Abstract
Human herpesvirus-8 (HHV-8) replication is a key factor in Kaposi sarcoma, primary effusion lymphoma, and Castleman’s disease pathogenesis. In vitro data suggest that antivirals inhibit HHV-8 replication, but little data exist in humans. Daily oropharyngeal swabs were analyzed from HIV/HHV-8 dually-infected men enrolled in three previous clinical trials of valacyclovir and famciclovir for HIV-1 and/or HSV-2 suppression. Fifty-eight participants contributed 6036 swabs. HHV-8 was detected in 1128 (19%) of 6036 swabs, including 618 (21%) of 2992 on placebo, 323 (15%) of 2221 on valacyclovir, and 187 (23%) of 823 on famciclovir. After adjusting for baseline HIV viral load and highly active antiretroviral therapy (HAART) use, an 18% reduction in HHV-8 shedding frequency (IRR 0.822; p=0.011) was found in participants on valacyclovir and a 30% reduction (IRR 0.700; p<0.001) on famciclovir. HAART was associated with an 89% (IRR 0.129; p=0.048) reduction in HHV-8 -shedding. Neither antiviral nor antiretroviral therapy was associated with decreased HHV-8 quantity. Valacyclovir and famciclovir were associated with modest but significant reductions in HHV-8 oropharyngeal shedding frequency. In contrast, HAART was a potent inhibitor of HHV-8 replication. Studies of whether antiviral therapy in combination with ART will prevent HHV-8-associated disease appear warranted.
Keywords: HHV-8, HIV, antiretroviral therapy, famciclovir, valacyclovir
Introduction
Human herpes virus-8 (HHV-8) is the cause of Kaposi sarcoma (KS), primary effusion lymphoma, and some forms of multicentric Castleman disease [Antman and Chang, 2000]. In the era of highly active antiretroviral therapy (HAART), KS remains the most common AIDS-associated malignancy in the United States [Eltom et al., 2002] and the most common overall malignancy in certain regions with high HHV-8 and HIV-1 seroprevalence [Chokunonga et al., 1999; Sitas et al., 1997; Sitas et al., 2000; Wabinga et al., 2000]. KS treatment with HAART and chemotherapy appears only modestly effective, with 51% of patients having persistent KS 36 months after diagnosis [Nguyen et al., 2008]. Therefore, there is a need for improved strategies for the prevention and treatment of HHV-8-associated diseases.
Antiviral therapy has been shown to be effective in the prevention of other viral-associated malignancies, including hepatitis-related hepatocellular carcinoma [Liaw et al., 2004; Shiratori et al., 2005] and Epstein Barr Virus (EBV)-associated lymphoma [Funch et al., 2005]. As in these cancers, replication of the causative viral agent, HHV-8, is thought to be central to the development of HHV-8 related cancers. This hypothesis is supported by findings that detection of HHV-8 in the peripheral blood is a strong predictor of KS development [Broccolo et al., 2002; Campbell et al., 2000; Cannon et al., 2003; Engels et al., 2003; Lorenzen et al., 2002; Whitby et al., 1995] and that a small amount of replicating virus is necessary for tumor initiation and maintenance [Cesarman et al., 2000]. Results from various in vitro and animal studies suggest that antiviral compounds inhibit HHV-8 replication, and, therefore, their use could offer a potential strategy for both prevention and treatment of HHV-8 associated diseases [Casper, 2006]. In these studies, ganciclovir, cidofovir, foscarnet, zidovudine and stavudine all yielded moderate reductions in HHV-8 replication; additionally, acyclovir treatment resulted in a slight reduction in detectable virus.
In a randomized, controlled trial, valganciclovir decreased HHV-8 oropharyngeal shedding by 46% [Casper et al., 2008]. However, whether other antiviral medications also inhibit HHV-8 replication in humans, and could therefore be used as synergistic or alternative treatments to valganciclovir, has not been studied. In this study, saliva samples were used from HHV-8 infected men obtained in three randomized, double-blind, placebo-controlled, cross-over clinical trials that studied valacyclovir and famciclovir in HIV-1 and HSV-2 co-infected persons to assess the effect of antiviral therapy on oral mucosal shedding of HHV-8.
