Abstract
Object
The effect of Deep Brain Stimulation (DBS) for Parkinson's disease (PD) on balance is unclear. The goal of this study was to investigate how automatic postural responses were affected in subjects randomized to either the Subthalamic Nucleus (STN) or the Globus Pallidus interna (GPi) surgery.
Methods
We tested 24 PD subjects who underwent bilateral DBS, 9 PD control subjects without DBS, and 17 age-matched control subjects. Electrode placement site was randomized and blinded to PD subjects and experimenters. Kinematic, kinetic and electromyographic recordings of postural responses to backward disequilibrium via forward translations of the standing surface were recorded in the week prior to surgery while off (OFF) and on antiparkinsonian medication (ON) and then 6 months after surgery in four conditions: off medication with DBS switched off (OFF/OFF), off medication with DBS on (DBS), on medication with DBS off (DOPA), and both medication and DBS on (DBS+DOPA). Stability of the automatic postural response (APR stability) was measured as the difference between the displacement of the center of pressure and the projected location of the center of body mass.
Results
PD subjects had worse APR stability than control subjects. Turning the DBS on at either site improved APR stability compared to the postoperative off condition by lengthening the tibialis response, whereas medication did not show an appreciable effect. The STN group had worse APR stability in their best functional state (DBS+DOPA) six months after the DBS procedure compared to their best functional state (ON levodopa) before the DBS procedure. In contrast, the GPi group and the PD control group showed no change over 6 months. APR stability impairment in the STN group was associated with smaller tibialis response amplitudes, but no change in response latency or co-activation with gastrocnemius.
Conclusions
Turning the DBS current on improved APR stability for both STN and GPi sites. However, there was a detrimental DBS procedural effect for the STN group and this effect was greater than the benefit of the stimulating current, making overall APR stability functionally worse after surgery for the STN group.
Keywords: Parkinson's Disease, DBS, Postural control
INTRODUCTION
Deep brain stimulation (DBS) for Parkinson's disease (PD) has proven to be an effective treatment for the cardinal motor symptoms of tremor, muscle rigidity and bradykinesia.1, 21, 30 However, DBS's effectiveness in alleviating balance problems associated with PD is unclear. Postural instability and gait disability (PIGD) items the Unified Parkinson's Disease Rating Scale (UPDRS III) are the commonly reported metric of balance in PD. Within the first year of DBS surgery most studies report some improvement in PIGD when patients are treated with DBS and medication 2, however our recent meta-regression shows that across five years the PIGD progressively worsens, even though cardinal motor symptoms remain steady.32 Furthermore, increased falls are reported after DBS surgery 38 particularly with STN DBS.13
Poor postural responses to external perturbations are associated with increased falls.14 Quantitative laboratory experiments of postural responses can offer insight into how balance mechanisms are affected by PD and how they are modified by medication and the DBS procedure. In PD, the latency of automatic postural reactions is normal but the size of the reactive force is reduced and is not typically not responsive to dopaminergic medications.5, 8, 18
There are as yet no published studies of perturbed balance in patients with DBS in GPi and the few studies in STN report inconsistent findings. For example, when comparing best treated states before (ON medication) and after surgery (ON medication and DBS) an improved ability to stand on a slowly oscillating surface with eyes closed is reported.9 In contrast, other studies found that when compared to the off treatment state post-surgery, responses to tilts of the standing surface do not show a convincing improvement with DBS.26, 37
The automatic postural responses to brief forward surface translations that force the body backwards were investigated in the current study because postural stability in the backward direction is known to be particularly affected in PD.8, 11, 20 When the body is pushed backwards, the automatic postural response to maintain stability involves bilateral activation of the tibialis anterior muscle. This muscle activation moves the ground reactive force backward (balancing on the heels in an extreme situation) in order to produce a stabilizing, forward torque to the body.
Comparing balance performance before and after the DBS procedure is important for determining the overall success of the therapy in treating balance problems. This was the first study to examine the effects of DBS and medication in both the OFF and ON treatment states pre- versus post-operatively on automatic postural responses in both STN and GPi DBS sites. Given that the improvements seen with DBS generally follow those seen with antiparkinsonian medication, it was hypothesized that DBS would not have a significant improvement on the automatic postural response.
METHODS
Subjects
The PD subjects who underwent surgery were a cohort from the VA/NINDS multi-center clinical trial,38 and recruited through Seattle and Portland locations. DBS site was randomized and blinded to subjects and experimenters. Post-operative testing was not performed on seven subjects: two due to surgical complications (1 stroke, 1 infection), one declined for unknown reasons and four had broken limbs from falls since surgery (2xSTN, 2xGPi). Of the subjects tested both before and after surgery, 13 had STN DBS and 11 had GPi DBS.
There were two control groups: 1) PD control subjects (n=9), who met the criteria for DBS surgery but had chosen not to undergo the surgery; and 2) healthy control subjects (n=17), with no neurological or musculoskeletal problems. The PD control subjects were tested to determine whether there was significant natural decline in APR over 6 months. The healthy control subjects were tested to determine the normal level of function. At baseline, the groups did not differ significantly in the demographic measures, although the GPi group tended to have slightly worse clinical ratings (Table). All subjects gave signed consent for the protocol that was approved by the Oregon Health & Science University and the Veterans Administration Medical Center Institutional Review Boards.
