Abstract
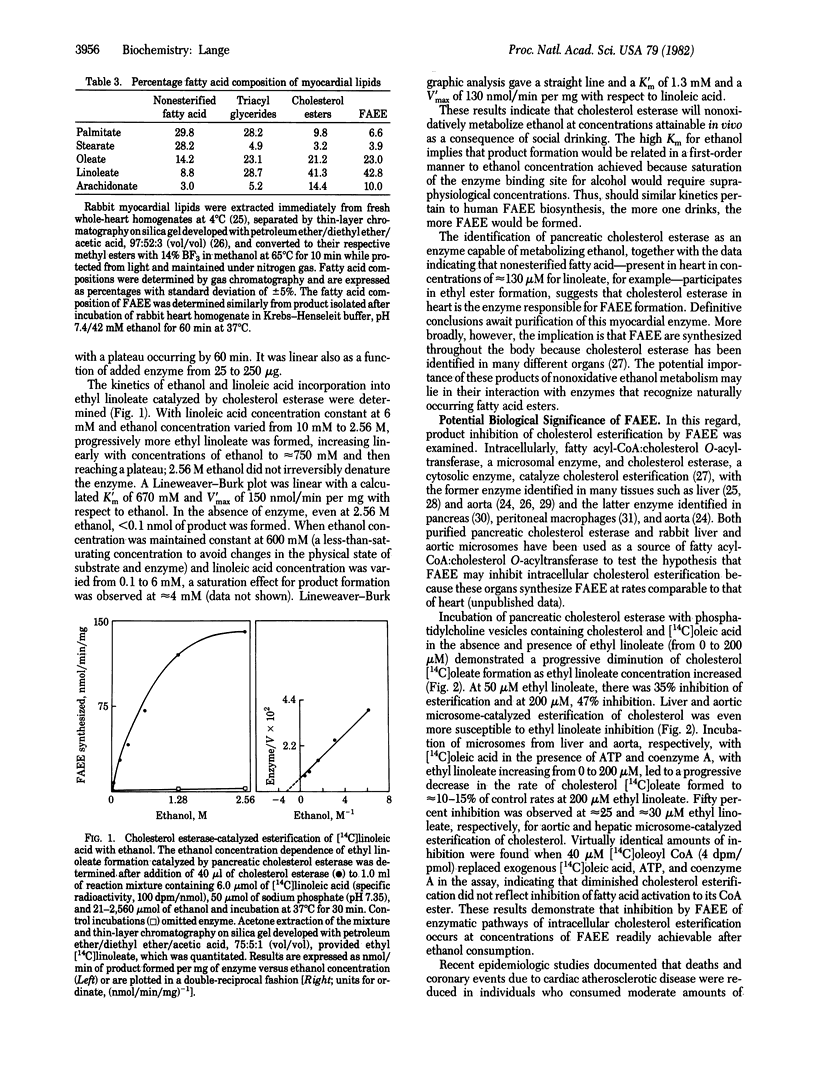
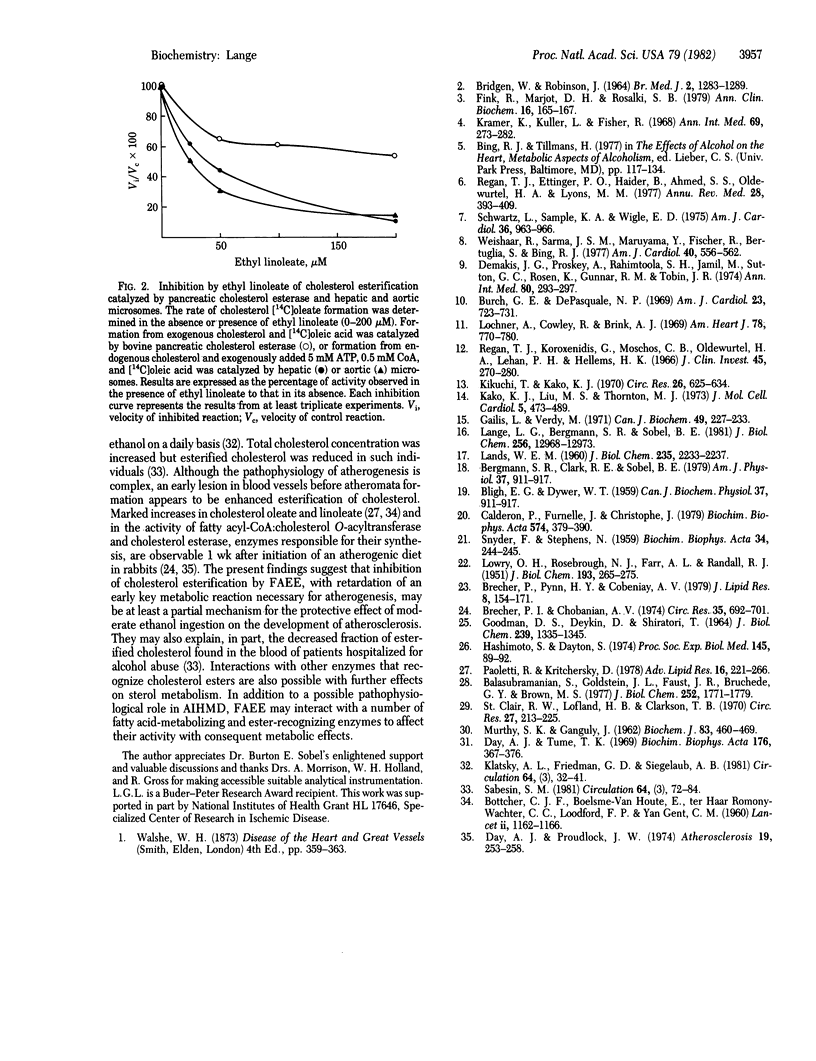
The recent identification of myocardial metabolites of ethanol--fatty acid ethyl esters--suggests that some of the pathophysiological derangements associated with alcohol-induced heart muscle disease may be a consequence of products of myocardial ethanol metabolism. The donor of the fatty acid moiety in the formation of fatty acid ethyl esters has been identified as nonesterified fatty acid. Fatty acid esterification with ethanol is shown to be mediated by cholesterol esterase (sterol-ester acylhydrolase, EC 3.1.1.13), a finding that identifies a singular nonoxidative ethanol metabolism by an enzyme. A potential basis for the protective effect of ethanol ingestion on atherogenesis is also suggested because fatty acid ethyl esters inhibit cholesterol esterification catalyzed by pancreatic cholesterol esterase and hepatic and aortic microsomal fatty acyl-CoA:cholesterol O-acyltransferase (EC 2.3.1.26).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BRIGDEN W., ROBINSON J. ALCOHOLIC HEART DISEASE. Br Med J. 1964 Nov 21;2(5420):1283–1289. doi: 10.1136/bmj.2.5420.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brunschede G. Y., Brown M. S. Lipoprotein-mediated regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesteryl ester metabolism in the adrenal gland of the rat. J Biol Chem. 1977 Mar 10;252(5):1771–1779. [PubMed] [Google Scholar]
- Brecher P. I., Chobanian A. V. Cholesteryl ester synthesis in normal and atherosclerotic aortas of rabbits and rhesus monkeys. Circ Res. 1974 Nov;35(5):692–701. doi: 10.1161/01.res.35.5.692. [DOI] [PubMed] [Google Scholar]
- Burch G. E., DePasquale N. P. Alcoholic cardiomyopathy. Am J Cardiol. 1969 May;23(5):723–731. doi: 10.1016/0002-9149(69)90036-8. [DOI] [PubMed] [Google Scholar]
- Calderon P., Furnelle J., Christophe J. In vitro lipid metabolism in the rat pancreas. I. Basal lipid metabolism. Biochim Biophys Acta. 1979 Sep 28;574(3):379–390. doi: 10.1016/0005-2760(79)90234-0. [DOI] [PubMed] [Google Scholar]