Methods
Study participants and design
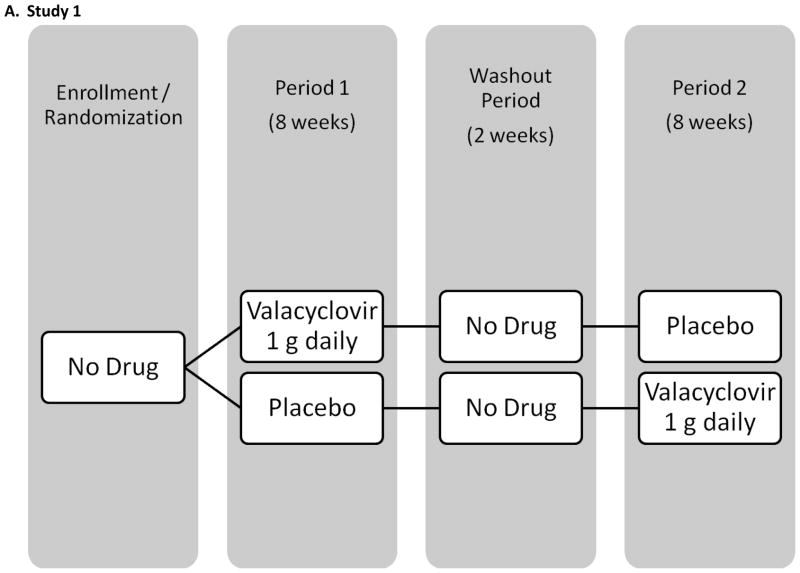
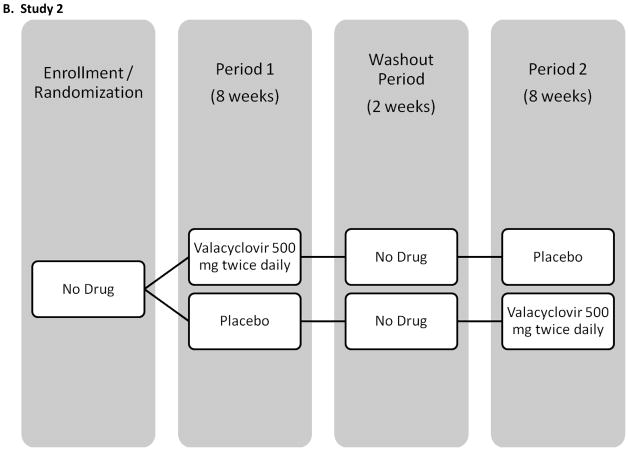
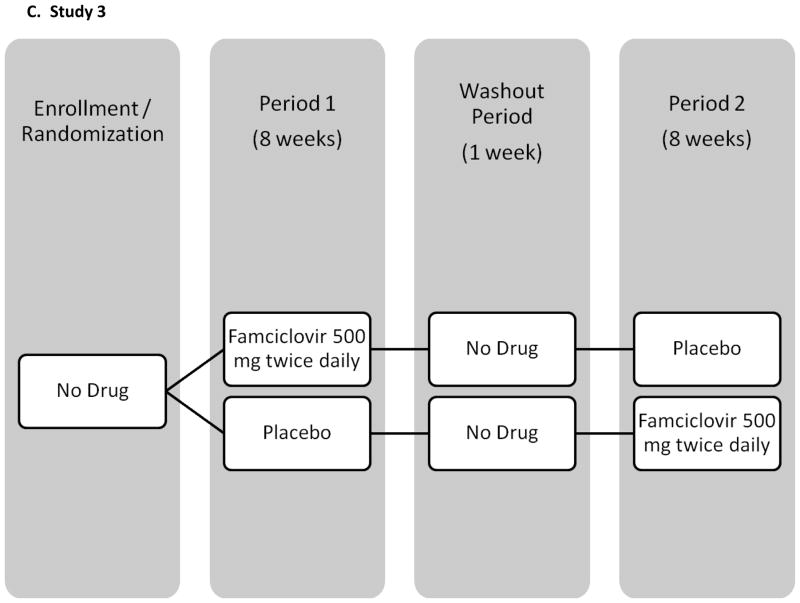
The baseline salivary samples of all participants enrolled in 3 previously conducted crossover trials of acyclovir, valacyclovir, and famciclovir were screened retrospectively for HHV-8 DNA. All participants were HIV-positive MSM and were followed for 18 weeks with daily genital and oral swabs (see Figure I for study schematics). Participants were randomized to receive study drug or placebo during the initial 8 weeks followed by 1 or 2 weeks of washout and 8 weeks of the alternate study regimen. Results from the genital swabs from all 3 studies and plasma HIV RNA in 2 studies have been previously published [Gupta R, October 2007; Schacker et al., 1998; Zuckerman et al., 2007]. None of the participants had clinical evidence of KS, lymphoma or Castleman syndrome during the study. Briefly, study 1 enrolled 60 participants in Seattle, WA, and used valacyclovir 1000 mg once daily [Gupta R, October 2007]. Thirty participants were on stable HAART for at least two months with a HIV viral load <1000 copies/ml and thirty participants were either not on HAART and had a CD4 count greater than 200 cells/μl, or were taking HAART and had a plasma HIV RNA >15000 copies/ml. Study 2 enrolled 20 participants in Lima, Peru, using valacyclovir 500 mg twice daily [Zuckerman et al., 2007]. All men were HAART naïve and had a CD4 count greater than 200 cells/μl. The third study was conducted prior to availability of HAART, and enrolled 48 participants in Seattle, WA, and used famciclovir 500 mg twice daily [Schacker et al., 1998].
Figure I.
The analysis was restricted to participants with either serologic or virologic evidence of HHV-8 infection, who completed both study arms, and collected at least 30 days of oral swabs in each study arm. Virologic evidence of HHV-8 infection was defined as greater than 150 copies of HHV-8 DNA/ml detected by polymerase chain reaction (PCR) from oropharyngeal swabs on 3 or more days. A total of 58 of the 128 patients enlisted in the 3 studies had evidence of HHV-8 infection.
The human experimentation guidelines of the US Department of Health and Human Services and the individual institutions were followed in the conduct of the clinical research in accordance with the Declaration of Helsinki. All participants provided signed informed consent. The institutional review boards of the University of Washington and/or the Asociación Civil Impacta Salud y Educación approved the protocols.
