Summary
The adult Drosophila midgut is maintained by intestinal stem cells (ISCs) that generate both self-renewing and differentiating daughter cells. How this asymmetry is generated is currently unclear. Here, we demonstrate that asymmetric ISC division is established by a unique combination of extracellular and intracellular polarity mechanisms. We show that Integrin-dependent adhesion to the basement membrane induces cell-intrinsic polarity and results in the asymmetric segregation of the Par proteins Par-3, Par-6, and aPKC into the apical daughter cell. Cell-specific knockdown and overexpression experiments suggest that increased activity of aPKC enhances Delta/Notch signaling in one of the two daughter cells to induce terminal differentiation. Perturbing this mechanism or altering the orientation of ISC division results in the formation of intestinal tumors. Our data indicate that mechanisms for intrinsically asymmetric cell division can be adapted to allow for the flexibility in lineage decisions that is required in adult stem cells.
Graphical Abstract
Highlights
► Par complex segregates asymmetrically in dividing Drosophila intestinal stem cells ► Par complex inhibition leads to tumor-like intestinal stem cell overproliferation ► aPKC overactivation alters levels of Notch activity, causing stem cell loss ► Integrins regulate spindle orientation and Par protein localization
This paper extends the role of Par proteins in asymmetric cell division to an adult stem cell lineage—the Drosophila intestinal stem cell—and implicates integrins in regulating Par asymmetry in this context.
Introduction
Stem cells require precise control in the balance between self-renewal and differentiation that needs to be maintained into adulthood to ensure tissue homeostasis while preventing tumorigenesis (Morrison and Spradling, 2008). Much of what is known about how stem cells control this balance is derived from experiments done in the fruit fly Drosophila melanogaster. In Drosophila, neuroblasts and germline stem cells use distinct mechanisms of asymmetric cell division to generate cellular diversity. How asymmetric cell division is controlled in Drosophila adult somatic stem cells, however, is not understood.
During development, neuroblasts undergo repeated rounds of asymmetric divisions, creating neurons in a highly stereotyped lineage (Doe, 2008; Wu et al., 2008; Knoblich, 2008). They arise from a polarized epithelium from which they delaminate while maintaining the apical localization of the so-called Par complex, consisting of the adaptor proteins Bazooka (Baz; Drosophila homolog of Par-3), Par-6, and the protein kinase aPKC (Suzuki and Ohno, 2006). During mitosis, the Par complex interacts with the mitotic spindle machinery to align the spindle orientation along the apicobasal axis, resulting in the asymmetric segregation of the Par complex into the apical daughter cell where it maintains self-renewal capacity (Wodarz et al., 1999, 2000; Schober et al., 1999; Rolls et al., 2003; Lee et al., 2006b). aPKC activity is required to restrict the localization of the protein factors Numb, Prospero (Pros), and Brat to the opposite, basal cortex and thereby ensures their segregation into the differentiating daughter cell (Knoblich, 2010; Doe, 2008; Lee et al., 2006b). Although evidence exists for extrinsic signals playing a role in this system (Siegrist and Doe, 2006), it is largely believed that neuroblasts can divide asymmetrically in a cell-autonomous manner.
Germline stem cells, in contrast, divide throughout adulthood and depend on a secreted signal from the surrounding stem cell niche (Fuller and Spradling, 2007; Kirilly and Xie, 2007). During mitosis, the mitotic spindle is oriented so that after cell division, only one of the two daughter cells can maintain niche contact while the other cell loses contact and undergoes differentiation. When stem cells are lost, niche contacts become available and both daughter cells can retain a stem cell fate (Xie and Spradling, 2000; Kai and Spradling, 2004; Sheng and Matunis, 2011). Therefore, the niche mechanism used by Drosophila germline stem cells allows more flexibility and is thought to prevail in adult stem cell lineages (Morrison and Spradling, 2008; Scadden, 2006) where stem cell numbers have to be adjusted after injury or during regeneration.
Recently, a novel population of multipotent intestinal stem cells (ISCs) has been described in the adult Drosophila midgut (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). These ISCs reside within an epithelial monolayer, and upon cell division, differential levels in Delta/Notch signaling between daughter cells result in the production of an undifferentiated enteroblast (EB), which directly differentiates into a large epithelial-like enterocyte (EC) or a hormone-secreting enteroendocrine (ee) cell (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007). Although this shows how Delta/Notch signaling influences cell fate choices in the adult Drosophila intestinal tract, the mechanism of how this is achieved remains unknown.
Recent studies in the mammalian intestinal tract have demonstrated by lineage tracing that mammalian ISCs behave in a stochastic manner called neutral drift (Lopez-Garcia et al., 2010; Snippert et al., 2010). Rather than cells dividing asymmetrically to generate daughter cells of a different cell fate, the mammalian intestinal epithelium is composed of stem cells that divide symmetrically. This leads to neutral competition within the equipotent stem cell population in the intestinal crypt whereby the stochastic loss of a stem cell results in its replacement and clonal expansion from its neighboring one, which eventually leads to crypt monoclonality (Lopez-Garcia et al., 2010; Snippert et al., 2010). This has lead to the proposal that stem cells are likely to use either an extrinsic or a neutral drift mechanism to maintain homeostasis during adulthood whereas the invariant cell-intrinsic asymmetric divisions are restricted to periods during development (Simons and Clevers, 2011; Klein and Simons, 2011).
Here, we analyze the mechanism that controls cell fate diversity in the adult Drosophila ISC lineage. We demonstrate that ISCs divide using an intrinsic polarity whereby the evolutionary conserved Par complex localizes asymmetrically to the apical daughter cell. Through aPKC activity, this promotes a differentiated cell fate. The apical localization of the Par complex, mitotic spindle orientation, and control of ISC proliferation are dependent on Integrins, indicating that ISCs use a combination of extrinsic and intrinsic mechanisms to regulate ISC self-renewal. Our data show that adult somatic stem cells can adopt a mechanism that regulates the localization of cell-intrinsic polarity to influence the outcome of cell lineages required in adult tissues.
Results
Adult Drosophila ISCs Divide Asymmetrically to Generate an ISC and an EB
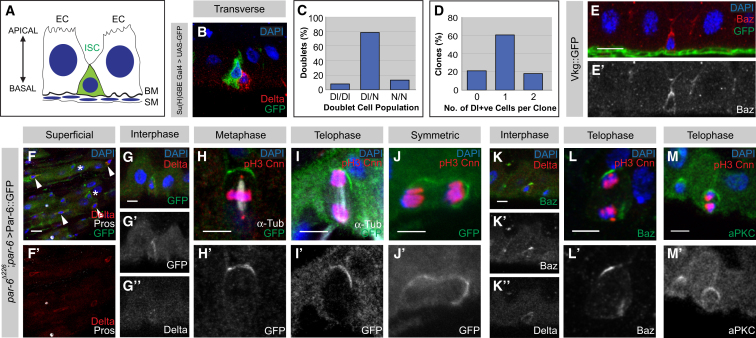
ISCs reside within an epithelial monolayer formed by the ECs with an underlying basement membrane, which is further surrounded by a visceral muscle sheath (Figure 1A) (Baumann, 2001; Ohlstein and Spradling, 2006; Micchelli and Perrimon, 2006). It has been previously described that ISCs divide asymmetrically to generate one self-renewing ISC and one differentiating EB (Ohlstein and Spradling, 2007; O’Brien et al., 2011). In Drosophila neuroblasts, the asymmetric segregation of specific cell fate determinants specifies an invariant stem cell lineage in which one cell will always remain a neuroblast while the other will differentiate. To determine the proportion of divisions where asymmetric cell fates are established in the ISC lineage, we analyzed lineage markers in cells that recently underwent mitosis. The Notch ligand Delta specifically marks ISCs while the EB is active for Notch and therefore expresses Su(H)GBE Gal4 > UAS-CD8::GFP (Figures 1B and Figures S1A and S1B available online) (Ohlstein and Spradling, 2007; Zeng et al., 2010). Assuming that all recent divisions form cell doublets, we were able to unambiguously distinguish EB and ISC fate in the two daughter cells. Although with slight discrepancies from previous studies (O’Brien et al., 2011), we found that approximately 79% of the divisions (Figure 1C, n = 137) resulted in asymmetric cell fate (ISC/EB) whereas symmetric self-renewal (ISC/ISC) and differentiation (EB/EB) was observed in approximately only 8% and 13% of the divisions, respectively. Consistent with this, we also observed similar results from clonal analysis on two-cell pairs (Figure S1C, n = 10). To further characterize the ISC lineage, we conducted a clonal analysis to analyze the cell populations produced over time. In 4–5 day positively marked clones, we observed clonal sizes ranging from typically 2–11 cells (data not shown). The majority of these clones (about 61%) contained only a single Delta-positive cell (Figure 1D, n = 33 cell clones in total) while only few clones had two or no Delta-positive cells. Together, this indicates that the majority of ISCs undergo an asymmetric cell division in terms of cell fate. Unlike neuroblasts, however, ISCs have the flexibility to also divide symmetrically and to generate two daughter cells of the same fate.
