Abstract
Enhanced histopathology (EH) of the immune system is a tool that the pathologist can use to assist in the detection of lymphoid organ lesions when evaluating a suspected immunomodulatory test article within a subchronic study or as a component of a more comprehensive, tiered approach to immunotoxicity testing. There are three primary points to consider when performing EH: (1) each lymphoid organ has separate compartments that support specific immune functions; (2) these compartments should be evaluated individually; and (3) semiquantitative descriptive rather than interpretive terminology should be used to characterize any changes. Enhanced histopathology is a screening tool that should be used in conjunction with study data including clinical signs, gross changes, body weight, spleen and thymus weights, other organ or tissue changes, and clinical pathology. Points to consider include appropriate tissue collection, sectioning, and staining; lesion grading; and diligent comparison with concurrent controls. The value of EH of lymphoid organs is to aid in the identification of target cell type, changes in cell production and cell death, changes in cellular trafficking and recirculation, and determination of mechanism of action.
Keywords: enhanced histopathology, lymphoid organs, immune system, immunomodulation, immunotoxicity, terminology
Introduction
Enhanced histopathology (EH) involves evaluation of the separate compartments of lymphoid organs using semiquantitative descriptive terminology to identify and define any changes in cellularity and/or architecture (Elmore 2010; Haley et al. 2005). However, this methodology also involves proper collection and processing of tissues; assessment of and comparison with clinical pathology; determination and consideration of body and organ weights; use of appropriate criteria for severity grading; careful evaluation of concurrent controls; and consideration of other factors such as spontaneous or induced lesions in nonlymphoid organs, environmental factors, overall animal health, nutritional status, antigen load, age, sex, steroid hormone status, and any areas of potential stress for the animals. Additionally, this methodology requires knowledge of organ structure, function, and histology; species differences in lymphoid organs; and an appreciation of the complex and dynamic nature of the immune system, including how one organ system may interact with another. The value of EH is that it can serve as a screening tool to determine whether there is a potential effect on the immune system, and it can also serve as an aid in the identification of the putative target cell population affected. This article will review certain highlights in the history of immunotoxicity testing and the role of histopathology as a screening tool and component of the tiered approach. The basics of EH will also be discussed as well as certain points to consider when evaluating and interpreting the data.
Historical Perspective
The immunotoxicology community has been constantly evaluating and updating the testing methods used in the assessment of xenobiotics on the immune system for more than thirty years. As early as 1980, Dr. Jeffrey Vos, from the National Institute of Public Health, The Netherlands, discussed the use of screening tests such as growth, weight, and histology of lymphoid and endocrine organs as well as hematology and serum immunoglobulin concentrations (Vos 1980). He, and others in the field, discussed tests to assess cell-mediated immunity, humoral immunity, and phagocytosis by macrophages as ways to determine the functional significance of an effect on the immune system and to gain more insight into the mode of action of a chemical.
This approach to assess immunotoxicity was modified in subsequent years by several investigators (Krajnc-Franken et al. 1990; Schuurman et al. 1991; Van Loveren and Vos 1989). In 1988, Luster and colleagues described the development of a testing battery to assess chemical-induced immunotoxicity as a part of the National Toxicology Program’s guidelines for immunotoxicity evaluation in mice (Luster et al. 1988). The strategy was to develop and validate a simple two-tiered panel of assays to identify immunomodulation that may occur following chemical exposure. This battery was considered an adjunct to more traditional toxicity and carcinogenicity testing. The first tier was a screening panel and consisted of immunopathology (hematology, body and organ weights, spleen cellularity, histology of spleen, thymus, lymph node), and immune function assays to evaluate humoral-mediated immunity, cell-mediated immunity, and nonspecific immunity. If a test article was found to be a potential immunomodulatory agent, then it would progress to the second tier, which was a more comprehensive panel consisting of quantitation of splenic B and T cell numbers; additional tests for humoral, cell-mediated, and nonspecific immunity; and various tests for host resistance. In 1992, Luster and colleagues followed up with a report on the sensitivity and predictability of these immune tests by analyzing the database generated from previous studies (Luster et al. 1992). They reported that the tests or test combinations in the tier approach were able to detect immunotoxic compounds, and that the performance of only two or three immune tests was sufficient to predict immunotoxic compounds in rodents with > 90%concordance. In an effort to improve future testing strategies, this database was used to develop statistical models that examine the qualitative and quantitative relationships between the immune function and host resistance tests (Luster et al. 1993). Although it has undergone minor revisions over the years, this two-tier testing panel is still used today.
In 1994, Schuurman and colleagues published a manuscript that discussed histopathology of the immune system as a tool to assess immunotoxicity (Schuurman et al. 1994). This review described the practical aspects of sampling lymphoid organs and tissue processing and the application of conventional and advanced histologic techniques. The highly dynamic nature of the immune system was noted, as the histologic picture of lymphoid organs is not static but can be quite variable and dependent on the status of immune activation, antigenic load, and other intrinsic and extrinsic factors. The authors also discussed the proper evaluation and interpretation of histology in relation to modifying factors such as age, stress, sex, animal strain, housing conditions and nutritional status. The authors acknowledged that histopathology may prove to be less sensitive in “flagging” immunotoxicity when low doses are applied, whereas at higher doses histopathology is considered a sensitive indicator of toxicity.