- Day A. J., Proudlock J. W. Changes in aortic cholesterol-esterifying activity in rabbits fed cholesterol for three days. Atherosclerosis. 1974 Mar-Apr;19(2):253–258. doi: 10.1016/0021-9150(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Day A. J., Tume R. K. Cholesterol-esterifying activity of cell-free preparations of rabbit peritoneal macrophages. Biochim Biophys Acta. 1969 Mar 4;176(2):367–376. doi: 10.1016/0005-2760(69)90195-7. [DOI] [PubMed] [Google Scholar]
- Demakis J. G., Proskey A., Rahimtoola S. H., Jamil M., Sutton G. C., Rosen K. M., Gunnar R. M., Tobin J. R. The natural course of alcoholic cardiomyopathy. Ann Intern Med. 1974 Mar;80(3):293–297. doi: 10.7326/0003-4819-80-3-293. [DOI] [PubMed] [Google Scholar]
- Fink R., Marjot D. H., Rosalki S. B. Detection of alcoholic cardiomyopathy by serum enzyme and isoenzyme determination. Ann Clin Biochem. 1979 May;16(3):165–166. doi: 10.1177/000456327901600135. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S., DEYKIN D., SHIRATORI T. THE FORMATION OF CHOLESTEROL ESTERS WITH RAT LIVER ENZYMES. J Biol Chem. 1964 May;239:1335–1345. [PubMed] [Google Scholar]
- Gailis L., Verdy M. The effect of ethanol and acetaldehyde on the metabolism and vascular resistance of the perfused heart. Can J Biochem. 1971 Feb;49(2):227–233. doi: 10.1139/o71-033. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Dayton S. Cholesterol-esterifying activity of aortas from atherosclerosis-resistant and atherosclerosis-susceptible species. Proc Soc Exp Biol Med. 1974 Jan;145(1):89–92. doi: 10.3181/00379727-145-37754. [DOI] [PubMed] [Google Scholar]
- Kako K. J., Liu M. S., Thornton M. J. Changes in fatty acid composition of myocardial triglyceride following a single administration of ethanol to rabbits. J Mol Cell Cardiol. 1973 Oct;5(5):473–489. doi: 10.1016/0022-2828(73)90017-5. [DOI] [PubMed] [Google Scholar]
- Kikuchi T., Kako K. J. Metabolic effects of ethanol on the rabbit heart. Circ Res. 1970 May;26(5):625–634. doi: 10.1161/01.res.26.5.625. [DOI] [PubMed] [Google Scholar]
- Kramer K., Kuller L., Fisher R. The increasing mortality attributed to cirrhosis and fatty liver, in Baltimore (1957-1966). Ann Intern Med. 1968 Aug;69(2):273–282. doi: 10.7326/0003-4819-69-2-273. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D., Kothari H. V. Arterial enzymes of cholesteryl ester metabolism. Adv Lipid Res. 1978;16:221–266. doi: 10.1016/b978-0-12-024916-9.50010-x. [DOI] [PubMed] [Google Scholar]