Figure 1.
Adult Drosophila ISCs Divide Asymmetrically and Segregate Members of the Par Complex into the Apical Daughter Cell during ISC Division
(A) Schematic diagram of the Drosophila intestinal epithelium. EC, enterocyte; ISC, intestinal stem cell; BM, basement membrane; SM, surrounding musculature.
(B) Transverse view of Delta-positive ISC (red) and its immediate progeny, the EB (green), expressing Su(H)GBE Gal4 > UASCD8::GFP.
(C and D) Quantification of the percentage of cell doublet populations in terms of cell fate (C, n = 137) and the number of Delta-positive ISCs within 4–5 day clones (D, n = 33).
(E–G) Members of the Par complex are expressed and polarized apically. Endogenous Baz (E, red) apically localizes with respect to the basement membrane (E, Vkg::GFP, green) within small ISC-like diploid cells. Par-6::GFP (F, green) is expressed in Delta-positive ISCs (arrowheads, red) and EBs (asterisks), but not Pros-positive ee cells (white). Par-6::GFP (G, green) is polarized in Delta-positive ISCs (red) along the apicobasal axis at interphase.
(H–J) Localization of Par-6::GFP in mitotic ISCs. In metaphase (H), apical asymmetric localization of Par-6::GFP is maintained, and in telophase (I), it is segregated into the apical daughter cell. Par-6::GFP is mislocalized/symmetric (J) in a small fraction of ISC divisions.
(K–M′) Localization of other members of the Par complex. Endogenous Baz (K, green) is apical in interphase ISCs (red) and is apically asymmetric in telophase (L). Endogenous aPKC (M) is asymmetric in telophase ISCs.
All images were taken as confocal Z-stacks and were processed as maximum projections. Scale bars = 10 μm (E and F) and 5 μm (G–M). See also Figure S1.
The Par Complex Localizes Asymmetrically to the Apical Cell during ISC Divisions
Asymmetric cell division can be orchestrated either by intrinsic cell polarity or by extrinsic environmental cues. To address the relevance of intrinsic polarity, we next looked at how the intestinal epithelium is polarized. The apical membrane of the intestinal epithelium faces the gut lumen. Interestingly, we observed that the septate junction marker Discs-large (Dlg) localizes apicolaterally (Figures S1D and S1E) while the Drosophila β-Catenin homolog Armadillo, which marks adherens junctions, is located basolaterally (Figures S1F and S1G) (Baumann, 2001). Thus, the epithelial characteristics of the Drosophila adult midgut are distinct from other established epithelia, and polarity complexes may function differently in this tissue.
Asymmetric cell division in neuroblasts is oriented by the apical Par complex consisting of the PDZ domain proteins Baz and Par-6 and the kinase aPKC (Wodarz et al., 1999; Schober et al., 1999; Petronczki and Knoblich, 2001; Rolls et al., 2003; Wodarz et al., 2000). To ask whether the Par complex is expressed in ISCs, we analyzed the endogenous localization for Baz and aPKC and used a functional GFP-tagged genomic rescue construct in a par-6 null mutant background for Par-6 (Wirtz-Peitz et al., 2008). In the midgut, all members of the Par complex were expressed in small, basal cells that often, but not always, are in doublets (Figures 1E, S1F, and S1G). Furthermore, transverse sections through the intestinal epithelium reveal that within these small cells, all the Par complex members localize in a polarized fashion apically (Figures 1E and S1G). Staining for the ISC marker Delta and ee marker Pros shows that these cells are ISCs and EBs (Figure 1F). In Delta-positive interphase ISCs, Par-6 (Figure 1G) and Baz (Figure 1K) are apically enriched, and in mitotic ISCs, Par-6 (Figures 1H and 1I, 85% apical asymmetry, n = 33 anaphase/telophase cells), Baz (Figure 1L, 84% apical asymmetry, n = 31 prophase-telophase cells), and aPKC (Figure 1M, 78% apical asymmetry, n = 9 prophase-telophase cells) are concentrated in the cortical area overlying the apical centrosome. Similar observations were made during mitosis when we overexpressed Baz::GFP (Figure S1H). After division, it is inherited by the apical daughter cell, which will become the EB. Interestingly, in a small fraction of ISC divisions, we found mislocalization (Figure 1J, 15% mislocalized/symmetric, n = 33 anaphase/telophase cells) of the Par complex, which is consistent with what we observed in cell fate asymmetry in cells that have recently undergone mitosis (Figure 1C). Thus, ISCs display a cell-intrinsic polarity that might be similar to the one observed in neuroblasts.
In neuroblasts, aPKC phosphorylates Numb and Miranda, leading to their basal accumulation and their segregation into the basal daughter cell (Atwood and Prehoda, 2009; Smith et al., 2007; Wirtz-Peitz et al., 2008). Using a Numb::GFP fusion protein containing the PTB domain and all sequences necessary for asymmetric localization (NumbPTB::GFP) (Roegiers et al., 2001), we analyzed whether aPKC exhibits kinase activity during mitosis in ISCs. NumbPTB::GFP is uniformly cortical in interphase (Figure S1I), but it concentrates over the basal centrosome in prometa (Figure S1J) and metaphase (Figure S1K) so that it is segregated into the future stem cell upon division (Figure S1L, 79% basal asymmetry, n = 19 metaphase-two cell stage). Upon inhibition of the aPKC binding partner Par-6 by RNAi or upon overexpression of the constitutively active membrane-tethered form of aPKC (aPKC-CAAX), NumbPTB::GFP becomes uniformly cortical (Figure S1M, 69% symmetric localization, n = 13 metaphase-telophase cells) or is mislocalized into the cytoplasm (Figure S1N, n = 9 metaphase-telophase cells), respectively. This mislocalization is not seen in a kinase-dead version of aPKC-CAAX (aPKC-CAAXKD), indicating that aPKC kinase activity is required for the asymmetric localization of NumbPTB::GFP in the ISCs (Figure S1O, n = 16 prometaphase-two cell stage). In neuroblasts, loss of numb function results in overproliferation and tumor formation (Lee et al., 2006a; Wang et al., 2006). In ISCs, however, we were unable to detect asymmetrically localized endogenous Numb protein by immunofluorescence (data not shown). Furthermore, consistent with previous data (Bardin et al., 2010), we did not detect any striking lineage phenotype in numb mutant ISCs (data not shown). As the asymmetric localization of Numb is known to depend on direct phosphorylation by aPKC, these data suggest that aPKC is active in mitotic ISCs. Unlike in neuroblasts, however, the asymmetric segregation of Numb does not seem to be a major downstream target of aPKC in ISCs.
