Abstract
Background and Objectives
Azotobacter vinelandii, a gamma-proteobacterium, is an obligate aerobic free-living gram-negative soil bacterium capable of fixing nitrogen. Oxygen transfer rate into the cell is reduced by the increase of alginate concentrations during the course of A. vinelandii cultivation. This phenomenon provides a low intracellular oxygen concentration needed for nitrogenase activity. The aim of this study was to design a simple strategy to explain the alginate production, cell growth and nitrogenase activity correlation in A. vinelandii under aerobic conditions.
Material and Methods
Thirty-five different soil samples were taken from the rhizosphere of agricultural crops of Iran. Enrichment and isolation strategies were employed for microbial isolation. Physiological and biochemical characteristics were determined. Molecular identification was performed using selective nifH-g1 primers. Alginate production and nitrogenase activity assay by each isolate of Azotobacter were carried out. Bacterial growth, alginate production and Nitrogenase activity were conducted by time-coursed quantitative measurements.
Results
Total of 26 isolates were selected after enrichment, isolation, and screening. The isolate was identified by molecular tests as A. vinelandii. The highest alginate productions of 1.02 g/l and 0.91g/l were noted after 4 days in 8 isolates, cell biomass of which were estimated 4.88-5.26 g/l. Six of 8 isolates were able to fix atmospheric N2 on nitrogen-free medium. Rates obtained in isolates were in the range of 12.1 to 326.4 nmol C2H4 h-1 vial-1.
Conclusions
Nitrogen fixation and alginate production yielded significant and positive Pearson's correlation coefficient of R2 = 0.760, p ∼ 0.02. Finally association between bacterial growth, alginate production and nitrogenase activity almost noticeable yielded significant and positive Pearson's correlation coefficient R2= 0.723, p ∼ 0.04.
Keywords: Alginate, Azotobacter vinelandii, Nitrogenase, Nitrogen fixation
INTRODUCTION
Azotobacter vinelandii is a gamma-proteobacterium belonging to the family Pseudomonadaceae. It is an obligate aerobic free-living gram-negative soil bacterium capable of fixing nitrogen directly from the atmosphere that helps the plants for better grain production (1). The nitrogenase enzyme complex that catalyzes dinitrogen reduction to ammonium is composed of two highly conserved proteins: the iron (Fe) protein (encoded by the nifH gene) and the molybdenum iron (MoFe) protein (encoded by the nifDK genes) (2). Evolutionarily conserved amino acid sequences within the nifH gene have been oppressed to design PCR primers to detect the genetic potential for nitrogen fixation in the environment (3, 4).
Nitrogenase is highly sensitive to oxygen (2, 5), However, Nitrogen fixation occurs in A. vinelandii using three distinct nitrogenase systems under fully aerobic conditions that typically inactivate the nitrogenase enzyme (6). Obligate aerobes such as A. vinelandii are known to use two mechanisms for protecting the nitrogenase system against oxygen damage: (i) high respiration rate that the uncommonly high activities of cellular oxygen utilization, prevent the diffusion of oxygen into the cells and consequently to the nitrogenase (7), (ii) conformational protection of the enzyme or the switch-off of nitrogenase activity by shethna or FeSII protein (8). Recently, alginate formation is considered as a new protection mechanism for nitrogenase against oxygen (5).
Alginates are a family linear copolymers composed of variable amounts of (1–4) -β-D-mannuronic acid and its epimer, α-L-guluronic acid (9–11). Some bacteria especially Pseudomonas aeruginosa and A. vinelandii can produce exopolysaccharide alginate (9, 12). Alginate is important in various biotechnological and biomedical applications, e.g. for immobilizing cells in the pharmaceutical or as a stabilizing, thickening and gelling agent in food production (5). The species A. vinelandii seems to be the best candidate for the industrial production of alginate (13).
Azotobacter vinelandii produces the intracellular polymer polyhydroxybutyrate (PHB) and excretes alginate into the medium during vegetative growth. Synthesis and production of alginate and PHB by A. vinelandii is essential for cyst formation and differentiation. The mutant varieties of bacteria do not produce alginate and are unable to form mature cysts. The cyst is formed under unfavorable environmental conditions. The mature cysts are surrounded by two capsule-like layers containing a high proportion of the alginate. The intine (inner coat) and exine (outer coat) layers of the cyst contain different types of alginate. Under favourable conditions, the alginate coating swells and the cyst germinates (11).