Table.
Demographic and clinical characteristics of the groups
| Parameter | DBS | PD control | Healthy control | |
|---|---|---|---|---|
| STN, N=13 | GPi, N=11 | |||
| Sex M/F | 10/3 | 10/1 | 7/2 | 14/3 |
| Age (yrs) | 61.6 (5.8) | 62.8 (8.1) | 60.3 (7.8) | 65.6 (7.7) |
| Weight (kg) | 78.8(16.3) | 80.6(19.0) | 77.4 (16.1) | 80.7 (16.6) |
| Height (m) | 1.77 (0.09) | 1.78 (0.08) | 1.72 (0.10) | 1.76 (0.09) |
| Disease duration (yrs) | 12.8 (4.9) | 14.6 (7.6) | 11.6 (6.3) | |
| H&Y OFF baseline | 3.3 (0.8) | 3.8 (1.0) | 3.0 (1.0) | |
| ON baseline | 2.4 (0.7)* | 3.1 (0.7)* | 2.1 (0.6) | |
| OFF 6 months | 3.7 (1.2) | 3.3 (0.8) | 3.0 (1.0) | |
| ON (best)† 6 months | 2.3 (0.6) | 2.3 (0.8) | 2.2 (0.4) | |
| Cardinal Signs | ||||
| OFF baseline | 36.6 (8.1) | 40.0 (12.4) | 35.3 (12.6) | |
| ON baseline | 18.4 (9.0) | 23.6 (11.6) | 16.9 (7.0) | |
| OFF/OFF 6 months | 38.5 (12.4) | 39.6 (14.4) | 36.7 (10.1) | |
| ON (best)† 6 months | 15.4 (7.0) | 17.5 (10.1) | 18.8 (7.3) | |
| PIGD Signs | ||||
| OFF baseline | 6.5 (3.2) | 7.6 (4.2) | 6.2 (3.0) | |
| ON baseline | 2.7 (2.5) | 4.1 (2.7) | 3.2 (2.0) | |
| OFF/OFF 6 months | 8.0 (3.6) | 6.3 (4.5) | 6.0 (3.7) | |
| ON (best)† 6 months | 3.2 (2.1) | 2.5 (3.3) | 3.2 (2.8) | |
| ‡LEDD baseline | 1349 (668) | 1412 (887) | 1234 (450) | |
| LEDD 6 months | 908 (538) | 1123 (348) | 1144 (326) | |
| DBS pulse width (ms) | 90 × n=10, 60 × n=3 | 90 × n=9, 60 × n=2 | ||
| amplitude (V) | 3.3 (0.76) | 3.7 (0.80) | ||
| rate (Hz) | 166 (22.8) | 181 (11.7) | ||
Values in parentheses are standard deviations
P<0.05 by an independent t-test between adjacent groups.
ON (best) is ON DOPA for the PD controls and ON DOPA+DBS for the PD surgery groups.
Levodopa Equivalent Daily Dose, (Nutt et al., 2003)
H&Y= Hoehn and Yahr score range: 0 – 5
Tremor was the sum of UPDRS items 20 and 21 with a maximum score of 28.
Rigidity was the total for UPDRS item 22 with a maximum score of 20.
Bradykinesia was the sum of UPDRS items 23, 24, 25, and 26 with a maximum score of 32.
Surgery
Implantation of the DBS electrodes (Medtronic, 3387) was performed by author KJB under local anesthesia using a Leksell stereotactic frame and MRI guidance, employing an image-guidance platform (STEALTH FrameLink). A 3T 3D MRI FLAIR sequence was used for STN, and 3D T2 sequence was obtained for GPI. Initial targets were based on standard coordinates referenced to the mid-point of the anterior-posterior commissure (AC-PC) line: for STN (x=12, y=-4, z=-4mm) and for GPi (x=20-21, y=2, z=-4mm). Modification of the target coordinates were made, if necessary, after the initial target was examined on the image guidance platform. Using the NeuroTrek system (Alpha-Omega, Atlanta, Georgia) two microelectrodes were advanced simultaneously and recordings made for the purposes of target verification: one electrode was directed to the target, with one parallel track directed 2 mm anterior to the target, for both the STN and GPI targets. Electrodes were inserted bilaterally through two, pre-coronal, burr holes. Intra-operative microelectrode recordings were performed to confirm target localization, and corrections to the implant site were made accordingly. A single-channel (Medtronic, Soletra) or dual-channel (Medtronic, Kinetra) internal pulse generator was surgically implanted in the infra-clavicular area under general anesthesia one week following lead implantation. Once the stimulator was turned on, patients had at least 3 visits over 90 days to a movement disorders neurologist for DBS setting optimization and appropriate medication adjustment.
Experimental Conditions
Baseline
PD subjects reported to the laboratory in the morning and were initially tested in the practical OFF state - having withheld their antiparkinsonian medication for at least 12 hours. Subjects then took their prescribed antiparkinsonian medication and testing was repeated once subjects reported they were in the ON state (45 - 75 minutes later).