As a prelude to the Organisation for Economic Cooperation and Development’s (OECD) incorporation of “enhanced immunopathology” into the test guideline for the twenty-eight–day rat study, Basketter and colleagues published pathology considerations for, and subsequent risk assessment of, chemicals identified as immunosuppressive in routine toxicology studies (Basketter et al. 1995). This manuscript discussed the histological evaluation of the thymus, spleen, lymph nodes, and bone marrow; comparison with concurrent controls; and the need to identify whether effects are a primary or secondary effect of the chemical. Importantly, consideration of a holistic view of changes in lymphoid tissues or immune cell populations in the context of other toxic manifestations and the overall health status of the animals was noted.
In 2000, there was a more focused effort on histopathological evaluation of the lymphoid organs. Kuper and colleagues described histologic approaches to detect changes indicative of immunotoxicity and noted that the current two-tier testing strategy guidelines needed improvement and standardization of the histopathology procedures and provided suggestions to further improve the identification of histopathologic changes in lymphoid organs and tissues (Kuper et al. 2000). Lymphoid organ structure, cellular composition, and function were described along with proposed descriptive and semiquantitative diagnostic terms for identifying changes.
In 2004, Germolec and colleagues reported on the validation effort of a semiquantitative histopathology approach for examining specific structural and architectural changes in lymphoid organs to determine the utility of such an approach (Germolec, Kashon, et al. 2004). The conclusions of this study were that agreement between pathologists evaluating the same set of tissues was highest for cortical cellularity of the thymus, good for spleen follicular cellularity and spleen and lymph node germinal center development, and poorest for red pulp changes in the spleen. Importantly, the ability to accurately identify changes in lymphoid tissues was dependent on the experience and training of the individual pathologist and the apparent severity of the lesion. A follow-up study that used data from EH evaluations compared with more traditional immune test results (functional and nonfunctional) revealed that the ability to accurately identify immunotoxic chemicals using histologic end points decreased linearly as the level of stringency used to determine significant histopathological changes increased (Germolec, Nyska, et al. 2004). The use of a minimal classification for hazard identification was considered inappropriate because of the increased likelihood of false positives. Although functional assays were shown to be more accurate, EH was considered to provide a reasonable level of accuracy as a screening test to identify immunomodulatory compounds, provided the level of stringency used to score histologic lesions is carefully considered to allow for the detection of tissue changes while limiting false positives.
In 2005, both the Society for Toxicologic Pathology (STP) and the European Society of Toxicologic Pathology (ESTP) published guidelines on the routine pathology evaluation of the immune system (Haley et al. 2005; Ruehl-Fehlert et al. 2005). The STP Immunotoxicology Working Group prepared “best practice” recommendations for the collection, interpretation, and reporting of organ weights, gross and microscopic observations, and other pathology data relevant to the immune system. These guidelines state that (1) each lymphoid organ has separate compartments that support specific immune functions; (2) these compartments can and should be evaluated individually for changes; and (3) descriptive, rather than interpretive, terminology should be used to characterize changes within these compartments. Additionally, semiquantitative terms should be used to describe any changes, and the results should be interpreted in a meaningful pathobiologic context in the discussion section of the pathology report. The Guideline Committee of the ESTP also recommended that each compartment within the different lymphoid organs be evaluated separately and semiquantitatively since this approach has been shown to increase the sensitivity and specificity of immunohistopathology. This approach would allow one to (1) conclude whether a significant effect on the immune system is present (as a screening tool); (2) identify the critical site(s) or compartment affected within the immune organ/tissue; (3) provide some insights about the putative target cell population(s); and (4) characterize the dose-response relationships of immunomodulatory effects. In 2006, Elmore provided a series of articles that described more fully how EH should be performed for the lymph node, spleen, thymus, bone marrow, and mucosa-associated lymphoid tissues (Elmore 2006a, 2006b, 2006c, 2006d, 2006e).
When Should EH Be Performed?
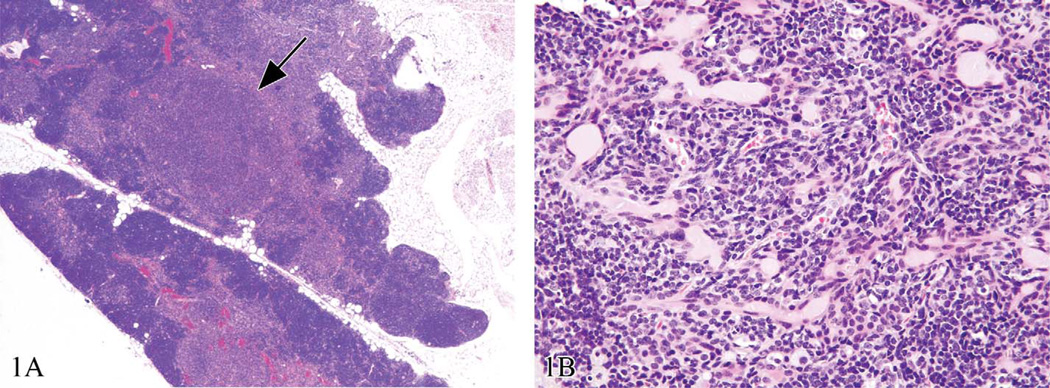
There are times when EH should always be performed, times when it may or may not be performed, and times when it is not advisable to use this type of evaluation. Enhanced histopathology should always be performed as a screening tool for specific immunomodulatory studies as a component of the tiered testing approach (Luster et al. 1988). For short-term or subchronic studies, EH may be a part of the initial evaluation or may be done if routine histopathology indicates a potential immunomodulatory effect. Also, if there is reason to suspect an immunomodulatory effect based on prior information, then EH may be considered for the initial review. However, for chronic studies, EH is not particularly useful and can lead to confusing results because the normal aging effects of lymphoid organs, particularly the thymus, can interfere with critical evaluation of the tissue. Importantly, the thymus is often a target tissue for immunotoxicity, so careful and accurate evaluation of this tissue is vital. Typical age-related changes in the thymus (also called physiological “involution”) are reduction in size; fatty infiltration in the capsule and interlobular septae; lymphocyte decreases in the cortex and thymus; loss of corticomedullary demarcation; medullary epithelial cell prominence, proliferation, or formation of tubules or cysts; and focal areas of medullary B cell hyperplasia (Figure 1). There is the added caveat that such age-related tissue changes can vary among animals within a study as well as between species, strains, and sexes. Evaluation of xenobiotic-related changes within specific tissue compartments that have concurrent age-related changes can thus be challenging and sometimes uninterpretable.