The alginate extracellular accumulation acts as a barrier to oxygen diffusion or heavy metals (10). In A. vinelandii, the increase of alginate concentrations during the course of cultivation in culture broth can reduce the oxygen transfer rate into the cell and consequently provide a low intracellular oxygen concentration that is essential for nitrogenase activity (6, 8).
The present study was aimed to design a simple strategy to explain the correlation between alginate production, cell growth and nitrogenase activity in A. vinelandii under aerobic conditions.
MATERIALS AND METHODS
Bacterial isolation and identification
Thirty five different soil samples from the rhizosphere of agricultural crops of Iran (Tehran, Qazvin and Guilan) were transferred to laboratory. Strategies used for isolation were:
Enrichment: For enrichment of A. vinelandii strains and the growth inhibition of other Azotobacter species, 1 g soil samples were added into 100 ml Erlenmeyer flasks containing 20 ml of Azotobacter broth medium with the following composition; K2HPO4 0.8 g, KH2PO4 0.2 g, MgSO4·7H2O 0.5 g, FeSO4·6H2O 0.10 g (or 0.05 g), CaCl2·2H2O 0.05 g or CaCO3 20.0 g, NaMoO4·2H2O 0.05 g per liter (Adjust to pH 7.4–7.6) (14). Ethylene glycol (1%) as sole source of carbon, 0.1% phenol and cycloheximide (100 µg/ml) were added into medium and were incubated at 37°C for 2-5 days (15).
Isolation: Serial dilutions were prepared from enrichment culture followed by streaking and incubation at 37°C. All the isolates were subcultured on selective nitrogen-free specific medium Azotobacter Agar plates and were purified.
Physiological and biochemical characteristics was performed according to Bergey's Manual of Systematic Bacteriology instructions (1), including colony morphology, the gram, cyst and PHB granules staining as well as production of pigment.
Molecular identification was performed with PCR using selective nifH-g1 primer from Azotobacter (GenBank accession nos. M11579, M20568) (16):
fD1 (5'GGTTGTGACCCGAAAGCTGA-3’), rP1 (5’-GCGTACATGGCCATCATCTC-3’)
Reference strain Azotobacter sp. PTCC 1658 used as the control for comparison.
Alginate production
The medium for alginate production by A. vinelandii contained 20 g sucrose, 0.6 g (NH4)2SO4, 2 g Na2HPO4, 0.3 g MgSO4·7H2O, and 6 g yeast extract per liter of distilled water at pH 7.2 (17). Erlenmeyer flasks containing 25 ml alginate production medium were inoculated with ∼104CFU/ml of each isolates of Azotobacter and were incubated at 28°C at 180 rpm for 96 h.
Separation of cell biomass
Separation of A. vinelandii cells from the culture broth was achieved in the following manner.
Five ml for each sample was centrifuged at 8400 rpm at 15°C for 30 min in pre weighed tubes,
The supernatant was removed and the residue was suspended in NaCl (5M) and Na4EDTA (0.5M).
Centrifuging as in step 1.
The harvested biomass was washed with deionized water and then dried at 60°C for 24 h in an oven to estimate the biomass concentration (18).
Alginate determination
Alginate was measured by Gravimetric Method (17) as the following procedure:
The supernatant of previous step was removed and equal volume of ice-cold 95% ethanol was added, stirring slowly.
The mixture was centrifuged at 12,000 rpm at 4°C for 20 min.
The supernatant was carefully removed. The tubes were dried at 105°C for 24 h and alginate dry weight and concentration were determined.
Nitrogen fixation and nitrogenase activity
The nitrogenase activity assay was carried out according to the method of acetylene reduction (19). Five ml of the Azotobacter broth medium in 12 ml vials was inoculated with ∼104CFU/ml of each isolate and incubated for 48-96 h at 28 °C. Once visible growth was observed, each vial was sealed with rubber stopper. By means of a disposable plastic syringe, 10% of air from the head space (7 ml) was removed and an equal amount of acetylene was injected into vials (20, 21). Gas samples (0.7 ml) were removed after 24 h incubation, and were assayed for ethylene production with a gas chromatograph in triplicate (GOW MAC - GM 816 model). The chromatograph was fitted with Poropak N column and a H2-FID detector.