6 months
PD subjects were evaluated after six months to allow the effects of surgery in the PDDBS group to stabilize.6 PD-DBS subjects arrived at the laboratory in the practical-off medication state with their DBS on. Following testing in the DBS only condition, the stimulator was turned off and after 60 minutes the subject was retested in the OFF/OFF condition. Subjects then took their prescribed antiparkinsonian medication dose and were tested approximately an hour later in the on medication only condition (DOPA). In the final condition, the stimulator was turned on and approximately 30 minutes later testing was performed in the DBS+DOPA condition. PD control subjects were tested 6 months later with the same procedure as baseline. Healthy control subjects were tested four times on a single visit to the laboratory with similar time delays as PD-DBS subjects to evaluate effects of repeated testing.
Experimental Procedure
Subjects stood with arms folded and gaze directed at an art poster 5 meters ahead. Feet were placed a comfortable distance apart on two servo-driven platforms that were under computer control. To reduce startle responses associated with surface motion a series of six backward surface translations were delivered prior to the test trials. In the test trials, subjects were perturbed backwards without a cue, by forward surface translation of 11.77 cm (s.d. 0.05) in 1.06 s (s.d. 0.04) with a constant velocity of 11.1 cm/s. Subjects were instructed to maintain standing without stepping. Trials in which a step occurred were noted and the postural response prior to the step was included in the analysis.
The Hoehn and Yahr scale15 and the motor Unified Parkinson's Disease Rating Scale (UPDRS III),12 were administered to the PD subjects in each experimental condition.
Data collection
Recordings were triggered 600 ms before platform motion and lasted four seconds. A 3-D body representation was calculated from recordings of 23 reflective-markers placed on body landmarks at 60 Hz (Motion Analysis, Santa Rosa, CA). Displacement of whole-body center of mass (CoM) was calculated from the weighted-sum of the CoM location of individual body segments.36 The center of pressure (CoP) displacement was calculated from floor ground reaction force vectors measured by strain-gauges in the surface sampling at 480 Hz. Bilateral EMG activity of the tibialis anterior (TIB) and medial gastrocnemious (GAS) was sampled at 480 Hz, amplified at a gain of 5–10K, band-pass filtered from 75–2000 Hz, and full-wave rectified. To normalize EMG amplitude, maximal voluntary contractions (MVCs) of these muscles held isometrically were recorded at the completion of each testing session.
To assure that subjects maintained their weight evenly between the feet and consistently in the anterior-posterior direction prior to each perturbation, the CoP was monitored during testing on an oscilloscope and directions for adjustment were given to the subject. For confirmation, the mean position of the CoP relative to the malleolus was calculated as a percentage of foot length for 500 ms before platform translation and was not significantly different among conditions.
Data Analysis
The displacement between the anterior/posterior CoP and CoM positions projected vertically to the ground provides a measure of the active acceleration through the feet, generated by the automatic postural response (APR) to arrest the falling CoM.40 The CoP and CoM traces were zeroed to their means over the first 500 ms of the trial and movement of the standing surface was subtracted from the CoM displacement for equivalent reference frames. The dependent measure, APR stability, was the area (normalized to subject height) between the CoP-CoM time-series over 300 ms (Figure 1A), starting 50 ms after the onset of the first TIB burst to allow for electromechanical coupling delay.18 This time window was chosen as it encompassed the short, medium and long latency APR18 and therefore provided a good overall performance score. The fixed time-window allowed the APR stability score to reflect both speed of force generation as well as magnitude, with a higher score reflecting a fast and strong APR (Figure 1B).
Figure 1.
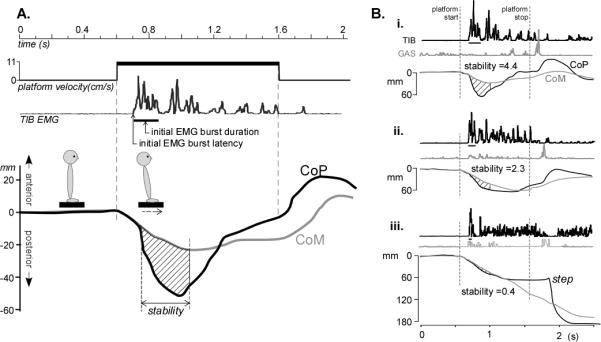
A. Quantifying automatic postural responses to backward disequilibrium from forward surface translations. CoP and CoM displacement in the anterior/posterior direction and tibialis anterior muscle (TIB) EMG response to the 1 sec-platform translation from a control subject are shown. APR stability was calculated as the area between the CoP and CoM traces for a 300 ms window (hashed area) starting 50ms after TIB onset. The duration of the initial TIB burst is indicated by the horizontal line under the TIB trace. B. Examples of automatic postural responses: i) The response of a control subject is characterized by an initial activation of the TIB that moves the CoP backward, beyond the falling CoM, which is quickly stabilized. Note that when the platform stops moving there is an activation of the GAS to stabilize the body in the anterior direction; ii) In this PD subject, a lower APR stability score was the result of a slower initial change in the CoP; iii) In this PD subject, the displacement of the CoP and the TIB burst area was so small that the response failed to arrest the falling CoM and so a step was required.