Figure 1.
Control thymuses from chronic Sprague Dawley rat bioassays. These are examples of how age-related physiological involution could interfere with the critical evaluation of the thymus. In (a), there is fatty infiltration, cortical lymphocyte decrease, and focal medullary hyperplasia (arrow); (b) illustrates medullary epithelial proliferation and cystic structures associated with age. Hematoxylin and eosin.
Basics of EH
Enhanced histopathology should always be performed with a thorough knowledge of lymphoid organ structure and function (Cesta 2006a; Cesta 2006b; Kuper et al. 2000; Pearse 2006; Travlos 2006; Willard-Mack 2006), species differences (Haley 2003), and pattern of draining lymph nodes (Tilney 1971). Because of the dynamic nature of the immune system, there is the potential for a wide “range of normal,” especially in the tissues exposed to dietary antigens. Therefore, a thorough review of all concurrent controls is necessary. To avoid diagnostic drift, it is best to evaluate one organ system at a time. For example, evaluate all thymus controls, then high-dose thymuses, then low- and mid-dose thymuses. This systematic review is then repeated for each of the lymphoid organs. Re-evaluation of controls or referring to examples of control slides that exhibit the normal range of tissue variation is necessary to maintain the “range of normal” reference. A secondary blinded review can be performed if lesions are subtle or if there is a need to further confirm findings. If available, information on animal strain, dosing regimen, clinical signs, organ weights, body weights, clinical chemistry, gross lesions, and so on should be reviewed before starting the microscopic evaluation.
The minimum recommended lymphoid tissues for evaluation include the thymus, spleen, bone marrow, most proximal draining lymph node, distal peripheral lymph node, and any gross lesions. A distal peripheral lymph node, such as the popliteal node, can be useful to determine whether there is a systemic, rather than a local, effect of treatment. When using histopathology, the thymus has been shown to be the most sensitive predictor of immunotoxicity; however, it is also the primary lymphoid tissue affected by stress. The cortical cells of the adrenal glands may become more metabolically active after exposure to stress and subsequent increase in glucocorticoid production and may thus be useful in differentiating stress from a primary effect of treatment. The adrenal gland may increase in weight and the cortical cells may increase in size with an increase in cytoplasm that appears foamy as a result of increased lipid content and steroid production. Other select nonlymphoid organs and tissues may also be evaluated since components of the immune system and immune events can occur within other locations such as the liver, kidney, and lung. It is also imperative to rule out lesions in nonlymphoid organs, such as inflammation, that may have an effect on the immune system.
As described in the STP best practices document, EH should be performed by identifying and evaluating the individual compartments within lymphoid organs (Haley et al. 2005). The identification and reporting of specific cellular changes within tissue compartments may provide insight into the type of cell affected and thus mechanism of action. The compartments to be evaluated include the cortex and medulla of the thymus (Elmore 2006a); the periarteriolar lymphoid sheaths (PALS), follicles, marginal zone, and red pulp of the spleen (Elmore 2006b); and the cortex (follicles, subcapsular sinus), paracortex, and medulla (cords, sinuses) of the lymph nodes (Elmore 2006c). Although anatomic pathologists do not recognize different compartments within the bone marrow, the individual cell populations should be evaluated and the myeloid:erythroid ratio determined (Elmore 2006d). Any potential treatment-related effects observed in the bone marrow should be followed up with clinical pathology. The mucosa-associated lymphoid tissues (MALT) are aggregates of nonencapsulated organized lymphoid tissue that are associated with immune responses at mucosal surfaces. If present, the compartments to evaluate within the MALT are the follicles and interfollicular areas (Elmore 2006e). High endothelial venules and the overlying follicle-associated epithelium should also be a part of the evaluation. Examples of checklists that the pathologist can use to record findings have been previously published (Elmore 2006a, 2006b, 2006c, 2006d, 2006e).
Descriptive semiquantitative terminology, rather than interpretive terminology, should be used to describe the tissue changes, as illustrated in Figures 2–4. For example, instead of using lymphoid atrophy, involution, hypoplasia, or depletion to describe decreased lymphocyte cellularity, a descriptive term such as “decreased lymphocytes” should be used. Likewise, “increased lymphocytes” should be used instead of lymphocyte hyperplasia or proliferation. Any increase or decrease in area or cellularity of a specific compartment within a lymphoid organ should be noted in a similar fashion. By reporting such changes with descriptive terminology, the pathologist is not suggesting that the change in cellularity is the result of a reactive change in the resident cell population, a developmental change in the resident cell population, or a change in cellular trafficking. This point is of particular importance since the mechanism behind the change in cellularity cannot always be determined.
