability for the receipt of that drug based on his/her baseline characteristics. Propensity score for the receipt of ACE inhibitors, ARBs, beta-blockers and aldosterone antagonists will be separately calculated using four separate non-parsimonious multivariable logistic regression models, checking for plausible interactions [21–24]. In each model, the drug under study will be the dependent variable and all measured prognostically important confounders will be used as covariates. Importantly, this model does not include outcomes data and as in RCTs, investigators are blinded to outcomes during study design [9].
The efficacy of propensity score models will be assessed by estimating post-match absolute standardized differences for baseline characteristics that directly quantify biases in their means (or proportions) [25, 26]. Absolute standardized differences will be expressed as percentages of pooled standard deviations and will be presented in Love plots. Values <10% are considered inconsequential and a value of 0% indicates no residual bias. A greedy matching protocol will be used to identify and match patients with similar propensity scores who received and did not receive a drug [27–30]. Clinical effects of each of the four drugs will be estimated in the assembled balanced cohorts using survival analyses.
3. Results
Of the 248 non-VA OPTIMIZE-HF hospitals included 48,116 hospitalizations. There was no missing data for sex, and dates of birth and admission. After excluding 1812 hospitalizations with missing data on dates of discharge, 46,304 hospitalizations had data on all 4 variables. We excluded 337 hospitalizations that exactly matched by all 4 variables with >1 hospitalizations (Figure 1). Using the remaining 45,967 hospitalizations in 248 OPTIMIZE-HF hospitals, we were able to identify 33,476 hospitalizations occurring in 244 hospitals to one or more hospitalizations in the Medicare data by exact “4-variable keys” (Figure 1). Of the 248 OPTIMIZE-HF hospitals, 238 (96% of 248) could be linked and assigned Medicare provider numbers, of which 212 hospitals were linked based on exact matching on ≥5 hospitalizations in both data and 26 hospitals were manually linked (Figure 1).
Figure 1.
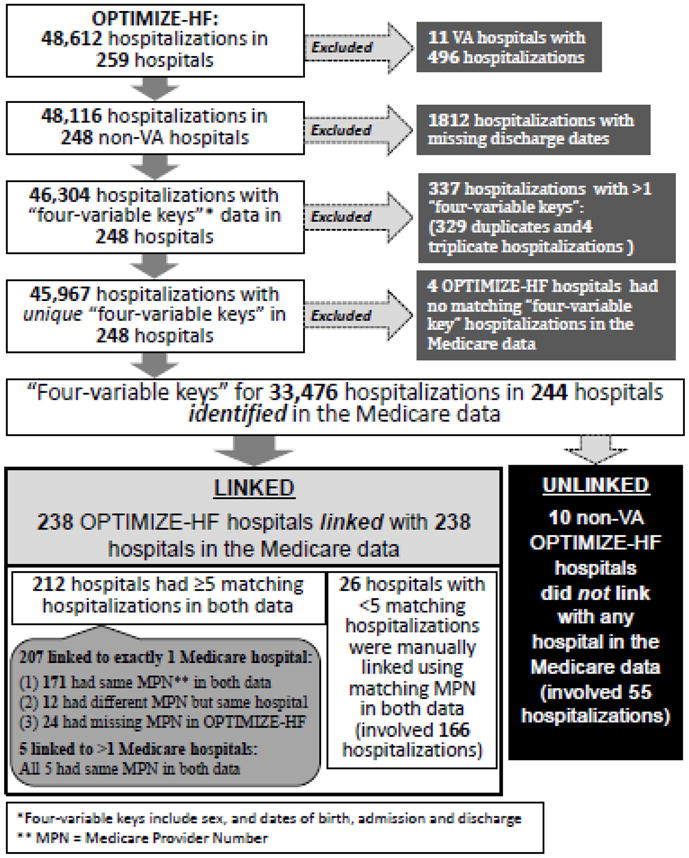
Hospital-level linking between OPTIMIZE-HF and Medicare claims data
Of the 48,612 OPITMIZE-HF hospitalizations occurring in 259 hospitals, 20,839 (43%) were due to HF-PEF. Of these, 13,270 (64% of 20,839) hospitalizations were identified in Medicare data, which occurred in 11,997 unique patients. A total of 11,035 (92% of 11,997) patients were linked by exact matches on all 5 variables (Table 1). Of the 20,839 OPITMIZE-HF hospitalizations due to HF-PEF, 16,477 (79%) occurred in patients ≥65 years of age. Of these hospitalizations, 12,040 (73% of 16,477) were identified in Medicare data that occurred in 10,889 unique older patients (Table 1).