The mechanism underlying the effects of DBS and levodopa on the APR was investigated by examining postural EMG activity. Baseline EMG activity was recorded over 500 ms before platform motion. The latencies of the TIB and GAS muscle bursts from the onset of platform motion were identified as the first, sustained EMG activity (> 25 ms) greater than two SD above baseline EMG. The time for this reactive EMG burst to return to baseline level was the initial EMG burst duration. The size of the agonist, TIB response was calculated as the area under the first 75 ms EMG burst, calculated as a percentage of each subjects’ MVC. A Co-activation Index was defined as the duration of the antagonist, GAS EMG activity above baseline within the initial 75 ms of the TIB EMG burst. Data analysis was performed using MATLAB (Matlab R2009b, The Mathworks Inc, Natick MA).
Statistical Analysis
Within the PD-DBS subjects, to determine the effects of: group (STN, GPi), DBS (ON or OFF), medication (ON or OFF), and surgery (baseline or 6 months) on the dependent variables (APR stability score and EMG parameters), a linear mixed-model with an auto-regressive covariance structure was used, including each of these fixed factors and subject as a random-effects factor.39 The distributions of the dependent measures for each group, in each condition, were not different to normal (by Shapiro-Wilk test) and the variance was homogenous (by Levene's test). Unless otherwise stated in the results, the statistical tests reported are contrast tests between particular combinations of factors in the model to determine the apriori effects of interest.
Stimulation effect: Does turning the DBS stimulator on (DBS) improve APR stability compared to the OFF/OFF state post-surgery?
Medication effect: Is APR stability better when antiparkinsonian medication is taken?
Therapeutic effect: Is APR stability improved in the “best-treated” state postoperatively (DOPA+DBS) compared to preoperatively (DOPA only)?
Procedural effect: Is APR stability different between the off treatment state before surgery (OFF) and after surgery (OFF/OFF)?
Repeated measures ANOVAs were used to assess APR stability changes over six months in the PD control group, and over repeated sessions within a day in the healthy control group. The healthy control group was compared to the PD groups with non-parametric statistics as the variance between the groups was different. Statistical analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
APR Stability
While all control subjects maintained standing balance, 19% of PD trials resulted in a compensatory step. Taking a step was strongly associated with low APR stability (p<0.001 by Spearman correlation). In addition, a better APR stability score had a small, but significant, correlation with better performance on the postural stability item of the UPDRS (“pull” test item #30, p=0.023, Spearman).
The mean APR stability scores across conditions for each group are presented in Figure 2. The control group had higher APR stability scores than PD subjects before surgery in the off state. Despite randomization, at baseline the GPi group had worse APR stability scores than the STN group (p=0.018).
Figure 2.
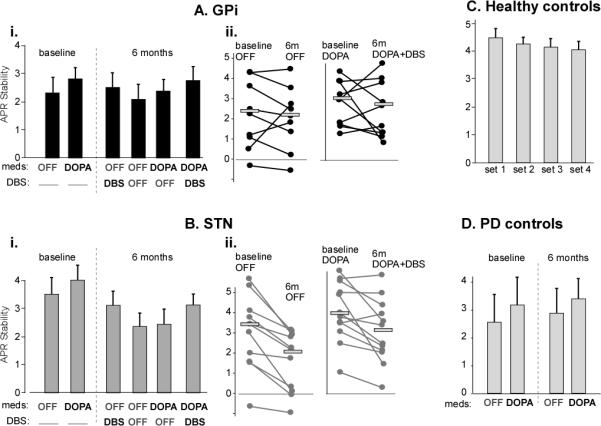
APR stability scores of the postural response for the surgery and control groups. Mean scores (±s.e.m) in each of the six conditions for the GPi group (Ai) and STN group (Bi) are shown. Individual subject changes between the un-medicated states pre- and post-operatively and the best-treated state pre- and post-operatively in the GPi and STN group are plotted in Aii and Bii, with the horizontal line representing the group mean. Some subjects are not shown because they requested not to be testing in the OFF state. C shows the APR stability scores of the healthy control subjects for each of the four sets repeated on a single day. D shows the scores of the PD control subjects tested 6 months apart.
Stimulation effect
Turning the DBS stimulator on, either in STN or GPi, significantly improved APR stability when compared to the OFF/OFF state after surgery (p=0.002), with no site interaction (p=0.97).
Medication effect
There was a small improvement in APR stability with levodopa, however, this effect did not reach significance (p=0.067) and there was no medication by site (p=0.47), or medication by surgery interaction (p=0.70). There was no additional benefit of medication with DBS with the difference between the DBS and DOPA+DBS conditions non-significant (p=0.86).
Therapeutic effect
The mean APR stability score was reduced in the STN group in the best-treated state, 6-months post-surgery compared to baseline (DOPA+DBS versus baseline-DOPA, p=0.012). In contrast, the GPi group had similar levels of APR stability at baseline and 6 months in the treated states (p=0.239).
Procedural effect
The change between APR stability pre-surgery OFF compared to post-surgery OFF/OFF showed a significant site by surgery interaction effect (p=0.037). The STN group had significantly worse APR stability post-surgery compared to baseline (p=0.024), whereas there was no change for the GPi group (p=0.32).