The Par Complex Is Required and Sufficient to Induce Differentiation in ISCs
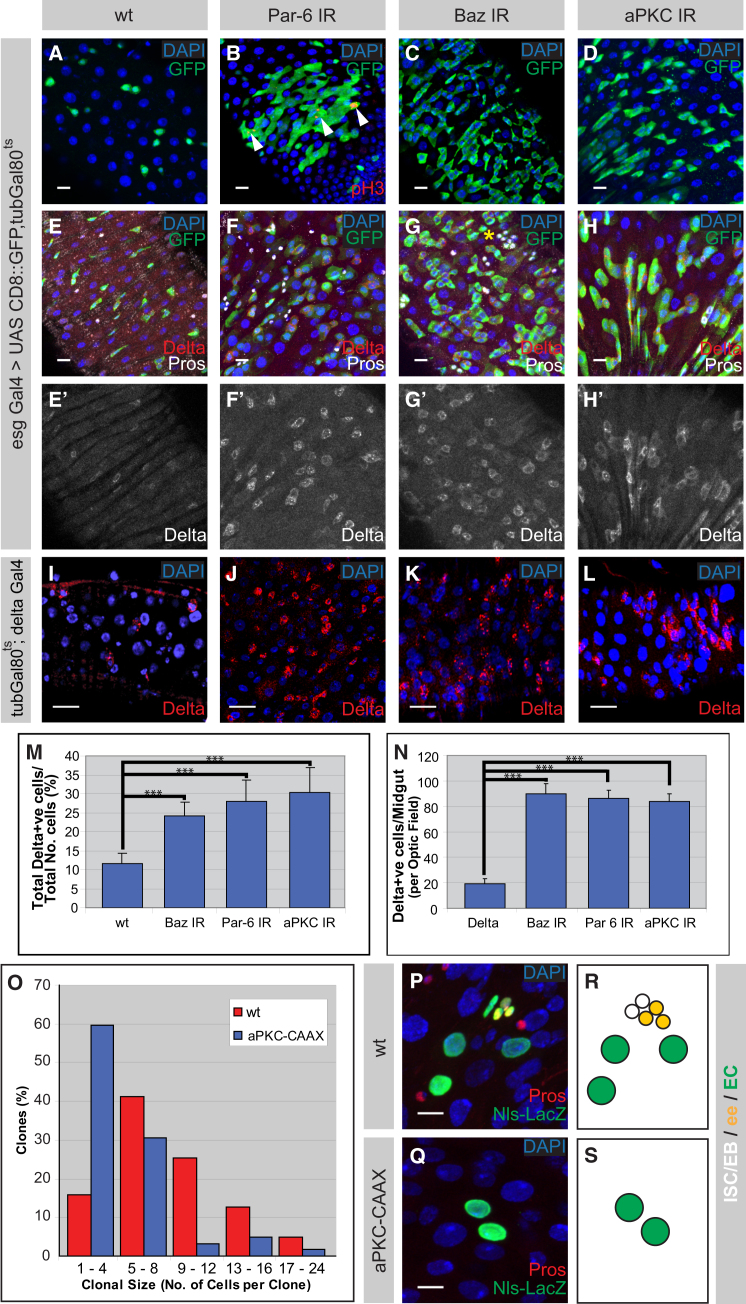
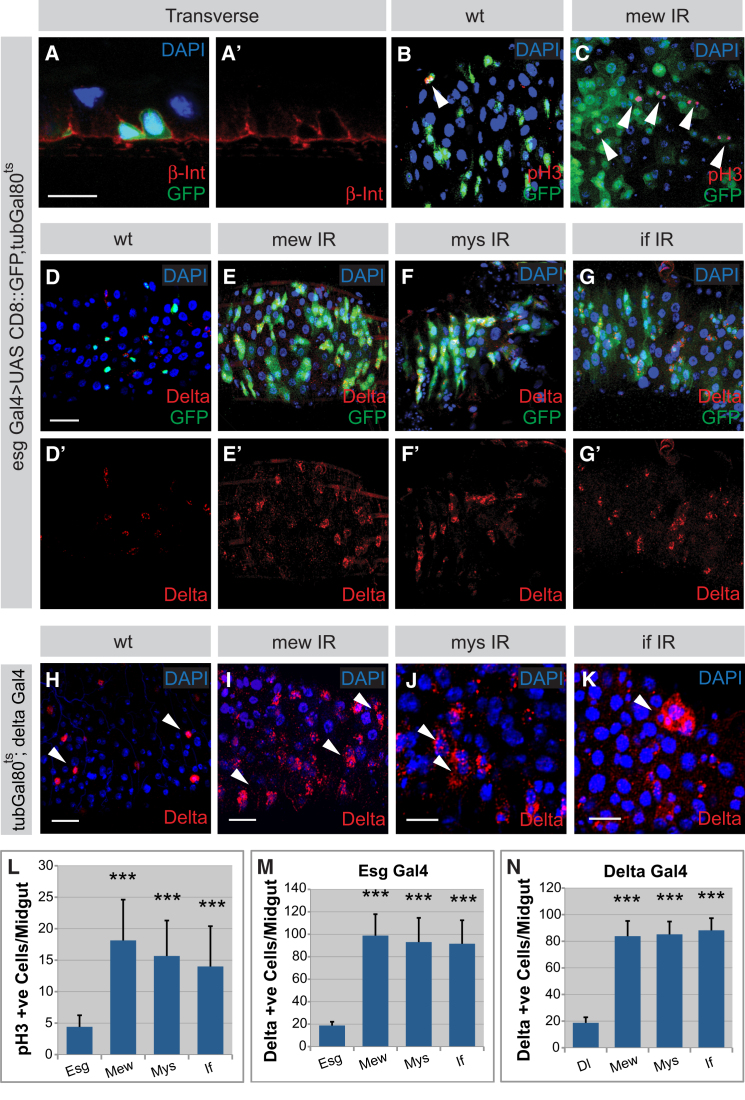
To determine the function of the Par complex, we utilized the Temporal and Regional Gene Expression Targeting (TARGET) system to spatially and temporally regulate RNAi knockdown in ISCs and EBs using the escargot (esg) Gal4 driver (McGuire et al., 2003). The functionality of the RNAi lines was confirmed in neuroblasts and in the adult midgut (Figures S2A–S2J). In a control midgut, stem cells exist individually or (rarely) as doublets, together with their daughter EB cell (Figures 2A and 2E). Upon silencing of Baz, Par-6, or aPKC, we observed an increase of small esg-positive cells, which are therefore ISCs and/or EBs (Figures 2A–2D). Some of these cells are mitotically active (anti-phospho H3-positive, arrows Figure 2B) and form small tumor-like clusters that are positive for the stem cell marker Delta (Figures 2F–2H and 2M). Interestingly, we also occasionally observed clustering in small groups of Pros-positive ee cells (Figure 2G, asterisk), similar to the Notch loss-of-function phenotypes previously described (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Overproliferation phenotypes were confirmed using alternative secondary/tertiary RNAi lines (Figures S2K–S2O). This is further supported by the increased size of par-6 or baz mutant clones (Figure S2P). The number of Delta-positive cells was increased in those clones (data not shown). Of note, we also observed an increase in Delta-positive cells outside the clones, which could be explained by the slight dominant phenotype that was seen in the stocks used to generate the clones (data not shown). Although non-cell-autonomous effects cannot be completely excluded, the basolateral localization of Arm upon knockdown of Par-6 was unaffected (Figure S2Q), thus indicating that the phenotype does not arise from defects in epithelial polarity. To test whether the Par complex is required in ISCs, we made use of Delta Gal4, which is specifically expressed in ISCs, combined with the TARGET system (Zeng et al., 2010). Consistent with the knockdown effects seen using esg Gal4, expression of Baz, Par-6, or aPKC RNAi constructs specifically in the ISCs resulted in a similar increase in Delta-positive ISCs (Figures 2I–2L and 2N). Thus, silencing of the Par complex in the ISCs leads to the increase in undifferentiated cells in the Drosophila intestinal epithelium.
Figure 2.
The Par Complex Is Required for Differentiation of EBs and the Kinase Activity of aPKC Is Sufficient to Induce Differentiation in ISCs
(A–H) RNAi knockdown of Par-6 (B and F), Baz (C and G), and aPKC (D and H [KK line, VDRC]) results in the ectopic clusters of undifferentiated ISCs and EBs (green) to varying extents in comparison to wild-type (A and E). Arrowheads (B) show mitotic ISCs (red). Lineage staining using Delta (red) to label ISCs and Pros (white) to label ee cells shows an increase in Delta upon knockdown of Par complex members (F–H) in comparison to wild-type (E). ee lineages (E–H, white) are largely unaffected but occasionally also form clusters (G, asterisk).
(I–L) RNAi knockdown in the ISCs, specifically by Delta Gal4, of Par6 (J), Baz (K), or aPKC (L) leads to increased Delta-positive cells (red) in comparison to wild-type (I).
(M and N) Quantification of Par complex RNAi knockdown using esg Gal4 (M, n = 4 guts/genotype) and Delta Gal4 (N, n = 12 guts/genotype).
(O–S) Clonal analysis using the Act-FLP out-Gal4 system comparing aPKC-CAAX overexpression to that of wild-type clones. Histogram representing the percentage of clones with respect to clonal size, 4–5 days after clonal induction (O) is shown. Nuclear-LacZ (Nls-LacZ, green) positively labels mitotic clones in wild-type (P), and aPKC-CAAX overexpression (Q) with Pros (red) marks ee cells. Schematic diagram of ISC lineage identity in wild-type and aPKC-CAAX backgrounds (R and S) is shown.
Statistical significance was determined by Student’s t test (∗∗∗p < 0.001). All images were taken as confocal Z-stacks and were processed as maximum projections. Scale bar = 10 μm (A–L) and 5 μm (P and Q). See also Figure S2.