Laboratory assessments
HHV-8 was quantified using real-time PCR with primers to the orf73 gene by laboratory staff who were blinded to the study arm, as described previously [Casper et al., 2004]. All samples with greater than 150 copies of HHV-8 DNA/ml were characterized as positive. Serologic testing for study 2 was conducted using an HHV-8 whole-virus enzyme imunnoassay (EIA) with selective subsequent use of an HHV-8 immunofluorescence assay (IFA), as described previously [Casper et al., 2002a]. For studies 1 and 3, based on the availability of sera, one or a combination of the HHV-8 whole virus EIA, HHV-8 IFA, and/or a novel HHV-8 latency associated nuclear antigen (LANA) assay [Oda-Ikoma et al., 2007] was performed on stored serum collected at the time of study enrollment and immediately preceding virologic sample collection. Participants were considered to be seropositive if any of these three assays were positive.
Statistical Analysis
All data were managed and analyzed at the University of Washington. Participants that completed only one study arm or who collected fewer than 30 days of oral swabs in one or both study arms were excluded from the analysis, as infrequent sampling of mucosal sites may reduce the precision of the estimate of true herpesvirus shedding frequency[Magaret et al., 2009]. Samples from the first day a participant received either study drug or placebo and from days of the washout period were also excluded from the analysis. Data from the three parent studies were combined for analysis. Frequency of HHV-8 shedding in oropharyngeal swabs was defined as the number of swabs in which HHV-8 DNA was detected by PCR divided by the total number of oropharyngeal swabs collected.
Poisson regression with person-level random effects was used to examine the univariate association of HHV-8 shedding frequency and treatment (famciclovir, valacyclovir, or placebo). HHV-8 quantity (DNA copies/mL) was log10 transformed prior to analysis. A linear mixed effect model with person-level random intercepts was used to test the univariate association of HHV-8 quantity and treatment on days in which HHV-8 DNA was detected. The following covariates were also tested in univariate models to identify correlates of HHV-8 replication and quantity: HAART use (defined as any combination of ≥3 antiretroviral agents that included at least 1 non-nucleoside reverse transcriptase inhibitor or protease inhibitor; yes or no), CD4 count (cells/μl), age (years), race/nationality (white, black, Peruvian, and other), and HIV-1 plasma viral load (log10 copies/mL). Covariates associated with HHV-8 replication and quantity at the p < 0.2 level were included in multivariate models and assessed for significant association and confounding of each outcome. Two-sided p-values <0.05 were considered statistically significant. STATA, version 9.0, was used to perform all statistical analyses.
Results
Participant characteristics
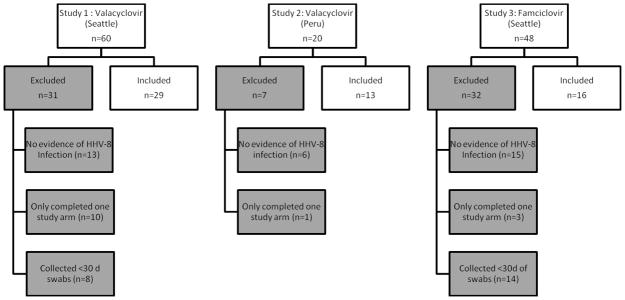
Fifty-eight of 128 participants enrolled in the 3 trials were eligible for inclusion in this analysis (Figure II). Thirty-one of 60 persons were excluded from study 1 (13 because they did not have evidence of HHV-8 infection, 10 because they only completed one study arm, and 8 because they collected less than 30 days of oral swabs in one or both study arms), 7 of 20 persons were excluded from study 2 (6 because they did not have evidence of HHV-8 infection and 1 because they only completed one study arm), and 32 of 48 persons were excluded from study 3 (15 because they did not have evidence of HHV-8 infection, 14 because they collected less than 30 days of oral swabs in one or both study arms, and 3 because they only completed one study arm).
Figure II.
Participants from study 1 tended to be older (median age of 44) than those from the other two studies (median ages of 31 for study 2 and 36 for study 3; Table I).
Table I.
Study participant demographic and clinical characteristics including those completing only one study arm.
| Valacyclovir | Famciclovir | ||
|---|---|---|---|
| Study 1 (n=29) | Study 2 (n=13) | Study 3 (n=16) | |
| Age (years), median (range) | 44 (28 – 53) | 31 (22 – 43) | 36 (29 – 48) |
| Race/Nationality, n (%) | |||
| White | 20 (69) | 3 (23) | 14 (88) |
| Peruvian | 0 | 10 (77) | 0 |
| Black | 7 (24) | 0 | 1 (6) |
| Other | 2 (7) | 0 | 1 (6) |
| Sexual preference, n (%) | |||
| Homosexual | 26 (90) | 13 (100) | 14 (88) |
| Bisexual | 3 (10) | 0 | 2 (13) |
| Days on treatment median (range) | |||
| Placebo | 55 (49 – 64) | 56 (56–56) | 55 (53 – 60) |
| Drug | 56 (51 – 64) | 56 (53 – 56) | 55 (46 – 63) |
| Years since diagnosis median (range) | 10.2 (0.2 – 20.1) | N/A | 5.2 (0.2 – 11.1) |
| Age at diagnosis (years) median (range) | 33 (18 – 47) | N/A | 30 (21 – 43) |
| Antiretroviral use, n (%) | |||
| None | 12 (41) | 13 (100) | 10 (63) |
| Non-HAART | 0 | 0 | 4 (25) |
| HAART | 17 (59) | 0 | 0 |
| Missing | 0 | 0 | 2 (13) |
| CD4 count (cells/μl) median (range) | 351 (85 – 1062) | 380 (232 – 584) | 420 (90 – 921) |
| HIV viral load (log10 copies/ml), median (range) | 1.2 (1.2 – 5.2) | 4.3 (3.4 – 5.1) | 3.8 (2.4 – 5.2) |
Participants in studies 1 and 3 were primarily white, while those from study 2 were Peruvian (reflecting study location). Ninety-two percent of participants in all three studies were men who have sex with men (MSM). Participants in study 1 tended to have a greater time since HIV diagnosis than those in study 3 (this information was not available for participants in study 2). Antiretroviral use was markedly different across studies, with 59% of participants from study 1 on HAART and the remaining participants not taking any antiretroviral treatment. Median CD4 count was similar across studies, whereas median HIV viral load was lower in study 1, reflecting inclusion criteria of that study.