PD subjects who did not have DBS surgery showed no change in the APR stability score over 6 months (p=0.67, by repeated-measures; Fig. 2C). Healthy control subjects showed a practice effect between sessions (p<0.001, by repeated measures; Fig 2D). Post-hoc comparisons showed only the first session had a larger APR stability score than the three subsequent sessions by a mean of 0.2 units (p<0.02 for each comparison), with no difference among the final three sessions.
APR EMG
Subjects responded to backward CoM perturbations with a burst of activation in bilateral TIB. The APR stability score was significantly correlated with both the TIB EMG area in the first 75 ms (R=0.44, p<0.001) and the TIB EMG duration of the first burst (R=0.23, p<0.001).
The latency of the initial TIB burst, at approximately 100 ms, did not differ between control and PD subjects (p=0.57) regardless of surgery, medication, or DBS state (Figure 3A).
Figure 3.
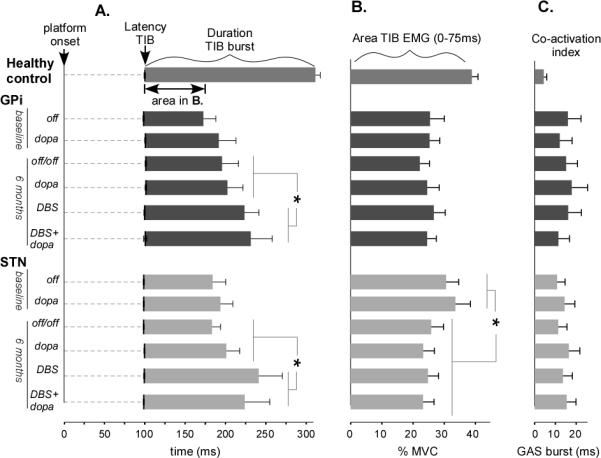
Mean (s.e.m.) EMG response in the six conditions. A. The TIB onset latencies are indicated by the vertical black lines on the time axis, the duration of this burst is shown in the proceeding bar graph. The asterisk (*) indicates a significant lengthening of the TIB burst duration for the DBS states in both the GPi and STN groups. B. The area under the first 75 ms of the TIB burst is significantly reduced for the STN group after surgery. C. The co-activation index is the length of time during the initial 75 ms of the TIB that the GAS was significantly above baseline level and was unaffected by conditions.
At baseline, the duration of the TIB burst was significantly shorter in PD subjects than healthy control subjects (Figure 3A). The duration of the TIB burst did not change between OFF baseline and OFF/OFF at 6 months for either GPi (p=0.64) or STN (p=0.81), despite worsening in the STN group's APR stability score. However, when the DBS was turned ON after surgery, the TIB burst duration increased by 32.5 ms compared to the DBS OFF states after surgery (p=0.03) for both the STN and GPi groups, with no site interaction (p=0.26; Figure 3A).
At baseline, TIB EMG area was significantly smaller in the PD group than the healthy control group (p<.01; Figure 3B). Consistent with poorer stability after surgery in the STN group, TIB area (Fig 3B) was significantly smaller post-surgery compared to pre-surgery (p=0.023), but this was not the case for the GPi group (p= 0.59).
The co-activation index of the antagonist gastrocnemius muscle (GAS) with the TIB burst was significantly larger in PD subjects than control subjects (p<0.01, Figure 3C) but the length of co-activation was not significantly affected by the DBS or DOPA conditions.
DISCUSSION
Maintaining stability following a perturbation, such as a push, slip, or trip, requires a fast postural response of appropriate size and direction. The results of this study showed that turning the DBS ON did improve the response to this type of postural threat relative to the OFF state on the same day, by increasing agonist burst duration for both STN and GPi stimulation. The improvement with DBS was greater than the improvement with medication, suggesting that nondopaminergic pathways used for in-place postural responses are activated by DBS. The data also showed that the APR stability of PD subjects in the best treated state worsened six months after DBS surgery in STN and was not improved nor worsened after GPi surgery. Worsening of APRs after STN surgery was consistent with smaller TIB burst amplitudes after surgery, suggesting exacerbated bradykinetic postural responses 19 not observed in the GPi group. This pattern of effects suggests two independent central processes are affecting postural responses; one related to the DBS procedure in STN and a second related to the stimulating current.