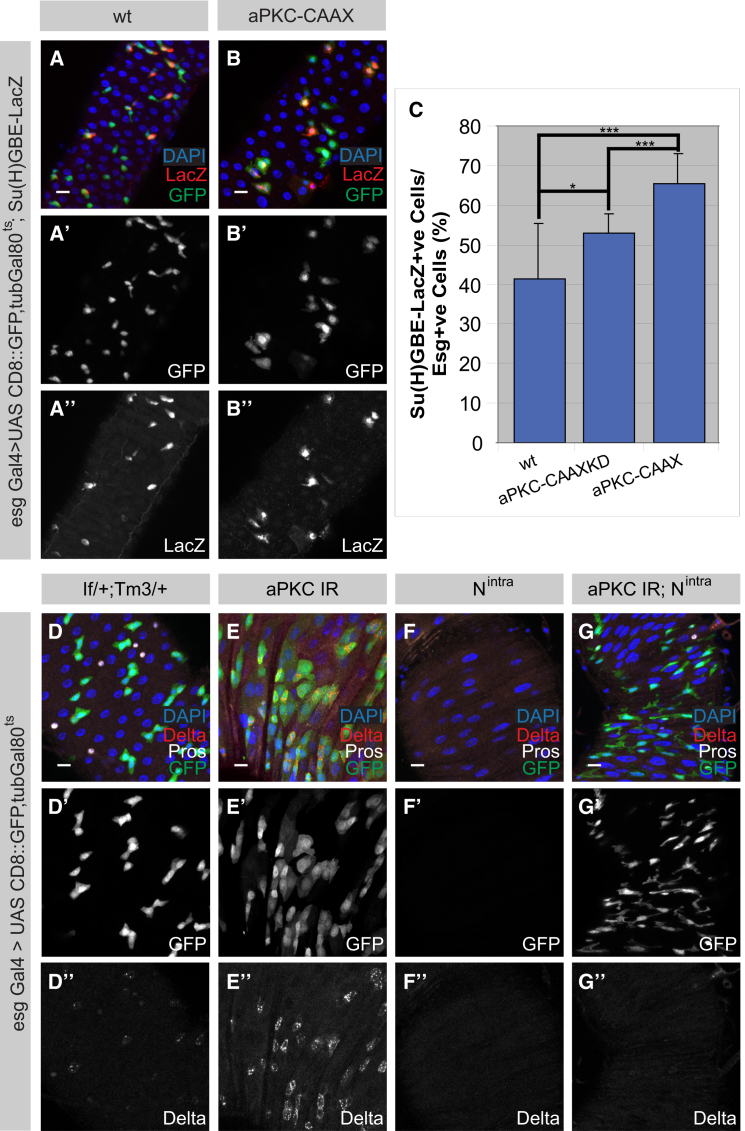
To ask whether the segregation of the Par complex into the differentiating daughter cell limits self-renewal capacity, we expressed aPKC-CAAX. In neuroblasts, aPKC-CAAX promotes self-renewal in both daughter cells and induces the formation of stem-cell-derived tumors (Lee et al., 2006b). Surprisingly, however, aPKC-CAAX expression in ISCs actually inhibits self-renewal capacity (Figures 2O–2S). Sixty percent of 4- to 5-day-old nuclear β-Galactosidase marked clones overexpressing aPKC-CAAX contain four or fewer cells while such low cell numbers are only observed in fifteen percent of the control clones (Figure 2O). Staining for the ee marker Pros reveals that in 46% (n = 37) of these clones, stem cells are lost and all cells have differentiated into polyploid ECs that can be uniquely identified by their large nuclear morphology (Figures 2P–2S). Upregulation of Delta/Notch signaling results in the differentiation of ISCs into EBs (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). To address whether Notch activity has altered under conditions in which aPKC is constitutively active, we used the Notch reporter Su(H)GBE-LacZ (Furriols and Bray, 2001). Upon overexpression of aPKC-CAAX, an increase in the number of Su(H)GBE-LacZ-positive cells was observed in comparison to either wild-type or aPKC-CAAXKD that was statistically significant (Figures 3A–3C and S3A–S3C). This indicates that the proportion of Notch-active cells, hence differentiating EBs, has increased in the intestinal tract. A slight increase in Notch activity was also seen in aPKC-CAAXKD overexpression, indicating that aPKC may also have alternative functions in this system (Figures 3C and S3A–S3C).
Figure 3.
aPKC Kinase Activity Acts on Notch Signaling and Notch Is Epistatic to the Par Complex
(A–C) Notch activity within the intestinal epithelium increases upon the overexpression of aPKC-CAAX. The subset of Su(H)GBE-LacZ-positive cells (red) within esg-positive cells (green) increases upon the aPKC-CAAX expression (B) in comparison to wild-type (A). Histogram of the percentage of Su(H)GBE-LacZ-positive cells within esg-positive cells (C) is shown.
(D–G″) Notch is epistatic to aPKC. In comparison to the internal control (D), aPKC IR (E, 1B1 aPKC IR) results in an increase in esg-positive (green) and Delta-positive (red) cells, whereas overexpression of Nintra leads to the depletion of esg-positive and Delta-positive cells (F). Overexpression of Nintra rescues the aPKC IR phenotype, reducing the number of esg-positive cells (G). These cells are not Delta-positive (G), indicating that they are EBs. Error bars, standard deviation; n = 12 guts in wild-type, n = 11 guts in aPKC-CAAX overexpression, n = 10 guts in aPKC-CAAXKD. Statistical significance was determined by Student’s t test (∗p < 0.05, ∗∗∗p < 0.001).
All images were taken as confocal Z-stacks and were processed as maximum projections. Scale bar = 10 μm. See also Figure S3.
Because aPKC activity regulates Notch in ISCs, we asked if Notch activity could rescue the overproliferation phenotype observed in the aPKC knockdowns. Using the esg Gal4 driver with the TARGET system, we found that upon the overexpression of an active form of Notch (Nintra), the depletion of ISCs (Figures 3F, S3F, and S3G) was similar to that observed in previously described studies (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2007), while the knockdown of aPKC results in an increase of ISCs (Figures 3E and S3E, n = 9/11 guts) in comparison to the internal control (Figure 3D and S3D, n = 7 guts). However, when Nintra was overexpressed in the background of aPKC knockdown, we observed a decrease in the number of esg-positive cells and Delta-positive ISCs (Figures 3G and S3H, n = 7/8 guts), similar to the levels observed in the weak Nintra phenotype (Figure S3F, n = 2/6 guts). Therefore Notch is epistatic to the Par complex in ISCs and thus, unlike in other Drosophila stem cell lineages, aPKC acts to induce differentiation and limits self-renewal.
Integrins Regulate the Proliferation of ISCs
Cell-Extracellular Matrix (ECM) interactions are required for extrinsic cues to establish epithelial polarity (Ekblom, 1989; Conder et al., 2007). Because the adult Drosophila intestinal epithelium directly contacts a basement membrane, we asked whether cell-ECM interactions are required in ISC self-renewal. Integrins are heterodimeric cell-matrix adhesion receptors consisting of an alpha and a beta subunit associated with numerous processes (Ellis and Tanentzapf, 2010; Shattil et al., 2010). In Drosophila there are three canonical Integrins, namely the αPS1 subunit multiple endematous wings (mew), the αPS2 subunit inflated (if), and the βPS subunit myospheroid (mys) (Brown, 2000). Using the esg Gal4 driver to identify ISCs and EBs with GFP, we found that the βPS subunit mys (Figure 4A) and the two alpha subunits (data not shown) localize basolaterally within ECs and cortically around the ISCs and EBs with an enrichment basally contacting the basement membrane.
Figure 4.
Integrins Regulate ISC Proliferation and Self-Renewal
(A and A′) Integrin localization in intestinal epithelium. βPS-Integrin (red) is in contact with the ISCs (A, green).
(B and C) ISC divisions increase in Integrin knockdown. Single ISCs (green) dividing (pH3, red) are compared with multiple dividing ISCs in mew knoc′kdown (C).
(D–G) Integrin knockdown in the ISCs and EBs leads to ectopic esg-positive cell clusters. Clusters of esg-positive (green) and Delta-positive (red) cells form in mew (E), mys (F), and if (G) RNAi knockdowns in comparison to wild-type (D).
(H–K) Integrin RNAi phenotype with Delta Gal4 driver. Confocal Z projections of Delta Gal4 driver line alone (H) and Delta Gal4 driving mew (I), mys (J), and if knockdown (K) in ISCs marked by Delta (red) is shown.
(L–N) Quantification of mitotic cells upon Integrin knockdown with the esg Gal4 driver line (L, n = 15 guts/genotype) and the quantification of the increase in Delta-positive cells in Integrin knockdown with the esg Gal4 (M, n = 15 guts/genotype) and Delta Gal4 (N, n = 10 guts/genotype) drivers.
Error bars, standard deviation. Statistical significance was determined by Student’s t test (∗∗∗p < 0.001). Scale bar = 10 μm. See also Figure S4.
