Abstract
Coronary restenosis, a major complication of percutaneous balloon angioplasty, results from neointimal proliferation of vascular smooth muscle cells (VSMCs). The sarco/endoplasmic reticulum calcium ATPase isoform 2a (SERCA2a), specific to contractile VSMCs, has been reported previously to be involved in the control of the Ca2+-signaling pathways governing proliferation and migration. Moreover, SERCA2a gene transfer was reported to inhibit in vitro VSMC proliferation and to prevent neointimal thickening in a rat carotid injury model.
The aim of this study was to evaluate the potential therapeutic interest of SERCA2a gene transfer for prevention of in-stent restenosis using a human ex vivo model of left internal mammary artery (hIMA) intimal thickening.
Left hIMAs, obtained at the time of aorto-coronary bypass surgeries, were subjected to balloon dilatation followed by infection for 30 min with adenoviruses encoding either human SERCA2 and GFP or control gene (beta-galactosidase) and GFP. Proliferation of subendothelial VSMCs and neointimal thickening were observed in balloon-injured hIMA maintained 14 days in organ culture under constant pressure and perfusion. SERCA2a gene transfer prevented vascular remodeling and significantly (p<0.01, n=5) reduced neointimal thickening in injured arteries (intima/media ratio was 0.07 ± 0.01 vs 0.40 ± 0.03 in βGal-infected arteries).
These findings could have potential implications for treatment of pathological in stent-restenosis.
Keywords: Restenosis, SERCA2a, Gene Transfer, Internal Mammary Artery
INTRODUCTION
Atherosclerosis and related cardiovascular accidents are the number one causes of mortality in the world (World Health Organization, 2008). These acute events are mainly caused by a build up of low-density lipoproteins (LDL) in the arterial wall of large and medium sized arteries, resulting in chronic inflammation and possibly blockage, interrupting blood flow to the heart, brain, and/or lower extremities. When atherosclerosis is symptomatic, the principal technique used for restoring blood flow is percutaneous transluminal coronary angioplasty (PCTA), known as balloon angioplasty, followed in more than 80% of cases by the implantation of stents. Nevertheless, post-interventional in-stent restenosis, the re-narrowing of the arteries caused by vascular smooth muscle cell (VSMCs) proliferation, occurs in 10 to 20 % of patients. Attempts at reducing this relapse, by placement of drug eluting stents (DES) delivering cell cycle inhibitors such as rapamycin to prevent intimal hyperplasia, have been plagued by side effects 1. The use of DES significantly reduces restenosis but impairs the re-endothelialization process and subsequently often induces late thrombosis 1,2. Trans-differentiation of contractile VSMCs towards a synthetic/proliferating inflammatory/migratory phenotype after PTCA in humans appears to be a fundamental process of vascular healing 3. Such phenotypic redifferentiation of neointimal VSMC after bare metal stent implantation was reported to be associated with a decline in platelet activation and inflammatory cell infiltration, and the regeneration of endothelial cell layer 4. Thus defining novel molecular target(s) of DESs that can simultaneously prevent VSMC proliferation and adverse vascular remodeling while facilitating re-endothelialization is necessary.
The primary function of VSMCs in the arteries is to maintain vascular tone. VSMCs in the media of normal human arteries exhibit a quiescent/contractile phenotype 5. We and others have pointed out the presence of undifferentiated (synthetic) VSMCs exhibiting a proliferating/migratory/inflammatory phenotype in the subendothelial intima of normal human coronary arteries 5,6. These subendothelial VSMCs were supposed to be the source of neointimal proliferation during physiological or pathological vascular remodeling 6. Of note, subendothelial VSMCs are not the only source of neointimal growth; trans-differentiation of medial VSMCs immediately adjacent to the site of injury was reported as soon as 2 days after PTCA 3. Similarly, in animal models of vascular remodeling, contractile VSMCs were shown to undergo trans-differentiation towards a synthetic phenotype 7-9. Trans-differentiation of VSMCs is characterized by the down-regulation of proteins regulating the excitation-contraction coupling process. These proteins include the L-type Ca2+ channels 10,11, the sarco(endo)plasmic reticulum (SR/ER) Ca2+ channels, the ryanodine receptor 2 (RyR2), and the SR/ER calcium ATPase 2a isoform (SERCA2a) 5,7,8. The disappearance of SERCA2a translates into the activation of the Ca2+-regulated calcineurin/NFAT (nuclear factor of activated T lymphocytes) transcription pathway, governing proliferation and migration in VSMCs 5,7,8. Indeed, we and others showed that i) blockade of NFAT signaling suppresses balloon injury-induced neo-intima formation in a rat carotid artery model; ii) a synthetic peptide inhibitor of NFAT has anti-restenotic potential; iii) SERCA2a gene transfer reduces rat vascular VSMC proliferation and neo-intima formation 7,12,13. Thus, SERCA2a gene transfer is potentially promising for prevention of post-angioplasty remodeling and restenosis.
Most anti-proliferative strategies working in animal models turn out to be inefficient in the clinic, highlighting the shortcomings of conventional animal models of arterial intervention and the gap in our knowledge of human disease 14-16. Our model of ex vivo human mammary artery organ culture could be useful to test any potentially promising therapeutic agents before initiating pre-clinical large animal trials or clinical trials. First described by Holt et al., (1992) 17, human internal mammary artery (hIMA) organ culture of endothelial denuded arteries reproduces the early wall changes seen in a stented vessels; furthermore, it responds to local delivery of anti-proliferative agents, as coronary vessels do in vivo 18. More recently, Guerin et al., (2005) successfully used this ex vivo hIMA organ culture model to characterize the molecular mechanism through which rapamycin reduces in-stent restenosis 19. Also significantly, hIMA, which is a potential clinical target of human gene therapy since it is used in aorto-coronary bypass graft, is amenable to gene transfer 6. Considering the advantages of hIMA organ culture mentioned above, we chose this model, improved it by perfusing the vessels intraluminally rather than opening them and pinning them to onto a dish, and demonstrate, for the first time, the relevance of considering SERCA2a gene transfer as a possible therapy of post-angioplasty restenosis in human arteries.
RESULTS
1. Structure of human coronary and mammary arteries
A difference between rat carotid artery and normal human coronary vessels is the presence of distinct subendothelial intima that consists of several layers of VSMCs and extracellular matrix 5,6.
