Abstract
Background and Objectives
Pseudomonas aeruginosa possesses a variety of virulence factors that may contribute to its pathogenicity. The aim of this study was to evaluate oprI, oprL and toxA genes for PCR identification of clinical P. aeruginosa. In order to find out any relation between special virulence factors and special manifestation of P. aeruginosa infections, we detected virulence factors among these isolates by PCR. Ribotyping was used to evaluate the clonal relationship between strains with the same genetic patterns of the genes studied.
Materials and Methods
In this study, 268 isolates of P. aeruginosa were recovered from burn, wound and pulmonary tract infections. The prevalence of oprI, oprL, toxA, lasB, exoS and nan1 genes was determined by PCR. One hundred and four isolates were selected randomly to investigate clonal diversity of the isolates with ribotyping using SmaI.
Results and Conclusions
All P. aeruginosa isolates in this study carried oprI, oprL and lasB genes. Difference between exoS prevalence in isolates from pulmonary tract and burn isolates was statistically significant. Prevalence of nan1 and toxA gene was significantly higher in pulmonary tract and burn isolates, respectively. Ribotyping showed that most of the isolates (87%) belonged to clone A and B.
Detection of oprI, oprL and toxA genes by PCR is recommended for molecular identification of P. aeruginosa. Determination of different virulence genes of P. aeruginosa isolates suggests that they are associated with different levels of intrinsic virulence and pathogenicity. Ribotyping showed that strains with the same genetic patterns of the genes do not necessarily have similar ribotype patterns.
Keywords: Pseudomonas aeruginosa, ribotyping, virulence factors
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen capable of infecting virtually all tissues. Pulmonary tract colonization with mucoid P. aeruginosa is a major cause of morbidity and mortality in patients with cystic fibrosis (1). P. aeruginosa infections in hospitals mainly affect the patients in intensive care units and those having catheterization, burn, and/or chronic illnesses (2).
Although conventional microbiological methods for identifying P. aeruginosa from clinical and environmental samples are reliable, they require several days to be completed. Rapid detection of isolates causing hospital infections is very important for consequent treatment decision of patients.
PCR has the potential for identifying microbial species rapidly by amplification of sequences unique to a particular organism (3). L and I lipoproteins are two outer membrane proteins of P. aeruginosa responsible for inherent resistance of P. aeruginosa to antibiotics and antiseptics. As these proteins are found only in this organism, they could be a reliable factor for rapid identification of P. aeruginosa in clinical samples (4–6).
P. aeruginosa also has a large number of virulence factors such as exotoxin A, exoenzyme S, elastase and sialidase which are tightly regulated by cell-to-cell signalling systems (7). Protein biosynthesis is inhibited by exotoxin A and virulence factor exoenzyme S is secreted by a type III section system (8, 9). A zinc metalloprotease called Las B has an elastolytic activity on lung tissue (10). The gene called nan1 encodes a sialidase that is responsible for adherence to the respiratory tract (11).
In epidemiologic studies, dissemination of resistant and highly virulent pathogens is also the main problem worldwide. Because of highly conserved rRNA sequences among the eubacteria, chromosomal DNA restriction fragment length polymorphism of rRNA genes (ribotyping) is a powerful approach to discriminate strains, between and within species (12).
In this study, we examined rapid identification of P. aeruginosa isolated from pulmonary tract, wound and burn samples based on PCR amplification of I lipoprotein (oprI) for detection of genus and L lipoprotein (oprL) for detection of species of this organism. The toxA gene was also examined to evaluate molecular detection of the isolates by this factor. Furthermore, in order to find any relation between special virulence factors and special manifestation of P. aeruginosa infections, we detected exoS, nan1, lasB virulence factors among these isolates by PCR. Ribotyping was also used to evaluate the clonal relationship between strains with the same genetic patterns of the genes studied in this research.
MATERIALS AND MTHODS
Bacterial strains and identification test
Totally 268 P. aeruginosa isolates including 100 strains recovered from burn, 50 from wound and 118 from pulmonary tract infections were obtained from patients admitted in four hospitals in Tehran, Iran. Each strain was identified on the basis of colony morphology and conventional biochemical testes (13).