Fifty-eight participants contributed 6036 oropharyngeal swabs for analysis (Table IIa). Thirty-four (59%) of 58 men had HHV-8 detected in at least three oral swabs. Of the 58 participants, 11 (19%) were PCR positive only, 24 (41%) were only positive by serology, and 23 (40%) were positive by both measures. The median person-level oral HHV-8 shedding rates during placebo sessions were 0% (IQR: 0 – 34%) for study 1, 2% (IQR: 0 – 4%) for study 2, and 8% (IQR: 1 – 69%) for study 3. The overall frequency of HHV-8 detection during placebo administration was 19% of days (269/1449) for study 1, 12% (85/716) for study 2, and 32% (264/827) for study 3.
Table II.
HHV-8 shedding rates and quantities detected in oropharyngeal swabs collected among participants completing both study arms, for A. by study arms, and B. by HAART use.
| A) | ||||
|---|---|---|---|---|
| Total (n=58) | Placebo (n=58) | Valacyclovir (n=42) | Famciclovir (n=16) | |
| Overall frequency of HHV-8 detection, oropharyngeal swabs with HHV-8 detected/all swabs (%) | 1128/6036 (19) | 618/2992 (21) | 323/2221 (15) | 187/823 (23) |
| Person-level frequency (%)of HHV-8 detection, median (IQR) | 2 (0–23) | 2 (0 – 34) | 2 (0 – 10) | 0 (0 – 51) |
| HHV-8 DNA quantity* (log10 copies/mL), median (IQR) | 4.68 (3.67–5.72) | 4.44 (3.61–5.31) | 5.26 (3.80–6.3) | 4.68 (3.80–5.56) |
| B) | ||
|---|---|---|
| HAART (n= 17) | No HAART (n= 41) | |
| Overall frequency of HHV-8 detection, oroparyngeal swabs with HHV-8 detected/all swabs (%) | 93/1761 (5) | 1034/4074 (25) |
| Person-level frequency (%) of HHV-8 detection, median (IQR) | 0 (0, 2) | 4 (0, 38) |
| HHV-8 DNA quantity* (log10 copies/mL), median (IQR) | 4.62 (3.42–5.53) | 4.69 (3.67–5.73) |
On days with detectable HHV-8
Effect of valacyclovir and famciclovir on the detection of HHV-8 in the oropharynx
HHV-8 was detected in 34 (59%) of 58 participants receiving placebo, 26 (62%) of 42 receiving valacyclovir, and 5 (31%) of 16 receiving famciclovir. Overall, HHV-8 was detected in 1128 (19%) of 6036 oropharyngeal swabs, including 618 (21%) of 2992 swabs from participants receiving placebo, 323 (15%) of 2221 swabs from those on valacyclovir treatment and 187 (23%) of 823 swabs from those on famciclovir (Table II).
There was a wide distribution of shedding frequencies among participants from all three groups (Figure III); however, median shedding frequency was slightly lower among participants on famciclovir compared to those on valacyclovir and placebo (Table IIa, Figure III).
Figure III.
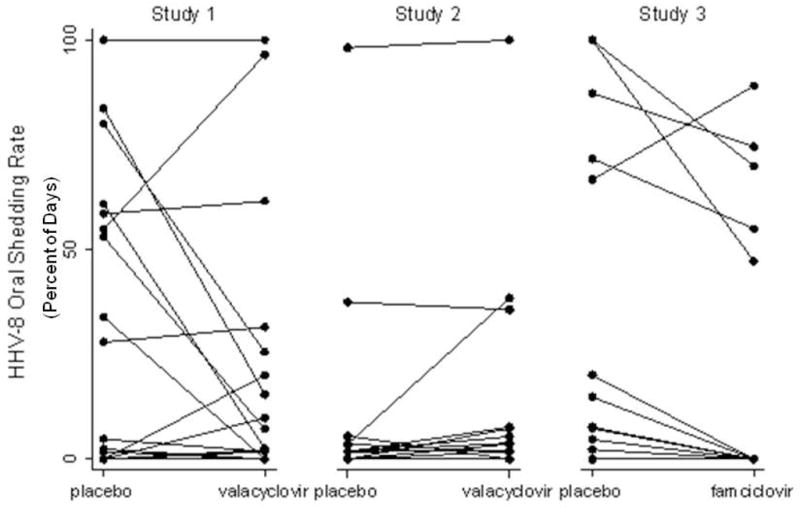
Change in HHV-8 Oropharyngeal Detection Rates between Placebo and Antiviral Drug Arms, by Participant
Valacyclovir decreased HHV-8 shedding frequency by 18% (95% confidence interval [CI], 4%–29%; P = 0.01) and famciclovir by 30% (95% CI, 16%–42%; P < 0.001; Table III), respectively, compared to placebo. These estimates did not substantially change in the multivariate model (Table III).