The rate of nitrogen fixation was calculated by Ravikumar formula (21) and Values were obtained nmoles C2H4 h-1 vial-1 (22):
Bacterial growth, alginate production and Nitrogenase activity
Time-coursed quantitative measurements were carried out in Erlenmeyer flasks containing 25 ml of broth medium. 100µl of bacterial suspension were inoculated into medium and incubated at 28°C and 180 rpm. Final population of bacterial suspension was ∼104 CFU/ml of isolates with the highest alginate production (A3 and A21). The uninoculated medium used as control in each case. Sampling was carried out within 120 h. For estimation of the growth rate, 100 μl of medium were removed every 6 hours, serial dilution were prepared and were contained colonies on solid medium and the growth curve was drawn, while the measurement of simultaneous alginate production and nitrogenase activity were done at 48 h, 72 h, 96 h and 120 h in triplicates. Cell numbers at log phase of Azotobacter were adjusted to 107 CFU/ ml.
Statistical analysis
Data analysis and graph drawing was carried out using statistics software GraphPad Prism (v5.0.4) and SPSS (v18). We used the Bivariate Pearson's correlation to estimate the correlation between the alginate production and nitrogenase activity. Pearson's correlation measure how variables or rank orders are related, according to correlation coefficient and significance level.
RESULTS
Isolation and Identification
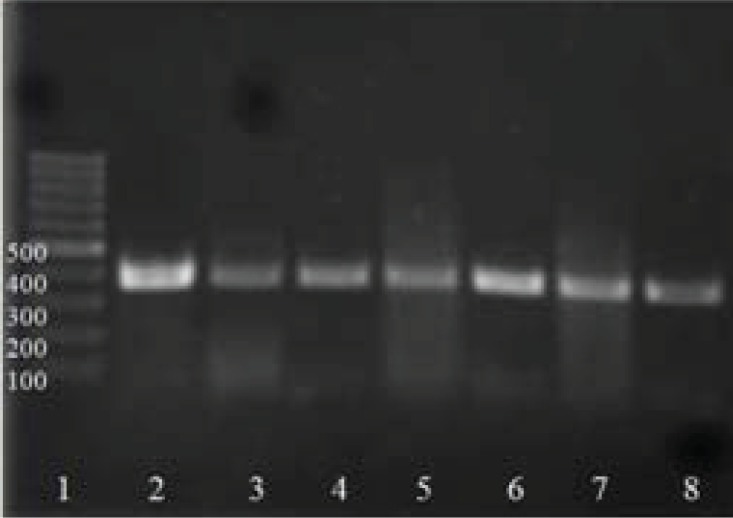
Total of 26 isolates named as A1 to A26, were selected after enrichment, isolation, and screening from 35 soil samples. The Azotobacter isolates were identified by molecular tests with PCR by specific primers for nifH-g1. The PCR reaction was carried out for all the 26 isolates in order to check the presence of nifH gene. PCR products were about 400 bp. The results showed that there was no significant difference in banding pattern compared to the reference Azotobacter strains (Fig. 1). The identification of isolates was completed according to biochemical characteristics from Bergey's Manual of Determinative Bacteriology (1). The isolate was identified as A. vinelandii.
Fig. 1.
The PCR product of reference Azotobacter strain and Azotobacter isolates as follows Lanes 2 and 8, line 1 = 100 bp marker, line 2 = Reference strain Azotobacter sp. PTCC 1658, Lane 3 to 8 = Respectively; A2, A3, A5, A8, A15, A21 isolates.