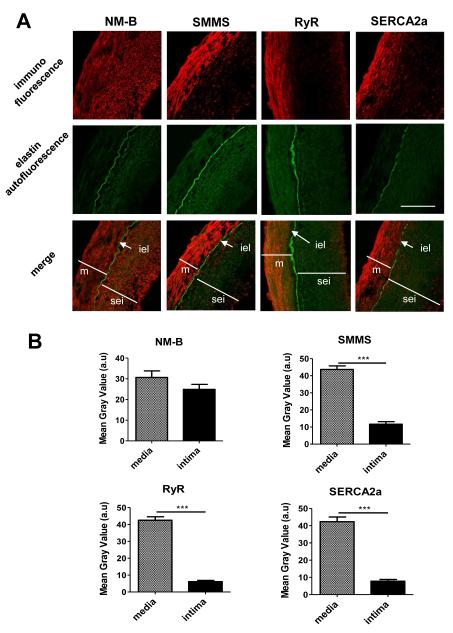
First, we examined the phenotype of VSMCs in healthy segments of human coronary arteries (CA) and hIMA. The media was visualized by the presence of elastin green autofluorescence and the endothelial cell (EC) layer by immunofluorescence with CD31. The space between the EC layer and the internal elastic lamina was defined as subendothelial intima. As emphasized in magnification and as expected 5,6, cross sections of CA and hIMA revealed a highly convoluted internal elastic lamina (iel) covered by an intimal layer of endothelial cells and several layers of sub-endothelial smooth muscle cells (5 to 10 layers in the coronary arteries (Fig. 1) and 1 to 3 layers in hIMA (Fig. 2)). Confocal immunofluorescence revealed that the contractile VSMC were localized in the medial layer, as attested by the presence of the smooth muscle MHC (SMMS), Ryanodine Receptor (RyR) and SERCA2a proteins (Fig. 1). By contrast, VSMCs from the sub-endothelial intima (sei) possessed the synthetic/proliferating phenotype characterized by the absence of SMMS, RyR and SERCA2a proteins (Fig 1). Non-muscular myosin (NM-B), a marker of both synthetic and contractile VSMCs, was expressed in medial and subendothelial VSMCs (Fig 1&2). Interestingly, the structure of the vessel wall was not preserved in injured vessels cultured 2 weeks without perfusion (not shown), highlighting the importance of luminal pressure and perfusion for maintenance of normal vessels physiology.
Figure 1. Healthy human coronary arteries are characterized by contractile VSMCs in the media and synthetic VSMCs in the sub-endothelial intima.
A. Confocal immunofluorescence (red) with indicated antibodies of human CA cross sections. Green - elastin autofluorescence. Abbreviations: NM-B - non muscular myosin B; SMMS - smooth muscle myosin heavy chain 1 and 2; RyR – ryanodine receptor; SERCA2a sarco/endoplasmic reticulum calcium ATPase isoform 2a; m - media; sei - subendothelial intima; iel - internal elastic lamina. Bar - 50 μm. B. Bar-graph representing the mean gray values (a.u.) of immunofluorescence in a media or an intima of CA cross sections. CA from 3 patients were analyzed, three independent sections from each patient were measured.
Figure 2. The structure of healthy human left IMA differs from that of the rat carotid artery.
A. VSMCs are detected in the sub-endothelial space of hIMA. B. Endothelial cells are directly apposed to the internal elastic lamina in the rat carotid artery, with no intercalated VSMCs. Confocal immunofluorescence (red) with indicated antibodies. Green - elastin autofluorescence. Abbreviations: CD31 - a marker of endothelial cells; m – media; iel-internal elastic lamina, SSMC - subendothelial smooth muscle cells; EC – endothelial cells. Bar – 50 μm (A) and 20 μm (B). The fourth panel is an enlarged section of the third panel indicated by the white square.
In human coronary and mammary arteries, the subendothelial VSMCs were suggested to be the most likely source of intimal growth in atherosclerosis, restenosis, and bypass graft intimal hyperplasia 20,21. Nonetheless, the classical in vivo animal model widely used to reproduce in-stent restenosis is the rat carotid injury model. However, the structure of the rat carotid artery differs from that of the human coronary artery by the absence of a subendothelial intima containing undifferentiated synthetic/proliferating VSMCs lying between endothelial cells and the internal elastic lamina. Indeed, as demonstrated on Fig 2B, in the rat carotid artery the endothelial cells (EC), indicated by immunolabeling with CD31, are seated directly on the internal elastic lamina. Moreover, all VSMCs in the media of the normal rat carotid arteries possess a contractile/differentiated phenotype (Fig. 1S). In this model, in vivo balloon injury destroys the EC layer, inducing trans-differentiation of medial VSMCs into a synthetic phenotype (Fig 1S). Vascular healing in rat carotid arteries is associated with proliferation and migration of synthetic VSMCs in both the media and the neointima, leading to an increase in the thickness of both VSMC layers and resulting in a global increase in vessel wall thickness and media/lumen area one month after injury (Fig 1S).
2. Post-injury vascular remodeling in an organ culture model of human internal mammary artery (hIMA)
To test the permanently perfused organ culture of balloon-injured hIMA as a possible human model of restenosis, we first evidenced the impact of an angioplasty on vascular remodeling and VSMC proliferation. To do so, one half of each artery was injured using an inflated PTCA catheter, while the other half remained undisturbed. Both segments were cultured for 2 weeks under permanent perfusion (see materials and methods section for detailed procedure). Of note: in this model tissue viability can be maintained for at least 14 days 17 and as long as 28 days 19. The media and neo-intima were delimited by elastin auto-fluorescence; the efficiency of the balloon angioplasty was attested by the destruction of the endothelial barrier, identified using an anti-CD31 antibody. Two weeks after injury, only small groups of CD31-positive cells were observed right above the internal elastic lamina within the injured arteries, whereas in non-injured arteries the endothelial barrier was intact (Fig. 3). As further evidenced in Fig. 3, permanently perfused injured arteries displayed a clear intimal thickening compared to the control (non-injured) counterpart segments, visualized by elastin autofluorescence and NM-B immunolabeling. In the vessel shown (Fig. 3), the intima/media area ratio in injured segment was as high as 0.81±0.09 compared with 0.21±0.01 in the non-injured contra segment (p<0.05, n=10) and with 0.09±0.01 in native non cultured contra segment (p<0.05 vs injured; p=ns vs non injured). Immunolabeling with anti-SMMS, a marker of contractile phenotype, demonstrated that at 2 weeks organ culture SMMS staining was significantly lower in medial VSMCs of the injured segment compared to that in the non-injured segment (Fig. 3c), suggesting that in human arteries, similarly to rat arteries, medial VSMCs undergo trans-differentiation after injury. Neointimal thickening in this model may very well depend, at least in part, on proliferation of synthetic subendothelial VSMCs, as suggested previously 20,21.