Table III.
Univariate and multivariate model of HHV-8 shedding rate for persons completing both study arms.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IRR | 95% CI | P-value | IRR | 95% CI | P-value | |||
|
| ||||||||
| Age | 0.95 | 0.88 | 1.03 | 0.22 | ||||
|
| ||||||||
| Ethnicity | ||||||||
| Black* | 1.23 | 0.19 | 8.21 | 0.83 | ||||
| Peruvian* | 2.35 | 0.45 | 12.39 | 0.31 | ||||
| Other* | 2.39 | 0.15 | 38.90 | 0.54 | ||||
|
| ||||||||
| Days when genital HSV detected compared to days when no genital HSV detected | 0.97 | 0.79 | 1.19 | 0.75 | ||||
|
| ||||||||
| CD4 count, for every 100 increase | 0.92 | 0.69 | 1.22 | 0.54 | ||||
|
| ||||||||
| HIV log10 copies/ml, for each log increase | 1.78 | 1.17 | 2.71 | 0.01 | 1.10 | 0.59 | 2.04 | 0.77 |
|
| ||||||||
| HAART vs. no HAART | 0.11 | 0.03 | 0.42 | 0.001 | 0.13 | 0.02 | 0.99 | 0.05 |
|
| ||||||||
| Valaciclovir vs. placebo | 0.82 | 0.71 | 0.95 | 0.01 | 0.82 | 0.71 | 0.96 | 0.01 |
|
| ||||||||
| Famciclovir vs placebo | 0.70 | 0.58 | 0.84 | <0.001 | 0.70 | 0.58 | 0.84 | <0.001 |
Compared to white race
The reduction in HHV-8 shedding frequency was not associated with a reduction in viral copy number on days with detectable shedding. On days that HHV-8 was detected, the median log10 copies of HHV-8 DNA/ml detected in oral swabs was somewhat greater among participants on valacyclovir (5.26) compared to placebo (4.44) and famciclovir (4.68, Table II). Valacyclovir and famciclovir increased HHV-8 log10 copies/ml by 0.132 (95% CI 0.016 lower to 0.280 higher; P = 0.081) and 0.218 (95% CI 0.049–0.387; P = 0.012), respectively, compared to placebo. No other covariates were associated with HHV-8 quantity.
Effect of antiretroviral therapy on the detection of HHV-8 in the oropharynx
Seventeen participants on HAART contributed 1761 oropharyngeal swabs for analysis. Four participants on non-HAART ART and 35 participants not taking any ART contributed 4074 oropharyngeal swabs for analysis. HAART regimens included 5 participants taking a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen, 11 participants taking a protease inhibitor (PI)-based regimen, and 1 participant who was on a NNRTI-based regimen during the placebo arm and a PI-based regimen during the drug arm. Among participants taking a PI-based regimen, 10 were on dual PIs and 1 was on three PIs. All participants taking a NNRTI-based regimen were on only one NNRTI.
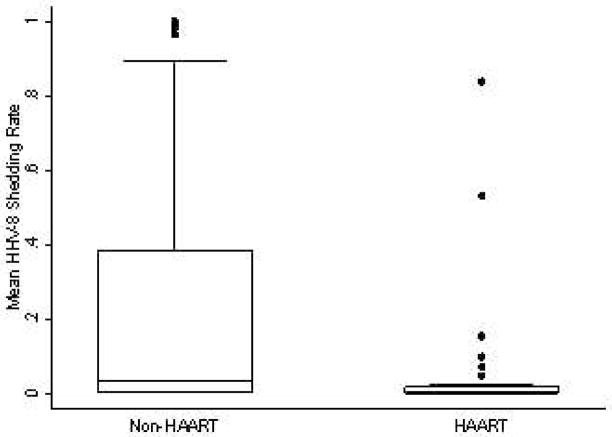
HHV-8 was detected in 93 (5%) of 1761 oropharyngeal swabs on HAART compared to 1034 (25%) of 4074 oropharyngeal swabs not on HAART (Table IIb, above).
HHV-8 oral shedding rates were 89% (95% CI 58%–97%; P = 0.001) lower among participants on HAART compared to those not taking HAART (Figure IV, Table III). This estimate did not change substantially in the multivariate model; however, the strength of the association was attenuated (Table III). Of note, HIV viral load was the only other covariate associated with HHV-8 shedding in a univariate model, but this association was not statistically significant after adjusting for HAART use and study drug (Table III). The combination of ART and antiviral therapy showed no synergistic benefit in reducing HHV-8 shedding though numbers of observations in each stratum were small.HHV-8 copy number on days with detectable shedding was significantly different by HAART use (Table IIb; data not shown).
Figure IV.
Change in HHV-8 Oropharyngeal Detection Rates by Highly Active Antiretroviral Therapy Use
Discussion
In this analysis of HHV-8 oropharyngeal shedding patterns, a modest decrease in HHV-8 oropharyngeal detection frequency was found with the use of valacyclovir or famciclovir. These findings are consistent with in vitro studies reporting a weak effect of acyclovir in preventing HHV-8 reactivation [Friedrichs et al., 2004; Kedes and Ganem, 1997; Medveczky et al., 1997; Neyts and De Clercq, 1997]. A striking finding of this analysis is that HAART was associated with an 89% decrease in HHV-8 shedding frequency. On days that HHV-8 was detected, anti-viral agents were associated with a reduction in frequency of detection but not in the titer of HHV-8. In fact, a slight increase in HHV-8 DNA was observed on both famciclovir and valacyclovir during breakthrough. The significance of these findings is not clear but may indicate that low-copy shedding is controlled more readily than higher copy shedding. Of note, valganciclovir has been shown in a previous study to reduce both HHV-8 detection frequency by 46% (P = 0.02) and quantity by 0.44 logs (P = 0.007) [Casper et al., 2008]. It may be the case that for anti-herpes agents, a more potent effect on HHV-8 replication is needed to decrease shedding quantity once reactivation has occurred.