We next used the TARGET system to silence Integrins in ISCs and EBs. Having confirmed the functionality of the RNAi lines by the characteristic wing blistering phenotype observed upon Integrin silencing in wing imaginal discs (Figures S4A–S4D) (Brower and Jaffe, 1989), we found that silencing of any of the canonical Integrins results in the formation of additional esg-positive cells (Figures 4D–4G), similar to the Par complex knockdown phenotype. The number of mitotic cells in the intestine upon Integrin knockdown resulted in an increase of approximately 3- to 4-fold in comparison to the control (Figures 4B, 4C, and 4L), and a subset of the additional cells express the stem cell marker Delta (Figures 4D–4G and 4M). This phenotype is specific because it can be recapitulated by secondary RNAi lines and in integrin mutants (Figures S4E–S4H and S4K–S4M). To test whether Integrins are required in ISCs, we again made use of Delta Gal4 with the TARGET system. Expression of Integrin RNAi lines using this driver resulted in the formation of ectopic Delta-positive cells (Figures 4H–4K and 4N), demonstrating that the Integrin knockdown phenotype originates from ISCs. Similar phenotypes are observed upon silencing of Talin and Tensin (Figures S4I and S4J), two proteins required for the signaling function of Integrins (Legate and Fässler, 2009). Knockdown of the noncanonical Integrin βν, however, did not cause this phenotype (data not shown), although this form has been reported to have a developmental role in the Drosophila midgut (Yee and Hynes, 1993). Furthermore, using the epithelia-like EC driver Myo1A Gal4 (Jiang et al., 2009) to silence the canonical Integrins also resulted in no ISC phenotype (Figures S5G–S5J). Thus, Integrins are required in ISCs to ensure that only one of the daughter cells retains stem cell fate. Because Integrin deletion in mammalian ISCs also results in intestinal hyperplasia (Jones et al., 2006), these results may indicate a conserved role for these proteins in limiting adult stem cell proliferation, and we went on to characterize their mode of action (Lin et al., 2008).
The surrounding visceral musculature of the adult Drosophila midgut has been associated with the secretion of ligands required to regulate ISC proliferation (Lin et al., 2008). To determine whether Integrins are required nonautonomously in the surrounding visceral musculature, we used the mef2 Gal4 driver to remove Integrins. Upon RNAi-mediated silencing of any of the three conventional Integrins, the number of Delta-expressing ISCs is increased (Figures S4N–S4R) and clusters of ISCs are formed that resemble the phenotype observed after RNAi in the stem cells themselves. We conclude that Integrins are required in the visceral musculature and ISCs to limit proliferation and self-renewal in the ISC lineage.
Integrin-Mediated Adhesion and Par Complex Are Required for Oriented ISC Divisions
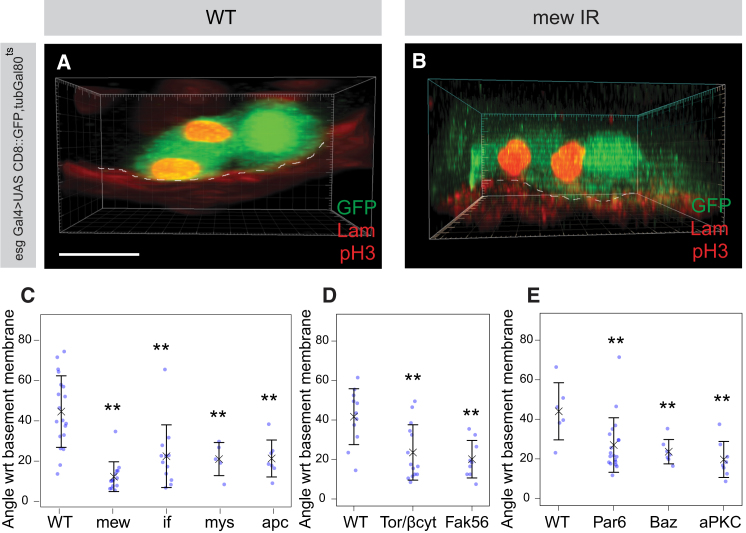
Our results indicate that asymmetric division of ISCs is induced by a combination of extrinsic Integrin signaling and intrinsic Par-protein polarity. To determine how those apparently distinct mechanisms cooperate, we analyzed the effects of Integrin RNAi on the orientation of ISC division and intrinsic ISC polarization. Previously, it was shown in the Drosophila follicular epithelium that Integrins regulate spindle orientation (Fernández-Miñán et al., 2007). Therefore we looked at mitotic ISCs and labeled the centrosomes with either anti-centrosomin (Cnn) or anti-gamma tubulin and analyzed angles between the spindle axis and the basement membrane after 3D reconstructions of confocal stacks. Consistent with previous observations (Ohlstein and Spradling, 2007), we found that most ISCs divide so that one daughter cell no longer contacts the basement membrane (Figures 5A and 5C). Upon RNAi silencing of the tumor suppressor adenomatous polyosis coli (APC), which is known to regulate spindle orientation (Yamashita et al., 2003), ISC divisions become parallel to the basement membrane, indicating that the spindle orientation machinery is involved (Figure 5C). Parallel spindle orientation was also seen upon RNAi of any conventional Integrin (Figures 5A–5C). To understand whether Integrin adhesion is required for the positioning of the mitotic spindle to direct asymmetric cell division in ISCs, we utilized the TorD/βcyt and FAK56wt dominant-negative adhesion constructs, which do not bind to the ECM (Palmer et al., 1999; Martin-Bermudo and Brown, 1999). The overexpression of either the chimeric TorD/βcyt, consisting of the βPS cytoplasmic tail fused to a dominant-active extracellular Torso receptor tyrosine kinase domain, or the Drosophila homolog of focal adhesion kinase, FAK56wt, resulted in defects in spindle orientation in ISCs (Figure 5D). Consistent with this, overexpression of these constructs resulted in localized ISC proliferation defects (Figures S5A–S5C). Therefore, Integrin-mediated adhesion to the basement membrane is required for spindle reorientation.
Figure 5.
Integrins Regulate Spindle Orientation and Localization of Asymmetric Proteins
(A and B) 3D reconstruction of confocal projections of mitotic esg-positive ISCs (green) labeled by pH3 (red) in wild-type (A) and mew (B) knockdown cells. Basement membrane is labeled by Laminin (red).
(C–E) Quantification of angles of spindle orientation away from the basement membrane upon knockdown of mew (n = 14), mys (n = 6), if (n = 12), and apc (n = 7) (C) cells. Overexpression of TorD/βcyt (n = 14) and FAK56wt (n = 9) dominant-negative adhesion constructs in shown in (D) and knockdowns of Par complex members Par-6 (n = 21), Baz (n = 7), and aPKC (n = 8) is shown in (E).
Statistical significance was determined by Wilcoxon rank sum test. Scale bars = 5 μm. See also Figure S5.
During asymmetric cell division, the Par complex interacts with the spindle machinery to orient the axis of division. To investigate whether this is the case in ISCs, we silenced members of the Par complex and found that cells divided more parallel (Figure 5E) across the basement membrane, similar to the Integrin RNAi phenotype. This is consistent with the role described recently for the Par complex in orienting spindle in epithelia (Hao et al., 2010; Durgan et al., 2011). Because Par complex activity has been shown in neuroblasts to bias Delta/Notch signaling (Wirtz-Peitz et al., 2008), which is also required for cell fate decisions in ISCs, we also looked at whether RNAi knockdown of Notch would result in ISC spindle misorientation but found no obvious defects (data not shown).