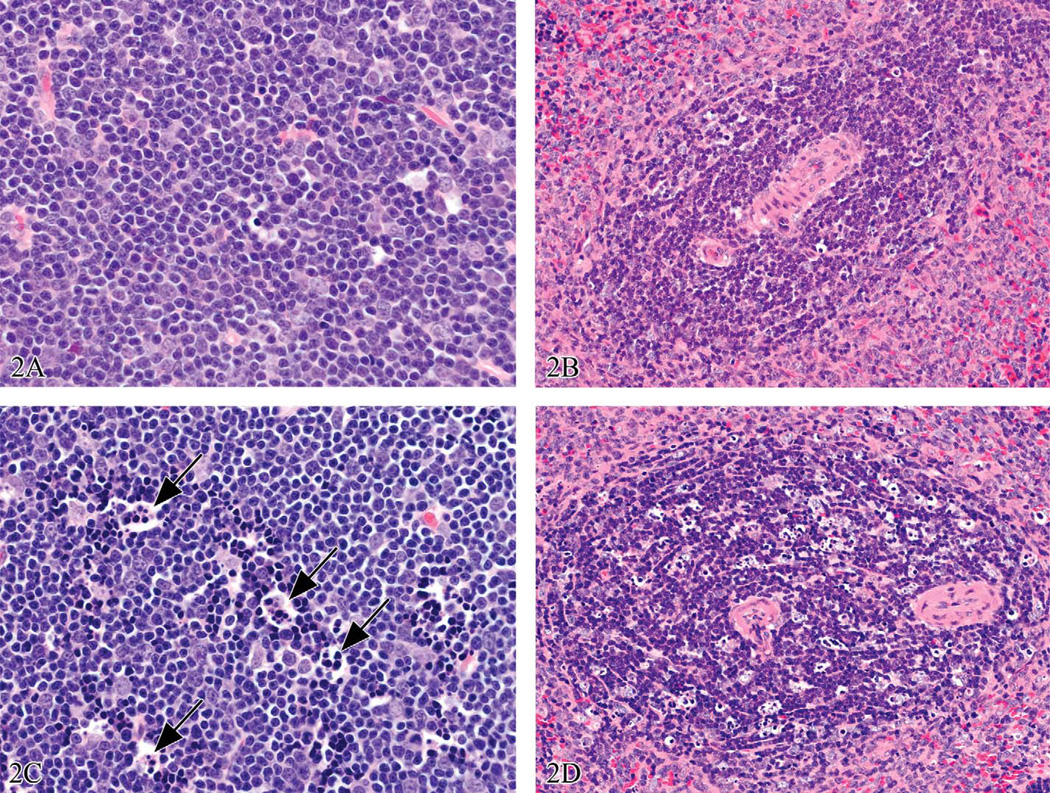
Figure 2.
Three-month-old male Sprague Dawley rat treated with 1 mg/kg bodyweight of dexamethasone; tissues were collected three hours later. (a) and (b) are thymus and spleen from a concurrent control animal. Using EH, the lesions in (c) would be diagnosed as thymus, cortex: increased apoptosis, increased tingible body macrophages (arrows), and decreased lymphocytes. For (d), the splenic periarteriolar lymphoid sheath would be the compartment specified and similar diagnoses would be noted. Appropriate laboratory-specific severity grades would be used. Hematoxylin and eosin.
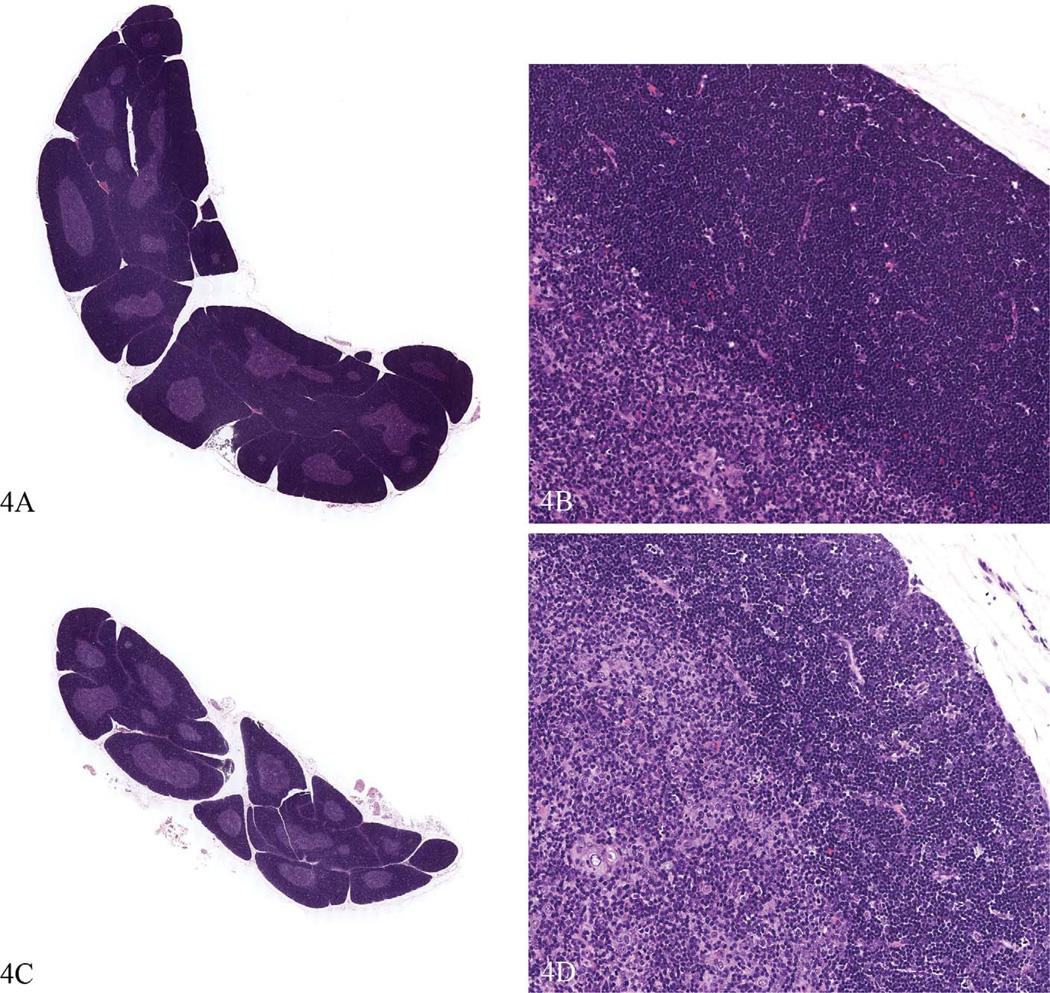
Figure 4.