Alginate production
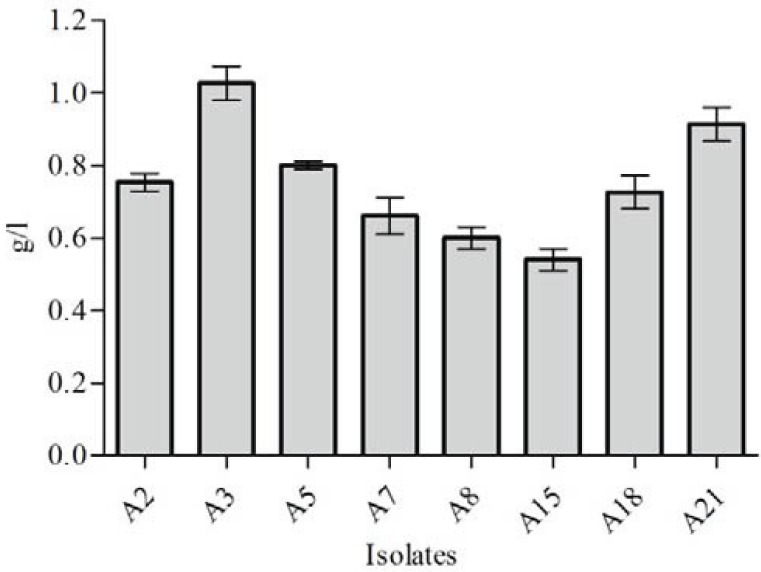
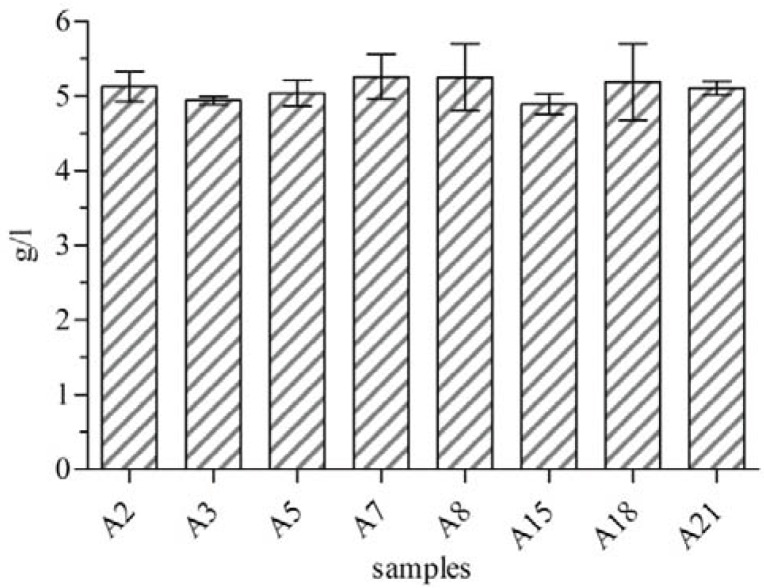
Among 26 isolated A. vinelandii, alginate production was measured in 8 isolates (A2, A3, A5, A7, A8, A15, A18, and A21) showing higher slimy and mucoid phenotype on solid medium compared to the other isolates. The highest alginate production was observed for A3 (1.02 g/l) followed by A21 (0.91 g/l) at during 4 days (Fig. 2). In this series of experiments, cell biomass of all the 8 isolates estimated were between 4.88 g/l and 5.26 g/l. The lowest rate was of A15 and both A7 and A8 had the highest values (Fig. 3).
Fig. 2.
Production of bacterial alginate by selected A. vinelandii isolates (A2, A3, A5, A7, A8, A15, A18 and A21). The isolates were cultivated at 28°C and 180 rpm for 4 days. Values are means of triplicates.
Fig. 3.
Cell biomass of selected A. vinelandii isolates (A2, A3, A5, A7, A8, A15, A18 and A21). Cell biomasses estimated were between 4.88 g/l and 5.26 g/l.
Nitrogen fixation and Nitrogenase activity
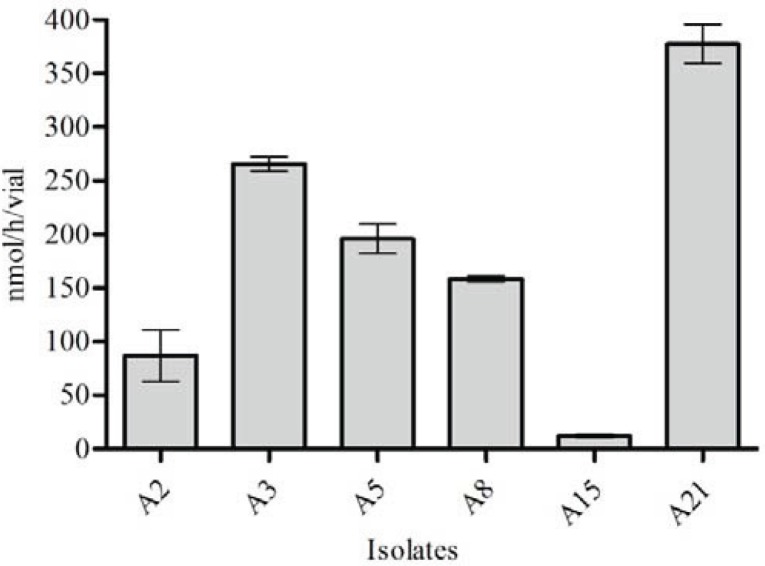
As shown in Fig. 4, among 8 isolates, only six isolates were found to be able to fix mesurable amount of atmospheric N2. Amounts of acetylene reduced by A. vinelandii isolates in samples were quite different. Rates obtained in isolates were in the range of 12.1 to 326.4 nmol C2H4 h-1 vial-1. Nitrogen fixation and alginate production yielded significant and positive Pearson's correlation coefficient (R2= 0.760, p ∼ 0.02).
