Abstract
Skin is the primary interface between health care providers and patients and is assessed clinically to predict physiological stability or instability. The biomechanical properties of human skin, most notably elasticity and viscoelasticity, are critical to its protective function. In this article, the authors describe the physiological basis for skin elasticity and viscoelasticity. The authors discuss the role of viscoelasticity in nursing science and consider avenues for scientific exploration of the skin’s biomechanical properties, including applications in pressure ulcer research, injury, and healing. They also discuss the Cutometer® as one option for measurement of viscoelasticity in clinical and bench research protocols.
Keywords: skin, viscoelasticity, elasticity, measurement, injury, cutometer®
Skin is the largest organ in the human body and serves as a primary interface between health care providers and patients. Paradoxically, skin biomechanics represent relatively underdeveloped areas of research in nursing science. Skin biomechanics are the physiological properties of the epidermis and dermis that (a) provide protection against biological invasion, injury, and ultraviolet (UV) radiation and (b) resist the loss of skin integrity that occurs with movement, stretching, and application of force (Barel, Lambrecht, & Clarys, 1998; Clancy, Nilsson, Anderson, & Leahy, 2010; Seehra & Silver, 2006).
Elasticity is generically defined as the physical property of a substance that enables it to change its length, volume, or shape in response to a force, followed by recovery toward its original form when the force is removed. Skin elasticity (SE) is a property of the skin that enables it to change and recover shape when stretched or deformed (Clancy et al., 2010). Skin viscoelasticity (SVE) incorporates the water content of the skin and adds the principle of viscosity, the internal resistance to flow when a shearing force or stress is applied to a fluid. In fluids, resistance to flow is caused by adhesion of molecules; viscosity is a measure of the effort to shear the fluid, or to overcome the friction between the layers of molecules. The viscoelastic property of the skin provides protection against injury, as it allows for additional movement (as compared to just elastic properties) of skin structures away from and returning toward baseline without breaking (Clancy et al., 2010; Seehra & Silver, 2006). In this article, we focus on SVE because of these protective properties and their role in protection versus injury.
The purpose of this article is to describe the physiologic basis of SVE, discuss a measurement strategy that scientists can use when SVE is a variable in research protocols, describe changes in SVE that occur during the life span, and analyze the relevance of SVE to knowledge development in nursing science.
Physiology of Viscoelasticity
Specific physiologic contributions to SVE by the layers of the skin can be found in Table 1. The skin acts as a reservoir for water and contains approximately 20% of the total body water (Clancy et al., 2010; Girard, Beraud, & Sirvent, 2000). Skin is a continuous matrix comprised mostly of extracellular rather than intracellular tissues; the structural and mechanical properties occur because the extracellular space is filled with water (60–72% of total weight of skin), collagen (30%), elastin (.2%), and glycosaminoglycans (GAGS; .03%) such as hyaluronic acid (Clancy et al., 2010; Seehra & Silver, 2006). Cellular components and noncollagenous proteins of skin comprise less than 1% of the total weight of skin (Seehra & Silver, 2006).
Table 1.
Relationship Between Anatomic Structure and Viscoelastic Function of the Skin
| Skin Layer | Structure | Role in Viscoelasticity |
|---|---|---|
| Stratum corneum—outermost layer of epidermis | Structure of up to 25–30 rows of corneocytes; includes fibrous keratin; “brick and mortar” arrangement, in conjunction with other stratified layers in the epidermis, increases tensile strength (resistance to longitudinal stress), and resistance to damage (Micali, Lacarrubba, Bongu, & West, 2001) Water content is 15–30% (Johnsen, Haugsnes, Martinsen, & Grimnes, 2010) |
Supports pliability (ease in change of shape from baseline) Promotes strength, elastic behavior, and resistance to loss of skin integrity with movement, stretching, and application of force |
| Basement membrane zone (BMZ) | Collection of three cell layers between the epidermis and the dermis (lamina lucida, lamina densa, and lamina propria; Bruckner-Tuderman & Stanley, 2007; Chan, 1997); comprised of proteins (primarily laminins, proteoglycans, and types IV and VII collagens; Chan, 1997) Desmosomes (cells responsible for adhesion) serve as binding cells between basal layer of skin and upper lamina lucida (Bruckner-Tuderman & Stanley, 2007; Chan, 1997) Anchoring fibrils and a matrix of fibers at varying stages of maturity connect thicker lamina densa layer to upper layer of dermis (Barland, Zettersten, Brown, Ye, Elias, & Ghadially, 2004; Chan, 1997) BMZ semipermeable to water; limits water passage to maintain skin hydration and support viscoelasticity |
Lamina layers extremely flexible due to construction of multiple-microfibrillar subdensa and protein-based supra-lamina desmosomes (Bruckner-Tuderman & Stanley, 2007; Chan, 1997) Supports epidermis and provides strong adhesion between the epidermal and dermal layers to protect against shearing forces (Chu, 2007); when force applied on parallel plane to skin, it has a viscoelastic response of expanding and then contracting fiber matrix and associated fluids Serves as an anchor to surrounding layers; disruption of BMZ leads to amorphous structure within epidermis and dermis causing skin structure breakage and reduced viscoelastic response |