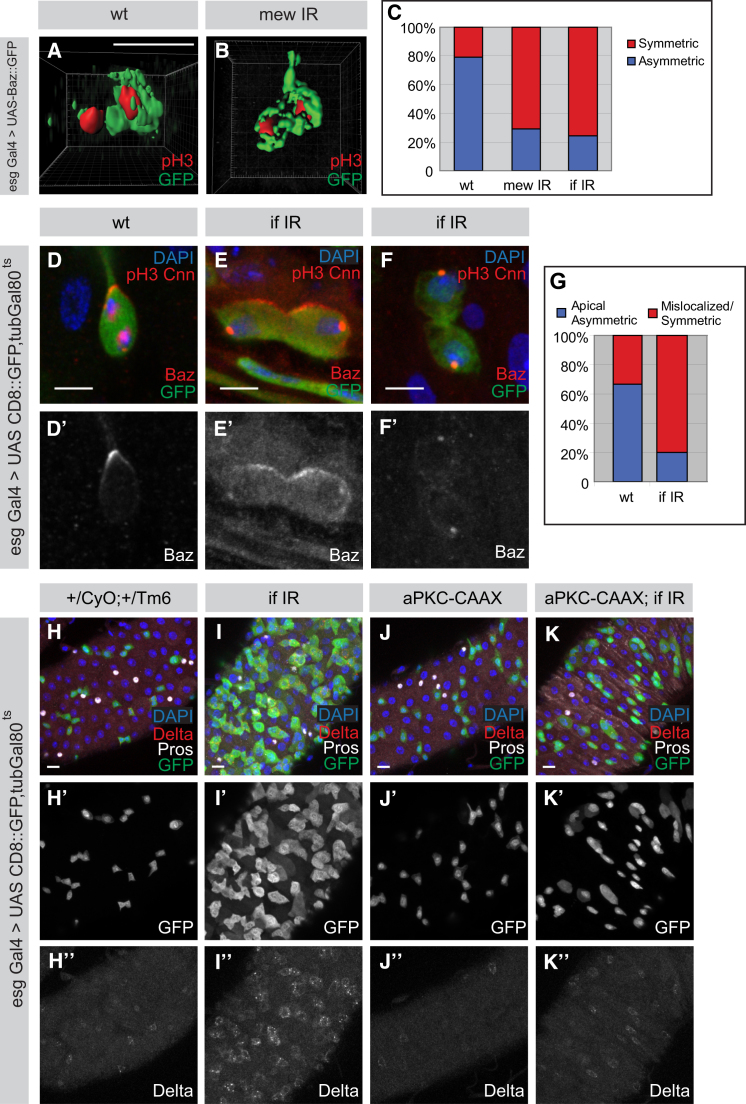
Integrins Are Required for the Asymmetric Localization of the Par Complex and Interact Genetically Upstream of the Par Complex
To ask whether intrinsic asymmetry is maintained upon silencing of Integrins, we analyzed Baz::GFP and endogenous Baz localization. The distinct apical localization of both Baz::GFP and endogenous Baz are lost in ISCs upon Integrin silencing (Figures 6A–6G). Based on these results, we would expect that the Par complex is genetically downstream of Integrins in ISCs. To investigate whether the Par complex is epistatic to Integrins, we overexpressed aPKC-CAAX in the Integrin knockdown background to see whether aPKC activity can rescue the phenotype resulting from Integrin RNAi silencing. While Integrin knockdown results in ectopic formation of ISCs (Figure 6I) and aPKC-CAAX overexpression causes the depletion of ISCs (Figure 6J) in comparison to the wild-type (Figure 6H), we found that the severe phenotype observed in Integrin RNAi (Figure 6I, n = 4/15 guts with severe phenotypes) is rescued upon the overexpression of aPKC-CAAX (Figure 6K, n = 0/10 guts with severe phenotype), although areas of local overproliferation are still occasionally observed (n = 3/10 guts). Therefore this indicates indeed that the Par complex is epistatic to Integrins. This is further supported by unaffected Integrin localization in the knockdown of the Par complex (Figure S5K). Because a disruption of spindle orientation by knockdown of Cnn or EB1 (Figures S5D–S5F) causes an overproliferation phenotype similar to the one observed upon Par complex silencing, we propose that the spindle orientation defects resulting from the mislocalization of the Par complex might be responsible for the apparent overproliferation phenotype caused by Integrin knockdown. Thus, a mechanism where extrinsic Integrin signaling establishes intrinsic polarity to orient the mitotic spindle machinery and asymmetric protein segregation seems to be responsible for asymmetric cell division in adult ISCs to bias the outcome of the cell lineages required during homeostasis.
Figure 6.
Integrins Regulate the Localization and Are Genetically Upstream of the Par Complex
(A–G) Asymmetric localization of the Par complex is affected upon Integrin knockdown. 3D reconstruction of mitotic (red) Baz::GFP (green) ISCs in wild-type (A) and Integrin knockdown (B) is shown. Statistical quantification of the percentage of asymmetric/symmetric Baz::GFP between wild-type (n = 24 cells) and Integrin knockdown (C, mew IR, n = 24 cells, if IR, n = 33 cells) is shown. Endogenous Baz localization (red) is also perturbed upon knockdown of if, leading to apical symmetric (E) or general mislocalization (F) (if IR, n = 20 cells metaphase/two-cell stage) in comparison to the wild-type (D, n = 15 cells metaphase/two-cell stage) in mitotic (red) ISCs (green). Histogram (G) of the localization of endogenous Baz between wild-type and if RNAi is shown.
(H–K″) The Par complex is epistatic to integrins. RNAi knockdown of if results in supernumerary Delta-positive ISCs (I) whereas aPKC-CAAX overexpression leads to the loss of ISCs (J) in comparison to the internal control (H). The phenotypic severity observed in if RNAi is rescued upon the overexpression of aPKC-CAAX (K).
Images (D–F and H–K) were taken as confocal Z-stacks and were processed as maximum projections. Scale bars = 5 μm (A, B, and D–F) and 10 μm (H–K).
Discussion
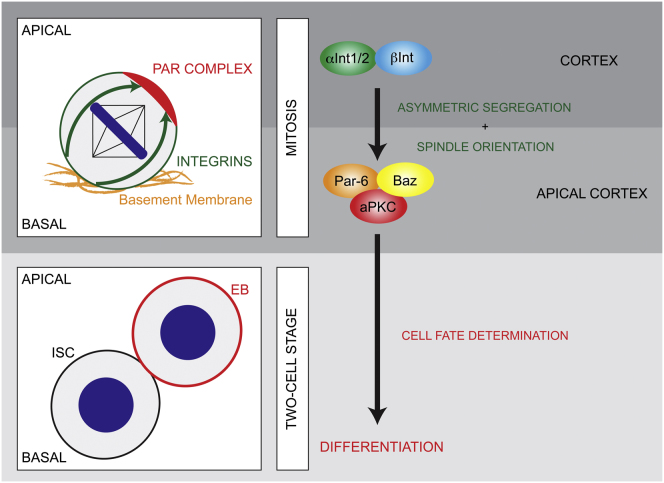
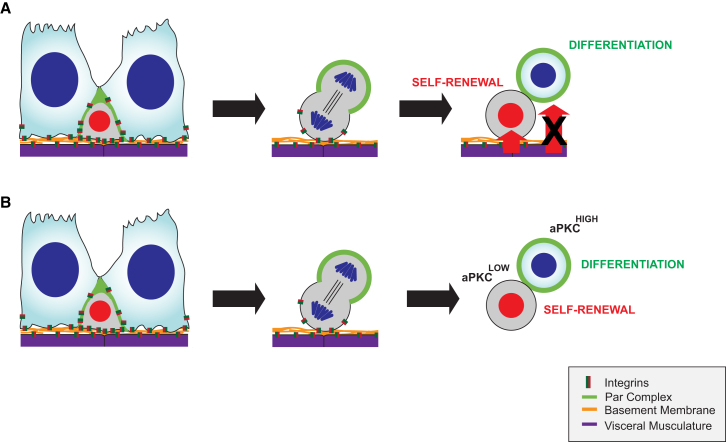
Our results suggest that adult Drosophila ISCs use a combination of extrinsic and intrinsic polarity cues to divide asymmetrically (Figure 7). We demonstrated that the polarity proteins used for asymmetric cell division in neuroblasts during development are also conserved in regulating the adult Drosophila ISC lineage. Remarkably, whereas neuroblasts produce an invariable cell lineage, we found that ISCs have adopted a mechanism to generate a variable cell lineage dependent on Par complex segregation between daughter cells. This localization required Integrin-mediated adhesion to orient the spindle in order to establish different cell fates in the two daughter cells.
Figure 7.
The Par Complex and Integrins Direct Asymmetric Cell Division in ISCs
(A and B) Adult Drosophila ISCs divide asymmetrically through Integrin-dependent asymmetric localization the Par complex during mitosis. Different fates might arise either because the two daughter cells reside in different microenvironments and receive different signals (A) or because levels of aPKC activity are higher in one of the two daughter cells (B).
Our data suggest a model in which adhesion to the basement membrane through Integrins provides positional information for the apical localization and asymmetric segregation of the Par complex. At the moment, we cannot strictly exclude the possibility that loss of Par proteins in stem cells triggers a nonautonomous effect stimulating stem cell proliferation. However, this is unlikely due to the phenotypes observed upon cell-type-specific knockdown. Instead, there are two more plausible ways to explain our data. One possibility is that the two daughter cells interact differently with the basement membrane due to the oblique orientation of ISC divisions (Figure 7A). In this scenario, the two daughter cells are placed in different microenvironments and only one daughter cell maintains contact with the basement membrane and retains stem cell fate. Consistent with this, knockdown of proteins affecting spindle orientation causes lineage defects and stem cell overproliferation. However, this would imply that the Par complex simply acts by orienting the mitotic spindle and is therefore not consistent with the opposing phenotypes observed upon Par complex knockdown and overexpression of the constitutive active form of aPKC.