Thymus glands from the same study as Figure 3. Compared to the control spleen (a and b) the high dose animals (c and d) had up to a 40% decrease in thymus weight, the thymuses appeared smaller at low magnification, and the cortical area appeared smaller. However, the tissue changes were very subtle, described as decreased area and cellularity of cortex (compare d to b). The cortex stained less intensely, a result of decreased cellularity. This lesion would be difficult to identify without careful comparison to control tissue. Hematoxylin and eosin.
Interpretation and Reporting
Interpretation and reporting of tissue findings is done within the discussion section of the pathology report. The consideration of the dynamic and complex nature of lymphoid organs and the relationship of lymphoid organs with other nonlymphoid organs and overall animal health are important. As examples, adrenal cortex hypertrophy may indicate high cortisol/corticosterone production associated with stress, and any inflammatory condition within an organ system is likely to illicit an immune response, especially within the lymph nodes draining that area. Thymus and spleen weights can be sensitive indicators of cellular changes within a tissue and are also reported to be reasonable indicators of systemic immunotoxicity (ICICIS Group Investigators 1998). Consideration of hematology and clinical chemistry results are also a critical component of the interpretation process.
It is particularly challenging to determine whether the tissue changes identified with EH are a primary or secondary effect of the chemical, or unrelated to treatment. As an example, stress and the associated thymus cortical lymphocyte apoptosis can be difficult to differentiate from a primary chemical effect and may, in some cases, occur concurrently. Examples of a primary effect of treatment would be dexamethasone targeting the glucocorticoid receptor on the cortical CD4+ CD8+ thymocytes or dioxin targeting the aryl hydrocarbon receptor on the thymus stromal cells with detrimental effects on T cell maturation. Examples of secondary effects of treatment would be unpalatability of the test article that results in a moderate to severe decrease in body weight or a repeated (i.e., daily) route of administration that is particularly stressful for the animal. Another example of a secondary effect of treatment would be a test article that results in an inflammatory reaction with drainage to, and stimulation of, the local lymph node(s). If the inflammation is severe or present in many locations, then the immune response may be systemic and thus seen in multiple lymph nodes. An example of a stress effect unrelated to treatment may be excessive noise in the animal room or inappropriate animal housing (with respect to sex and number).
To attempt to determine the underlying cause of tissue changes in lymphoid organs, there are certain questions that can be asked, such as:
Is there prior knowledge about the structure or mechanism of action of the chemical that might provide insight to questions such as targeted cell receptors?
Are there any known or potential stressors in the animals (such as infection) or in the environment (such as housing protocols)?
Are there changes in food intake?
What is the overall health status of the animals?
Is the same lesion seen in animals that are clinically healthy versus “poor-doers”?
Is the lesion related to sex, age, or strain?
What is the hormone status of the affected animals (i.e., pregnancy and lactation)?
Are there body weight decreases in the treatment groups that correlate with the lesion and, if so, is it dose-related?
Are there similar changes in concurrent controls?
Are there similar lesions in more than one lymphoid organ, and if so, is there a correlation in terms of targeted cells?
Are there lesions in nonlymphoid organs?
Is there an increase in acute-phase proteins?
Are there hematology changes that support the findings?
Since the causes of immunotoxicity may overlap, it is not always possible to determine whether the observed effect(s) is a result of a primary or secondary effect of treatment or a result of stress that is unrelated to treatment. Ultimately, a “weight of evidence” approach should be used with consideration of all the available study data.
Points to Consider
Proper tissue collection, sectioning, and processing are critically important for the accurate evaluation of rodent lymphoid organs. As an example, the entire mesenteric lymph node chain should be collected and evaluated, since different lobules drain different sections of the intestines. Lymph nodes should be sectioned longitudinally, and all lymphoid organs, including the spleen, should be sectioned at the point of greatest width. Superficial, tangential, or cross sections of lymph nodes may give the appearance of increased or decreased cortex, paracortex, or medulla, whereas superficial, tangential, or cross sections of thymus may result in similar perceived changes in the cortex and medulla. Special care should also be taken to avoid variability in tissue thickness and staining, which may give the appearance of increased or decreased cellularity (Figure 5). One way to decrease the potential for processing artifacts is by sectioning and staining all tissues together, preferably on the same day, by the same technician and without changes to staining solutions. However, this approach may not be practical, especially for large studies. As an alternative, tissue samples may be randomized before processing, thereby decreasing the likelihood that a processing artifact will occur within one group of animal tissues.