Figure 3. Vascular remodeling and neointimal proliferation after 2 weeks of hIMA organ culture.
A. Endothelial cells (CD31) are mostly absent from injured arteries, compared with non-injured vessels. Similarly, smooth muscle myosin heavy chain 1 and 2 (SMMS) expression is reduced in the media after injury, whereas non muscular myosin B (NM-B) is distributed equally in the media and neointima of all arteries, injured or not. Confocal immunofluorescence (red) with indicated antibodies; the media was identified by elastin autofluorescence (green). Abbreviations: m – media, ni- neointima, iel- internal elastic lamina. Bar – 50 μM. The second panel is an enlarged section of the first panel indicated by the white square. B. Bar graph representing the intima/media area ratio in control non cultured segment and in segments from the same artery, injured and non-injured, cultured for 2 weeks. At least 10 measurements were performed for each segment on independent cross sections (over 0.5 cm of length). ***p<0.001 C. Bar graph representing the mean gray values (a.u.) of red immunofluorescence in the media and intima of injured and non-injured hIMA from the same vessel after 2 weeks of organ culture. All microscope settings were kept constant to allow comparison. Three independent sections were quantified; three measurements were done for each section. ***p<0.001; *p<0.05.
3. SERCA2a gene transfer inhibits proliferation of hIMA VSMCs
We have previously demonstrated that SERCA2a gene transfer prevents proliferation of human synthetic CA VSMCs via inhibition of calcium-sensitive transcription factor NFAT 5. Now we analyzed the effects of SERCA2a gene transfer on the synthetic VSMCs obtained from hIMA. VSMCs were isolated from media of freshly dissected hIMA and induced to proliferate in culture. The contractile/differentiated phenotype of freshly isolated VSMCs issued from hIMA was attested by the presence of the smooth muscle MHC (SMMS), calponin and SERCA2a proteins (Fig. 4 A&B). After being cultured in the presence of 5% supplement mix, cultured VSMCs adopted a synthetic status compared with primary cells, since i) immuno-labeling of the contractile markers was no longer detectable, ii) the expression of NM-B was maintained or even increased as visualized in western blot (Fig. 4B), and iii) Cyclin D1, the marker of proliferating cells, was highly increased (Fig. 4B). Of note, SERCA2b was expressed in both contractile and proliferating hIMA VSMCs. As an initial approach to understanding the molecular mechanism by which SERCA2a prevents VSMC proliferation, freshly isolated VSMCs from hIMA were infected with Ad-S2a, Ad-βgal or Ad-VIVIT (delivering an inhibitory peptide that blocks the calcineurin/NFAT interaction). As evidenced in Fig. 4C-E, BrdU incorporation and cyclin D1 expression in cultured hIMA VSMCs was significantly increased by 5% supplement mix compared with 0.1% supplement mix. Adenovirus-directed gene transfer of SERCA2a or VIVIT in presence of 5% supplement mix prevented cell proliferation compared with βGal-infected cells (Fig. 4C-E), confirming previous observations made for CA VSMCs 5. These data confirm the involvement of NFAT signaling in human mammary VSMC proliferation associated with SERCA2a downregulation. Noteworthy: i) Ad-βGal infection did not significantly alter growth medium-induced proliferation (BrdU incorporation was 100.00 ±6.33 in control vs 87.78±4.97, n=3 in Ad βGal infected cells, ns); ii) Infection of hIMA VSMC with adenovirus at indicated concentrations results in negligible cytotoxic effect estimated as ~2.5% of dead cells in virus infected cultures vs ~1% of dead cells in non infected culture (Fig 2S); however, ~ 97% of cells in virus infected cultures were fully functional.
Figure 4. Forced SERCA2a expression prevents proliferation of hIMA VSMCs in culture.
A&B. Primary VSMCs exhibit a contractile phenotype, characterized by expression of SMMS (smooth muscle myosin heavy chain 1 et 2) and calponin, whereas synthetic, cultured cells express only NM-B (non muscular myosin B heavy chain). SERCA2a is expressed essentially in primary VSMCs, whereas SERCA2b is detected in both primary and cultured VSMCs. Confocal immunofluorescence (A) and immunoblot analysis (B) of freshly isolated or cultured (passage 2) hIMA VSMCs. For immunoblot, 50μg of total protein extracts were loaded. N=5. Bar - 5μM. C. Confocal microscopy of cultured hIMA VSMCs, control or infected for 3 days with indicated adenovirus. Cells were cultured in SMCBM2 with 5% of Supplement Mix. Cyclin D1 (red) – a marker of cell proliferation; DAPI (blue) – nuclei; GFP (green) – GFP autofluorescence in adenovirus-transduced cells. Bar – 5 μM. D. Representative immunoblot (50 μg/lane) demonstrating the effect of SERCA2a gene transfer on Cyclin D1 expression. Cells were infected with indicated adenovirus for 4 days in the presence of 5% Supplement Mix (S). Control non infected cells were cultured in the presence of 0.1% or 5% Supplemented Mix. E. Effect of serum, Ad S2a, Ad βGal or Ad VIVIT on VSMC proliferation. Cells were infected and cultured for 48 h in the presence of adenovirus. Infection was controlled by GFP fluorescence. Thereafter, cells were cultured in virus-free medium containing BrdU for a further 48 hours. Each point represents the mean of 8 independent experiments performed on VSMCs from 4 donors. Values are plotted as the percentage of change with respect to control (0.1% Supplement Mix). ***p<0.01 vs Ad βGal infected.
4. SERCA2a gene transfer prevents balloon angioplasty-induced neointimal thickening in hIMA ex vivo model
We next evaluated whether maintaining SERCA2a expression could attenuate restenosis in human arteries. Arteries were infected with an adenovirus encoding SERCA2a/GFP (Ad-S2a) or Ad βGal/GFP (Ad-βGal) right after ballooning. Infection was controlled by real-time PCR analysis of total DNA extracted from cross sections of MA cultured for 2 weeks after virus infection (Fig. 5A). Fluorescence quantification analysis of cross sections revealed an overall increase of 488 nm (green) fluorescence in Ad-S2a/GFP and Ad-βGal/GFP infected arteries (Fig. 5B), demonstrating the efficacy of gene transfer in hIMA. The transgenes expression was also confirmed by imaging GFP fluorescence (green) and visualizing anti-GFP immuno-labeling (red) to avoid any false positives due to elastin auto-fluorescent (Fig. 5C). Of note, to preserve the natural fluorescence of GFP, for these experiments the arteries were fixed with paraformaldehyde (4%). As documented on figure 5C, in injured vessels adenovirus was able to transduce the entire subendothelial intima and several layers of medial VSMCs.