The latency of the postural response was similar in PD and control subjects, as reported previously.11 However, the effectiveness of the postural response in countering backward body movement was reduced in PD, evidenced by smaller amplitude and shorter duration of the agonist activity and increased co-activation of the antagonist. Although APRs are peripherally triggered by proprioceptive stimuli, the processing of the postural responses are centrally organized in the brainstem and scaled by descending signals from the basal ganglia.19 This could explain the fixed gain of postural responses that underlies postural inflexibility in PD.4, 17
Postural reactions are typically not responsive to dopaminergic medications.5, 8, 18 It is generally considered that the PD symptoms that respond to levodopa will also respond to DBS, implying a common mode of action. However, the results here suggest that turning the stimulators on improved the APR more than taking levodopa. The stimulation may be influencing nondopamingeric systems that contribute to sustaining reactive muscle contractions. The notion of an independent action of DBS and levodopa, at least for postural control, is consistent with a report that STN DBS improved voluntary CoM movements and abnormal sensory aspects of postural instability that did not respond to medication.31
The stimulating current did not show an appreciable effect on the size of the short latency (<75ms) response – indicating the central set response is unaffected. However DBS lengthened the burst duration of the agonist muscle resulting in an increased reactive CoP and more effective reversal of the falling CoM. The effect of DBS on mid to late phases of the APR response indicates that longer latency pathways that use sensory feedback are improved and DBS may be acting through descending brainstem connections from both STN and GPi sites to the pedunculopontine nucleus (PPN) and other brainstem postural areas 34. The DBS did not show an appreciable effect on the excessive co-activation of the antagonist muscle however, which is known to be common during PD movements.7, 11, 22
The deficit at 6 months in the STN group cannot be accounted for by a change in levodopa dose, as this did not change significantly pre- to post-surgery between STN and GPi groups (Table 1). Despite the randomization, the STN group did have better APR stability before surgery compared to both the GPi and PD control groups. Because the STN showed similar performance to the GPi group at 6 months the surgery may have had some normalizing effect – with better performers more negatively affected than worse performers. However all STN subjects, regardless of baseline function, were worse after surgery (Fig 2Bii). The consistent responses of the PD control group over 6 months suggest that there is negligible natural decline in postural responses over this time. Another possibility is that the surgical procedure created a microlesion in an area that contributes to the scaling of postural responses. Most surgeons are aware that prior to the initiation of stimulation, surgical tracts may cause microlesioning effects in STN surgery.10 Cardinal PD signs of tremor, rigidity and bradykinesea often show improvements from microlesions 23 still present after 6 months.25 Negative microlesion effects are not unheard of however. A STN microlesion has been suggested to account for reduced verbal fluency 28 and reduced jaw velocity29 tested 6 months after STN DBS surgery. These recent studies showed reduced oromotor function was independent of stimulating current or levodopa and was apparent in STN but not GPi surgery. Further testing is required to establish whether a microlesion is responsible for the reduced automatic postural responses in subjects with STN DBS shown in the present study. This could be investigated by testing automatic postural responses after implantation surgery but before stimulation is turned on.
A recent study showed the microlesion effect applies to the STN site but not the GPi.25 Perhaps the smaller STN (158 mm3) compared to the larger GPi (458 mm3)33 renders this structure more susceptible to a lesion effect. Or perhaps lesions in the GPi have a beneficial effect on postural responses of some patients, as studies of pallidotomy suggest (Melnick, 1999). Another explanation is that there is damage caused to different areas of the brain as the points of entry and trajectories of the electrodes differ between STN and GPi surgery. Recent work shows that the microlesion effect in STN is correlated with the number of microelectrode tracks, suggesting that the effect depends on tissue changes along the entirety of surgical trajectories. 24
The post-operative deficit in the STN group could be specific to the surgical procedure used at this center. However, the fact that pooled data across seven different centers in the VA/NIND multi-center clinical trial showed a significant worsening of UPDRS III scores in STN compared to GPi sites in the without stimulation/without medication condition post-surgery,13 suggests the STN surgical effect may be more generalizable. A break-down of the UPDRS scores in this larger study would determine whether this effect was driven by a worsening of PIGD components of the scale.
The STN deficit may relate to the off and on timing of the stimulation, and this is acknowledged as a limitation of the experimental design. The time it takes to alter particular signs after turning DBS on and off varies.3 Tremor is affected almost instantly, followed by rigidity and bradykinesia, whereas maximal locomotion and balance changes are delayed for a few hours.35 As testing in the post surgery OFF/OFF state was performed approximately 60 minutes after DBS was turned off, some residual benefit of the stimulation may be expected. However, the residual effect would be acting to improve the APR, which was the opposite of the direction recorded.
Although the results of this study showed that the GPi site had fewer deleterious effects on in-place automatic postural responses than the STN site, the clinical decision about the surgical site should be made in the context of all PD symptoms, medication tolerance, and the concerns and expectations of the patient. The DBS effects seen in this experiment may not be even be generalized to other aspects of balance control, as balance is the outcome of a number of different processes that may be differentially affected by the procedure. Other DBS targets, such as in the pedunculopontine nucleus in the brainstem also show some promise for improving balance and gait function in PD (Stefani, 2007; Ferraye, 2010). Further controlled, randomized studies are required to determine the optimal risk-benefit ratio of each DBS target site for treating particular PD symptoms.
In the healthy control subjects the APR stability was slightly higher for the first set of trials compared to the following three sets of trials. This effect is probably through learning to optimize their response.16 A similar session effect is possible in the PD group however it may be less likely because PD subjects have less capacity for motor learning than control subjects 27 and the PD response was below the optimal response initially. If there was a similar practice effect occurring in the PD-DBS group, there would have been an over estimation in the baseline OFF and 6 month DBS stability scores, as these were always tested first. Such a practice effect would not affect the main finding that DBS improved APR stability as the other condition with DBS (DBS+DOPA) was the last condition tested and also showed significant improvement.