Preparation of bacterial DNA
All isolates were inoculated aerobically on tryptose soy broth for 18–24 hour at 37° C. Bacterial DNA extraction was performed using phenol-chloroform method as previously described (14).
Detection of virulence genes by PCR
PCR amplifications of the oprI, oprL, toxA, exoS, nan1 and lasB genes were performed in 25 µl reaction mixture containing 0.5 µl of dNTPs (10 mM), 0.5 µl of each primer (10 pmol), 1.5 µl MgCl2 (25 mM), 0.2 µl Taq DNA polymerase (5 U/µl) (Fermentas, Lithuania) (3, 4, 15). Each gene was amplified separately. Pseudomonas aeruginosa ATCC 27853 and E. coli ATCC 25922 were used as positive and negative control respectively. PCR products were visualized by electrophoresis using a 1% agarose gel stained with ethidium bromide.
Ribotyping
Ribotyping was performed as described previously (16). In brief, the extracted DNA from P. aeruginosa strains was cleaved by SmaI restriction endonuclease (Fermentas, Lithuania). The fragments were separated by electrophoresis and then transferred to nylon membrane by vacuum blotter (Bio-Rad Laboratories, Hercules, CA). Hybridization was performed by probes labeled with digoxigenenin. The membrane was then visualized by NBT (nitroblue tetrazolium) and BCIP (5-bromo-4-chloro-3-indolyl phosphate).
Statistical method
The distribution of virulence genes with respect to strain origin was compared using the Chi square test.
RESULTS
The oprI and oprL genes were detected in all of 268 P. aeruginosa isolates collected. However, presence of toxA gene in clinical samples was different. According to Table 1 the presence of toxA gene in isolates from burn was significantly higher than pulmonary tract (P < 0.05).
Table 1.
Prevalence of toxA, exoS and nan1 among P. aeruginosa obtained from various sources.
| 1 | 2 | 3 | ||
|---|---|---|---|---|
|
|
||||
| Virulence gene | Wound (%) (n= 50) | Burn (%) (n = 100) | Pulmonary tract (%) (n = 118) | P value (Chi square test) |
| toxA+ | (45) 90 | (97) 97 | 101 (85.6) | *0.004 = (2,3) |
| exos+ | 31 (62) | (67) 67 | 56 (47.4) | *0.004 = (2,3) |
| nan1+ | 15 (30) | (4) 4 | 55 (46.6) | (1,2) = 0.000 = (1,3)* *0.000 = (2,3)* 0.04 |
Our results showed that all tested isolates harbored lasB gene. However, difference between exoS prevalence in isolates from pulmonary tract and burn isolates was statistically significant (P < 0.05). The nan1 gene, other virulence factor studied in this research, was found in 55 (46.6%) of 118 isolates from pulmonary tract, 15 (30%) of 50 from wound and 4 (4%) of 100 from burn specimens. The prevalence of nan1 gene was significantly higher in isolates of pulmonary tract than burn specimens (P < 0.05). There was a borderline significant difference in the prevalence of nanI gene among the isolates from pulmonary tract and wound infections. Furthermore, the prevalence of nan1 among wound isolates was significantly higher than burn isolates (P < 0.05) (Table 1).
The isolates were divided into 8 genetic groups (I-VIII) based on the presence of six genes amplified by PCR (Table 2).
Table 2.
Various genetic groups of P. aeruginosa isolates according to the presence of virulence genes. oprI, oprL and lasB occurred in all isolates.
| Isolate | Wound | Burn | Pulmonary tract | Total |
|---|---|---|---|---|
| Genetic group | ||||
| I | 14 | 3 | 16 | 33 |
| II | 1 | 1 | 31 | 33 |
| III | 17 | 36 | 33 | 86 |
| IV | 13 | 57 | 21 | 91 |
| V | 2 | 2 | 2 | 6 |
| VI | 3 | 1 | 7 | 11 |
| VII | 0 | 0 | 5 | 5 |
| VIII | 0 | 0 | 3 | 3 |
| Total | 50 | 100 | 118 | 268 |
group I: presence toxA, exoS and nan1; group II: presence toxA and nan1; group III: presence toxA and exoS; group IV: presence toxA; group V: presence exoS; group VI: nonexistence toxA, exoS and nan1; group VII: presence exoS and nan1; group VIII: presence nan1.