| Dermis—layer between the epidermis and subcutaneous tissues | Within papillary region (uppermost layer of dermis), a networking of thin elastin protein fibers (oxytalan fibers and the elaunin fibers cross-linked via desmosomes) is in loose matrix with procollagen (a precursor to collagen that originates within ground substance) and ground substance (Chu, 2007; Haake et al., 2001; Schafer, Pandy, Ferguson, & Davis, 1985; Uitto, Chu, Gallo, & Eisen, 2007) Reticular region (below papillary region and above hypodermis) is comprised of ground substance and a thicker mesh of collagen fibers wound among thicker elastic fibers assembled from elastin and microfibrils (Haake et al., 2001; Schafer et al., 1985; Uitto et al., 2007) |
With force, elastin molecules stretch in linear pattern, cross links maintain structure; quick elastic reaction provides immediate response to force, followed by slower viscous response and then full return to baseline Elastic fibers are thinner in papillary region and used for quick response but break more easily; elastic fibers in reticular region thicker, more bundled with collagen, and provide slower, viscoelastic behavior and greater tensile strength (Uitto et al., 2007; Wysocki, 1999) |
| Hypodermis—innermost and thickest layer of skin; connects dermis to bone or connective tissue | Adipose tissue is present in the hypodermis, but thickness of this layer may vary (Agache, 2006; Agache & Diridollou, 2006; Tortora & Grabowski, 1993) | Thickness of adipose deposits maintains shape of skin, protects it from underlying (bony) structures, and is positively correlated with skin strength and elasticity (Agache & Varchon, 2004; Smalls et al., 2006); positive and protective effects may negated in obesity (Yosipovitch, DeVore, and Dawn (2007) Problems with obesity include impaired skin barrier repair, decreased lymphatic flow, decreased strength of collagen structures, impaired circulation, decreased wound healing, and skin disorders that change the structure and impair the function of the skin (Yosipovitch, DeVore, & Dawn, 2007) |
Fibroblasts are vital to skin maintenance and healing. They produce two types of collagen (I and III), elastin, and ground substance; contribute to wound granulation tissue; and serve as key components in wound contraction (Falabella & Falanga, 2001). Collagen is a key source of support and mechanical strength. Extracellularly, fibroblasts secrete collagen, which is then assembled into fibrils and amassed into an anisotropic (directional) network of fiber bundles. These bundles form a collagen mesh, the primary structural support in the skin (Clancy et al., 2010; Seehra & Silver, 2006). Collagen fibers have “greater tensile strength than an equal cross section of steel wire,” supporting “more than ten thousand times their own weight” (Wysocki, 1999, p. 783). This strength provides support when skin is subjected to force.
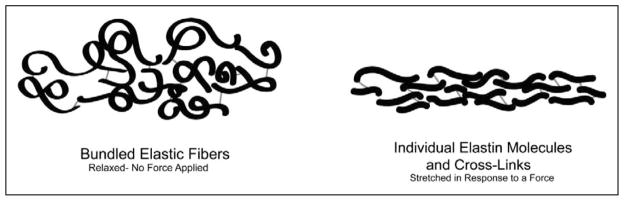
When elastin is formed from fibroblasts, the enzyme lysyl oxidase serves as a catalyst to form cross-links that join collagen and elastic fibers (Lewis, Bercovitch, Dill, & Robinson-Bostom, 2004). These fibers are in a condensed matrix (or bundle) when relaxed, but when force is applied, the fibers stretch in a more linear pattern (see Figure 1). The cross-links maintain structure and allow the elastic fibers to stretch and relax (rather than breaking) during application and release of force, providing for return toward baseline once the force is released. When faced with force, the skin’s thin elastic fibers provide the immediate response. This quick elastic reaction is followed by a slower viscous reaction involving the fluid components of the skin and thick elastic fibers to facilitate an even greater extension of the components as well as a full return to the original state (Prost-Squarcioni, Fraitag, Heller, & Boehm, 2008; Uitto, 2008). This viscoelastic response provides more “give” without structural failure.
Figure 1.
Bundled elastic fibers and individual elastin molecules with cross-links. Modified from Alberts et al. (1994).
Ground substance, comprised of many components, is an essential contributor to SVE. GAGs, such as hyaluronic acid, are hydrophilic polysaccharide chains that bind with water in volumes up to 1,000 times their own, expanding the extracellular matrix (Eisenbeiss, Welzel, Eichler, & Klotz, 2001; Haake, Scott, & Holbrook, 2001). Hyaluronic acid absorbs water, forming a thick gel that impedes the bulk flow of fluid and creates hydrostatic pressure and skin turgor; the hydrostatic pressure resists compressive forces, protects the solid structures of the skin, and contributes to the viscoelastic behaviors of the skin. Blood cells in ground substance (neutrophils, macrophages, mast cells, plasma cells, adipose cells, and erythrocytes) add volume to ground substance and thus add to the thickness, viscoelasticity, and resilience of the skin (Eisenbeiss et al., 2001; Oikarinen & Knuutinen, 2002). While sufficient water levels support homeostasis, an overabundance of water within the extracellular space can disrupt the arrangement of collagen within the skin, which can alter the viscoelastic response to force (Eisenbeiss et al., 2001; Wu, van Osdol, & Dauskardt, 2006).
SVE Changes Across the Life Span
Changes in SVE are relevant to health and disease, from both from research and clinical standpoints. Researchers have found that aging and sun/UV light exposure can decrease SVE (Suwabe, Serizawa, Kajiwara, Ohkido, & Tsutsumi, 1999; Thakur, Batheja, Kaushik, & Michniak, 2008). Many of the physiologic changes associated with aging impair skin structure and function. The most important of these include (a) decreased estrogens, testosterone, dehydroepiandrosterone, melatonin, and thyroxine; (b) increased glucocorticoids; (c) increased estrone in women, which results in decreased collagen and lipid content with accompanying skin dryness, increased extensibility, and decreased elasticity; (d) decreased ground substance volume with loss of fibroblasts and mast cells and decreased capillary flow; (e) loosening of the collagen fiber matrix; and (f) retraction of surfaces of the dermis and epidermis via the basement membrane zone, thereby decreasing nutrient and fluid exchange between the layers (Brincat, Baron, & Galea, 2005; Hall & Phillips, 2005; Yaar, 2006; Zouboulis & Makrantonaki, 2006).