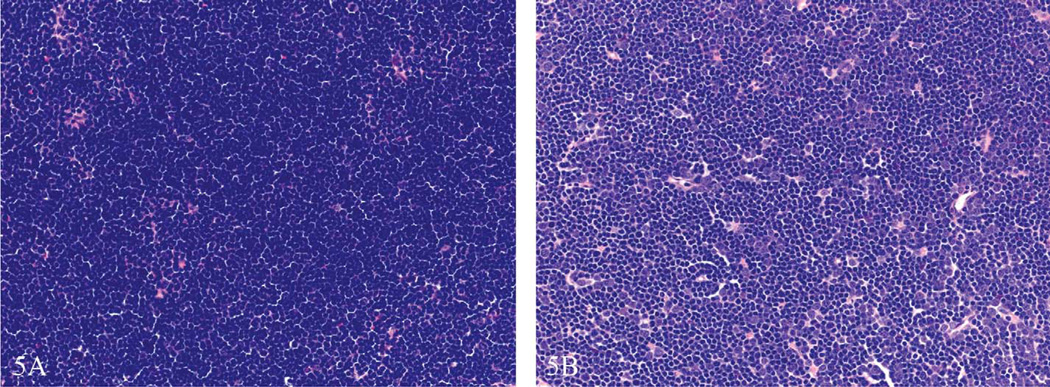
Figure 5.
Thymus cortex from two 3-month-old control Sprague Dawley rats within the same study. This is an example of how sectioning and/or staining artifact can interfere with enhanced histopathology evaluation. The thymus in (a) was sectioned thicker and thus appeared more cellular than the thymus in (b). The ideal section thickness is 5 µm. Without knowledge of tissue thickness differences, the tissue in (a) might be misdiagnosed as increased cellularity or the tissue in (b) might be misdiagnosed as decreased lymphocyte cellularity. Hematoxylin and eosin.
There is a normal variability of some lymphoid organs, particularly those that are exposed to dietary antigens such as the Peyer’s patches, mesenteric lymph nodes, submandibular lymph nodes, and so on. For this reason, very subtle lesions may not be easily detectable in these tissues. However, careful consideration and comparison to the entire “range of normal” in concurrent controls will help to avoid calling false positives in these tissues.
Knowledge of strain, sex, and species differences in the structure and function of the immune system should also be considered (Haley 2003). As one example, the number and drainage field of lymph nodes can vary by animal species, and most species of laboratory animals have significantly smaller numbers of lymphatic trunks and nodes than humans (Haley 2003). The mouse has relatively fewer lymph nodes that drain larger fields compared with larger species with more numerous lymph nodes organized into more complex chains that drain proportionately smaller areas. As another example, the mouse spleen is a major source of hematopoietic activity throughout life and will have more extramedullary hematopoiesis compared to other species such as the rat, dog, nonhuman primate, and human. Ultimately, the utility of the final data may be affected by the choice of animal model. There is an increasing tendency for pharmaceutical companies to produce immunomodulatory molecules that demonstrate site- or species-specific pharmacology. Therefore, chemicals that test positive in one species may test negative in another species. However, the consideration and use of some well-defined species/strain differences may prove advantageous to delineate specific immunologic mechanisms.
In the study by Germolec et al., the ability to accurately identify changes in lymphoid organs was dependent upon the experience and training of the individual pathologist and the apparent severity of the lesion (Germolec, Kashon, et al. 2004; Germolec, Nyska, et al. 2004). These investigators determined that the ability to accurately identify immunotoxic chemicals using histologic end points decreased linearly as the level of stringency used to determine significant histopathological changes increased. Therefore, care should be taken to set appropriate severity-grading schemes for each individual laboratory or institution in order to accurately detect immunomodulatory changes while decreasing the likelihood of false positives. A stringent pathology peer review process will also help to ensure the accuracy and consistency of the results.
Preventing diagnostic drift is another point to consider when performing EH. One recommended method of evaluation is to review all concurrent control tissues from one lymphoid organ first to determine the “range of normal” for overall tissue architecture and cellularity within that group of animals. Next one would evaluate the high-dose group, and then the low-and mid-dose groups, constantly referring back to tissues from the control group to prevent diagnostic drift. Once all tissues from one lymphoid organ have been reviewed, the evaluation of another organ would be done in the same manner. The need to evaluate the concurrent controls first and determine the range of normal indicates that a blinded evaluation should not be initially performed. However, this can be a useful tool to identify subtle tissue changes between groups or confirm diagnoses after the initial read has been performed. Similarly, specialized techniques such as immunohistochemistry, morphometry, and flow cytometry may be valuable for clarifying specific issues or answering a specific question but are not recommended as routine screening tools.