Therefore, the most likely explanation for our data is that higher levels of aPKC in one of the two daughter cells drive this cell toward differentiation (Figure 7B). This is similar to neuroblasts where the apical segregation of aPKC is a key determinant for the establishment of correct cell fates (Cabernard and Doe, 2009; Lee et al., 2006b; Rolls et al., 2003). In contrast to neuroblasts, however, aPKC prevents rather than induces ISC self-renewal. This establishes aPKC as a tumor suppressor and contrasts the previous findings where aPKC overexpression has resulted in tumor formation (Lee et al., 2006b; Grifoni et al., 2007). Interestingly, a similar discrepancy of aPKC function is seen in vertebrates where aPKC is upregulated in human non-small cell lung cancer cells (Regala et al., 2005), ovarian cancer (Zhang et al., 2006; Eder et al., 2005), and breast cancer (Kojima et al., 2008), but inhibits Ras-induced lung carcinogenesis (Galvez et al., 2009) and is required for differentiation in mammary glands (McCaffrey and Macara, 2009).
Although we have demonstrated that the asymmetric segregation of the Par complex is required and sufficient for the differentiation of adult Drosophila ISCs, the mechanism through which aPKC acts on Notch signaling to establish differentiated cell fate remains unclear. In neuroblasts, apical segregation of aPKC is always accompanied by the segregation of the cell fate determinants Numb, Pros, and Brat into the differentiating daughter cell. The Notch repressor Numb and the adaptor protein Miranda, which binds Pros and Brat, are directly phosphorylated by aPKC (Smith et al., 2007; Wirtz-Peitz et al., 2008; Atwood and Prehoda, 2009). This results in the loss of cortical attachment of the cell fate determinants from the apical domain, restricting their activity in only the basal ganglion mother cell (GMC). In ISCs, the machinery for Numb localization is in place as NumbPTB::GFP localizes basally and segregates asymmetrically when expressed in ISCs. Because endogenous Numb was not detected in ISCs (data not shown) and numb mutations do not cause lineage defects (Bardin et al., 2010) (data not shown), it seems unlikely that Numb is a key downstream factor of aPKC in ISCs. We also were not able to demonstrate a loss-of-function phenotype or asymmetric segregation for any other of the known segregating determinants (data not shown), although we cannot exclude the possibilities of functional redundancy and the existence of another not yet identified basal determinant. For these reasons, we conclude that the apical segregation of aPKC is not accompanied by the basal segregation of cell fate determinants into the opposite daughter cell in ISCs.
Asymmetric cell division in ISCs is remarkably similar to what has been described for the mouse epidermis. Mouse epidermal stem cells contact the basement membrane via Integrins, can divide along the apical-basal axis of a polarized epithelium, and undergo an asymmetric division in which Par-3 is segregated into the apical daughter cell (Lechler and Fuchs, 2005). Promotion of Notch signaling is required for the differentiation of suprabasal cells (Williams et al., 2011), indicating surprising functional parallels between Drosophila ISCs and mouse epidermal stem cells. Unlike in neuroblasts, the asymmetric segregation of Numb does not seem to be required for asymmetric cell division in both of these tissues.
Our data suggest that Drosophila and mouse ISCs use different mechanisms to establish distinct fates among their daughter cells. In the mouse intestine, a population of equipotent stem cells competes for contact with the Paneth cells, which provide a short-range stem cell competence signal (Sato et al., 2011). Elegant clonal analyses have shown that each ISC division is symmetric, implying that the daughter cells of each division have an equal probability of undergoing differentiation (Snippert et al., 2010). Asymmetry is established at the population level in the intestinal crypt as stochastic loss of stem cells is compensated by the symmetric division of others. A similar mechanism has been observed for germline stem cells in the mouse testes and has also been proposed for Drosophila ISCs (Simons and Clevers, 2011). Our data instead indicate that ISCs undergo an intrinsically asymmetric division in which extrinsic cues leading to intrinsic polarization are important for regulating self-renewal. Unlike neuroblasts, however, where asymmetric protein segregation establishes an invariant lineage, we found that ISCs are able to divide both asymmetrically and symmetrically. This leads us to propose that imprecision in establishing asymmetric protein inheritance provides the lineage flexibility observed in ISCs. Therefore, our data indicate that intrinsically asymmetric cell divisions occur in adult stem cell lineages, although the precise mechanism differs from neuroblasts. It will be exciting to determine whether intrinsic asymmetry contributes to fate specification in adult mammalian stem cell lineages as well.
Experimental Procedures
Immunohistochemistry
Female adult intestinal tracts were dissected in PBS and fixed immediately in 4% PFA in PBS for 15–20 min. Fix was washed with PBS and transferred to blocking solution (2% NGS [Sigma] in 0.1% PBS/Triton X-100) for 1 hr at RT or overnight at 4°. After blocking, specimens were incubated with the primary antibodies diluted in blocking solution for 3 hr at RT or overnight at 4°. Primary antibodies were rinsed twice in 0.1% PBS/Triton X-100 and washed twice for at least 15 min each before the addition of the secondary antibodies for 1 hr at RT or overnight at 4°. Secondary was rinsed and washed with 0.1% PBS/Triton X-100, transferred to PBS, and exchanged with 50% PBS/glycerol before being mounted on slides with Vector Shield mounting medium with DAPI (Vector Laboratories). Alternatively, dissections were done in Grace’s Insect Medium, fixed and stained as described previously (Lin et al., 2008), and mounted on Vector Shield.
TARGET System and Temperature Shifting
The TARGET system was used for temporal and spatial control of transgene expression in adult flies. Crosses were kept at 18° or 22°. Adult flies were transferred to new vials with fresh food 1–3 days after eclosion (AE) and kept in 29° incubators for approximately 10 days before dissection unless specified.
Clonal Analysis
Clones were induced in adult flies 1–3 days AE by inducing hs-Flp for 2 × 30 min (with 30 min resting period) in a 37° water bath. After heat shock, flies were transferred to fresh food and kept for 4–5 days at 25° before dissection.
Calculation of Spindle Orientation
To calculate the angle of ISC divisions, midguts were dissected and stained for GFP, phospho-H3, Cnn, and Laminin. Confocal Z-stacks of whole cells were taken with 0.75 mm optical sections and reconstituted in 3D using IMARIS software, and movies were made from the 3D images. Using Laminin to label the basement membrane, the angle of division was determined through bisecting the chromatin and centrosomes of late mitotic cells and measuring the angle between the two vectors. Significance was calculated by Wilcoxon rank sum test.
Acknowledgments
We thank Heike Harzer and Christian Berger for generating rabbit and guinea pig anti-Pros; Christoph Jueschke for comments on the manuscript and data analysis; all Knoblich lab members for discussion; Norbert Perrimon, Bruce Edgar, Andreas Wodarz, Ruth Palmer, Nick Brown, Alana O’Reilly, Michael Simon, Guy Tanentzapf, Thom Kaufman, Xiankun Zeng, Steven Hou, the Bloomington Drosophila stock center, the Vienna Drosophila RNAi center (VDRC), and the Developmental Studies Hybridoma Bank (DSHB) for fly stocks and reagents; and Karin Aumayr and Pawel Pasierbek for imaging support. R.C. is supported by an EMBO Long Term Fellowship. Work in J.A.K.’s laboratory is supported by the Austrian Academy of Sciences, the Austrian Science Fund (FWF), the EU FP7 network EuroSystems, and an advanced grant from the European Research Council (ERC). Experiments were conceived and designed by S.G., R.C., and J.A.K and performed and analyzed by S.G and R.C. The manuscript was written by S.G., R.C., and J.A.K.
Published: October 4, 2012
Footnotes
Supplemental Information for this article includes five figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.stem.2012.06.017.