As expected, linked HF-PEF patients were older than unlinked HF-PEF patients (Table 2) as many younger OPTIMIZE-HF patients were not Medicare-eligible. Other baseline characteristics of linked and unlinked HF-PEF patients are displayed in Table 2. Key baseline characteristics and mortality of Medicare beneficiaries with HF-PEF in OPTIMIZE-HF were comparable to those in the population-based data (Table 3).
Table 2.
Characteristics of younger and older heart failure patients with preserved ejection fraction in OPTIMIZE-HF, by linking to the Medicare claims data
| n (%) or mean (±SD) | Overall hospitalizations (n=20839) | Unlinked hospitalizations (N=7569) | Patients linked to Medicare data (n=11997) | P value* |
|---|---|---|---|---|
| Age (years) | 75 (±14) | 69 (±16) | 78 (±11) | <0.001 |
| Female | 12884 (62%) | 4551 (60%) | 7505 (63%) | 0.001 |
| African–American | 3115 (15%) | 1609 (21%) | 1364 (11%) | <0.001 |
| Left ventricular ejection fraction | 55 (±9) | 54 (±9) | 55 (±9) | <0.001 |
| Medical history | ||||
| No known prior heart failure | 2781 (13%) | 1195 (16%) | 1577 (13%) | <0.001 |
| Coronary artery disease | 9490 (46%) | 3156 (42%) | 5615 (47%) | <0.001 |
| Acute myocardial infarction | 3675 (18%) | 1254 (17%) | 2159 (18%) | 0.010 |
| Hypertension | 15808 (76%) | 5730 (76%) | 9114 (76%) | 0.673 |
| Diabetes | 8910 (43%) | 3311 (44%) | 4973 (42%) | 0.002 |
| Atrial fibrillation | 6866 (33%) | 2094 (28%) | 4227 (35%) | <0.001 |
| Stroke or transient ischemic attack | 3433 (17%) | 1076 (14%) | 2114 (18%) | <0.001 |
| Chronic kidney disease | 13455 (65%) | 4419 (58%) | 8031 (67%) | <0.001 |
| Medications prior to admission | ||||
| Angiotensin-converting enzyme inhibitor | 7500 (36%) | 2784 (37%) | 4236 (35%) | 0.036 |
| Angiotensin receptor blocker | 2623 (13%) | 857 (11%) | 1590 (13%) | <0.001 |
| β-Blocker | 10752 (52%) | 3759 (50%) | 6232 (52%) | 0.002 |
| Aldosterone antagonists | 985 (5%) | 362 (5%) | 522 (4%) | 0.157 |
| Diuretic | 13264 (64%) | 4631 (61%) | 7646 (64%) | <0.001 |
| Digoxin | 3518 (17%) | 1082 (14%) | 2174 (18%) | <0.001 |
| Hydralazine | 599 (2.9%) | 224 (3.0%) | 299 (2.5%) | 0.048 |
| Nitrate | 4328 (21%) | 1428 (19%) | 2506 (21%) | 0.001 |
| Aspirin | 7974 (38%) | 2741 (36%) | 4694 (39%) | <0.001 |
| Admission clinical presentation | ||||
| Pulse (beats per minute) | 85 (±21) | 86 (±22) | 84 (±21) | <0.001 |
| Systolic blood pressure (mmHg) | 149 (±34) | 150 (±35) | 149 (±33) | 0.001 |
| Diastolic blood pressure (mmHg) | 76 (±20) | 79 (±21) | 75 (±19) | <0.001 |
| Dyspnea on exertion | 12954 (62%) | 4712 (62%) | 7483 (62%) | 0.866 |
| Dyspnea at rest | 9218 (44%) | 3328 (44%) | 5290 (44%) | 0.863 |
| Orthopnea | 5484 (26%) | 2154 (29%) | 3015 (25%) | <0.001 |
| Paroxysmal nocturnal dyspnea | 2891 (14%) | 1126 (15%) | 1592 (13%) | 0.002 |
| Râles | 6597 (64%) | 4709 (64%) | 7734 (65%) | 0.006 |
| Edema | 13903 (67%) | 5126 (69%) | 7955 (66%) | 0.041 |
| Laboratory data | ||||
| Creatinine (mg/dL) | 1.7 (±1.6) | 1.7 (±1.5) | 1.7 (±1.6) | 0.011 |
| Sodium (mEq/L) | 137 (±11) | 137 (±11) | 137 (±11) | 0.281 |
| Hemoglobin (g/dL) | 12 (±4) | 12 (±5) | 12 (±3) | <0.001 |
| B-type natriuretic peptide (pg/mL) | 973 (±1085) | 954 (±1079) | 962 (±1064) | 0.682 |