The extrinsic factors of sun and other UV and infrared radiation exposure have well-documented effects on the skin as well. Skin chromophores react to UV light by initiating cellular changes, including cell death and the increased production and secretion of cytokines, that can directly reduce elasticity (Kochevar, Taylor, & Krutman, 2007). Short-term effects of UV exposure include inflammation and changes in skin pigmentation, which affect subsequent UV absorption. Long-term effects include photoaging of the skin, with dermal elastosis, decreased skin hydration, and decreased SE (Goh, 2006; Lavker, Gerberick, Veres, Irwin, & Kaidbey, 1995). Both aging and sun exposure are important variables in studies of viscoelasticity, and their relationships to SVE (and thus skin protection and injury) warrant further study.
Measuring SVE
The Cutometer® (Courage and Khazaka [C+K] Electronic GmbH, Köln, Germany, cost $12,000–$15,000 with probe) uses a dynamic method for in vivo measurement of SVE. Dynamic methods involve multiple applications of a stress, or force, to induce a skin response. Generally, the responses from dynamic measurements are a combination of frequency and magnitude of response with a variety of units or parameters (Cal, Zakowiecki, & Stefanowska, 2010). In contrast, static methods for in vivo testing apply a stress, or force (stretching, squeezing, twisting, or suction), with a single outcome measure such as distance/time or the value of the force required to induce a response (Edwards & Marks, 1995).
Several characteristics of the Cutometer® instrument make it ideal for research and clinical measurements of SVE. It is the most commonly used instrument in a wide number of studies across diverse populations (Barel et al., 1998; Cua, Wilhelm, & Maibach, 1990; Fong, Hung, & Cheng, 1997; Smalls, Wickett, & Visscher, 2006). The Cutometer is noninvasive, portable, and lightweight and may be used in either the laboratory or hospital/clinic setting without interrupting clinical care. Once the researcher selects software settings, Cutometer operation is uncomplicated. Use of the Cutometer requires little (approximately 2 hr) training, and a series of measurements performed by an experienced operator at four skin sites takes about 5 min.
The Cutometer MPA580 is the most up-to-date version of the instrument; there is no comparable instrument available from other vendors. The Cutometer allows the investigator to measure the amount of skin raised into a suction probe upon application of an operator-defined amount of constant negative pressure over an operator-defined amount of time and number of application/release cycles (time-strain mode). Changes in skin deformation are recorded optically (Cal et al., 2010), and analysis of the recorded measurement curves (see Figure 2) makes it possible to determine the elastic and plastic characteristics of the skin (C+K, 2005; Cua et al.,1990). The Cutometer software allows for four operational modes, with the only difference being the means of negative pressure application, from Mode 1 with constant negative pressure to Mode 4 with pressure rising linearly and then stopping abruptly; researchers have used Mode 1 most extensively (C+K, 2005; Dobrev, 2005, 2007; Smalls et al., 2006).
Figure 2.
Cutometer® MPA580 waveforms.
Suction can range from 20 to 500 mbar (millibar, a unit of pressure), and the time of suction from 0.1 to 60 s (C+K, 2005). This procedure is typically repeated for a series of three or more cycles at a time, and the change in shape of the skin over time, whether deviation from or return toward baseline, is recorded every 0.1 s for the duration of the measurement cycle. The number of application/release cycles is dependent on the defined time of suction and release, which is limited to a maximum total of 320 s.
Cutometer probes have apertures that range in size from 2 to 8 mm in diameter. Smaller (2–4 mm) apertures are used to evaluate the uppermost layers of the epidermis, while the largest aperture (8 mm) allows for measurement of dermal skin. Output from the Cutometer includes measures of elastic deformation (in hundredths of millimeters, total displacement from initial position at maximum negative pressure), immediate retraction (in hundredths of millimeters, .1 s after release of negative pressure), and biological elasticity (ratio of elastic recovery to elastic deformation; Dobrev, 2007; Smalls et al., 2006). The applied negative pressure is measured in mbar, which is 1/1,000 of the unit of pressure known as a “bar,” roughly equal to atmospheric pressure on Earth at sea level.
Table 2 provides an explanation of Cutometer measures. The immediate changes are tied to the elastic response of the skin and reflect the function of the solid structures (elastin and collagen) alone, not the viscous response. The slower viscous responses, termed “creep,” are products of the solid elastic structures combined with the fluid that facilitates the additional movement and greater extension of the skin in response to force. These are reflected in the delayed distension (Uv), the final deformation (Uf), delayed retraction (Ua–Ur) and final retraction (Ua) measurements; decreased hydration decreases the magnitude of these creep responses.
Table 2.
Measurements of Skin Viscoelasticity Obtained From a Cutometer®
| Measurand | Description |
|---|---|
| Immediate deformation/skin extensibility (Ue) | Quick response to force; change in shape from baseline in response to force (suction) |
| Delayed distention (Uv) | Incremental change in shape or viscous “creep” that occurs after the quick elastic response (Ue) reacting to force over time |
| Final deformation (Uf) | Total viscoelastic deviation of the skin from baseline in response to force over time (Ue + Uv) |
| Immediate retraction (Ur) | Quick return of the skin toward baseline when force is removed |
| Final retraction (Ua) | Ultimate return of the skin from Uf toward baseline after removal of force. Final retraction is Ur plus the viscous “downward incremental creep” and final measurement of deformation before another measurement cycle begins |
| Gross elasticity (R2) | Ratio (Ua/Uf) that includes the viscous changes of the skin for both deformation and retraction. Includes effects of both elasticity of the skin’s solid components {elastic fibers, etc.) and viscosity from the liquid content of the skin. Variable viscous retraction rates may result in an incomplete measurement of skin function with this value |
| Net elasticity (R5) | Ratio (Ur/Ue) that excludes viscous changes, focusing the measure on the solid components of the skin |
| Biological elasticity (R7) | Ratio (Ur/Uf) that includes measures of viscoelastic deformation on application of force and elastic retraction on release; provides a practical means for evaluating the viscous and anatomical components of skin elasticity |
Anatomic Sites and Measurement Values
Ideal anatomic sites for assessment of SVE are those that are dry (moisture damages the skin probes) and stable, often with bone near the surface to provide support. Examples of these locations include the volar forearm, forehead, cheek, and sacrum. Sites that are less firm because of adiposity or soft tissues, such as the breasts or stomach, are prone to greater deviation between measurements and are not commonly used. We were unable to locate publications with normed values in healthy populations or those with acute or chronic conditions. We present a list of mean SE and SVE values for four anatomical locations in 233 healthy adult women, stratified by age, in Table 3 (Everett, Fargo, & Sommers, 2011).
