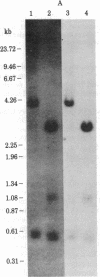
Abstract
Lactoprotein clones have been isolated from a rat mammary gland recombinant library of cDNA plasmids. Clones p-Wp 52 and p-Wp 47 were shown by hybrid selection, in vitro translation, and immunoprecipitation to represent a cloned DNA sequence encoding rat whey phosphoprotein. We report here the nucleotide sequence of the cDNA insert of p-Wp 52 and shows that it encodes the complete whey phosphoprotein sequence. The encoded sequence shows a high content of half-cystine, glutamic acid, aspartic acid, and serine but an absence of tyrosine. The half-cystines appear in unique arrangements and are repeated in two domains of the protein. The second domain has striking similarities with the second domain of the red sea turtle protease inhibitor. Clone p-Wp 52 has allowed the study of expression of whey phosphoprotein mRNA during functional differentiation of rat mammary gland and in mammary tumors. The whey phosphoprotein mRNA is detected during midpregnancy and lactation in the rat mammary gland but is barely detected in mammary tumors in which other milk protein mRNAs are expressed. The whey phosphoprotein gene in these tumors is hypermethylated, correlating with the reduced expression of this gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabartty P. K., Qasba P. K. Partial purification of rat alpha-lactalbumin mRNA. Nucleic Acids Res. 1977 Jun;4(6):2065–2074. doi: 10.1093/nar/4.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar A. M., Qasba P. K. Rat alpha-lactalbumin has a 17-residue-long COOH-terminal hydrophobic extension as judged by sequence analysis of the cDNA clones. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4853–4857. doi: 10.1073/pnas.78.8.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J. The structure of neurophysin. J Biol Chem. 1981 Mar 25;256(6):2601–2602. [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Losonczy I., Berghoffer B. Mammary tumor regression. I. Physiopathologic characteristics of hormone-dependent tissue. J Natl Cancer Inst. 1972 Nov;49(5):1333–1348. [PubMed] [Google Scholar]
- Henriksen O., Robinson E. A., Appella E. Purification and chemical characterization of papain-solubilized histocompatibility-2 antigens from mouse liver. J Biol Chem. 1979 Aug 25;254(16):7651–7658. [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie R. M., Larson B. L. Isolation and partial characterization of rate casein proteins. J Dairy Sci. 1978 Jul;61(7):885–889. doi: 10.3168/jds.s0022-0302(78)83666-2. [DOI] [PubMed] [Google Scholar]
- McKenzie R. M., Larson B. L. Purification and partial characterization of a unique group of phosphoproteins from rat milk whey. J Dairy Sci. 1978 Jun;61(6):723–728. doi: 10.3168/jds.S0022-0302(78)83639-X. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Haze G., Gaye P., Hue D. Amino terminal sequence of the precursor of ovine beta-lactoglobulin. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1236–1245. doi: 10.1016/0006-291x(78)90320-0. [DOI] [PubMed] [Google Scholar]
- Nicholas K. R., Topper Y. J. Enhancement of alpha-lactabumin-like activity in mammary explants from pregnant rats in the absence of exogenous prolactin. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1424–1431. doi: 10.1016/0006-291x(80)90578-1. [DOI] [PubMed] [Google Scholar]
- Prasad R., Hudson B. G., Butkowski R., Hamilton J. W., Ebner K. E. Resolution of the charge forms and amino acid sequence and location of a tryptic glycopeptide in rat alpha-lactalbumin. J Biol Chem. 1979 Nov 10;254(21):10607–10614. [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Qasba P. K., Chakrabartty P. K. Purification and properties of two forms of rat alpha-lactalbumin. J Biol Chem. 1978 Feb 25;253(4):1167–1173. [PubMed] [Google Scholar]
- Qasba P. K., Gullino P. M. alpha-Lactalbumin content of rat mammary carcinomas and the effect of pituitary stimulation. Cancer Res. 1977 Oct;37(10):3792–3795. [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Richards D. A., Blackburn D. E., Rosen J. M. Restriction enzyme mapping and heteroduplex analysis of the rat milk protein cDNA clones. J Biol Chem. 1981 Jan 10;256(1):533–538. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]