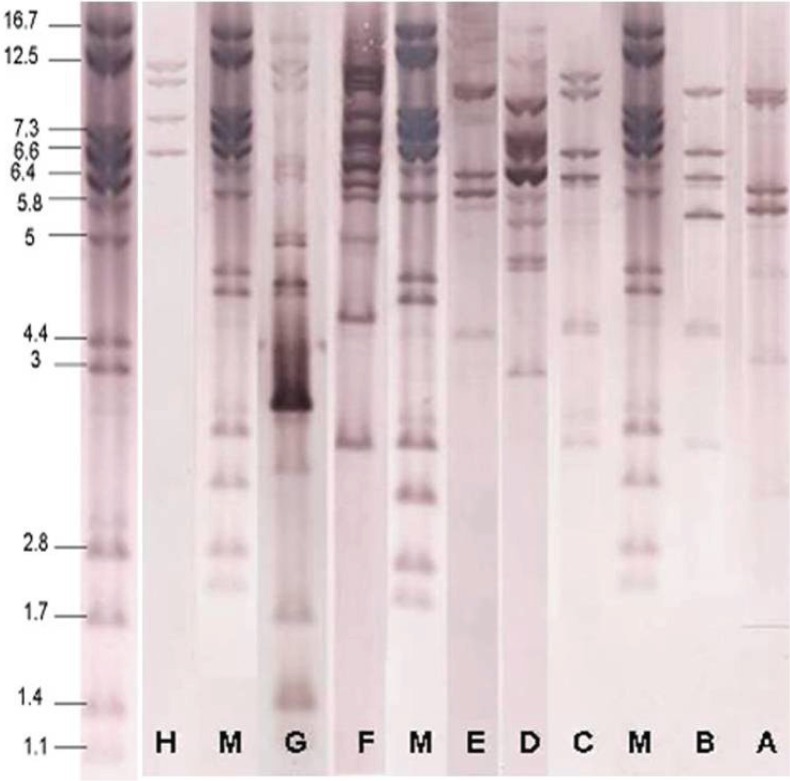
Of 8 genetic groups obtained from PCR results, 104 isolates were randomly selected to investigate clonal diversity of the isolates by ribotyping. Ribotyping patterns of the isolates compared by visual inspection. Ribotyping analysis generated 8 distinct patterns (A-H) (Fig. 1). These isolates were distributed in ribotypes A (53 isolates), B (28 isolates), C (5 isolates), D (4 isolates), E (1 isolate), F (1 isolate), G (1 isolate) and H (1 isolate) patterns (Table 3).
Fig. 1.
Ribotyping of P. aeruginosa. M ribotype is related to Citrobacter koseri CIP 105177 (Grimont 32) that selected as marker.
Table 3.
Numbers and ribotype patterns for genetic groups of P. aeruginosa isolates.
| Genetic group | I | II | III | IV | V | VI | Total |
|---|---|---|---|---|---|---|---|
| Isolate | |||||||
| Wound | 11 (8A,3B) | 1 (1B) | 11 (6A,4B,1E) | 13 (2A,10B,1C) | 1 (1D) | 3 (2C,1F) | 40 (16 A,18B,3C,1D,1E,1F) |
| Burn | 2 (2A) | 0 | 19 (17A,2B) | 10 (7A,3B) | 0 | 3 (1G,1A,1H) | 34 (27A,5B,1G,1H) |
| Pulmonary tract | 4 (4A) | 12 (4A,6B,2C) | 4 (4B) | 4 (1A, 2B,1D) | 1 (1B) | 5 (1A,2B,2D) | 30 (10A,15B,2C,3D) |
| Total | 17 (14A,3B) | 13 (4A, 7B,2C) | 34 (23A,10B,1E) | 27 (10A,15B,1C,1D) | 2 (1D,1B) | 11 (2A,2B,2C,2D,1F,1G,1H) | 104 (53A,38B,5C,4D,1E,1F,1G,1H) |
DISCUSSION
Identification of P. aeruginosa has traditionally relied on phenotypic methods. This still is the most accurate standard when dealing with typical isolates of P. aeruginosa. In cystic fibrosis (CF) patients, P. aeruginosa isolates display unusual phenotypic reactions (17). Moreover, biochemical testing takes long time to perform and requires extensive hands-on work by the technologist, both for setup and for ongoing evaluation. Molecular methods have been reported to be superior to the phenotypic methods for identification of P. aeruginosa (17).