Table 3.
Mean Skin Elasticity (SE) and Viscoelasticity (SVE) Ratios by Anatomical Location and Age in 233 Healthy Female Volunteers (Everett et al., 2011)
| Site, SVE Ratio, and Age (In Years) | N | M | SD |
|---|---|---|---|
| Forearm R5 | |||
| 18–30 | 134 | 1.05 | .15 |
| 31–50 | 83 | 1.03 | .17 |
| 51–65 | 16 | .84 | .17 |
| All | 233 | 1.03 | .17 |
| Forearm R7 | |||
| 18–30 | 134 | .67 | .07 |
| 31–50 | 83 | .63 | .08 |
| 51–65 | 16 | .54 | .12 |
| All | 233 | .65 | .09 |
| Upper arm R5 | |||
| 18–30 | 134 | 1.01 | .16 |
| 31–50 | 83 | .96 | .19 |
| 51–65 | 16 | .88 | .13 |
| All | 233 | .99 | .17 |
| Upper arm R7 | |||
| 18–30 | 134 | .68 | .07 |
| 31–50 | 83 | .64 | .09 |
| 51–65 | 16 | .60 | .08 |
| All | 233 | .66 | .08 |
| Upper inner thigh R5 | |||
| 18–30 | 134 | 1.04 | .16 |
| 31–50 | 83 | 1.01 | .19 |
| 51–65 | 16 | 1.00 | .20 |
| All | 233 | 1.02 | .17 |
| Upper inner thigh R7 | |||
| 18–30 | 134 | .74 | .08 |
| 31–50 | 83 | .69 | .10 |
| 51–65 | 16 | .64 | .09 |
| All | 233 | .71 | .09 |
| Sacrum R5 | |||
| 18–30 | 134 | 1.09 | .24 |
| 31–50 | 83 | 1.15 | .33 |
| 51–65 | 16 | 1.12 | .21 |
| All | 233 | 1.11 | .27 |
| Sacrum R7 | |||
| 18–30 | 134 | .66 | .11 |
| 31–50 | 83 | .64 | .13 |
| 51–65 | 16 | .60 | .10 |
| All | 233 | .65 | .12 |
Note. R5 = net elasticity (SE), R7 = biological elasticity (SVE). Higher mean values indicate greater SE or SVE.
Accuracy and Precision of Measurements
The construct validity and precision of Cutometer data have been extensively evaluated, and findings support the validity of Cutometer measurements (Cua et al., 1990; Goh, 2006; Imokawa, 2009; Langton, Sherratt, Griffiths, & Watson, 2010). Barel, Lambrecht, and Clarys (1998) described the technology of the instrument and assessed the accuracy, repeatability, and reproducibility of the data under well-controlled and standardized conditions. They found that SE ratios decreased significantly (p < .05) in elderly men and women as compared to their younger counterparts. Other researchers reported similar results when comparing aging and youthful skin (Ahn, Kim, Lee, Moon, & Chang, 2007; Cua et al., 1990; Dobrev, 2005; Ryu, Joo, Kim, Park, & Youn, 2008; Smalls et al., 2006). Further support for construct validity comes from a series of studies demonstrating the decrease in SE with sun exposure (Barel et al., 1998; Ryu et al., 2008) and scarring of tissue (Draaijers et al., 2004; Fong et al., 1997; Nedelec, Correa, Rachelska, Armour, & LaSalle, 2008).
Investigators comparing Cutometer measurements to other strategies that assess skin biomechanics generally report high levels of agreement. Draaijers et al. (2004) found statistically significant concurrent validity between the Cutometer measures and subjective evaluation of scar tissue by experts (r ≥ −.046 except for Uv, when r = −.29). Nedelec, Correa, Rachelska, Armour, and LaSalle (2008) found that, in less severe scarring, the Cutometer data provided “moderate” validity as compared with the pliability subscale, a subjective measure (Spearman’s rho −.57, p < .0001 and −.47, p < .006 for nonsevere scar sites). In a study of 44 women, Ahn, Kim, Lee, Moon, and Chang (2007) reported correlations of r = .594 – .711 in several elasticity and viscoelasticity values assessed by both the Cutometer and the Moire topography system. In Moire topography, researchers create and measure contour lines to quantitatively assess characteristics of skin.
With respect to precision, Barel et al. (1998) found repeatable measurements at the same skin location with a coefficient of variation ranging between 4% and 6%. At different skin locations unexposed to sun, they found coefficients of variation between 12% and 22%, and at sun-exposed skin sites, coefficients of variation ranged from 23% to 32%. Draaijers et al. (2004) found low intraobserver variability with intraclass correlations (ICC) of the elasticity and extension parameters of the Cutometer, r = .76 and r = .74, respectively, thereby increasing our confidence in the reliability of the measurements. Fong, Hung, and Cheng (1997) found similar ICC results. A well-developed body of science shows that, across populations and skin conditions, Cutometer data have robust construct and concurrent validity and considerable repeatability across operators (Ahn et al., 2007; Barel et al., 1998).