Despite the stimulating current improving the size of the postural response, postural responses were worse in the STN group in the best medical therapy state after the procedure (DBS+DOPA) compared to the best medical therapy state before the procedure (DOPA) whereas there was no change in the GPi group. These different postural control outcomes in the daily functional state between STN and GPi DBS sites may be reflected by adverse events of large-population studies. Rodriguez-Oroz et al. report significantly more falls, balance disturbances and gait disorders in an STN group (n=79) after DBS compared to a GPi group (n=20).30 Similarly, the randomized VA/NINDS multi-center study reports significantly more serious falls 24 months after DBS surgery in the STN (n=147) compared to GPi (n=152).13 The reason for the increased falls after DBS is unknown, the worsening postural responses after surgery shown in this study may be one explanation.
Conclusions
APR stability in PD subjects improved with both STN and GPi DBS through more sustained agonist contractions compared to conditions with the DBS turned off and with medication. This suggests DBS can affect non-dopaminergic pathways that are involved in postural stabilization. However, APR stability did not improve in PD subjects when comparing the best-treated state before, and 6 months after, the DBS procedure. In fact, the STN group was worse after the surgery due to a worsening in postural bradykinesia. This result may account for the increased number of falls reported in PD subjects with DBS in STN.
ACKNOWLEDGEMENTS
We acknowledge the help of Triana Nagel for subject scheduling & data collection; Marilee Stephens and Lesley Silar for data collection, Tara Phillips for data processing and Dr Ali Samii for referring patients for study from the Seattle location.
Funding: This research was supported by the National Institute on Aging grants AG19706 and AG006457. The Parkinson's Alliance contributed to author RStG's salary.
Footnotes
Portions of this work were presented in poster form at the Society of Neuroscience meeting, Chicago, USA, October 19 2009.
DISCLOSURE
Author K. Burchiel has received an educational grant from and holds stock in Medtronic Inc.
REFERENCES
- 1.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP, Anderson VC, et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Archives of Neurology. 2005;62:554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 2.Bakker M, Esselink RA, Munneke M, Limousin-Dowsey P, Speelman HD, Bloem BR. Effects of stereotactic neurosurgery on postural instability and gait in Parkinson's disease. Movement Disorders. 2004;19:1092–1099. doi: 10.1002/mds.20116. [DOI] [PubMed] [Google Scholar]
- 3.Beuter A, Modolo J. Delayed and lasting effects of deep brain stimulation on locomotion in Parkinson's disease. Chaos. 2009;19 doi: 10.1063/1.3127585. [DOI] [PubMed] [Google Scholar]
- 4.Bloem BR, Beckley DJ, Remler MP, Roos RA, van Dijk JG. Postural reflexes in Parkinson's disease during ‘resist’ and ‘yield’ tasks. Journal of the Neurological Sciences. 1995;129:109–119. doi: 10.1016/0022-510x(94)00253-k. [DOI] [PubMed] [Google Scholar]
- 5.Bloem BR, Beckley DJ, van Dijk JG, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease. Movement Disorders. 1996;11:509–521. doi: 10.1002/mds.870110506. [DOI] [PubMed] [Google Scholar]
- 6.Burchiel K, Anderson V, Favre J, Hammerstad F. Comparison of pallidal and subthalamic nucleus deep brain stimulation for advanced Parkinson's disease: results of a randomized, blinded pilot study. Neurosurgery. 1999;45:1375–1384. doi: 10.1097/00006123-199912000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Burleigh A, Horak F, Nutt J, Frank J. Levodopa reduces muscle tone and lower extremity tremor in Parkinson's disease. Canadian Journal of Neurological Sciences. 1995;22:280–285. doi: 10.1017/s0317167100039470. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter MG, Allum JH, Honegger F, Adkin AL, Bloem BR, Allum JHJ. Postural abnormalities to multidirectional stance perturbations in Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:1245–1254. doi: 10.1136/jnnp.2003.021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colnat-Coulbois S, Gauchard GC, Maillard L, Barroche G, Vespignani H, Auque J, et al. Bilateral subthalamic nucleus stimulation improves balance control in Parkinson's disease.[see comment]. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:780–787. doi: 10.1136/jnnp.2004.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deuschl G, Herzog J, Kleiner-Fisman G, Kubu C, Lozano AM, Lyons KE, et al. Deep brain stimulation: postoperative issues. Movement Disorders. 2006;21(Suppl 14):S219–237. doi: 10.1002/mds.20957. [DOI] [PubMed] [Google Scholar]
- 11.Dimitrova D, Horak FB, Nutt JG, Dimitrova D, Horak FB, Nutt JG. Postural muscle responses to multidirectional translations in patients with Parkinson's disease. Journal of Neurophysiology. 2004;91:489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S, Elton R. Unified Parkinson's disease rating scale. Macmillan Healthcare Information; Florham Park (NJ): 1987. [Google Scholar]