Supplemental Information
References
- Atwood S.X., Prehoda K.E. aPKC phosphorylates Miranda to polarize fate determinants during neuroblast asymmetric cell division. Curr. Biol. 2009;19:723–729. doi: 10.1016/j.cub.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin A.J., Perdigoto C.N., Southall T.D., Brand A.H., Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O. Posterior midgut epithelial cells differ in their organization of the membrane skeleton from other drosophila epithelia. Exp. Cell Res. 2001;270:176–187. doi: 10.1006/excr.2001.5343. [DOI] [PubMed] [Google Scholar]
- Brower D.L., Jaffe S.M. Requirement for integrins during Drosophila wing development. Nature. 1989;342:285–287. doi: 10.1038/342285a0. [DOI] [PubMed] [Google Scholar]
- Brown N.H. Cell-cell adhesion via the ECM: integrin genetics in fly and worm. Matrix Biol. 2000;19:191–201. doi: 10.1016/s0945-053x(00)00064-0. [DOI] [PubMed] [Google Scholar]
- Cabernard C., Doe C.Q. Apical/basal spindle orientation is required for neuroblast homeostasis and neuronal differentiation in Drosophila. Dev. Cell. 2009;17:134–141. doi: 10.1016/j.devcel.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Conder R., Yu H., Zahedi B., Harden N. The serine/threonine kinase dPak is required for polarized assembly of F-actin bundles and apical-basal polarity in the Drosophila follicular epithelium. Dev. Biol. 2007;305:470–482. doi: 10.1016/j.ydbio.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Doe C.Q. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- Durgan J., Kaji N., Jin D., Hall A. Par6B and atypical PKC regulate mitotic spindle orientation during epithelial morphogenesis. J. Biol. Chem. 2011;286:12461–12474. doi: 10.1074/jbc.M110.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder A.M., Sui X., Rosen D.G., Nolden L.K., Cheng K.W., Lahad J.P., Kango-Singh M., Lu K.H., Warneke C.L., Atkinson E.N. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc. Natl. Acad. Sci. USA. 2005;102:12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 1989;3:2141–2150. doi: 10.1096/fasebj.3.10.2666230. [DOI] [PubMed] [Google Scholar]
- Ellis S.J., Tanentzapf G. Integrin-mediated adhesion and stem-cell-niche interactions. Cell Tissue Res. 2010;339:121–130. doi: 10.1007/s00441-009-0828-4. [DOI] [PubMed] [Google Scholar]
- Fernández-Miñán A., Martín-Bermudo M.D., González-Reyes A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-epithelium monolayer. Curr. Biol. 2007;17:683–688. doi: 10.1016/j.cub.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Fuller M.T., Spradling A.C. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Furriols M., Bray S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr. Biol. 2001;11:60–64. doi: 10.1016/s0960-9822(00)00044-0. [DOI] [PubMed] [Google Scholar]
- Galvez A.S., Duran A., Linares J.F., Pathrose P., Castilla E.A., Abu-Baker S., Leitges M., Diaz-Meco M.T., Moscat J. Protein kinase Czeta represses the interleukin-6 promoter and impairs tumorigenesis in vivo. Mol. Cell. Biol. 2009;29:104–115. doi: 10.1128/MCB.01294-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni D., Garoia F., Bellosta P., Parisi F., De Biase D., Collina G., Strand D., Cavicchi S., Pession A. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene. 2007;26:5960–5965. doi: 10.1038/sj.onc.1210389. [DOI] [PubMed] [Google Scholar]
- Hao Y., Du Q., Chen X., Zheng Z., Balsbaugh J.L., Maitra S., Shabanowitz J., Hunt D.F., Macara I.G. Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr. Biol. 2010;20:1809–1818. doi: 10.1016/j.cub.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Patel P.H., Kohlmaier A., Grenley M.O., McEwen D.G., Edgar B.A. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.G., Li X., Gray P.D., Kuang J., Clayton F., Samowitz W.S., Madison B.B., Gumucio D.L., Kuwada S.K. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J. Cell Biol. 2006;175:505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T., Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kirilly D., Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- Klein A.M., Simons B.D. Universal patterns of stem cell fate in cycling adult tissues. Development. 2011;138:3103–3111. doi: 10.1242/dev.060103. [DOI] [PubMed] [Google Scholar]
- Knoblich J.A. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Knoblich J.A. Asymmetric cell division: recent developments and their implications for tumour biology. Nat. Rev. Mol. Cell Biol. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Akimoto K., Nagashima Y., Ishiguro H., Shirai S., Chishima T., Ichikawa Y., Ishikawa T., Sasaki T., Kubota Y. The overexpression and altered localization of the atypical protein kinase C lambda/iota in breast cancer correlates with the pathologic type of these tumors. Hum. Pathol. 2008;39:824–831. doi: 10.1016/j.humpath.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Lechler T., Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y., Andersen R.O., Cabernard C., Manning L., Tran K.D., Lanskey M.J., Bashirullah A., Doe C.Q. Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev. 2006;20:3464–3474. doi: 10.1101/gad.1489406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y., Robinson K.J., Doe C.Q. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- Legate K.R., Fässler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J. Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- Lin G., Xu N., Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C., Klein A.M., Simons B.D., Winton D.J. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo M.D., Brown N.H. Uncoupling integrin adhesion and signaling: the betaPS cytoplasmic domain is sufficient to regulate gene expression in the Drosophila embryo. Genes Dev. 1999;13:729–739. doi: 10.1101/gad.13.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey L.M., Macara I.G. The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 2009;23:1450–1460. doi: 10.1101/gad.1795909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S.E., Le P.T., Osborn A.J., Matsumoto K., Davis R.L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Micchelli C.A., Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Spradling A.C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien L.E., Soliman S.S., Li X., Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Palmer R.H., Fessler L.I., Edeen P.T., Madigan S.J., McKeown M., Hunter T. DFak56 is a novel Drosophila melanogaster focal adhesion kinase. J. Biol. Chem. 1999;274:35621–35629. doi: 10.1074/jbc.274.50.35621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M., Knoblich J.A. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat. Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- Regala R.P., Weems C., Jamieson L., Khoor A., Edell E.S., Lohse C.M., Fields A.P. Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res. 2005;65:8905–8911. doi: 10.1158/0008-5472.CAN-05-2372. [DOI] [PubMed] [Google Scholar]
- Roegiers F., Younger-Shepherd S., Jan L.Y., Jan Y.N. Two types of asymmetric divisions in the Drosophila sensory organ precursor cell lineage. Nat. Cell Biol. 2001;3:58–67. doi: 10.1038/35050568. [DOI] [PubMed] [Google Scholar]
- Rolls M.M., Albertson R., Shih H.P., Lee C.Y., Doe C.Q. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J. Cell Biol. 2003;163:1089–1098. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden D.T. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schober M., Schaefer M., Knoblich J.A. Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature. 1999;402:548–551. doi: 10.1038/990135. [DOI] [PubMed] [Google Scholar]
- Shattil S.J., Kim C., Ginsberg M.H. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X.R., Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138:3367–3376. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist S.E., Doe C.Q. Extrinsic cues orient the cell division axis in Drosophila embryonic neuroblasts. Development. 2006;133:529–536. doi: 10.1242/dev.02211. [DOI] [PubMed] [Google Scholar]
- Simons B.D., Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Smith C.A., Lau K.M., Rahmani Z., Dho S.E., Brothers G., She Y.M., Berry D.M., Bonneil E., Thibault P., Schweisguth F. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J. 2007;26:468–480. doi: 10.1038/sj.emboj.7601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert H.J., van der Flier L.G., Sato T., van Es J.H., van den Born M., Kroon-Veenboer C., Barker N., Klein A.M., van Rheenen J., Simons B.D., Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Ohno S. The PAR-aPKC system: lessons in polarity. J. Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Wang H., Somers G.W., Bashirullah A., Heberlein U., Yu F., Chia W. Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 2006;20:3453–3463. doi: 10.1101/gad.1487506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.E., Beronja S., Pasolli H.A., Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz-Peitz F., Nishimura T., Knoblich J.A. Linking cell cycle to asymmetric division: Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell. 2008;135:161–173. doi: 10.1016/j.cell.2008.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A., Ramrath A., Kuchinke U., Knust E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–547. doi: 10.1038/990128. [DOI] [PubMed] [Google Scholar]
- Wodarz A., Ramrath A., Grimm A., Knust E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 2000;150:1361–1374. doi: 10.1083/jcb.150.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.S., Egger B., Brand A.H. Asymmetric stem cell division: lessons from Drosophila. Semin. Cell Dev. Biol. 2008;19:283–293. doi: 10.1016/j.semcdb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Xie T., Spradling A.C. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Yamashita Y.M., Jones D.L., Fuller M.T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Yee G.H., Hynes R.O. A novel, tissue-specific integrin subunit, beta nu, expressed in the midgut of Drosophila melanogaster. Development. 1993;118:845–858. doi: 10.1242/dev.118.3.845. [DOI] [PubMed] [Google Scholar]
- Zeng X., Chauhan C., Hou S.X. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in drosophila. Genesis. 2010;48:607–611. doi: 10.1002/dvg.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Huang J., Yang N., Liang S., Barchetti A., Giannakakis A., Cadungog M.G., O’Brien-Jenkins A., Massobrio M., Roby K.F. Integrative genomic analysis of protein kinase C (PKC) family identifies PKCiota as a biomarker and potential oncogene in ovarian carcinoma. Cancer Res. 2006;66:4627–4635. doi: 10.1158/0008-5472.CAN-05-4527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.