Measurement Error
Several strategies help the investigator control for measurement error. To maintain accuracy and precision, the participant’s skin needs to be free of lotions and other substances that may interfere with the instrument. The Cutometer may not be used on broken skin, as fluids may enter the instrument and disrupt measurement. To reduce operator-induced random error, the probe needs to be held gently but firmly and perpendicular to the surface of unbroken skin. The same site should be used for repeated measurements. Additional potential sources of random error include electrical interference between the instrument and other devices, temperatures outside of the recommended range (68–76 °F), and operator or participant movement during testing. Instrument bias that has the potential to introduce nonrandom error includes dirty or damaged optics or loss of vacuum pressure due to instrument malfunction.
Application to Nursing Science
While assessment of the skin has been fundamental to the professional practice of nursing for decades, the protective role of viscoelasticity in injury and conceptual discussions of SVE are virtually absent in the nursing literature. A search of the Cochrane Library (2010; http://www.cochrane.org/) produced no reviews that focus on SVE in spite of a well-developed collection of reviews about diseases of the skin, pressure ulcers, and wounds. The Braden (1987) pressure ulcer risk assessment scale is comprised of six subscales (sensory perception, moisture, activity, mobility, nutrition, and friction/shear), none of which directly addresses viscoelasticity. Given that the biomechanical properties of the skin are intrinsic to skin health, it is critical that nurse scientists begin to work in this area.
Compelling reasons exist for broadening skin science in nursing to include SVE. According to the latest pressure ulcer statistics in the United States, approximately 159,000 nursing home residents have pressure ulcers (Park-Lee & Caffrey, 2009). The human and economic costs of pressure ulcers are high, as they may lead to health-compromising conditions such as sepsis, cellulitis, infectious arthritis, and squamous cell carcinoma. New strategies for prevention and treatment of pressure ulcers are needed.
SVE represents a promising avenue of exploration in determining the role of skin biomechanics in the development and management of pressure ulcers. Andersen and Karlsmark (2008) and Deprez, Brusseau, Fromageau, Cloutier, and Basset (2011) reported that tissues in early and later stages of breakdown are stiffer and less pliable than healthy tissues and suggest further research to explicate their findings. By learning more about SVE during skin breakdown and healing, clinicians can implement strategies to improve detection, prevention, and care.
The potential link between SVE and body mass also bears exploration. The relationship between pressure ulcers and body weight has been a topic of interest for a number of years (Compher, Kinosian, Ratcliffe, & Baumgarten, 2007; VanGilder, MacFarlane, Meyer, & Lachenbruch, 2009). Using data from the 2006 (N = 88,743) and 2007 (N = 79,193) International Pressure Ulcer Prevalence Surveys, VanGilder, MacFarlane, Meyer, and Lachenbruch (2009) did not find that Braden scores varied by body mass index (BMI; Braden & Bergstrom, 1987). However, they did find significant (p < .001) differences in pressure ulcer prevalence by body weight; participants in the lowest and normal BMI classes had the highest prevalence of pressure ulcers, 24.9% and 17.1%, respectively, as compared to an overall prevalence of 13.7%. Obese patients exhibited some degree of protection from pressure ulcers, but the role of viscoelasticity in the protective function is unknown. If, indeed, SVE explains the differences in prevalence, interventions to enhance viscoelasticity have promise in pressure ulcer prevention.
The role of nutrition in pressure ulcer prevention and healing has been documented by a recent meta-analysis of 15 studies reporting on oral or enteral nutritional supplements and pressure ulcer development (Biesalski, 2010). We could not locate any studies by investigators who reported on the relationship among SVE, nutrition, and BMI and its effect on pressure ulcer prevention, development, or healing; such studies could guide clinicians for improved pressure ulcer screening, prevention, and management.
The relationship between skin color and protection from skin injury is another area of interest to nursing science. The obstetrics literature reveals subtle indications that racial and/or ethnic differences may affect the prevalence of injury to the genital skin. Investigators reporting on differences in genital injury following vaginal births note that African Americans are less likely than Whites to have third- and fourth-degree perineal lacerations and tears (Howard, Davies, DeLancey, & Small, 2000; Robinson, Norwitz, Cohen, McElrath, & Lieberman, 1999). In several large studies of sexual assault and rape cases, authors have reported that White females have significantly more genital injury as compared to African American females (Cartwright, 1987; Coker & Richter, 1998). Sommers et al. (2009, 2008) found that, following consensual sexual intercourse, while race/ethnicity was a significant predictor of skin injury and prevalence, skin color itself confounded the relationship between race/ethnicity and injury and was a better predictor of injury than was race/ethnicity. Their sample with light skin had significantly more skin injuries than their sample with dark skin (p < .05). Baker, Fargo, Shambley-Ebron, and Sommers (2010) replicated these findings in female sexual assault victims. While several explanations exist for the differences in skin injury in women with light as compared to dark skin, one explanation is that viscoelasticity differs depending on skin color, and yet little is known about this phenomenon (Berardesca & Maibach, 1996). Clinically, if SVE varies by skin color, nursing assessment, injury detection, and pressure ulcer prevention also need to vary by skin color.
In short, there are multiple avenues for scientific exploration in the area of viscoelasticity that are relevant to nursing science and practice, including the associations of BMI, nutrition, and skin color with SVE and with injury, protection, and skin health. By highlighting their importance, we intend to engage researchers in these areas of evolving skin science. We also hope to engage clinicians to consider SVE as a parameter of interest when planning and providing patient care.
