Abstract
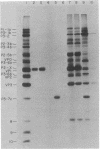
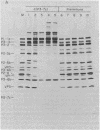
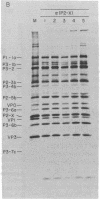
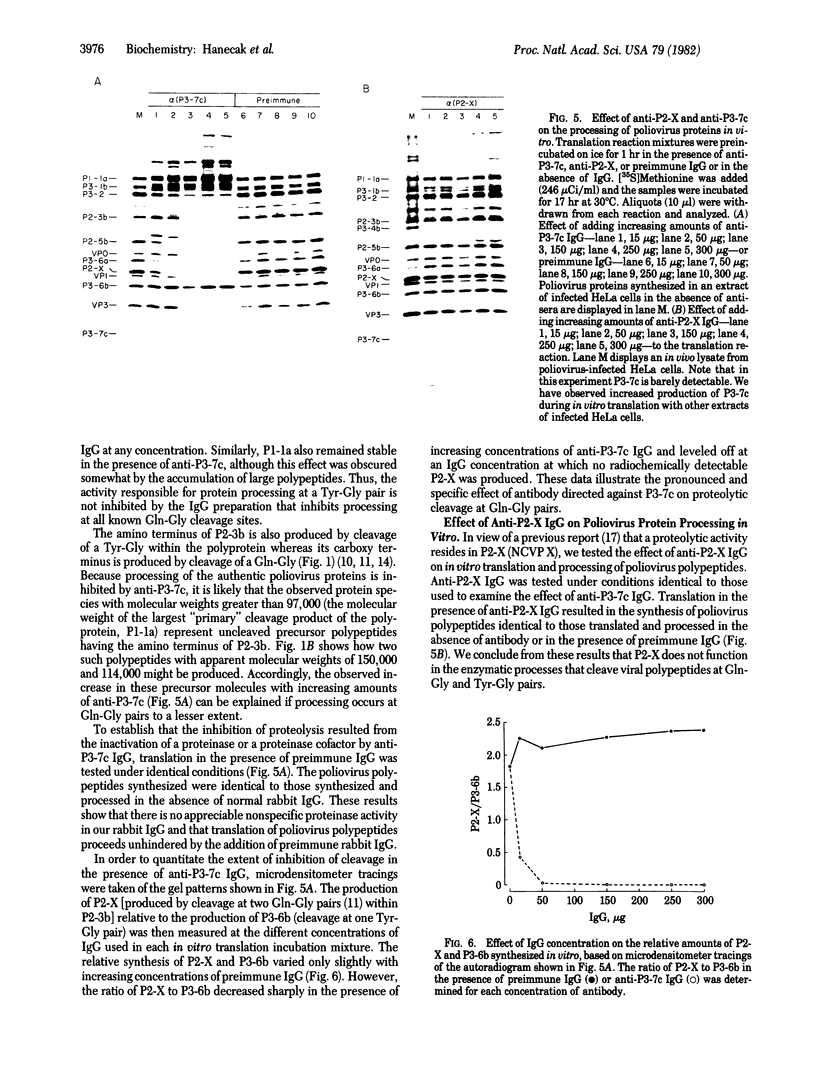
Proteolytic processing of poliovirus polypeptides was examined by the addition of antibodies directed against the viral proteins P3-7c and P2-X to a cell-free translation extract prepared from infected HeLa cells. Antisera to P3-7c specifically inhibited in vitro processing at Gln-Gly pairs. Partial amino acid sequence analysis revealed a second Tyr-Gly pair that is utilized in protein processing. Neither Tyr-Gly cleavage is affected by antibody to P3-7c. Anti-P3-7c antibodies react not only with P3-7c but also with P3-6a and P3-2, two viral polypeptides NH2-coterminal with P3-7c. Preimmune and anti-P2-X antibodies had no effect on the processing of poliovirus proteins in vitro. We conclude that the activity responsible for processing poliovirus polypeptides at Gln-Gly pairs resides in the primary structure of P3-7c (or P3-2 and P3-6a) and not in P2-X.