Figure 5. Analysis of efficacy of gene transfer in injured hIMA.
A. Quantitative PCR validating adenovirus infection of MA arteries: relative quantification of GFP/reference ratio in DNA extracted from control non-infected group (n=3), Ad-βGal infected group (n=5) and Ad-SERCA2a-infected group (n=5). Human Notch-1 gene was used as a reference control. B. Bar graph representing the mean gray values (a.u.) of green immunofluorescence in a media or an intima of hIMA cross sections of control (non-infected) arteries and arteries infected with indicated adenovirus. All microscope settings were kept constant to allow comparison. Arteries from 5 patients were analyzed; three independent sections for each patient were quantified. ***p<0.001. C. Confocal immunofluorescence detecting anti-GFP-Alexa 546 (red), and GFP and elastin autofluorescence (green). To preserve natural GFP fluorescence the sections were fixed in 4% paraformaldehyde before immunolabeling. Bar – 50 μM.
Hematoxylin/eosin staining of cross sections revealed a significant increase in intima thickness after 2 weeks of organ culture in injured Ad-βGal-infected segments, compared to injured Ad-S2a-infected segments (Fig. 6A). Of note: in each experiment, vessels were harvested, injured, then separated into two segments to test the effects of SERCA2a and βGal infection on intimal proliferation within the same artery. Quantification of hematoxylin/eosin stained sections revealed a ~5 fold lower intima/media (I/M) ratio in Ad S2a-infected arteries as compared to Ad βGal-infected ones (I/M: 0.07 ± 0.01, n=5 vs 0.40 ± 0.03, n=5, p<0.01; Fig. 6B). Although the medial thickness varied between different patients, the decrease in the intima/media ratio is mainly due to the inhibition of the intima formation; indeed, the intima thickness was significantly decreased in SERCA2a infected arteries (7.69 ± 0.60 μm vs 27.09± 1.30 μm, p <0.01).
Figure 6. SERCA2a gene transfer prevents balloon injury-associated neointimal thickening in an ex vivo hIMA model.
A. Representative hematoxylin-eosin staining cross sections of hIMA injured and infected with either Ad-S2a or Ad-βgal. The both segments were obtained from the same patient. After infection, arteries were perfused ex vivo for 14 days. Bar −50 μM. B. Quantification of ex vivo arterial remodeling after balloon injury, comparing effects of Ad-S2a and Ad-βgal. Bars represent mean ± SEM of intima and intima/media thickness ratio. N=5. Abbreviations: m - media; i – neointima; ad – adventitia; iel – internal elastic lamina.
Consistent with a cause to effect relationship between SERCA2a expression and the proliferation status of VSMCs in human vessels 5, SERCA2a gene transfer prevented synthetic VSMCs from proliferating (Fig. 7). Interestingly, proliferating cells (Cyclin D1 positive cells) were observed almost exclusively in the subendothelial intima from injured βGal-infected vessels, whereas absence of Cyclin D1 or poorly positive cells were observed in the medial layer (Fig. 7). These data fit with previous observations made for human coronary arteries 5, and suggest an important role for subendothelial VSMCs in neointima formation. Consistent with neo-intimal VSMCs being in a de-differentiated state, the smooth muscle myosin heavy chain (SMMS), a marker of terminal VSMC differentiation 22 was poorly expressed in the neo-intima layer, as compared to the medial layer, whereas the NM-B myosin heavy chain isoform, barely detectable in contractile/medial cells, was highly expressed in the neointima. SERCA2a protein expression was scarce in the neointimal layer in Ad-βGal infected injured hIMA, in accordance with the proliferative status of these cells (Fig. 7). By contrast, SERCA2a expression was high in subendothelial VSMC from injured SERCA2a-infected hIMA. The ubiquitous SERCA2b isoform was associated with both VSMC phenotypes, the differentiated/contractile and the synthetic. Here again, we corroborate previous in vitro and in vivo observations indicating that SERCA2a expression is associated with the quiescent status of VSMCs 5,7,8.
Figure 7. Characterization of VSMCs in an organ culture of injured hIMA infected with either or Ad-βgal or Ad-S2a.
Representative confocal immunofluorescence (red) detection of different antibody stainings in hIMA cross sections after 2 weeks of ex vivo organ culture. The media was identified by elastin autofluorescence (green). Abbreviations: SMMS - smooth muscle myosin heavy chain 1 and 2; NM-B – non-muscle B myosin heavy chain; CycD1 – Cyclin D1; SERCA2a and SERCA2b - sarco/endoplasmic reticulum calcium ATPase 2a and 2b; m – media; ni – neointima; EC – endothelial cells; iel – internal elastic lamina; SSMC - subendothelial smooth muscle cells. Bar – 50 μM.
Discussion
We have demonstrated in the present paper that the normal human coronary and mammary arteries contain synthetic VSMC lacking SERCA2a in the subendothelial intima. Gene transfer of SERCA2a inhibits the proliferation of synthetic VSMCs in vitro and prevents neointimal VSMC proliferation and intimal thickening in a human ex vivo IMA model of balloon angioplasty-associated restenosis.
Abnormal intracellular calcium ion (Ca2+) handling caused by a defect in sarcoplasmic reticulum (SR) function has been reported to be the primary cause in both hypertrophic cardiac and proliferative vascular remodeling 23-26. Impaired Ca2+ uptake results from decreased activity of SERCA2a caused by a decreased protein expression 23,26 and/or altered post-translational protein modifications 27. Accordingly, normalization of SERCA2a function by gene transfer has proven to be effective in improving cardiac function in heart-failure patients, as well as in animal models 23,24,26.
In vascular disease, alteration of the differentiated contractile phenotype of smooth muscle cells is known to play an important role in the formation of the neo-intima which occurs post-angioplasty. Therefore, much effort is being made to define molecular targets underlying VSMC de-differentiation occurring in this pathological situation. With this in mind, we previously evidenced in a rat model of restenosis 7 and in primary culture of human CA VSMCs 5 that the full transformation of contractile-type cells into proliferating-type cells is dependent on the down-regulation of the SR Ca2+ handling protein SERCA2a. In line with this, SERCA2a gene transfer prevents VSMC migration and/or proliferation 5,7. The present study now extends those findings by showing for the first time, in human ex-vivo arteries, a causal relationship between balloon injury-induced intimal proliferation and SERCA2a expression. Indeed, SERCA2a expression is down-regulated when hIMA VSMCs proliferate to form the neo-intima; enhancing its expression using adenovirus-directed SERCA2a gene transfer strategy prevents neointima formation.