Conclusions
Enhanced histopathology is a tool that the pathologist can use in the identification of immunomodulatory agents and can be used as a component of a tiered approach that includes nonfunctional, functional, and host-resistance assays. However, EH is considered a screening tool and does not directly measure immune function. Enhanced histopathology should be used with a “weight of evidence” approach in conjunction with all study data such as gross changes, body weights, organ weights (spleen, thymus, adrenal gland), changes in other organ systems, and clinical pathology. By evaluating and recording the cellular changes within lymphoid compartments, and with knowledge of organ and cellular function, EH can be used as an aid in the identification of target cell population, changes in cell production and cell death, as well as changes in cellular trafficking and recirculation. However, final interpretations and conclusions should be made only after careful consideration of all available study data and with consideration of the dynamic and complex nature of the immune system.
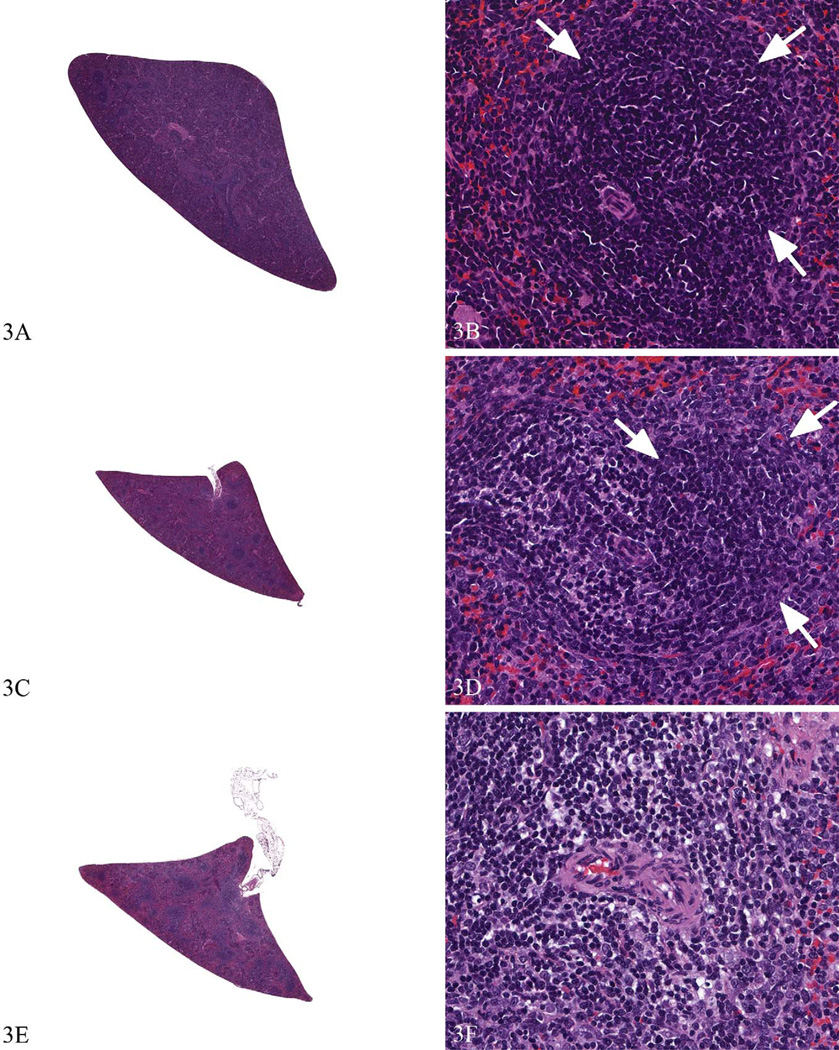
Figure 3.
Spleens from a Sprague Dawley rat study with more subtle immunotoxic effects to illustrate the use of enhanced histopathology. The spleen from a concurrent control (a and b) shows a densely cellular periarteriolar lymphoid sheath (PALS) region and adjacent follicle (arrows). The spleen from a low dose animal (c and d) would be diagnosed as PALS: lymphocyte decrease, minimal. The follicle in (d; arrow) is densely cellular and comparable with concurrent control (b). The spleen from the high dose animal (e and f) would be diagnosed as PALS: lymphocyte decrease, moderate. A follicle is not present in (f) for comparison. Hematoxylin and eosin.
Acknowledgments
The author wishes to thank Drs. Dori Germolec and Gordon Flake for critical review of this manuscript.
This research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). This article may be the work product of an employee or group of employees of the NIEHS, NIH, however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the United States government.