- 13.Follett K, Weaver F, Stern M, Hur K, Harris C, Luo P, et al. Pallidal versus Subthalamic Deep-Brain Stimulation for Parkinson's Disease. The New England Journal of Medicine. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 14.Hilliard MJ, Martinez KM, Janssen I, Edwards B, Mille ML, Zhang Y, et al. Lateral balance factors predict future falls in community-living older adults. Archives of Physical Medicine & Rehabilitation. 2008;89:1708–1713. doi: 10.1016/j.apmr.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 16.Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. Journal of Neurophysiology. 1989;62:841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- 17.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. Journal of the Neurological Sciences. 1992;111:46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 18.Horak FB, Frank JS, Nutt J. Effects of dopamine on postural control in Parkinsonian subjects: scaling, set and tone. Journal of Neurophysiology. 1996;75:2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 19.Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Smith JL, editor. Handbook of Physiology: Section 12: Exercise: Regulation and Integration of Multiple Systems. Oxford University Press; New York: 1996. pp. 255–292. [Google Scholar]
- 20.Horak FB, Dimitrova D, Nutt JG, Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson's disease. Experimental Neurology. 2005;193:504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. New England Journal of Medicine. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 22.Mak MK, Wong EC, Hui-Chan CW, Mak MKY, Wong ECY, Hui-Chan CWY. Quantitative measurement of trunk rigidity in parkinsonian patients. Journal of Neurology. 2007;254:202–209. doi: 10.1007/s00415-006-0327-4. [DOI] [PubMed] [Google Scholar]
- 23.Maltete D, Derrey S, Chastan N, Debono B, Gerardin E, Freger P, et al. Microsubthalamotomy: an immediate predictor of long-term subthalamic stimulation efficacy in Parkinson disease. Movement Disorders. 2008;23:1047–1050. doi: 10.1002/mds.22054. [DOI] [PubMed] [Google Scholar]
- 24.Maltete D, Chastan N, Derrey S, Debono B, Gerardin E, Lefaucheur R, et al. Microsubthalamotomy effect at day 3: screening for determinants. Movement Disorders. 2009;24:286–289. doi: 10.1002/mds.22380. [DOI] [PubMed] [Google Scholar]
- 25.Mann JM, Foote KD, Garvan CW, Fernandez HH, Jacobson CEt, Rodriguez RL, et al. Brain penetration effects of microelectrodes and DBS leads in STN or GPi. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80:794–797. doi: 10.1136/jnnp.2008.159558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer C, Mergner T, Xie J, Faist M, Pollak P, Lucking CH. Effect of chronic bilateral subthalamic nucleus (STN) stimulation on postural control in Parkinson's disease. Brain. 2003;126:1146–1163. doi: 10.1093/brain/awg100. [DOI] [PubMed] [Google Scholar]
- 27.Nieuwboer A, Rochester L, Muncks L, Swinnen SP, Nieuwboer A, Rochester L, et al. Motor learning in Parkinson's disease: limitations and potential for rehabilitation. Parkinsonism & Related Disorders. 2009;15(Suppl 3):S53–58. doi: 10.1016/S1353-8020(09)70781-3. [DOI] [PubMed] [Google Scholar]
- 28.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Annals of Neurology. 2009;65:586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson L, George RS, Carlson-Kuhta P, Hogarth P, Burchiel K, Horak F. Site of Deep Brain Stimulation Affects Jaw Velocity in Parkinson's Disease. Journal of Neurosurgery. 2011 doi: 10.3171/2011.7.JNS102173. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 31.Shivitz N, Koop MM, Fahimi J, Heit G, Bronte-Stewart HM, Shivitz N, et al. Bilateral subthalamic nucleus deep brain stimulation improves certain aspects of postural control in Parkinson's disease, whereas medication does not. Movement Disorders. 2006;21:1088–1097. doi: 10.1002/mds.20905. [DOI] [PubMed] [Google Scholar]
- 32.St George R, Nutt J, Burchiel K, Horak F. A Meta-analysis of the long-term effects of Deep Brain Stimulation on Balance and Gait in PD. Neurology. 2010;75:1292–1299. doi: 10.1212/WNL.0b013e3181f61329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudhyadhom A, Bova FJ, Foote KD, Rosado CA, Kirsch-Darrow L, Okun MS, et al. Limbic, associative, and motor territories within the targets for deep brain stimulation: potential clinical implications. Current Neurology & Neuroscience Reports. 2007;7:278–289. doi: 10.1007/s11910-007-0043-1. [DOI] [PubMed] [Google Scholar]
- 34.Takakusaki K, Tomita N, Yano M, Takakusaki K, Tomita N, Yano M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. Journal of Neurology. 2008;255(Suppl 4):19–29. doi: 10.1007/s00415-008-4004-7. [DOI] [PubMed] [Google Scholar]
- 35.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ, et al. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan CL, Andrews JG, Hay JG. Selection of body segment parameters by optimization methods. Journal of Biomedical Engineering (Guildford) 1982;104:38–44. doi: 10.1115/1.3138301. [DOI] [PubMed] [Google Scholar]
- 37.Visser JE, Allum JH, Carpenter MG, Esselink RA, Speelman JD, Borm GF, et al. Subthalamic nucleus stimulation and levodopa-resistant postural instability in Parkinson's disease. Journal of Neurology. 2008;255:205–210. doi: 10.1007/s00415-008-0636-x. [DOI] [PubMed] [Google Scholar]
- 38.Weaver F, Follett K, Stern M, Hur K, Harris C, Marks W, et al. Bilateral Deep Brain Stimulation vs best medical therapy for patients with advanced Parkinon Disease. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West B, Welch K, Galecki A. Linear mixed models: a practical guide using statistical software. Taylor & Francis Group; Boca Raton: 2007. [Google Scholar]
- 40.Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. Journal of Neurophysiology. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]