| Hospital characteristics | ||||
| Academic hospital | 109/247 (44%) | 21/25 (84%) | 88/221 (40%) | <0.001 |
| Interventional (CABG/PCI) capability | 152/247 (62%) | 19/25 (76%) | 133/221 (60%) | 0.232 |
| Heart transplant capability | 28/247 (11%) | 4/25 (16%) | 24/221 (11%) | 0.511 |
| Bed size >500 bed | 51/247 (21%) | 2/25 (8%) | 49/221 (22%) | 0.099 |
Abbreviations: CABG/PCI= coronary artery bypass graft surgery or percutaneous coronary intervention;
P values comparing linked patients with unlinked hospitalizations
Table 3.
Baseline characteristics of younger and older heart failure patients with preserved ejection fraction (HF-PEF) in OPTIMIZE-HF, compared with HF-PEF patients in clinical trials and epidemiological studies
| % or mean (±SD) |
Linked OPTIMIZE -HF (n=11997) |
National HF Project (n=6754) |
Mayo Clinic (n=2167) |
EFFECT (n=880) |
DIG- Ancillary* (n=988) |
PEP- CHF* (n=850) |
CHARM- Preservred* (n=3023) |
I- PRESERVE* (n=4128) |
|---|---|---|---|---|---|---|---|---|
| [11] | [17] | [16] | [14] | [15] | [13] | [12] | ||
| Age (years) | 78 (±11) | 80 (±8) | 74 (±14) | 75 (±12) | 67 (±10) | 75 | 67 (±11) | 72 (±7) |
| Female | 63% | 71% | 66% | 66% | 41% | 56% | 40% | 63% |
| African–American | 11% | 9% | N/A | N/A | 14%† | N/A | 4% | 2% |
| LVEF (%) | 55 (±9) | 55 (±9) | 61 (±7) | 62 | 55 (±8) | 64 | 54 (±9) | 59 (±9) |
| Medical history | ||||||||
| CAD | 47% | 46% | 53% | 34% | N/A | N/A | N/A | N/A |
| AMI | 18% | 21% | N/A | 17% | (50% | 27% | 44% | 23% |
| Hypertension | 76% | 69% | 63% | 55% | 60% | 79% | 64% | 88% |
| Diabetes mellitus | 42% | 37% | 33% | 32% | 32% | 21% | 28% | 27% |
| Atrial fibrillation | 35% | 36% | 41% | 32% | (excluded) | 20% | 29% | 35% |
| Medications prior to admission | ||||||||
| ACE inhibitors | 35% | N/A | N/A | N/A | 86% | N/A | 19% | 25% |
| ARBs | 13% | N/A | N/A | N/A | N/A | N/A | N/A | |
| β-Blockers | 52% | N/A | N/A | N/A | N/A | 54% | 56% | 59% |
| Aldosterone antagonists | 4% | N/A | N/A | N/A | N/A | N/A | 12% | 15% |
| Diuretics | 64% | N/A | N/A | N/A | 76% | 44%‡ | 75% | 83% |
| Digoxin | 18% | N/A | N/A | N/A | 35% | 12% | 28% | 14% |
| Admission clinical and laboratory data | ||||||||
| Heart rate (bpm) | 84 (±21) | N/A | N/A | N/A | N/A | 73 | 71(±12) | 71 (±11) |
| SBP (mm Hg) | 148 (±33) | N/A | N/A | N/A | N/A | 139 | 136 (±18) | 136 (±15) |
| DBP (mm Hg) | 75 (±19) | N/A | N/A | N/A | N/A | 80 | 78 (±11) | 79 (±9) |
| Creatinine (mg/dL) | 1.7 (±1.6) | N/A | 1.6 (±1.1) | N/A | 1.3 (±0.4) | 1.0 | N/A | 1.0 (±0.33) |
| One-year mortality | 30.9% | N/A | 29% | 22.5% | 6% | N/A | 5.4% | 5.2%§ |
Randomized clinical trials,
non-white,
loop diuretics,
estimate based on annual mortality rate
Discussion
Findings from this study demonstrate that by linking OPTIMIZE-HF patients with HF-PEF to Medicare claims data we were able to assemble one of the largest cohorts of HF-PEF patients with long-term outcomes data. These patients are in general representative of Medicare beneficiaries with HF-PEF, who represent nearly half of all HF patients. The assembled cohort will serve as the basis for four propensity-matched studies of neurohormonal blockade in HF-PEF for whom there is little or no RCT data available, and will serve as a resource for future similar studies.