Full text
PDF
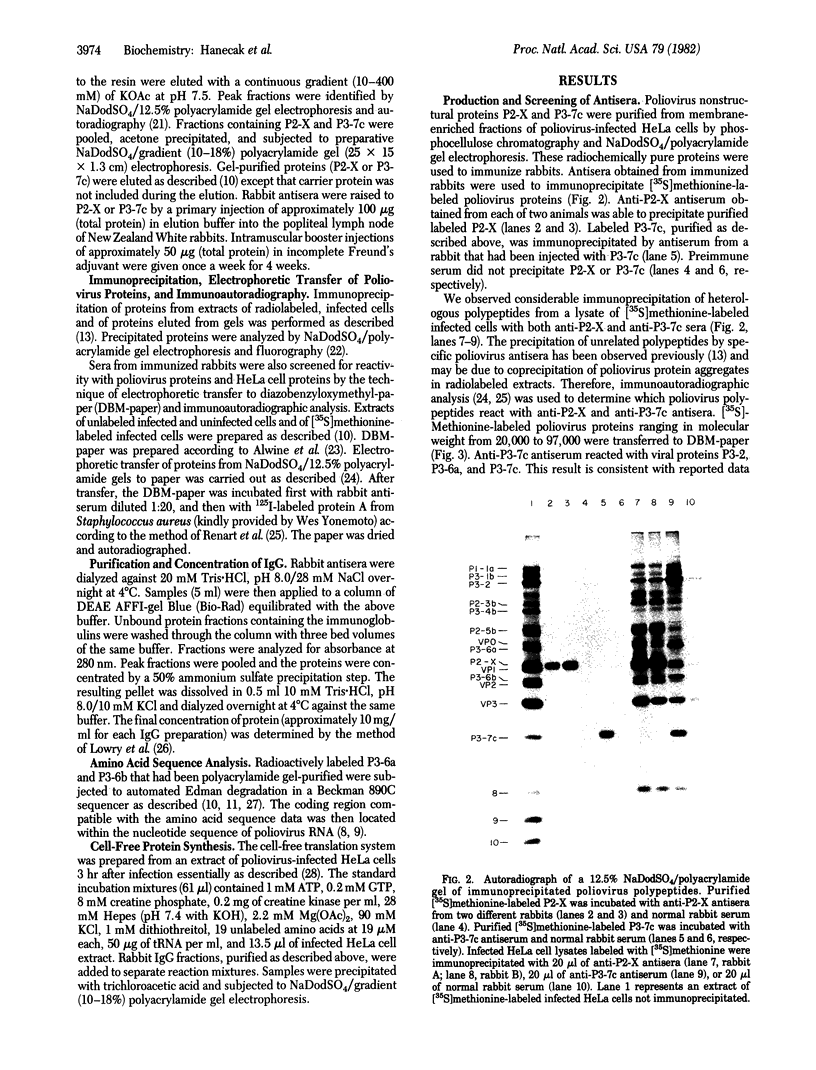


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B. Amino-terminal sequence of adenovirus type 2 proteins: hexon, fiber, component IX, and early protein 1B-15K. Virology. 1980 Jul 15;104(1):27–41. doi: 10.1016/0042-6822(80)90363-3. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Ehrenfeld E. Translation of poliovirus RNA in vitro: detection of two different initiation sites. J Mol Biol. 1975 Nov 15;98(4):761–780. doi: 10.1016/s0022-2836(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Rothberg P. G., Wimmer E. The fate of VPg during in vitro translation of poliovirus RNA. FEBS Lett. 1981 Sep 28;132(2):219–223. doi: 10.1016/0014-5793(81)81164-7. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Elzinga M., Wimmer E. Carboxy-terminal analysis of poliovirus proteins: termination of poliovirus RNA translation and location of unique poliovirus polyprotein cleavage sites. J Virol. 1982 Apr;42(1):194–199. doi: 10.1128/jvi.42.1.194-199.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Svitkin Y. V., Kazachkov Y. A., Agol V. I. Encephalomyocarditis virus-specific polypeptide p22 is involved in the processing of the viral precursor polypeptides. FEBS Lett. 1979 Dec 1;108(1):1–5. doi: 10.1016/0014-5793(79)81164-3. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Adler C., Wimmer E. Structure and expression of the picornavirus genome. Ann N Y Acad Sci. 1980;354:183–201. doi: 10.1111/j.1749-6632.1980.tb27967.x. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Korant B., Chow N., Lively M., Powers J. Virus-specified protease in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2992–2995. doi: 10.1073/pnas.76.6.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen G. R., Anderson C. W., Dorner A. J., Semler B. L., Wimmer E. Cleavage sites within the poliovirus capsid protein precursors. J Virol. 1982 Jan;41(1):340–344. doi: 10.1128/jvi.41.1.340-344.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Kitamura N., Golini F., Wimmer E. The 5'-terminal structures of poliovirion RNA and poliovirus mRNA differ only in the genome-linked protein VPg. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5345–5349. doi: 10.1073/pnas.74.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Rueckert R. R. Evidence for intramolecular self-cleavage of picornaviral replicase precursors. J Virol. 1982 Jan;41(1):244–249. doi: 10.1128/jvi.41.1.244-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Flanegan J. B., Rose J. K., Baltimore D. 5'-Terminal nucleotide sequences of polio virus polyribosomal RNA and virion RNA are identical. Nature. 1977 Jul 21;268(5617):270–272. doi: 10.1038/268270a0. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Hanecak R., Dorner L. F., Wimmer E. A membrane-associated precursor to poliovirus VPg identified by immunoprecipitation with antibodies directed against a synthetic heptapeptide. Cell. 1982 Feb;28(2):405–412. doi: 10.1016/0092-8674(82)90358-0. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Kitamura N., Rothberg P. G., Wishart W. L., Wimmer E. Poliovirus replication proteins: RNA sequence encoding P3-1b and the sites of proteolytic processing. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3464–3468. doi: 10.1073/pnas.78.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Anderson C. W., Wimmer E. Cleavage sites in the polypeptide precursors of poliovirus protein P2-X. Virology. 1981 Oct 30;114(2):589–594. doi: 10.1016/0042-6822(81)90242-7. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Kew O., Pallansch M., Rueckert R., Kaesberg P. Cell-free synthesis and processing of the proteins of poliovirus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5807–5811. doi: 10.1073/pnas.75.12.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. IV. Amino- and carboxyl-terminal analyses of the major capsid polypeptides. Virology. 1976 May;71(1):111–121. doi: 10.1016/0042-6822(76)90098-2. [DOI] [PubMed] [Google Scholar]