Abbreviations
- EH
enhanced histopathology
- ESTP
European Society of Toxicologic Pathology
- MALT
mucosa-associated lymphoid tissue
- NIEHS
National Institute of Environmental Health Sciences
- NIH
National Institutes of Health
- OECD
Organisation for Economic Cooperation and Development
- PALS
periarteriolar lymphoid sheaths
- STP
Society for Toxicologic Pathology
References
- Basketter DA, Bremmer JN, Buckley P, Kammuller ME, Kawabata T, Kimber I, Loveless SE, Magda S, Stringer DA, Vohr HW. Pathology considerations for, and subsequent risk assessment of, chemicals identified as immunosuppressive in routine toxicology. Food Chem Toxicol. 1995;33:239–243. doi: 10.1016/0278-6915(94)00128-b. [DOI] [PubMed] [Google Scholar]
- Cesta MF. Normal structure, function, and histology of mucosaassociated lymphoid tissue. Toxicol Pathol. 2006a;34:599–608. doi: 10.1080/01926230600865531. [DOI] [PubMed] [Google Scholar]
- Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006b;34:455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the lymph nodes. Toxicol Pathol. 2006a;34:634–647. doi: 10.1080/01926230600939997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the spleen. Toxicol Pathol. 2006b;34:648–655. doi: 10.1080/01926230600865523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the thymus. Toxicol Pathol. 2006c;34:656–665. doi: 10.1080/01926230600865556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the bone marrow. Toxicol Pathol. 2006d;34:666–686. doi: 10.1080/01926230600939971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006e;34:687–696. doi: 10.1080/01926230600939989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA. In: Enhanced Histopathology Evaluation of Lymphoid Organs in: Methods in Molecular Biology; Immunotoxicity Testing. Rod Dietert, editor. Chapter 22. New York, NY: Humana Press; 2010. pp. 323–339. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Kashon M, Nyska A, Kuper CF, Portier C, Kommineni C, Johnson KA, Luster MI. The accuracy of extended histopathology to detect immunotoxic chemicals. Toxicol Sci. 2004;82:504–514. doi: 10.1093/toxsci/kfh271. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Nyska A, Kashon M, Kuper CF, Portier C, Kommineni C, Johnson KA, Luster MI. Extended histopathology in immunotoxicity testing: interlaboratory validation studies. Toxicol Sci. 2004;78(1):107–115. doi: 10.1093/toxsci/kfh049. [DOI] [PubMed] [Google Scholar]
- Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188:49–71. doi: 10.1016/s0300-483x(03)00043-x. [DOI] [PubMed] [Google Scholar]
- Haley P, Perry R, Ennulat D, Frame S, Johnson C, Lapointe JM, Nyska A, Snyder P, Walker D, Walter G. STP position paper: best practice guideline for the routine pathology evaluation of the immune system. Toxicol Pathol. 2005;33:404–407. doi: 10.1080/01926230590934304. discussion 408. [DOI] [PubMed] [Google Scholar]
- International Collaborative Immunotoxicity Study (ICICIS) Group Investigators. Report of validation study of assessment of direct immunotoxicity in the rat. Toxicology. 1998;125:183–210. doi: 10.1016/s0300-483x(97)00166-2. [DOI] [PubMed] [Google Scholar]
- Krajnc-Franken MAM, Van Loveren H, Schuurman HJ, Vos JG. The immune system as a target for toxicity. A tiered approach of testing with special emphasis on histopathology. In: Dyan AD, Hertel RF, Heseltine E, Kazantis G, Smith EM, Van Der Venne MT, editors. Immunotoxicity of Metals and Immunotoxicology. New York, NY: Plenum Press; 1990. pp. 241–264. [Google Scholar]
- Kuper CF, Harleman JH, Richter-Reichelm HB, Vos JG. Histopathologic approaches to detect changes indicative of immunotoxicity. Toxicol Pathol. 2000;28:454–466. doi: 10.1177/019262330002800317. [DOI] [PubMed] [Google Scholar]
- Luster MI, Munson AE, Thomas PT, Holsapple MP, Fenters JD, White KL, Jr, Lauer LD, Germolec DR, Rosenthal GJ, Dean JH. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program’s guidelines for immunotoxicity evaluation in mice. Fundam Appl Toxicol. 1988;10:2–19. doi: 10.1016/0272-0590(88)90247-3. [DOI] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, Rosenthal GJ, Germolec DR, Corsini E, Blaylock BL, Pollock P, Kouchi Y, Craig W, White L, Munson AE, Comment CE. Risk assessment in immunotoxicology. II. Relationships between immune and host resistance tests. Fundam Appl Toxicol. 1993;21:71–82. doi: 10.1006/faat.1993.1074. [DOI] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, White KL, Jr, Gennings C, Munson AE, Rosenthal GJ. Risk assessment in immunotoxicology I. Sensitivity and predictability of immune tests. Fundam Appl Toxicol. 1992;18:200–210. doi: 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- Pearse G. Normal structure, function and histology of the thymus. Toxicol Pathol. 2006;34:504–514. doi: 10.1080/01926230600865549. [DOI] [PubMed] [Google Scholar]
- Ruehl-Fehlert C, Bradley A, George C, Germann PG, Bolliger AP, Schultee A. Harmonization of immunotoxicity guidelines in the ICH process—pathology considerations from the guideline Committee of the European Society of Toxicological Pathology (ESTP) Exp Toxicol Pathol. 2005;57:1–5. doi: 10.1016/j.etp.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Schuurman HJ, Krajnc-Franken MAM, Kuper CF, Van Loveren H, Vos JG. Immune system. In: Haschek WM, Rousseaux CG, editors. Handbook of Toxicologic Pathology. San Diego, CA: Academic Press; 1991. pp. 421–487. [Google Scholar]
- Schuurman HJ, Kuper CF, Vos JG. Histopathology of the immune system as a tool to assess immunotoxicity. Toxicology. 1994;86:187–212. doi: 10.1016/0300-483x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971;109(Pt 3):369–383. [PMC free article] [PubMed] [Google Scholar]
- Travlos GS. Normal structure, function, and histology of the bone marrow. Toxicol Pathol. 2006;34:548–565. doi: 10.1080/01926230600939856. [DOI] [PubMed] [Google Scholar]
- Van Loveren H, Vos JG. Immunological considerations: a practical approach to immunotoxicity testing in the rat. In: Dayan AD, Paine AJ, editors. In Advances in Applied Toxicology. London, UK: Taylor & Francis Ltd.; 1989. pp. 143–163. [Google Scholar]
- Vos JG. Immunotoxicity assessment: screening and function studies. Arch Toxicol Suppl. 1980;4:95–108. doi: 10.1007/978-3-642-67729-8_25. [DOI] [PubMed] [Google Scholar]
- Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34:409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]