Baseline characteristics and outcomes of patients in OPTIMIZE-HF have been shown to be similar to that of Medicare beneficiaries with HF [31]. Findings from the current study suggest that OPTIMIZE-HF patients with HF-PEF are also similarly representative of a broader population of Medicare beneficiaries with HF-PEF. The younger age, fewer female and more African American patients in our data compared to the National HF Project is likely due to our inclusion of younger patients [11]. Despite these apparent demographic dissimilarities, HF-PEF patients in our study had similar comorbidity burden and one-year mortality to those in the other two epidemiological cohorts. However, the lower mortality in those enrolled in RCTs of HF-PEF may be explained by the selection bias associated with RCT enrollment.
Several key aspects of the modification of the Duke approach of linking employed in this study deserve further discussion. Our inclusion of younger patients allowed us to link 1100 younger HF-PEF patients. HF is a qualifying disability for Medicare beneficiaries <65 years, and nearly half of these younger Medicare beneficiaries with HF are African American, who are at a higher risk for HF hospitalization [2]. We also used stricter criteria for the hospitalization-level linkage and did not relax the criteria for date of birth and hospital. Although this may have resulted in the smaller size of our assembled cohort, we were able to link 73% (12,040/16,477) of hospitalizations due to HF-PEF among those ≥65 years, which is comparable to the 75% linking reported by Hammill et al. [2]. Finally, our manual collection of Medicare provider numbers allowed us to link additional OPTIMIZE-HF patients to who did not link by probabilistic linking.
Our study has several limitations. The mean age of non-linked patients was 69 years (9 years younger than those linked) suggesting that a large number of patients ages ≥65 years were not linked. This is in part explained by patients enrolled in the Medicare managed care programs, those admitted to VA hospitals, those not using Medicare as primary insurance and other administrative and human errors involved in the collection and processing of large datasets. Finding based on this study may not be generalizable to all Medicare beneficiaries as MedPAR Files do not include data on about 15 to 25% of Medicare beneficiaries who are enrolled in Medicare managed care plans. Finally, we had no data on diastolic function for those with HF-PEF.
In conclusion, the OPTIMIZE-HF cohort of HF-PEF patients linked to Medicare outcomes data is one of the largest HF-PEF cohorts with long-term outcomes data that will provide unique opportunities to examine the effectiveness of neurohormonal antagonists in HF-PEF in studies designed using propensity scores which, as in RCTs, would allow outcome-blinded assembly of balanced cohorts.
Acknowledgments
Funding Sources: Dr. Ahmed is supported by grants (R01-HL085561 and R01-HL097047) from the National Heart, Lung, and Blood Institute (NHLBI), Bethesda, Maryland and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [32].