The importance of evaluating the benefit of adenovirus-directed SERCA2a gene transfer in human vessels is based on the fact that human coronary and mammary arteries exhibit distinct sub-endothelial intima when compared to rat carotid arteries (Fig. 1&2, and histological data published by Rekhter et al. 6). Indeed, we detected several layers of synthetic VSMCs lying between the external elastic lamina and the endothelial cells (from 1 to 3 in hIMA and 5 to 10 layers in coronary arteries). These subendothelial synthetic VSMCs are good potential targets for gene therapy, since they are “ready to proliferate” immediately after the destruction of the protective endothelial barrier.
Although the hIMA model of restenosis does not reproduce faithfully the in vivo situation met by surgeons – the vessel is not susceptible to atherosclerotic plaque formation (28), and humoral factors and circulating cells that affect restenosis are absent - these data can be considered as a first step towards clinical studies. In agreement with this remark: i) neo-intimal proliferation was only observed in injured vessels; this observation was in accordance with data described by Guerin et al. 19,29; ii) hIMAs (as opposed to rat carotids) present a caliber similar to that of coronary arteries, where stents are the most frequently implanted; hence, hIMA organ culture is often used to evaluate in-stent intimal hyperplasia and efficacy of different DESs 18,19,29,30; iii) the intima/media ratio measured here is comparable to that of displayed in vivo, in human arteries (Fig 1&7); iv) the VSMC phenotype changes observed in the cultured vessels are very similar to those taking place during post-angioplasty restenosis in vivo 3,31,32. Based on these several observations, we suggest considering this model as one that could be used for testing the efficacy of anti-restenotic agents before undertaking more expensive and time-consuming pre-clinical large animal trials or human randomized controlled clinical trials.
In non-injured vessels, adenovirus or adeno-associated virus transduction is mostly restricted to the endothelial cells. This is well illustrated by Rekhter et al 6, who found low numbers of Ad-infected medial cells in intact hIMA (1.3±0.4%), saphenous veins (1.4±1.0%), and coronary arteries (3.8±0.8%). Similar results were obtained by Cable et al. 33 who showed, performing immunohistochemical studies, a high level of transduction of Ad-eNOS in the endothelium and adventitia, but low transduction in the media (in this study, the vessel was infected in a culture dish, explaining the adventitial infection). Finally, intra-coronary injection of adeno associated virus (AAV) encoding for β-gal in pig resulted in transduction of endothelial cells only 34. Abundant extracellular matrix, endothelial cells, and internal elastic lamina act as a barrier, restraining virus infection and gene delivery to the medial cells. Consistently, Rekhter et al. 6 reported an improved efficacy of medial cell transduction in human vessels after collagenase or elastase treatment. Here we have demonstrated that adenovirus-directed gene transfer following balloon injury results in efficient transduction of the subendothelial intima and several layers of medial VSMCs. SERCA2a gene transfer is potentially promising to prevent in-stent restenosis after interventional balloon angioplasty in patients. Our results demonstrate the feasibility of transducing human vessels post angioplasty with a potential therapeutic outcome.
Numerous studies now show that neo-intimal VSMCs present in vascular lesions derived from cells of diverse origins including pre-existing medial/synthetic VSMCs, medial/contractile cells, and hematopoietic stem cells 35-38. Consistent with synthetic VSMCs arising from a pre-existing subpopulation of VSMCs, we detected synthetic cells in normal vessels, in this and other studies 5,9,39. The subendothelial VSMCs are believed to be the most likely source of intimal growth in atherosclerosis, restenosis, and bypass graft intimal hyperplasia 20,21. Whether bone-marrow derived cells actually contribute to intimal hyperplasia remains elusive. In our model, no hematopoietic or blood cells were available to home to the site of injury. Taking this observation into account, the development of the neointima in human arteries appears to be mainly due to pre-existing sub-endothelial synthetic VSMCs. We and others observed that sections of 14 days old hIMA organ culture showed proliferating VSMCs predominantly in the neointimal layer with few dividing cells in the media (Fig. 7) and 17,19,40. Because no Cyclin D1 labeling could be detected in the media, it is unlikely that medial cells contribute significantly to neointimal growth in human vessels.
The present results obtained in ex vivo hIMA indicate that the virus vector transduces the adult human vessel wall and acts on arterial smooth muscle cells to reduce neointimal thickening. The characteristic of neointimal thickening obtained in organ culture and the observed inhibitory effect of SERCA2a gene transfer suggest that the neointimal formation process in organ culture resembles that occurring in vivo. Blood factors could play an amplifying but not necessary role in vivo, including the recruitment of immune/inflammatory cells. However, our results indicate that neointimal formation comprises an intrinsic component, resulting from proliferation of subendothelial VSMCs.
We have demonstrated here that SERCA2a expression prevented proliferation of synthetic VSMCs and neointimal thickening in an ex vivo organ culture of hIMA, confirming previous observations made in a rat carotid injury model in vivo and in cultured rat and human CA VSMCs 5,7. The number of dead cells in SERCA2a infected culture was not different from that observed in control or Ad-βGal infected culture, ruling out the possibility that growth arrest was due to cytotoxic effects of gene transfer. However, analysis of cell viability in injured hIMA after 2 weeks of organ culture revealed the presence of some apoptotic cells in the medial layer of injured vessels (not shown), infected either with Ad-βGal/GFP or Ad-SERCA2a/GFP. The presence of apoptotic cells in the media can be a consequence of balloon injury of medial VSMCs, as was previously described in animal balloon injury models 7,41,42. The safety of SERCA2a gene transfer at the cellular, tissue and organism levels has been reported by numerous investigators over a dozen years, making the possibility of cytotoxic effects of SERCA2a on subendothelial VSMCs in hIMA organ culture highly unlikely. For example, cardiac function studies addressing potential adverse effects of SERCA2a gene transfer in animal models indicate that: i) long-term expression of SERCA2a in transgenic animals improves contractile function without adverse effects 43; ii) adenovirus-mediated gene transfer of SERCA2a improves cardiac hemodynamics and increased survival in a rat model of heart failure 44; iii) within two studies of adeno associated virus (AAV) directed SERCA2a gene transfer in porcine and sheep models of heart failure, safety-related parameters including histopathology analysis, hematology, and clinical chemistry indicate that the treatment does not cause any organ damage or inflammatory response 45,46; iv) in a special study designed to evaluate the potential toxicological effect of SERCA2a gene therapy, normal Göttingen minipigs received by transcoronary perfusion a dose of AAV1.SERCA2a at least 3-fold higher that the highest dose intended for administration in clinical trials; no signs of toxicity, clinical pathology or histology were observed in these animals 47. The overall conclusion from animal studies is that SERCA2a gene transfer appears to be a safe, well-tolerated, and effective treatment for cardiovascular diseases.