Fig. 4.
Nitrogenase activity (Acetylene Reduction Assay) by isolates that were able to fix N2 (A2, A3, A5, A8, A15 and A21). Values are means of triplicates followed by the standard deviation.
Bacterial growth, alginate production and Nitrogenase activity
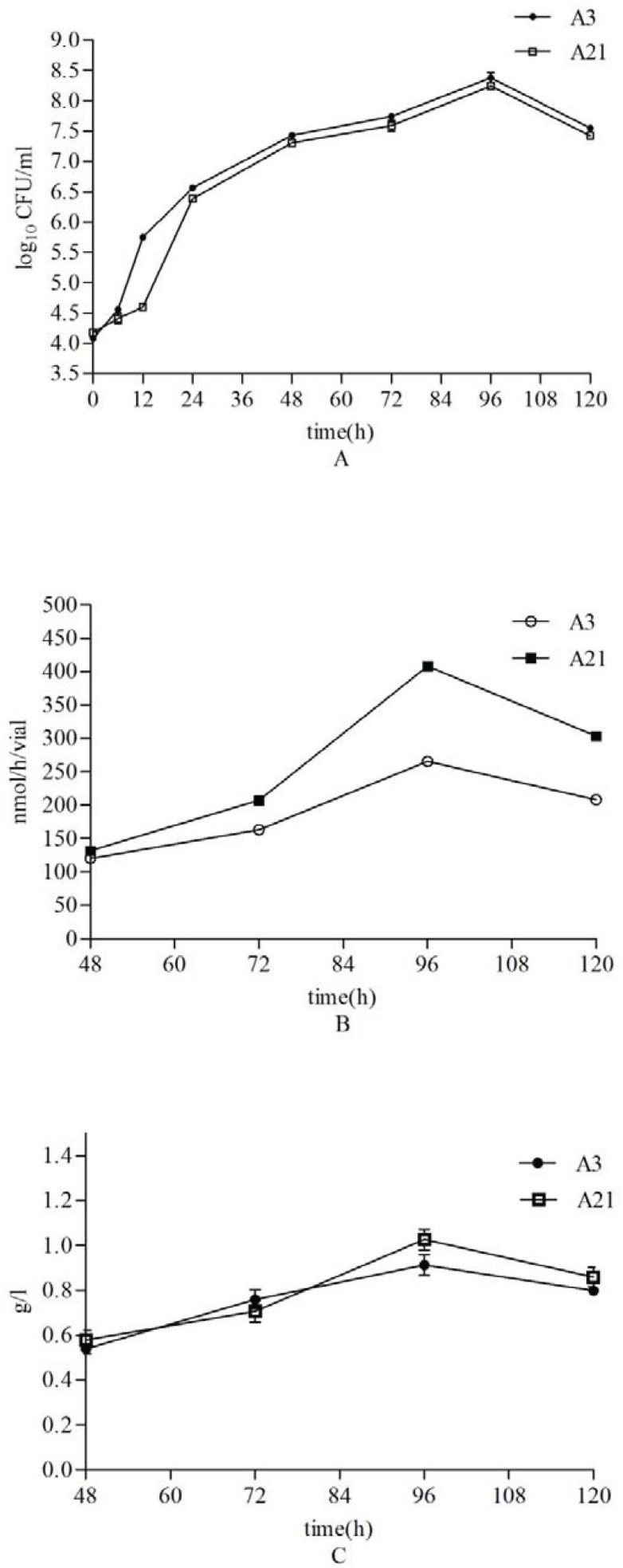
Two A. vinelandii isolates viz, A3 and A21, with the highest alginate production were chosen for detailed investigation to examine association between bacterial growths (which is the logarithm of CFU/ml of culture) (23), alginate production and nitrogenase activity. In both strains, almost noticeable association yielded significant and positive Pearson's correlation coefficient (R2= 0.723, p ∼ 0.04). The maximum levels of alginate production and nitrogen fixation for both A3 and A21 were reached after 96 h of incubation at 28°C while exponential growth phase of both strains delayed for 6 h and arrived at stationary phase after 96 h (Fig. 5A).
Fig. 5.