Summary
SVE is a physiologic concept grounded in the theories of skin biomechanics and reflects the complex interplay between microcellular structures of the human body and the physical forces that are regularly imposed on the skin. While several measurement strategies to quantify viscoelasticity are available to scientists, use of the Cutometer is feasible across bench and clinical settings, and scientists have confidence that the data are accurate and precise when error is controlled. As we learn more about SVE and how it is influenced by factors such as age, BMI, hydration, skin color, or disease processes, nurses can work within new standards of care that reflect varied injury (or healing) potential among individuals. Exploring the role of viscoelasticity in studies of the skin is a critical area for nursing science knowledge development.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge support from the National Institute of Nursing Research and the National Institute of Mental Health from the National Institutes of Health, Department of Health and Human Services, grants R01NR05352, R01NR011589, and F31NR011106, and the American Nurses Foundation, International Association of Forensic Nursing, and Emergency Nurses Association.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Agache P. Subcutis histophysiology. In: Agache P, Humbert P, editors. Measuring the skin. Berlin, Germany: Springer; 2006. pp. 401–409. [Google Scholar]
- Agache P, Diridollou S. Subcutis metrology. In: Agache P, Humbert P, editors. Measuring the skin. Berlin, Germany: Springer; 2006. pp. 410–424. [Google Scholar]
- Agache P, Varchon D. Mechanical behavior assessment. In: Agache P, Humbert P, editors. Measuring the skin. Berlin, Germany: Springer; 2004. pp. 447–467. [Google Scholar]
- Ahn S, Kim S, Lee H, Moon S, Chang I. Correlation between a Cutometer ((R)) and quantitative evaluation using Moire topography in age-related skin elasticity. Skin Research and Technology. 2007;13:280–284. doi: 10.1111/j.1600-0846.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular biology of the cell. 3. New York, NY: Garland Science; 1994. p. 116. [Google Scholar]
- Andersen ES, Karlsmark T. Evaluation of four non-invasive methods for examination and characterization of pressure ulcers. [Comparative Study Evaluation Studies] Skin Research and Technology. 2008;14:270–276. doi: 10.1111/j.1600-0846.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- Baker RB, Fargo JD, Shambley-Ebron D, Sommers MS. A source of healthcare disparity: Race, skin color, and injuries after rape among adolescents and young adults. Journal of Forensic Nursing. 2010;6:144–150. doi: 10.1111/j.1939-3938.2010.01070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barel AO, Lambrecht R, Clarys P. Mechanical function of the skin: State of the art. Skin Bioengineering. 1998;26:69–83. doi: 10.1159/000060577. [DOI] [PubMed] [Google Scholar]
- Barland CO, Zettersten E, Brown BS, Ye J, Elias PM, Ghadially R. Imiquimod-induced interleukin-1 alpha stimulation improves barrier homeostasis in aged murine epidermis. Journal of Investigative Dermatology. 2004;122:330–336. doi: 10.1046/j.0022-202X.2004.22203.x. [DOI] [PubMed] [Google Scholar]
- Berardesca E, Maibach H. Racial differences in skin pathophysiology. Journal of the American Academy of Dermatology. 1996;34:667–672. doi: 10.1016/s0190-9622(96)80070-3. [DOI] [PubMed] [Google Scholar]
- Biesalski H. Micronutrients, wound healing, and prevention of pressure ulcers. Nutrition. 2010;26:858. doi: 10.1016/j.nut.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Braden B, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehabilitation Nursing. 1987;12:8–12. doi: 10.1002/j.2048-7940.1987.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Brincat MP, Baron YM, Galea R. Estrogens and the skin. Climacteric. 2005;8:110–123. doi: 10.1080/13697130500118100. [DOI] [PubMed] [Google Scholar]
- Bruckner-Tuderman L, Stanley J. Epidermal and epidermal-dermal cohesion. In: Wolff K, Goldsmith L, Katz S, Gilchrest B, Paller A, Leffell D, editors. Fitzpatrick’s dermatology in general medicine. 7. Vol. 2. New York, NY: McGraw Hill Medical; 2007. pp. 447–459. [Google Scholar]
- C+ K. Information and operating instructions for the Cutometer MPA 580 and its probes. Koln, Germany: CK electronic GmbH; 2005. [Google Scholar]
- Cal K, Zakowiecki D, Stefanowska J. Advanced tools for in vivo skin analysis. International Journal of Dermatology. 2010;49:492–499. doi: 10.1111/j.1365-4632.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- Cartwright PS. Factors that correlate with injury sustained by survivors of sexual assault. Obstetrics and Gynecology. 1987;70:44–46. [PubMed] [Google Scholar]
- Chan LS. Human skin basement membrane in health and in autoimmune diseases. Frontiers in Bioscience. 1997;2:343–352. doi: 10.2741/a196. [DOI] [PubMed] [Google Scholar]
- Chu D. Overview of biology, development, and structure of skin. In: Wolff K, Goldsmith L, Katz S, Gilchrest B, Paller A, Leffell D, editors. Fitzpatrick’s dermatology in general medicine. 7. Vol. 1. New York, NY: McGraw-Hill Medical; 2007. pp. 57–72. [Google Scholar]
- Clancy NT, Nilsson GE, Anderson CD, Leahy MJ. A new device for assessing changes in skin viscoelasticity using indentation and optical measurement. Skin Research and Technology. 2010;16:210–228. doi: 10.1111/j.1600-0846.2010.00433.x. [DOI] [PubMed] [Google Scholar]