Using SERCA2a as a target to treat heart failure, two clinical trials have been initiated: a Phase I, randomized, double-blinded, placebo-controlled, dose escalation trial of intracoronary administration of AAV1.SERCA2a (MYDICAR™) in patients with congestive heart failure (Celladon Corp., CA, USA) and a Phase I study evaluating the safety and biological effects of AAV6.SERCA2a in non-ischemic patients undergoing left ventricular assist device placement. A first-in-human, Phase 1 trial was completed, demonstrating safety and feasibility of SERCA2a gene transfer 47,48. More recently, a Phase 2 trial performed to further evaluate the effects of 3 escalating doses of AAV1.SERCA2a versus placebo in a similar, advanced heart failure population, demonstrated safety and suggested benefit of AAV1.SERCA2a gene transfer in advanced heart failure 49.
Current therapies for heart disease are not directed towards vascular remodeling. Each year, more than 1.5 million patients worldwide undergo percutaneous coronary interventions followed by stent implantation for atherothrombotic lesions. Restenosis which occurs in 15-60% of patients remains the major impediment hampering this procedure. The advent of drug eluting stents (DES) has significantly reduced restenosis but the use of DES impairs the re-endothelialization process and subsequently often induces late thrombosis 1. Therefore a two-pronged strategy is necessary to prevent VSMC proliferation and adverse vascular remodeling while facilitating re-endothelialization. We have demonstrated that SERCA2a gene transfer prevents i) in vitro proliferation and migration of human CA VSMC 5; ii) neointimal proliferation and vascular remodeling while allowing re-endothelialization in a rat model of carotid injury 7; iii) neointimal proliferation in a hIMA ex vivo model of restenosis (this paper). Thus, SERCA2a could be a possible target for minimizing VSMC proliferation in the setting of large artery remodeling such as restenosis.
Materials and Methods
Materials
All media and sera were purchased from PromoCell (PromoCell GmbH, Heidelberg, Germany). The following primary antibodies were used: anti-SERCA2a and anti-SERCA2b (provided by Dr F. Wuytack, University of Leuven, Belgium)50, anti-RyR2 (provided by I. Marty, Inserm U607, Grenoble, France) 51, anti-non muscular myosin B heavy chain (NM-B) (Ab 684, Abcam), anti-smooth muscle myosin heavy chain (SMMS-1) (M3558, Dako Cytomation), anti-SM22 (Abcam), anti-calponin (C2687, Sigma-Aldrich), anti-cyclin D1 (556470, BD Biosciences), anti-CD31 (MAB1393; Chemicon International). The anti-rabbit and anti-mouse secondary antibodies used were coupled with fluorochromes Alexa 546 (1/500; Abcam).
Human samples
Coronary arteries were obtained from explanted hearts. Unused segments of left IMA were obtained during coronary artery bypass from the surgical department of the cardiology institute at Pitié-Salpêtrière Hospital, Paris, France, in accordance with French ethical laws (L.1211-3-9). The artery segments were immediately immersed in physiological saline solution, placed at 4°C and used within a few hours.
Adenovirus
The following adenovirus were used: Ad-S2a, encoding human SERCA2a and green fluorescence protein (GFP) under cytomegalovirus (CMV) promoter 52; Ad-βGal, encoding β-galactosidase and GFP under CMV promoter 52; Ad-VIVIT, encoding NFAT competing peptide VIVIT and GFP under CMV promoter 53. Cells were infected with adenovirus at 1 to10 pfu/cell. The efficacy of infection was controlled by GFP fluorescence.
Injury of the mammary artery, adenovirus mediated gene delivery and ex-vivo organ culture
The internal mammary artery was injured using a PTCA catheter (Cordis, 2.25 mm diameter). The catheter was introduced in the vessel’s lumen, inflated at 15 atmospheres and withdrawn 3 times by rotating it. The artery was then divided into 2 segments; both were cannulated at both extremities as previously described 54 and injected intra-lumenally with 1010 pfu of Ad SERCA2a/GFP (Ad-S2a) or βGal/GFP (Ad-βGal). After 30 minutes of infection, the arteries were flushed and connected to a closed perfusion circuit consisting of a 3-port reservoir, a peristaltic pump (Masterflex, Cole Palmer Instrument Co), and a pressure chamber as previously described 55. This experimental setting allowed the application of controlled intra-luminal hydrostatic pressure and flow (here of 80 mmHg and 0.2 ml/min, respectively). The perfusion liquid was smooth muscle cells basal medium 2 (SMCBM2) containing 1% antibiotics-antimycotics (15240-062, Gibco) supplemented with 5% of Supplement Mix (C-39262, PromoCell). The organ culture of hIMA was carried out under sterile conditions in an incubator containing 5% CO2 at 37°C during 2 weeks. At the end of the experiments, the arteries were collected, flushed with saline, included in cryomatrix and frozen at −80°C. The cryo-sections were fixed in 4% paraformaldehyde or in methanol and used for morphometric analysis and immuno-fluorescence, respectively. Gene transfer efficiency was assessed by staining with anti-GFP, or anti-SERCA2a antibody followed by an Alexa 546-labelled secondary antibody (Invitrogen).
Morphometric analysis
Neointimal thickening was visualised in hematoxylin/eosin stained mammary artery cross-sections and measured with a computer-based morphometric system (Lucia, Nikon). The intima-to-media ratio was calculated. For each vessel, 5 discontinuous sections were analyzed and, on each section, 25 measurements were done.
Culture of human VSMCs, measurement of proliferation and cytotoxicity
Human VSMCs were isolated from the medial layer of mammary arteries by enzymatic digestion. After dissection, the fragments of media were incubated in SMCBM2 with collagenase (CLS2, 50 U/mL, Worthington) and pancreatic elastase (0.25 mg/mL, Sigma) for 4-6 hours at 37°C. After periods of 30 minutes, the suspension was centrifuged at 1000 rpm for 3 minutes, and the cells were collected and placed in SMCBM2 + 20% Supplement Mix. The cells obtained in the first 30 minutes period were discarded. Those obtained in the other cycles were pooled and cultured at 37°C in an incubator containing 5% CO2 in SMCBM2 supplemented with 5% of Supplement Mix and antibiotics as described for ex-vivo organ culture. Cells were used between passages 2 to 8, and some were infected with adenovirus for 48 hours at 1 to 10 pfu/cell. Proliferation was monitored using Cell Proliferation ELISA, BrdU (colorimetric) assay kit (Roche). Cytotoxicity was analyzed using Live/Dead Viability/Cytotoxicity Kit (Invitrogen) according to manufacturer’s instructions. Live cells are characterized by the presence of intracellular esterase activity determined by the enzymatic conversion of cell permeable Calcein AM to calcein producing green fluorescence. Dead cells were identified by red fluorescence with ethidium homodimer EthD-1.