The growth curve (Panel A), nitrogen fixation (Panel B) and alginate production (Panel C) by A3 and A21 isolates. Sampling was carried out within 120 h. After 96 h, both strains arrived at stationary phase as well as the maximum levels of alginate production and nitrogen fixation. All data points are the means of three replicates. Standard errors are shown by vertical bars.
Alginate production and nitrogenase activity of A21 (1 g/l) were increased in comparison with A3 after 96 h (0.91 g/l) despite alginate production value of the isolate A3 was obtained higher than A21 in early experiment (section 3.2). This phenomenon provides that the increase in nitrogen-fixation dependent on alginate production and not bacterial count because the number of A21 was less than A3.
DISCUSSION
The method employed in the present work was described by different authors as feasible for A. vinelandii strains isolation from other Azotobacter species and other free-living nitrogen-fixing soil bacteria. Claus and Hempel (24) observed that ethylene glycol in 0.1 or 0.2% (wt/vol) concentration is a very selective carbon source for A. vinelandii. More common Azotobacter species apparently cannot utilize it. It has been shown that the use of 0.1% phenol in enrichment cultures will inhibit the growth of other Azotobacter species and incubation at 37°C will particularly suppress the development of genus Azomonas and makes A. vinelandii selectively dominant (25). The precise identification was achieved based on universal PCR detection of the nifH marker gene that has been applied to describe diazotroph populations in the environment, but they could not successfully separate Azetobacters from other diazotrophs. Helmut et al. (16) confirmed the nifH-g1 primer set that was designed to amplify nifH genes of Azotobacter species. Rajeswari and Mangai (4) reported that nifH-g1 primer targets Azotobacter spp. Combination of morphological, biochemical and molecular methods in this study confirm our identification of Azotobacter.
Clementi et al. (17) assessed the minimum alginate concentration 0.1–0.5 g/ml and speculated that gravimetric method lacks sensitivity because of precipitation of salts and peptones. In a similar study, Sabra et al. (6) reported 0.2–0.9 g/l for alginate production at different agitation speeds (300 to 1,000 rpm) by gravimetrical method. We obtained alginate production between 0.54–1.02 g/l by same method.
Alginate production at high concentrations by A. vinelandii depends on cultural conditions (26). The use of appropriate carbon and nitrogen sources, air-flow rate (agitation speed), temperature, pH allowed obtaining a maximum production. Emtiazi et al. (18) isolated strain AC2 with maximal production (7.5 mg/ml) in an optimized medium, 30°C and 200 rpm shaking during 4 days in 1% sucrose. Vermani et al. (27) suggested that A. vinelandii MTCC 2459 produce optimum alginate at 30°C, 110 rpm shaking, 50 g/l sucrose and 0.1 g/l NH4Cl at pH:7 during 72 h. Chen et al. (3) was obtained the largest amount of bacterial alginate at 34°C and 170 rpm shaking speed and 2% sucrose in about 110 h on optimum medium. In this work it was shown that A3 isolate produced maximum of 1.02 g/l exopolymer in media with 2% sucrose as the carbon source, at 28°C, 180 rpm shaking during 4 days.
Of several methods available for measuring nitrogen fixation, the Acetylene Reduction Assay (ARA) is simpler and faster than the other methods (28). The results presented in this paper are in agreement with the Rodelas et al. (29) who reported 9.70 to 257.73 nmol C2H4 h-1 vial-1 for ARA rate of A. vinelandii.
A. vinelandii has three distinct nitrogenases (3): the Mo, V and Fe-containing nitrogenase called nitrogenase 1, nitrogenase 2, and nitrogenase 3, respectively (30). The absence of vanadium in culture or mutation in nif gene was probably effective factors due to which we couldn't assay nitrogen fixation by isolates A7 and A18.
A possible link between alginate formation and protection of nitrogenase in this organism has not so far been examined. In fact, the biological function of alginate formation in bacteria is not fully understood (6). Nitrogen fixation is inhibited by oxygen since dinitrogenase reductase is rapidly and irreversibly inactivated by O2. For example at 4% oxygen level, A. vinelandii fixed 23.5 mg nitrogen per g sucrose supplied; at 20% oxygen the fixation was 8.1 mg nitrogen (28). A. vinelandii is known to produce alginate under aerobic conditions (6) while a few oxygen concentration is necessary for nitrogen fixation. Increasing biomass and alginate concentrations increase the nitrogenase activity because it reduces oxygen transferring into the cell. Sabra et al used transmission electron microscopy and clearly showed that the A. vinelandii cells grown diazotrophically at pO2 values of 20% formed capsules significant the thickness than pO2 values of 2.5% (6). The alginate concentrations and nitrogenase activity increased upon A3 and A21 reaching exponential phase. The largest values of nitrogen fixation and alginate were obtained in the termination of logarithmic phase. When both strains arrived at stationary phase, bacterial growth and nitrogen fixation values were reduced as expected.