- Coker AL, Richter DL. Violence against women in Sierra Leone: Frequency and correlates of intimate partner violence and forced sexual intercourse. African Journal of Reproductive Health. 1998;2:61–72. [PubMed] [Google Scholar]
- Compher C, Kinosian B, Ratcliffe S, Baumgarten M. Obesity reduces the risk of pressure ulcers in elderly hospitalized patients. Journal of Gerontology. 2007;62A:1310–1312. doi: 10.1093/gerona/62.11.1310. [DOI] [PubMed] [Google Scholar]
- Cua AB, Wilhelm KP, Maibach HI. Elastic properties of human skin: Relation to age, sex, and anatomical region. Archives of Dermatological Research. 1990;282:283–288. doi: 10.1007/BF00375720. [DOI] [PubMed] [Google Scholar]
- Deprez JF, Brusseau E, Fromageau J, Cloutier G, Basset O. On the potential of ultrasound elastography for pressure ulcer early detection. Medical Physics. 2011;38:1943–1950. doi: 10.1118/1.3560421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev H. Application of Cutometer area parameters for the study of human skin fatigue. Skin Research and Technology. 2005;11:120–122. doi: 10.1111/j.1600-0846.2005.00090.x. [DOI] [PubMed] [Google Scholar]
- Dobrev H. In vivo study of skin mechanical properties in Raynaud’s phenomenon. Skin Research and Technology. 2007;13:91–94. doi: 10.1111/j.1600-0846.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Draaijers LJ, Botman YAM, Tempelman FRH, Kreis RW, Middelkoop E, van Zuijlen PPM. Skin elasticity meter or subjective evaluation in scars: A reliability assessment. Burns. 2004;30:109–114. doi: 10.1016/j.burns.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Edwards C, Marks R. Evaluation of biomechanical properties of human skin. Clinics in Dermatology. 1995;13:375–380. doi: 10.1016/0738-081x(95)00078-t. [DOI] [PubMed] [Google Scholar]
- Eisenbeiss C, Welzel J, Eichler W, Klotz K. Influence of body water distribution on skin thickness: Measurements using high-frequency ultrasound. British Journal of Dermatology. 2001;144:947–951. doi: 10.1046/j.1365-2133.2001.04180.x. [DOI] [PubMed] [Google Scholar]
- Everett JS, Fargo JD, Sommers MS. Mean skin elasticity and viscoelasticity ratios by anatomical location and age in 233 healthy female volunteers. 2011. Unpublished raw data. [Google Scholar]
- Falabella A, Falanga V. Wound healing. In: Freinkel RK, Woodley DT, editors. The biology of the skin. New York, NY: Parthenon Publishing Group; 2001. pp. 281–297. [Google Scholar]
- Fong SS, Hung LK, Cheng JC. The Cutometer and ultrasonography in the assessment of postburn hypertrophic scar—a preliminary study. Burns. 1997;23:S12–S18. doi: 10.1016/s0305-4179(97)90095-4. [DOI] [PubMed] [Google Scholar]
- Girard P, Beraud A, Sirvent A. Study of three complementary techniques for measuring cutaneous hydration in vivo in human subjects: NMR spectroscopy, transient thermal transfer and corneometry—application to xerotic skin and cosmetics. Skin Research and Technology. 2000;6:205–213. doi: 10.1034/j.1600-0846.2000.006004205.x. [DOI] [PubMed] [Google Scholar]
- Goh BK. Seasonal variations and environmental influences on the skin. In: Serup J, Jemec G, Grove G, editors. Handbook of non-invasive methods and the skin. 2. Boca Raton, FL: Taylor & Francis; 2006. pp. 33–36. [Google Scholar]
- Haake A, Scott GA, Holbrook KA. Structure and function of the skin: Overview of the epidermis and dermis. In: Freinkel RK, Woodley DT, editors. The biology of the skin. New York, NY: Parthenon Publishing Group; 2001. pp. 19–46. [Google Scholar]
- Hall G, Phillips TJ. Estrogen and skin: The effects of estrogen, menopause, and hormone replacement therapy on the skin. Journal of the American Academy of Dermatology. 2005;53:555–568. doi: 10.1016/j.jaad.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Howard D, Davies PS, DeLancey JO, Small Y. Differences in perineal lacerations in black and white primiparas. Obstetrics and Gynecology. 2000;96:622–624. doi: 10.1016/s0029-7844(00)00956-x. S0029-7844(00) 00956-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G. Mechanism of UVB-induced wrinkling of the skin: Paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. Journal of Investigative Dermatology Symposium Proceedings. 2009;14:36–43. doi: 10.1038/jidsymp.2009.11. jidsymp200911. [DOI] [PubMed] [Google Scholar]
- Johnsen GK, Haugsnes AB, Martinsen OG, Grimnes S. A new approach for an estimation of the equilibrium stratum corneum water content. Skin Research and Technology. 2010;16:142–145. doi: 10.1111/j.1600-0846.2009.00412.x. [DOI] [PubMed] [Google Scholar]
- Kochevar I, Taylor C, Krutman J. Fundamentals of cutaneous photobiology and photoimmunology. In: Wolff K, Goldsmith L, Katz S, Gilchrest B, Paller A, Leffell D, editors. Fitzpatrick’s dermatology in general medicine. 7. Vol. 2. New York, NY: McGraw Hill Medical; 2007. pp. 797–808. [Google Scholar]
- Langton AK, Sherratt MJ, Griffiths CE, Watson RE. A new wrinkle on old skin: The role of elastic fibres in skin ageing. International Journal of Cosmetic Science. 2010;32:330–339. doi: 10.1111/j.1468-2494.2010.00574.x. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Gerberick GF, Veres D, Irwin CJ, Kaidbey KH. Cumulative effects from repeated exposures to suber-ythemal doses of UVB and UVA in human skin. Journal of the American Academy of Dermatology. 1995;32:53–62. doi: 10.1016/0190-9622(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: Part I. Increased elastic tissue and solar elastotic syndromes. Journal of the American Academy of Dermatology. 2004;51:1–21. doi: 10.1016/j.jaad.2004.03.013S0190962204007728. [DOI] [PubMed] [Google Scholar]