De Vos et al. (1997) by designing a multiplex PCR assay based on oprI and oprL genes for molecular detection of P. aeruginosa showed that the specificity and sensitivity of the PCR assay were 74 and 100%, respectively (4). Lavenir et al. also noted that all of P. aeruginosa strains contained the oprI and oprL genes (sensitivity = 100%, specificity = 80%). Similarly in this study, all of the 268 isolates were remarkably positive for both oprI and oprL genes (18).
According to these studies, detection of P. aeruginosa by PCR of oprI and oprL genes has a high sensitivity but a low specificity. The reason of low specificity of oprI and oprL genes is that, although the entire genome of P. aeruginosa has been sequenced, the genomes of its closest relatives have not. Thus, presence of false positive results among other species of bacteria during PCR assay of oprI and oprL genes indicate that they may have some similar sequences to oprI and oprL genes in their genomes (17). Consequently, use of only single gene target for molecular identification of P. aeruginosa potentially suffers from the same polymorphisms that complicate biochemical identification of this organism.
Khan and Cerniglia also developed a PCR procedure to detect P. aeruginosa by amplifying the toxA gene (3). They reported that of 130 tested P. aeruginosa isolates, 125 (96%) contained the toxA gene (sensitivity = 96%), whereas other species of bacteria did not yield any positive results (specificity = 100%). Qin et al. and Lavenir et al. also reported similar results. These studies indicate that, unlike oprI and oprL genes, detection of P. aeruginosa by PCR of toxA gene has a high specificity but a low sensitivity (17, 18). In this study our results also showed that 243 (90.7%) of 268 isolates harbored toxA gene.
The ptxR gene, expression enhancer of toxA gene, was only detected in P. aeruginosa isolates; whereas other species of Pseudomonas did not yield any positive results (19). Low sensitivity with toxA PCR screening is due to the fact that some isolates of P. aeruginosa do not carry this gene naturally.
Pathogenicity of P. aeruginosa is clearly multifactorial. LasB is one of the most important proteases of P. aeruginosa (20). In this study all isolates examined harbored lasB gene. This finding is in agreement with previous reports (20, 21). Mutation of lasB gene reduces markedly P. aeruginosa invasion (22). Prevalence of the lasB gene in all the environmental and clinical isolates implies the importance of LasB factor to survival of P. aeruginosa in various settings.
P. aeruginosa isolates generally express cytotoxicity or invasion phenotypes which is correlated with presence of exoU (encoding exotoxin U) or exoS (encoding exotoxin S) respectively (23). In our study difference between exoS prevalence in the isolates from pulmonary tract and burn infections was statistically significant (P < 0.05) (Table 1).
The proportion of isolates from pulmonary tract infections that exhibited exoS (47.4%) in this study was lower than that previously reported (20, 15, 24, 25). The conflicting results of these studies may be due to differences in the number of clinical isolates from different sites or due to the isolates from patients with different clinical and physiological conditions (20, 24)
About the nan1 gene, the other virulence factor studied in this research, we found that the prevalence of nan1 was significantly higher in isolates from pulmonary tract than isolates from burn (P < 0.05). Furthermore, the prevalence of nan1 among the isolates from wound was significantly higher than burn (P < 0.05). Similar to our results, Lanotte et al. reported that 7 (41.2%) of 17 wound isolates and 12 (48%) of 25 pulmonary tract isolates contained nan1 gene. This gene has probably A role in CF pulmonary disease evolution as previously described (15). The low prevalence of this factor among isolates from burn infections may show that the role of this gene in the burn infections is less important than wound and pulmonary tract infections.