Clinical data suggesting a direct effect of HAART on HHV-8 replication has been reported in one other study [Casper et al., 2004]. This study has the advantage of data obtained through intensive mucosal sampling and suggests that HAART may potently prevent HHV-8 reactivation. The mechanisms by which HAART may exert an anti-HHV-8 effect remain unclear. The sample size or heterogeneity in HAART regimens was insufficient to evaluate whether specific components of HAART are associated with an inhibition of HHV-8 replication, as has recently been described[Gantt et al., 2011], but the mechanisms by which HAART may indirectly inhibit HHV-8 infection through immune reconstitution, suppression of HIV replication, etc. have been reviewed elsewhere[Casper and Wald, 2007]. Further studies using prospective cohorts to assess the effect of specific HAART components and other antiretroviral drugs on HHV-8 are needed.
This study had several limitations. First, the parent studies were initially designed to evaluate different hypotheses, and HHV-8 infection was not a criterion for study entry. Thus it is possible that the selection of only HHV-8-infected persons for the analysis may have resulted in a population which differed from the general HHV-8-infected population in factors apart from their randomization to active drug or placebo first and may also influence the observed efficacy of antiviral medications in inhibiting replication. Second, different assays were used to detect HHV-8 between the studies in part because of improved methodology over time and the limited availability of sera to analyze retrospectively. This is potentially an important source of selection bias. Third, three disparate studies were pooled to ascertain the effect of antiviral medications on HHV-8 replication. This approach, while increasing the power to observe an effect of HHV-8 replication, may have introduced additional bias. Fourth, it is not known whether once versus twice daily administration of valacyclovir could have differential effects on HHV-8 which would have been missed by combining these two groups. Fifth, the population for all study groups was inclusive only of MSM, and the effects of anti-viral treatment might be expected to differ in other populations, such as those with endemic HHV-8 infections. Finally, the relevance of a reduced frequency of HHV-8 detection at the oropharynx to public health or clinical medicine remains unknown. Only circumstantial evidence supports a relationship between HHV-8 salivary shedding and disease transmission or acquisition[Casper et al., 2006; Casper et al., 2002b; Pauk et al., 2000]. However, among a cohort of HHV-8-infected Ugandan adults, HHV-8 oropharyngeal replication is highly correlated with detection in the peripheral blood, which in turn has been associated with the risk of incident[Engels et al., 2003] or relapsed[Bihl et al., 2009] KS.
Multiple lines of evidence suggest that HAART alone may be insufficient in management of HHV-8 related disease. These include persistence of KS despite HAART use [Nguyen et al., 2008], development of KS in persons stable on HAART [Maurer et al., 2007], and poor response to HAART in cases of primary effusion lymphomas and multicentric Castleman’s disease [Casper, 2006]. The response to HAART in areas with a high burden of KS also remains uncertain. Antiviral therapy targeting HHV-8 might play an important role in KS prevention among HIV and HHV-8 co-infected individuals. Additionally, although antiviral therapy alone was ineffective in treating classic KS[Little et al., 2003], the use of antiviral therapy may be indicated as an adjunct to chemotherapy in the treatment of refractory KS, or in treatment of primary effusion lymphoma and multicentic Castleman disease, which currently have no effective treatment [Casper, 2006]. Identifying the most potent combinations of HAART and anti-herpes agents against HHV-8 will have important treatment implications. These results indicate that further studies to evaluate the effect of anti-HSV drugs, alone or in combination with HAART, on progression of KS and other HHV-8-related diseases are warranted.
Acknowledgments
We would like to thank Drs Richard Zuckerman, Jorge Sanchez, and Aldo Lucchetti, along with the study staff at the Virology Research Clinic in Seattle, WA, and in Lima, Peru, for their work in running the studies, and Ms. Christa Ward for her assistance with critical review and technical preparation of the manuscript.
Grant Support: Support for this study was provided by a research grant from GlaxoSmithKline (research grant R 103) and by the National Institutes of Health (Centers for AIDS Research), Clinical Research and Laboratory Core Grants AI-27757 and AI-38858, HD-40540, R37 AI-42528, P30 AI 027757, UL1 RR025014, PO1 AI-30731 and K24 AI-071113.
Footnotes
Conflict of Interest: Connie Celum has received research grant support from the GlaxoSmithKline (GSK), which did not include salary support.
Contributor Information
Ashok Cattamanchi, University of Washington (UW). Present Affiliation: Department of Medicine, Inova Fairfax Hospital.
Misty Saracino, Department of Laboratory Medicine, UW.
Stacy Selke, Department of Laboratory Medicine, UW.
Meei-Li Huang, Department of Laboratory Medicine, UW.
Amalia Magaret, Department of Laboratory Medicine, UW.
Connie Celum, UW.
Lawrence Corey, Fred Hutchinson Cancer Research Center (FHCRC).
Anna Wald, UW.
Corey Casper, Vaccine and Infectious Disease, Public Health Sciences, and Clinical Research Divisions, FHCRC.