Decreasing alginate concentration after 120 h (Fig. 5C) shows dependence of alginate on bacterial metabolism. Synthesis of alginate depends on carbon sources and it is important for cyst formation (5, 9, 11). Alginate produces during the exponential phases of growth while the cyst has form after exponential growth (6, 9). Therefore under the experimental conditions it is probable that carbon source utilized for alginate production in during log phase and respectively, alginate is used in cyst formation in stationary phase. Increased alginate elevates the viscosity of the culture broth and hence reduces aerobic conditions, leading to a decreased cell growth.
In conclusion, due to correlation between bacterial growth, alginate production and nitrogenase activity almost noticeable yielded significant, the results presented in this work hint to possible role of alginate in nitrogenase activity and it is suggested that how the quantity and quality of alginate at different O2 concentration regulate nitrogen fixation at physiological and genetic levels.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Salimi (National Research Council of I.R. Iran) for molecular experiments. We thank Ms. Shakiba Darvish Alipour Astaneh (Department of Microbiology, Shahed University, Tehran-Iran) for her technical assistance. We are most grateful to Dr. Isar Nasiri (Institute of Biochemistry and Biophysics and COE in Biomathematics, University of Tehran) for the statistical analysis.
REFERENCES
- 1.Staley J, Brenner D, Krieg N. The Proteobacteria, Part B The Gammaproteobacteria . In: Garrity GM, editor. Bergey's manual of systematic bacteriology. 2nd ed. Michigan: Springer; 2005. pp. 384–402. [Google Scholar]
- 2.Dixon R, Kahn D. Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol. 2004;2:621–631. doi: 10.1038/nrmicro954. [DOI] [PubMed] [Google Scholar]
- 3.Chen WP, Chen JY, Chang SC, Su CL. Bacterial alginate produced by a mutant of Azotobacter vinelandii . Appl Environ Microbiol. 1985;49:543–546. doi: 10.1128/aem.49.3.543-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajeswari K, Kasthuri M. Molecular characterization of Azotobacter spp. nifH gene Isolated from marine source. Afr J Biotechnol. 2009;8:6850–6855. [Google Scholar]
- 5.Daiz-Barrera A, Soto E. Biotechnological uses of Azotobacter vinelandii Current state, limits and prospects. Afr J Biotechnol. 2010;9:5240–5250. [Google Scholar]
- 6.Sabra W, Zeng AP, Lunsdorf H, Deckwer WD. Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Appl Environ Microbiol. 2000;66:4037–4044. doi: 10.1128/aem.66.9.4037-4044.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Post E, Kleiner D, Oelze J. Whole cell respiration and nitrogenase activities in Azotobacter vinelandii growing in oxygen controlled continuous culture. Arch Microbiol. 1983;134:68–72. doi: 10.1007/BF00429410. [DOI] [PubMed] [Google Scholar]
- 8.Moshiri F, Crouse BR, Johnson MK, Maier RJ. The ‘nitrogenase protective’ FeSII protein of Azotobacter vinelandii: overexpression,characterisation and crystallization. Biochem. 1995;34:12973–12982. doi: 10.1021/bi00040a007. [DOI] [PubMed] [Google Scholar]
- 9.Remminghorst U, Rehm BHA. Bacterial alginates: from biosynthesis to applications. Biotechnol Lett. 2006;28:1701–1712. doi: 10.1007/s10529-006-9156-x. [DOI] [PubMed] [Google Scholar]
- 10.Adriana NdS, Crispin HG-C. Biopolymers by Azotobacter vinelandii . In: InTech. Magdy Elnashar., editor. Biopolymers. 2010. pp. 413–438. [Google Scholar]
- 11.Sabra W, Zeng AP. Microbial production of alginates: physiology and process aspects. In: Rehm BHA, editor. Alginates: Biology and Applications. Springer; 2009. pp. 153–173. [Google Scholar]
- 12.Sabra W, Zeng AP, Deckwer WD. Bacterial alginate: physiology, product quality and process aspects. Appl Microbiol Biotechnol. 2001;56:315–325. doi: 10.1007/s002530100699. [DOI] [PubMed] [Google Scholar]