- LANDS W. E. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J Biol Chem. 1960 Aug;235:2233–2237. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange L. G., Bergmann S. R., Sobel B. E. Identification of fatty acid ethyl esters as products of rabbit myocardial ethanol metabolism. J Biol Chem. 1981 Dec 25;256(24):12968–12973. [PubMed] [Google Scholar]
- Lochner A., Cowley R., Brink A. J. Effect of ethanol on metabolism and function of perfused rat heart. Am Heart J. 1969 Dec;78(6):770–780. doi: 10.1016/0002-8703(69)90443-8. [DOI] [PubMed] [Google Scholar]
- MURTHY S. K., GANGULY J. Studies on cholesterol esterases of the small intestine and pancreas of rats. Biochem J. 1962 Jun;83:460–469. doi: 10.1042/bj0830460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan R. J., Koroxenidis G., Moschos C. B., Oldewurtel H. A., Lehan P. H., Hellems H. K. The acute metabolic and hemodynamic responses of the left ventricle to ethanol. J Clin Invest. 1966 Feb;45(2):270–280. doi: 10.1172/JCI105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan T. J., Ettinger P. O., Haider B., Ahmed S. S., Oldewurtel H. A., Lyons M. M. The role of ethanol in cardiac disease. Annu Rev Med. 1977;28:393–409. doi: 10.1146/annurev.me.28.020177.002141. [DOI] [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- Schwartz L., Sample K. A., Wigle D. E. Severe alcoholic cardiomyopathy reversed with abstention from alcohol. Am J Cardiol. 1975 Dec;36(7):963–966. doi: 10.1016/0002-9149(75)90090-9. [DOI] [PubMed] [Google Scholar]
- St Clair R. W., Lofland H. B., Clarkson T. B. Influence of duration of cholesterol feeding on esterification of fatty acids by cell-free preparation of pigeon aorta. Circ Res. 1970 Aug;27(2):213–225. doi: 10.1161/01.res.27.2.213. [DOI] [PubMed] [Google Scholar]
- Weishaar R., Sarma J. S., Maruyama Y., Fischer R., Bertuglia S., Bing R. J. Reversibility of mitochondrial and contractile changes in the myocardium after cessation of prolonged ethanol intake. Am J Cardiol. 1977 Oct;40(4):556–562. doi: 10.1016/0002-9149(77)90071-6. [DOI] [PubMed] [Google Scholar]