- Micali G, Lacarrubba F, Bongu A, West D. The skin barrier. In: Freinkel R, Woodley DT, editors. The biology of the skin. New York, NY: Parthenon; 2001. pp. 219–232. [Google Scholar]
- Nedelec B, Correa JA, Rachelska G, Armour A, LaSalle L. Quantitative measurement of hypertrophic scar: Intrarater reliability, sensitivity, and specificity. Journal of Burn Care and Research. 2008;29:489–500. doi: 10.1097/BCR.0b013e3181710869. [DOI] [PubMed] [Google Scholar]
- Oikarinen A, Knuutinen A. Mechanical properties of human skin: Biochemical aspects. In: Elsner P, Berardesca E, Wilhelm K, Maibach HI, editors. Bioengineering of the skin: Skin biomechanics. Boca Raton, FL: CRC Press; 2002. [Google Scholar]
- Park-Lee E, Caffrey C. Pressure ulcers among nursing home residents: United States, 2004. NCHS Data Brief. 2009:1–8. [PubMed] [Google Scholar]
- Prost-Squarcioni C, Fraitag S, Heller M, Boehm N. Functional histology of dermis. Annales De Dermatologie Et De Venereologie. 2008;135:S5–S20. doi: 10.1016/S0151-9638(08)70206-0. [DOI] [PubMed] [Google Scholar]
- Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Epidural analgesia and third-or fourth-degree lacerations in nulliparas. Obstetrics and Gynecology. 1999;94:259–262. doi: 10.1016/s0029-7844(99)00259-8. S0029-7844(99)00259-8. [DOI] [PubMed] [Google Scholar]
- Ryu HS, Joo YH, Kim SO, Park KC, Youn SW. Influence of age and regional differences on skin elasticity as measured by the Cutometer (R) Skin Research and Technology. 2008;14:354–358. doi: 10.1111/j.1600-0846.2008.00302.x. [DOI] [PubMed] [Google Scholar]
- Schafer IA, Pandy M, Ferguson R, Davis BR. Comparative observation of fibroblasts derived from the papillary and reticular dermis of infants and adults: Growth kinetics, packing density at confluence and surface morphology. Mechanisms of Ageing and Development. 1985;31:275–293. doi: 10.1016/0047-6374(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Seehra GP, Silver FH. Viscoelastic properties of acid-and alkaline-treated human dermis: A correlation between total surface charge and elastic modulus. Skin Research and Technology. 2006;12:190–198. doi: 10.1111/j.0909-752X.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- Smalls LK, Wickett RR, Visscher MO. Effect of dermal thickness, tissue composition, and body site on skin biomechanical properties. Skin Research and Technology. 2006;12:43–49. doi: 10.1111/j.0909-725X.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Fargo JD, Baker RB, Fisher BS, Buschur C, Zink TM. Health disparities in the forensic sexual assault examination related to skin color. Journal of Forensic Nursing. 2009;5:191–200. doi: 10.1111/j.1939-3938.2009.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers MS, Zink TM, Fargo JD, Baker RB, Buschur C, Shambley-Ebron DZ, Fisher BS. Forensic sexualassault examination and genital injury: Is skin color a source of health disparity? American Journal of Emergency Medicine. 2008;26:857–866. doi: 10.1016/j.ajem.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwabe H, Serizawa A, Kajiwara H, Ohkido M, Tsutsumi Y. Degenerative processes of elastic fibers in sun-protected and sun-exposed skin: Immunoelectron microscopic observation of elastin, fibrillin-1, amyloid P component, lysozyme and anti-trypsin. Pathology International. 1999;49:391–402. doi: 10.1046/j.1440-1827.1999.00889.x. [DOI] [PubMed] [Google Scholar]
- Thakur R, Batheja P, Kaushik D, Michniak . Structural and biochemical changes in aging skin and their impact on skin permeability barrier. In: Dayan N, editor. Skin aging handbook. Norwich, NY: William Andrew; 2008. pp. 55–90. [Google Scholar]
- Tortora GJ, Grabowski SR. Principles of anatomy and physiology. 7. New York, NY: Harper Collins; 1993. [Google Scholar]
- Uitto J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. Journal of Drugs in Dermatology. 2008;7:s12–s16. [PubMed] [Google Scholar]
- Uitto J, Chu D, Gallo R, Eisen A. Collagen, elastic fibers, and the extracellular matrix of the dermis. In: Wolff K, Goldsmith L, Katz S, Gilchrest B, Paller A, Leffell D, editors. Fitzpatrick’s dermatology in general medicine. 7. Vol. 2. New York, NY: McGraw Hill Medical; 2007. pp. 517–542. [Google Scholar]
- VanGilder C, MacFarlane G, Meyer S, Lachenbruch C. Body mass index, weight, and pressure ulcer prevalence: An analysis ofthe2006–2007international pressure ulcer prevalence surveys. Journal of Nursing Care Quality. 2009;24:127–135. doi: 10.1097/01.NCQ.0000347449.83052.1a. [DOI] [PubMed] [Google Scholar]
- Wu KS, van Osdol WW, Dauskardt RH. Mechanical properties of human stratum corneum: Effects of temperature, hydration, and chemical treatment. Biomaterials. 2006;27:785–795. doi: 10.1016/j.biomaterials.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Wysocki A. Skin anatomy, physiology, and pathophysiology. Nursing Clinics of North America. 1999;34:777–797. [PubMed] [Google Scholar]
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest B, Krutman J, editors. Skin aging. Dusseldorf, Germany: Springer; 2006. pp. 9–22. [Google Scholar]
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: Skin physiology and skin manifestations of obesity. Journal of the American Academy of Dermatology. 2007;56:901–916. doi: 10.1016/j.jaad.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Zouboulis C, Makrantonaki E. The role of hormones in intrinsic aging. In: Gilchrest B, Krutman J, editors. Skin aging. Dusseldorf, Germany: Springer; 2006. pp. 55–64. [Google Scholar]