- 13.Clementi F, Crudele MA, Parente E, Mancini M, Moresi M. Production and characterisation of alginate from Azotobacter vinelandii . J Sci Food Agric. 1999;79:602–610. [Google Scholar]
- 14.Atlas RM. Handbook of media for environmental microbiology. 2nd ed. Boca Raton: CRC Press; 2005. [Google Scholar]
- 15.Becking J. The Family Azotobacteraceae . In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The Prokaryotes. New York: Springer; 2006. pp. 759–783. [Google Scholar]
- 16.Burgmann H, Widmer F, Von Sigler W, Zeyer J. New molecular screening tools for analysis of free-living diazotrophs in soil. Appl Environ Microbiol. 2004;70:240–247. doi: 10.1128/AEM.70.1.240-247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clementi F, Moresi M, Parente E. Alginate from Azotobacter vinelandii . In: Bucke C, editor. Carbohydrate biotechnology protocols. NJ Totowa: Springer, Humana Press Inc; 1999. pp. 23–42. [Google Scholar]
- 18.Emtiazi G, Ethemadifar Z, Habibi MH. Production of extra-cellular polymer in Azotobacter and biosorption of metal by exopolymer. Afr J Biotechnol. 2004;3:330–333. [Google Scholar]
- 19.Hardy RWF, Holsten RD, Jackson EK, Burns RC. The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol. 1968;43:1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthukumarasamy R, Revathi G, Lakshminarasimhan C. Influence of N fertilisation on the isolation of Acetobacter diazotrophicus and Herbaspirillum spp from Indian sugarcane varieties. Biol Fertil Soils. 1999;29:157–164. [Google Scholar]
- 21.Ravikumar S, Kathiresan K, Ignatiammal S, Babu Selvam M, Shanthy S. Nitrogen-fixing azotobacters from mangrove habitat and their utility as marine biofertilizers. J Exp Mar Biol Ecol. 2004;312:5–17. [Google Scholar]
- 22.Hawkes V. Methods for Studing Biological Soil Crusts, Methodology Paper of the 4th International Conference on IL TER East Asian and Pacific Region; Mongolia: Ulaanbaatar-Hatgal; 2001. pp. 36–45. [Google Scholar]
- 23.Malboobi MA, Owlia P, Behbahani M, Sarokhani E, Moradi S, Yakhchali B, et al. Solubilization of organic and inorganic phosphates by three highly efficient soil bacterial isolates. World J Microbiol Biotechnol. 2009;25:1471–1477. [Google Scholar]
- 24.Claus D, Hempel W. Specific substrates for isolation and differentiation of Azotobacter vinelandii . Arch Microbiol. 1970;73:90–96. doi: 10.1007/BF00409955. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JP, Skerman VBD. Azotobacteraceae: the taxonomy and ecology of the aerobic nitrogen-fixing bacteria. Academic Press Inc.(London) Ltd; 1979. [Google Scholar]
- 26.Pena C, Trujillo-Roldan MA, Galindo E. Influence of dissolved oxygen tension and agitation speed on alginate production and its molecular weight in cultures of Azotobacter vinelandii . Enzyme Microb Techno. 2000;27:390–398. doi: 10.1016/s0141-0229(00)00221-0. [DOI] [PubMed] [Google Scholar]
- 27.Vermani MV, Kelkar SM, Kamat MY. Studies in polysaccharide production and growth of Azotobacter vinelandii MTCC 2459, a plant rhizosphere isolate. Lett Appl Microbiol. 1997;24:379–383. [Google Scholar]
- 28.Sarbay GF. Growth and nitrogen fixation dynamic of Azotobacter chroococcum in nitrogenfree and omw containing medium; Department of Food Engineering,The Middle East Technical University; 2003. M.S.c Dissertation. [Google Scholar]
- 29.Rodelas B, Gonzalez-Lopez J, Pozo C, Salmeron V, Martinez-Toledo MV. Response of faba bean (Vicia faba L.) to combined inoculation with Azotobacter and Rhizobium leguminosarum bv. Viceae . Appl Soil Ecol. 1999;12:51–59. [Google Scholar]
- 30.Betancourt DA, Loveless TM, Brown JW, Bishop PE. Characterization of diazotrophs containing Moindependent nitrogenases, isolated from diverse natural environments. Appl Environ Microbiol. 2008;74:3471–3480. doi: 10.1128/AEM.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]