References
- Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- Bihl F, Berger C, Chisholm JVI, Henry LM, Bertisch B, Trojan A, Nadal D, Speck RF, Flepp M, Brander C, Mueller NJ Study tSHC. Cellular immune responses and disease control in acute AIDS-associated Kaposi’s sarcoma. Aids. 2009;23:1918–1922. doi: 10.1097/QAD.0b013e3283300a91. [DOI] [PubMed] [Google Scholar]
- Broccolo F, Bossolasco S, Careddu AM, Tambussi G, Lazzarin A, Cinque P. Detection of DNA of lymphotropic herpesviruses in plasma of human immunodeficiency virus-infected patients: frequency and clinical significance. Clinical and diagnostic laboratory immunology. 2002;9:1222–1228. doi: 10.1128/CDLI.9.6.1222-1228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TB, Borok M, Gwanzura L, MaWhinney S, White IE, Ndemera B, Gudza I, Fitzpatrick L, Schooley RT. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi’s sarcoma clinical stage. Aids. 2000;14:2109–2116. doi: 10.1097/00002030-200009290-00006. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Dollard SC, Black JB, Edlin BR, Hannah C, Hogan SE, Patel MM, Jaffe HW, Offermann MK, Spira TJ, Pellett PE, Gunthel CJ. Risk factors for Kaposi’s sarcoma in men seropositive for both human herpesvirus 8 and human immunodeficiency virus. Aids. 2003;17:215–222. doi: 10.1097/00002030-200301240-00012. [DOI] [PubMed] [Google Scholar]
- Casper C. Defining a role for antiviral drugs in the treatment of persons with HHV-8 infection. Herpes. 2006;13:42–47. [PubMed] [Google Scholar]
- Casper C, Carrell D, Miller KG, Judson FD, Meier AS, Pauk JS, Morrow RA, Corey L, Wald A, Celum C. HIV serodiscordant sex partners and the prevalence of human herpesvirus 8 infection among HIV negative men who have sex with men: baseline data from the EXPLORE Study. Sex Transm Infect. 2006;82:229–235. doi: 10.1136/sti.2005.016568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper C, Krantz E, Taylor H, Dalessio J, Carrell D, Wald A, Corey L, Ashley R. Assessment of a combined testing strategy for detection of antibodies to human herpesvirus 8 (HHV-8) in persons with Kaposi’s sarcoma, persons with asymptomatic HHV-8 infection, and persons at low risk for HHV-8 infection. J Clin Microbiol. 2002a;40:3822–3825. doi: 10.1128/JCM.40.10.3822-3825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper C, Krantz EM, Corey L, Kuntz SR, Wang J, Selke S, Hamilton S, Huang ML, Wald A. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. The Journal of infectious diseases. 2008;198:23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper C, Redman M, Huang ML, Pauk J, Lampinen TM, Hawes SE, Critchlow CW, Morrow RA, Corey L, Kiviat N, Wald A. HIV Infection and Human Herpesvirus-8 Oral Shedding Among Men Who Have Sex with Men. J Acquir Immune Defic Syndr. 2004;35:233–238. doi: 10.1097/00126334-200403010-00003. [DOI] [PubMed] [Google Scholar]
- Casper C, Wald A. The use of antiviral drugs in the prevention and treatment of Kaposi sarcoma, multicentric Castleman disease and primary effusion lymphoma. Curr Top Microbiol Immunol. 2007;312:289–307. doi: 10.1007/978-3-540-34344-8_11. [DOI] [PubMed] [Google Scholar]
- Casper C, Wald A, Pauk J, Tabet SR, Corey L, Celum CL. Correlates of prevalent and incident Kaposi’s sarcoma-associated herpesvirus infection in men who have sex with men. J Infect Dis. 2002b;185:990–993. doi: 10.1086/339605. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Mesri EA, Gershengorn MC. Viral G protein-coupled receptor and Kaposi’s sarcoma: a model of paracrine neoplasia? J Exp Med. 2000;191:417–422. doi: 10.1084/jem.191.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokunonga E, Levy LM, Bassett MT, Borok MZ, Mauchaza BG, Chirenje MZ, Parkin DM. Aids and cancer in Africa: the evolving epidemic in Zimbabwe. Aids. 1999;13:2583–2588. doi: 10.1097/00002030-199912240-00012. [DOI] [PubMed] [Google Scholar]
- Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi’s sarcoma and non-Hodgkin’s lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94:1204–1210. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- Engels EA, Biggar RJ, Marshall VA, Walters MA, Gamache CJ, Whitby D, Goedert JJ. Detection and quantification of Kaposi’s sarcoma-associated herpesvirus to predict AIDS-associated Kaposi’s sarcoma. Aids. 2003;17:1847–1851. doi: 10.1097/00002030-200308150-00015. [DOI] [PubMed] [Google Scholar]
- Friedrichs C, Neyts J, Gaspar G, De Clercq E, Wutzler P. Evaluation of antiviral activity against human herpesvirus 8 (HHV-8) and Epstein-Barr virus (EBV) by a quantitative real-time PCR assay. Antiviral Res. 2004;62:121–123. doi: 10.1016/j.antiviral.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Funch DP, Walker AM, Schneider G, Ziyadeh NJ, Pescovitz MD. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. Am J Transplant. 2005;5:2894–2900. doi: 10.1111/j.1600-6143.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- Gantt S, Carlsson J, Ikoma M, Gachelet E, Gray M, Geballe AP, Corey L, Casper C, Lagunoff M, Vieira J. The HIV protease inhibitor nelfinavir inhibits Kaposi sarcoma-associated herpesvirus replication in vitro. Antimicrob Agents Chemother. 2011:AAC.01295–01210. doi: 10.1128/AAC.01295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RSR, Kuntz S, Selke S, Huang ML, Corey L, Wald A. Daily Valacyclovir Reduced Plasma HIV RNA among Men Having Sex with Men in the United States. IDSA; San Diego, California: Oct, 2007. p. P-1293. [Google Scholar]