The differences in the distributions of virulence factor genes in the populations strengthen the probability that some P. aeruginosa strains are better adapted to the specific conditions found in specific infectious sites (15).
The isolates in this study were divided into 8 genetic groups (I-VIII) based on presence of the investigated virulence genes in the isolates. There was no correlation between clinical origin of P. aeruginosa isolates and their distribution in the 8 genetic groups (Table 2).
Although ribotyping is slightly less discriminatory than pulsed field gel electrophoresis (PFGE) (26), the high rate of interlaboratory reproducibility and the speed of the generation of results make this method a valuable approach for characterization of clinical bacteria (12). Ribotypoing demonstrated 8 distinct ribotype patterns (A-H). Fifty-three (51%) and 38 (36%) isolates belonged to ribotype pattern A and B, respectively. Indeed, most of the isolates (87%) belonged to ribotype pattern A and B. There was no significant meaningful correlation between genetic groups and ribotype patterns.
In conclusion, it seems that simultaneous use of oprI, oprL and toxA genes provides more confident detection of P. aeruginosa by PCR. Determination of different virulence genes of P. aeruginosa isolates suggests that they are associated with different levels of intrinsic virulence and pathogenicity. This may have different consequence on the outcome of infections. Significant correlations between some virulence genes and source of infections obtained in this research indicates that more further studies is required for finding out the actual role of these genes in different clinical infectious caused by P. aeruginosa. Ribotyping showed that strains with similar virulence genes do not necessarily have similar ribotype patterns. However, clonal spread of highly virulent isolates of P. aeruginosa within hospitals needs to apply additional precautions in clinical settings.
REFERENCES
- 1.Fegan M, Francis P, Hayward AC, Davis GH, Furest JA. Phenotypic conversion of Pseudomonas aeruginosa in cystic fibrosis. J Clin Microbiol. 1990;28:1143–1146. doi: 10.1128/jcm.28.6.1143-1146.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yetkin G, Otlu B, Cicek A, Kuzucu C, Durmaz R. Clinical, microbiologic, and epidemiologic characteristics of Pseudomonas aeruginosa infections in a university hospital, Malatya, Turkey. Am J Infect Control. 2006;34:188–192. doi: 10.1016/j.ajic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Khan AA, Cerniglia CE. Detection of Pseudomonas aeruginosa from clinical and environmental samples by amplification of the exotoxin A gene using PCR. Appl Environ Microbiol. 1994;60:3739–3745. doi: 10.1128/aem.60.10.3739-3745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vos D, Lim A, Pirnay JP, Struelens M, Vandenveld C, Duinslaeger L, et al. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane genes, oprI and oprL . J Clin Microbiol. 1997;35:1295–1299. doi: 10.1128/jcm.35.6.1295-1299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa . Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 7.Van Delden C, Iglewski BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yahr TL, Hovey AK, Kulich SM, Frank DW. Transcriptional analysis of the Pseudomonas aeruginosa exoenzyme S structural gene. J Bacteriol. 1995;177:1169–1178. doi: 10.1128/jb.177.5.1169-1178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleiszig SM, Wiener-kronish JP, Miyazaki H, Valias V, Mostov KE, Kanada D, et al. Pseudomonas aeruginosa- mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffar-Bandjee MC, Lazdunski A, Bally M, Carrere J, Chazalette JP, Galabert C. Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary tract exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aeruginosa . J Clin Microbiol. 1995;33:924–929. doi: 10.1128/jcm.33.4.924-929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryan R, Kube D, Perez A, Davis P, Prince A. Overproduction of the CFTR R domain leads to increased levels of asialoGM1 and increased Pseudomonas aeruginosa binding by epithelial cells. Am J Respir Cell Mol Bio. 1998;19:269–277. doi: 10.1165/ajrcmb.19.2.2889. [DOI] [PubMed] [Google Scholar]