Protein analysis
Total cell lysates were prepared according to a standard protocol (Upstate). Proteins were separated by SDS-PAGE and blotted on Hybond-C membranes (Amersham Biosciences). Proteins were visualized by using the Super Signal West Pico Chemiluminescent Substrate (34080, Pierce Biotechnology).
Statistical analysis
All quantitative data are presented as mean of at least 3 independent experiments ± SEM. Data were analyzed by using GrafPad Prism 5 software. A one-way ANOVA followed by Tukey’s multiple comparisons test was performed for comparison of multiples values. Statistical comparison of 2 groups was done by an unpaired Student’s t-test. Differences were considered significant when P<0.05.
Supplementary Material
Acknowledgments
This work is supported by AHA SDG 0930116N (LL), by NIH (1K01HL103176) (LH), by Leducq Foundation through the Caerus network (05 CVD 03 to AML and RJH), by NIH R01 HL078691, HL057263, HL071763, HL080498, & HL083156 (RJH). We thank Dr Alain Tedgui (Paris Cardiovascular Research Center, HEGP, Paris, France) for helpful discussion; Drs Susan Kraner and Christopher M. Norris (Sanders-Brown Center on Aging, Lexington, KY-USA) for providing AdVIVIT, and Dr Frank Wuytack (University of Leuven, Belgium) for the anti-SERCA2a and anti-SERCA 2b antibodies.
Footnotes
Conflict of interest: All authors declare having no competing financial interests in relation to the work described.
Supplementary information accompanies the paper on Gene Therapy’s website (http://nature.com/gt).
References
- 1.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda D, Sata M, Tanaka K, Nagai R. Potent inhibitory effect of sirolimus on circulating vascular progenitor cells. Circulation. 2005;111:926–931. doi: 10.1161/01.CIR.0000155612.47040.17. [DOI] [PubMed] [Google Scholar]
- 3.Ueda M, Becker AE, Naruko T, Kojima A. Smooth muscle cell de-differentiation is a fundamental change preceding wound healing after percutaneous transluminal coronary angioplasty in humans. Coronary artery disease. 1995;6:71–81. doi: 10.1097/00019501-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa M, Naruko T, Ikura Y, Komatsu R, Iwasa Y, Kitabayashi C, et al. A decline in platelet activation and inflammatory cell infiltration is associated with the phenotypic redifferentiation of neointimal smooth muscle cells after bare-metal stent implantation in acute coronary syndrome. Journal of atherosclerosis and thrombosis. 2010;17:675–687. doi: 10.5551/jat.3426. [DOI] [PubMed] [Google Scholar]
- 5.Bobe R, Hadri L, Lopez JJ, Sassi Y, Atassi F, Karakikes I, et al. SERCA2a controls the mode of agonist-induced intracellular Ca2+ signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol. 2011;50:621–633. doi: 10.1016/j.yjmcc.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rekhter MD, Simari RD, Work CW, Nabel GJ, Nabel EG, Gordon D. Gene transfer into normal and atherosclerotic human blood vessels. Circ Res. 1998;82:1243–1252. doi: 10.1161/01.res.82.12.1243. [DOI] [PubMed] [Google Scholar]
- 7.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, et al. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97:488–495. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- 8.Lipskaia L, Pourci ML, Delomenie C, Combettes L, Goudouneche D, Paul JL, et al. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ Res. 2003;92:1115–1122. doi: 10.1161/01.RES.0000074880.25540.D0. [DOI] [PubMed] [Google Scholar]
- 9.Gueguen M, Keuylian Z, Mateo V, Mougenot N, Lompre AM, Michel JB, et al. Implication of adenylyl cyclase 8 in pathological smooth muscle cell migration occurring in rat and human vascular remodelling. J Pathol. 2010;221:331–342. doi: 10.1002/path.2716. [DOI] [PubMed] [Google Scholar]
- 10.Gollasch M, Haase H, Ried C, Lindschau C, Morano I, Luft FC, et al. L-type calcium channel expression depends on the differentiated state of vascular smooth muscle cells. Faseb J. 1998;12:593–601. doi: 10.1096/fasebj.12.7.593. [DOI] [PubMed] [Google Scholar]
- 11.Quignard JF, Harricane MC, Menard C, Lory P, Nargeot J, Capron L, et al. Transient down-regulation of L-type Ca(2+) channel and dystrophin expression after balloon injury in rat aortic cells. Cardiovasc Res. 2001;49:177–188. doi: 10.1016/s0008-6363(00)00210-8. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Zhang C, Dronadula N, Li Q, Rao GN. Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model. J Biol Chem. 2005;280:14700–14708. doi: 10.1074/jbc.M500322200. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Sliedregt-Bol K, Overkleeft H, van der Marel GA, van Berkel TJ, Biessen EA. Therapeutic potential of a synthetic peptide inhibitor of nuclear factor of activated T cells as antirestenotic agent. Arterioscler Thromb Vasc Biol. 2006;26:1531–1537. doi: 10.1161/01.ATV.0000225286.30710.af. [DOI] [PubMed] [Google Scholar]
- 14.Sedighiani F, Nikol S. Gene therapy in vascular disease. Surgeon. 2011;9:326–335. doi: 10.1016/j.surge.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Van Assche T, Huygelen V, Crabtree MJ. Targeting vascular redox biology through antioxidant gene delivery: a historical view and current perspectives. Recent patents on cardiovascular drug discovery. 2011;6:89–102. doi: 10.2174/157489011795933873. [DOI] [PubMed] [Google Scholar]
- 16.Chen SH, Zhaori G. Potential clinical applications of siRNA technique: benefits and limitations. European journal of clinical investigation. 2011;41:221–232. doi: 10.1111/j.1365-2362.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- 17.Holt CM, Francis SE, Rogers S, Gadsdon PA, Taylor T, Clelland C, et al. Intimal proliferation in an organ culture of human internal mammary artery. Cardiovasc Res. 1992;26:1189–1194. doi: 10.1093/cvr/26.12.1189. [DOI] [PubMed] [Google Scholar]
- 18.Swanson N, Javed Q, Hogrefe K, Gershlick A. Human internal mammary artery organ culture model of coronary stenting: a novel investigation of smooth muscle cell response to drug-eluting stents. Clin Sci (Lond) 2002;103:347–353. doi: 10.1042/cs1030347. [DOI] [PubMed] [Google Scholar]