- Kedes DH, Ganem D. Sensitivity of Kaposi’s sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J Clin Invest. 1997;99:2082–2086. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw Y-F, Sung JJY, Chow WC, Farrell G, Lee C-Z, Yuen H, Tanwandee T, Tao Q-M, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J the Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for Patients with Chronic Hepatitis B and Advanced Liver Disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- Little RF, Merced-Galindez F, Staskus K, Whitby D, Aoki Y, Humphrey R, Pluda JM, Marshall V, Walters M, Welles L, Rodriguez-Chavez IR, Pittaluga S, Tosato G, Yarchoan R. A pilot study of cidofovir in patients with kaposi sarcoma. J Infect Dis. 2003;187:149–153. doi: 10.1086/346159. [DOI] [PubMed] [Google Scholar]
- Lorenzen T, Albrecht D, Paech V, Meyer T, Hoffmann C, Stoehr A, Degen O, Stellbrink HJ, Meigel WN, Arndt R, Plettenberg A. HHV-8 DNA in blood and the development of HIV-associated Kaposi’s sarcoma in the era of HAART--a prospective evaluation. Eur J Med Res. 2002;7:283–286. [PubMed] [Google Scholar]
- Magaret AS, Johnston C, Wald A. Use of the designation “shedder” in mucosal detection of herpes simplex virus DNA involving repeated sampling. Sex Transm Infect. 2009;85:270–275. doi: 10.1136/sti.2008.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. The New England journal of medicine. 2007;357:1352–1353. doi: 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- Medveczky MM, Horvath E, Lund T, Medveczky PG. In vitro antiviral drug sensitivity of the Kaposi’s sarcoma-associated herpesvirus. Aids. 1997;11:1327–1332. doi: 10.1097/00002030-199711000-00006. [DOI] [PubMed] [Google Scholar]
- Neyts J, De Clercq E. Antiviral drug susceptibility of human herpesvirus 8. Antimicrob Agents Chemother. 1997;41:2754–2756. doi: 10.1128/aac.41.12.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HQ, Magaret AS, Van Rompaey SE, Kitahata MM, Wald A, Casper C. Persistent Kaposi sarcoma in the era of HAART: characterizing the predictors of clinical response. AIDS. 2008;22:937–945. doi: 10.1097/QAD.0b013e3282ff6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda-Ikoma M, Casper C, Johnston C, Selke S, Morrow R, Corey L, Vieira J. KSHV LANA serological assay in the identification of immunocompetent asymptomatic KSHV infected individuals, and the development of improved methods for assaying neutralizing antibodies to KSHV. 10th International Workshop on Kaposi’s Sarcoma Associated Herpesvirus (KSHV) and Rlated Agents; Portland, OR. 2007. [Google Scholar]
- Pauk J, Huang ML, Brodie SJ, Wald A, Koelle DM, Schacker T, Celum C, Selke S, Corey L. Mucosal Shedding of Human Herpesvirus 8 in Men. N Engl J Med. 2000;343:1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- Schacker T, Hu HL, Koelle DM, Zeh J, Saltzman R, Boon R, Shaughnessy M, Barnum G, Corey L. Famciclovir for the suppression of symptomatic and asymptomatic herpes simplex virus reactivation in HIV-infected persons. A double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:21–28. doi: 10.7326/0003-4819-128-1-199801010-00004. [DOI] [PubMed] [Google Scholar]
- Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, Arakawa Y, Hashimoto E, Hirota K, Yoshida H, Ohashi Y, Omata M for the Tokyo-Chiba Hepatitis Research Group*. Antiviral Therapy for Cirrhotic Hepatitis C: Association with Reduced Hepatocellular Carcinoma Development and Improved Survival. Ann Intern Med. 2005;142:105–114. doi: 10.7326/0003-4819-142-2-200501180-00009. [DOI] [PubMed] [Google Scholar]
- Sitas F, Bezwoda WR, Levin V, Ruff P, Kew MC, Hale MJ, Carrara H, Beral V, Fleming G, Odes R, Weaving A. Association between human immunodeficiency virus type 1 infection and cancer in the black population of Johannesburg and Soweto, South Africa. Br J Cancer. 1997;75:1704–1707. doi: 10.1038/bjc.1997.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitas F, Pacella-Norman R, Carrara H, Patel M, Ruff P, Sur R, Jentsch U, Hale M, Rowji P, Saffer D, Connor M, Bull D, Newton R, Beral V. The spectrum of HIV-1 related cancers in South Africa. Int J Cancer. 2000;88:489–492. doi: 10.1002/1097-0215(20001101)88:3<489::aid-ijc25>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82:1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby D, Howard MR, Tenant-Flowers M, Brink NS, Copas A, Boshoff C, Hatzioannou T, Suggett FE, Aldam DM, Denton AS, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Zuniga R, Magaret AS, Wald A, Corey L, Celum C. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]