Footnotes
Conflict of Interest Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed A. Chronic heart failure in older adults. Med Clin North Am. 2011;95:439–61. ix. doi: 10.1016/j.mcna.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feller MA, Mujib M, Zhang Y, et al. Baseline characteristics, quality of care, and outcomes of younger and older Medicare beneficiaries hospitalized with heart failure: Findings from the Alabama Heart Failure Project. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–77. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–9. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin PC. Primer on statistical interpretation or methods report card on propensity-score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:62–7. doi: 10.1161/CIRCOUTCOMES.108.790634. [DOI] [PubMed] [Google Scholar]
- 7.Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32:1704–8. doi: 10.1093/eurheartj/ehr031. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 9.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 10.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–23. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 12.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 17.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 18.Ekundayo OJ, Adamopoulos C, Ahmed MI, et al. Oral potassium supplement use and outcomes in chronic heart failure: a propensity-matched study. Int J Cardiol. 2010;141:167–74. doi: 10.1016/j.ijcard.2008.11.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed A, Young JB, Love TE, Levesque R, Pitt B. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. Int J Cardiol. 2008;125:246–53. doi: 10.1016/j.ijcard.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed A, Pitt B, Rahimtoola SH, et al. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: a propensity-matched study of the DIG trial. Int J Cardiol. 2008;123:138–46. doi: 10.1016/j.ijcard.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gheorghiade M, Flaherty JD, Fonarow GC, et al. Coronary artery disease, coronary revascularization, and outcomes in chronic advanced systolic heart failure. Int J Cardiol. 2011;151:69–75. doi: 10.1016/j.ijcard.2010.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed MI, Lainscak M, Mujib M, et al. Gender-related dissociation in outcomes in chronic heart failure: reduced mortality but similar hospitalization in women. Int J Cardiol. 2011;148:36–42. doi: 10.1016/j.ijcard.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitt B, Zannad F, Gheorghiade M, et al. Transatlantic similarities and differences in major natural history endpoints of heart failure after acute myocardial infarction: a propensity-matched study of the EPHESUS trial. Int J Cardiol. 2010;143:309–16. doi: 10.1016/j.ijcard.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giamouzis G, Agha SA, Ekundayo OJ, et al. Incident coronary revascularization and subsequent mortality in chronic heart failure: a propensity-matched study. Int J Cardiol. 2010;140:55–9. doi: 10.1016/j.ijcard.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamopoulos C, Pitt B, Sui X, Love TE, Zannad F, Ahmed A. Low serum magnesium and cardiovascular mortality in chronic heart failure: a propensity-matched study. Int J Cardiol. 2009;136:270–7. doi: 10.1016/j.ijcard.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sui X, Gheorghiade M, Zannad F, Young JB, Ahmed A. A propensity matched study of the association of education and outcomes in chronic heart failure. Int J Cardiol. 2008;129:93–9. doi: 10.1016/j.ijcard.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekundayo OJ, Dell’Italia LJ, Sanders PW, et al. Association between hyperuricemia and incident heart failure among older adults: a propensity-matched study. Int J Cardiol. 2010;142:279–87. doi: 10.1016/j.ijcard.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed MI, Mujib M, Desai RV, et al. Outcomes in younger and older adults with chronic advanced systolic heart failure: A propensity-matched study. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie C, Ekundayo OJ, Muchimba M, et al. Effects of diabetes mellitus in patients with heart failure and chronic kidney disease: a propensity-matched study of multimorbidity in chronic heart failure. Int J Cardiol. 2009;134:330–5. doi: 10.1016/j.ijcard.2008.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alper AB, Campbell RC, Anker SD, et al. A propensity-matched study of low serum potassium and mortality in older adults with chronic heart failure. Int J Cardiol. 2009;137:1–8. doi: 10.1016/j.ijcard.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis LH, Greiner MA, Hammill BG, et al. Representativeness of a national heart failure quality-of-care registry: comparison of OPTIMIZE-HF and non-OPTIMIZE-HF Medicare patients. Circ Cardiovasc Qual Outcomes. 2009;2:377–84. doi: 10.1161/CIRCOUTCOMES.108.822692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1–2. [Google Scholar]