- 19.Guerin P, Sauzeau V, Rolli-Derkinderen M, Al Habbash O, Scalbert E, Crochet D, et al. Stent implantation activates RhoA in human arteries: inhibitory effect of rapamycin. J Vasc Res. 2005;42:21–28. doi: 10.1159/000082873. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 21.Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W, Jr., Richardson M, et al. American Heart Association A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis. Arterioscler Thromb. 1992;12:120–134. doi: 10.1161/01.atv.12.1.120. [DOI] [PubMed] [Google Scholar]
- 22.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kawase Y, Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nature clinical practice. 2008;5:554–565. doi: 10.1038/ncpcardio1301. [DOI] [PubMed] [Google Scholar]
- 24.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther. 2010;10:29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipskaia L, Hulot JS, Lompre AM. Role of sarco/endoplasmic reticulum calcium content and calcium ATPase activity in the control of cell growth and proliferation. Pflugers Arch. 2008 doi: 10.1007/s00424-007-0428-7. [DOI] [PubMed] [Google Scholar]
- 26.Tilemann L, Ishikawa K, Weber T, Hajjar RJ. Gene therapy for heart failure. Circ Res. 2012;110:777–793. doi: 10.1161/CIRCRESAHA.111.252981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477:601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sisto T, Isola J. Incidence of atherosclerosis in the internal mammary artery. Ann Thorac Surg. 1989;47:884–886. doi: 10.1016/0003-4975(89)90027-1. [DOI] [PubMed] [Google Scholar]
- 29.Guerin P, Rondeau F, Grimandi G, Heymann MF, Heymann D, Pillet P, et al. Neointimal hyperplasia after stenting in a human mammary artery organ culture. J Vasc Res. 2004;41:46–53. doi: 10.1159/000076245. [DOI] [PubMed] [Google Scholar]
- 30.Hlawaty H, Jacob MP, Louedec L, Letourneur D, Brink C, Michel JB, et al. Leukotriene receptor antagonism and the prevention of extracellular matrix degradation during atherosclerosis and in-stent stenosis. Arterioscler Thromb Vasc Biol. 2009;29:518–524. doi: 10.1161/ATVBAHA.108.181750. [DOI] [PubMed] [Google Scholar]
- 31.Morimoto S, Mizuno Y, Hiramitsu S, Yamada K, Kubo N, Nomura M, et al. Restenosis after percutaneous transluminal coronary angioplasty--a histopathological study using autopsied hearts. Japanese circulation journal. 1990;54:43–56. doi: 10.1253/jcj.54.43. [DOI] [PubMed] [Google Scholar]
- 32.Aikawa M, Sakomura Y, Ueda M, Kimura K, Manabe I, Ishiwata S, et al. Redifferentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation. 1997;96:82–90. doi: 10.1161/01.cir.96.1.82. [DOI] [PubMed] [Google Scholar]
- 33.Cable DG, Caccitolo JA, Caplice N, O’Brien T, Simari RD, Daly RC, et al. The role of gene therapy for intimal hyperplasia of bypass grafts. Circulation. 1999;100:II392–396. doi: 10.1161/01.cir.100.suppl_2.ii-392. [DOI] [PubMed] [Google Scholar]
- 34.Hadri L, Bobe R, Kawase Y, Ladage D, Ishikawa K, Atassi F, et al. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther. 2010;18:1284–1292. doi: 10.1038/mt.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res. 2001;38:113–119. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- 36.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 38.Zalewski A, Shi Y, Johnson AG. Diverse origin of intimal cells: smooth muscle cells, myofibroblasts, fibroblasts, and beyond? Circ Res. 2002;91:652–655. doi: 10.1161/01.res.0000038996.97287.9a. [DOI] [PubMed] [Google Scholar]
- 39.Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res. 1997;81:940–952. doi: 10.1161/01.res.81.6.940. [DOI] [PubMed] [Google Scholar]
- 40.Guerin P, Goueffic Y, Heymann MF, Pillet P, Al Habash O, Crochet D, et al. Direct stenting limits sirolimus-eluting stent edge neointimal thickening. J Vasc Surg. 2007;46:354–359. doi: 10.1016/j.jvs.2007.04.063. [DOI] [PubMed] [Google Scholar]
- 41.Malik N, Francis SE, Holt CM, Gunn J, Thomas GL, Shepherd L, et al. Apoptosis and cell proliferation after porcine coronary angioplasty. Circulation. 1998;98:1657–1665. doi: 10.1161/01.cir.98.16.1657. [DOI] [PubMed] [Google Scholar]
- 42.Durand E, Mallat Z, Addad F, Vilde F, Desnos M, Guerot C, et al. Time courses of apoptosis and cell proliferation and their relationship to arterial remodeling and restenosis after angioplasty in an atherosclerotic rabbit model. J Am Coll Cardiol. 2002;39:1680–1685. doi: 10.1016/s0735-1097(02)01831-4. [DOI] [PubMed] [Google Scholar]
- 43.He H, Giordano FJ, Hilal-Dandan R, Choi DJ, Rockman HA, McDonough PM, et al. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. The Journal of clinical investigation. 1997;100:380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene therapy. 2008;15:1550–1557. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 46.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. Journal of cardiac failure. 2008;14:355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. Journal of cardiac failure. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eggermont JA, Wuytack F, Verbist J, Casteels R. Expression of endoplasmic-reticulum Ca2(+)-pump isoforms and of phospholamban in pig smooth-muscle tissues. Biochem J. 1990;271:649–653. doi: 10.1042/bj2710649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marty I, Robert M, Villaz M, De Jongh K, Lai Y, Catterall WA, et al. Biochemical evidence for a complex involving dihydropyridine receptor and ryanodine receptor in triad junctions of skeletal muscle. Proc Natl Acad Sci U S A. 1994;91:2270–2274. doi: 10.1073/pnas.91.6.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan PG. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 54.Bardy N, Karillon GJ, Merval R, Samuel JL, Tedgui A. Differential effects of pressure and flow on DNA and protein synthesis and on fibronectin expression by arteries in a novel organ culture system. Circ Res. 1995;77:684–694. doi: 10.1161/01.res.77.4.684. [DOI] [PubMed] [Google Scholar]
- 55.Lehoux S, Esposito B, Merval R, Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation. 2005;111:643–649. doi: 10.1161/01.CIR.0000154548.16191.2F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.