- 12.Grattard F, Pozzetto B, Ros A, Gaudin OG. Differentiation of Pseudomonas aeruginosa strains by ribotyping: high discriminatory power by using a single restriction endonuclease. J Med Microbiol. 1994;40:275–281. doi: 10.1099/00222615-40-4-275. [DOI] [PubMed] [Google Scholar]
- 13.Lennette EH, Balows A, Hausler WJ, Shadom HJ. 4th ed. Washington D.C: 1985. Manual of clinical Microbiology. [Google Scholar]
- 14.Shahcheraghi F, Nikbin VS, Feizabadi MM. Prevalence of ESBLs genes among multidrug-resistant isolates of Pseudomonas aeruginosa isolated from patients in Tehran. Microb Drug Resist. 2009;15:37–39. doi: 10.1089/mdr.2009.0880. [DOI] [PubMed] [Google Scholar]
- 15.Lanotte P, Mereghetti L, Dartiguelongue N, Rastegar-Lari A, Gouden A, Quentin R. Genetic features of Pseudomonas aeruginosa isolates cystic fibrosis patients compared with those of isolates from other origins. J Med Microbiol. 2004;53:73–81. doi: 10.1099/jmm.0.05324-0. [DOI] [PubMed] [Google Scholar]
- 16.Regnault B, Grimont F, Grimont PAD. Universal ribotyping method using a chemically labeled oligonucleotide probe mixture. Res Microbiol. 1997;148:649–659. doi: 10.1016/S0923-2508(99)80064-3. [DOI] [PubMed] [Google Scholar]
- 17.Qin X, Emerson J, Stapp J, Stapp L, Abe P, Burns L. Use of real-time PCR with multiple targets to identify Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli from patients with cystic fibrosis. J Clin Microbiol. 2003;4:4312–4317. doi: 10.1128/JCM.41.9.4312-4317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavenir R, Jocktane D, Laurent F, Nazaret S, Cournoyer B. Improved reliability of Pseudomons aeruginosa PCR detection by the use of the specific ecfx gene target. J Microbiol Methods. 2007;70:20–29. doi: 10.1016/j.mimet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Vasil ML, Chamberlain C, Grant CCR. Molecular studies of Pseudomonas exotoxin A gene. Infect Immun. 1986;52:538–548. doi: 10.1128/iai.52.2.538-548.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lomholt JA, Poulsen K, Kilian M. Epidemic population structure of Pseudomons aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factor. Infect Immun. 2001;69:6284–6295. doi: 10.1128/IAI.69.10.6284-6295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicas TI, Iglewski BH. Production of elastase and other exoproducts by environmental isolates of Pseudomonas aeruginosa . J Clin Microbiol. 1986;23:967–969. doi: 10.1128/jcm.23.5.967-969.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowell BA, Twining SS, Hobden JA, Kwong MSF, Fleiszig SM. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology. 2003;149:2291–2299. doi: 10.1099/mic.0.26280-0. [DOI] [PubMed] [Google Scholar]
- 23.Winstanley C, Kaye SB, Neal TJ, Miksch S, Hart CA. Genotypic and phenotypic characterization of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J Med Microbiol. 2005;54:519–526. doi: 10.1099/jmm.0.46005-0. [DOI] [PubMed] [Google Scholar]
- 24.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa . Microbiology. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 25.Schulert GS, Feltman H, Rabin SDP, Martin CG, Battle SE, Rello J, et al. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis. 2003;188:1695–1706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- 26.Botes J, Williamson G, Sinickas V, Gürtler V. Genomic typing of Pseudomonas aeruginosa isolates by comparison of Riboprinting and PFGE: correlation of experimental results with those predicted from the complete genome sequence of isolate PAO1. J Microbiol Methods. 2003;55:231–240. doi: 10.1016/s0167-7012(03)00156-8. [DOI] [PubMed] [Google Scholar]