Abstract
Chlamydia trachomatis (Ct) is an obligate intracellular bacterial pathogen. Previously, we showed that infection of human trophoblast cells by Ct triggers the secretion of the pro-inflammatory cytokine, IL-1β. The aim of this study was to understand the innate immune pathways involved in trophoblast production of IL-1β after Ct infection. The approach we took was to inhibit the expression or function of the key Toll-like receptors (TLRs), Nod-like receptors (NLRs) and inflammasome components that have been associated with chlamydia infection. In this study we report that Ct-induced trophoblast IL-1β secretion is associated with the transcription of IL-1β mRNA, the translation and processing of pro-IL-1β, and the activation of caspase-1. In addition, we demonstrate that Ct-induced IL-1β production and secretion by the trophoblast is independent of TLR2, TLR4, MyD88, and the Nalp3/ASC inflammasome. Instead we report, for the first time, the importance of Nod1 for mediating trophoblast IL-1β secretion in response to a Ct infection.
INTRODUCTION
Chlamydia trachomatis (Ct) is an obligate intracellular gram-negative bacterium that is the leading cause of bacterial sexually transmitted infections1. Because it is largely asymptomatic, many individuals without access to routine healthcare go untreated. If untreated, Ct infection can lead to pelvic inflammatory disease, sterility, and ectopic pregnancy2. Moreover, chlamydia is increasingly becoming seen as an emerging pathogen during pregnancy because of its association with adverse pregnancy outcomes and vertical transmission3, 4. While Chlamydia mainly infects epithelial mucosa, it can also infect other cell types. Indeed, clinical studies have shown that Ct, as well as other strains like Chlamydia pneumoniae (C. pneumoniae), can be detected in placental tissue5–9, and experimental studies have demonstrated that Ct can infect human placental trophoblast cells10, 11. Thus, characterizing the innate immune response to Ct infection is important for understanding host-pathogen interactions. Furthermore, understanding the mechanisms by which Ct impacts trophoblast function may have clinical significance by helping us to better appreciate the progression of a Ct infection during pregnancy.
Microbes are initially sensed by cells of the innate immune system through binding to pattern recognition receptors (PRRs), such as the Toll-like receptors (TLRs) and Nod-like receptors (NLRs), resulting in a range of responses that include the secretion of pro-inflammatory cytokines and chemokines, the production of anti-microbial factors, and the induction of cell death by apoptosis, autophagy or pyroptosis12–14. However, non-immune cells, such as the trophoblast, also express TLRs and NLRs, and through these, can mount innate-immune responses towards pathogens and infectious components15, 16.
The TLRs are transmembranal PRRs, which allow for the extracellular and endosomal recognition of microbes or infectious components, and all but TLR3 signal through the adapter protein, MyD8817. The cytoplasmic-based NLRs function as intracellular receptors that respond to microbes or microbial components which have gained access to the cell’s cytoplasm18. The most well characterized NLRs in terms of microbial sensing are the Nod proteins, Nod1 and Nod2, and Nalp3 (Nlrp3). Nod1 and Nod2 induce inflammatory responses through the common adapter protein, Rip218. Nalp3 activation leads to the formation of the inflammasome, a protein platform that also contains the adapter protein, ASC (apoptosis-associated speck-like protein containing a CARD) and the non-apoptotic, caspase-1. Activation of the inflammasome specifically mediates the processing of intracellular pro-IL-1β (and pro-IL-18) into its active, secreted form19.
Several TLRs and NLRs have been reported to sense Ct and the related murine strain, C. muridarum. TLR2, which is normally located on the plasma membrane, and MyD88, are specifically recruited to the inclusion membrane during a productive infection and are required for Ct-induced IL-8 secretion20. Other studies have also implicated TLR2, TLR4 and MyD88 in chlamydia-mediated inflammation and pathology21–26. In addition to the TLR/MyD88 pathway, intracellular Nod1 has been shown to play a role in Ct-induced inflammation27, 28, while Nod1 and Nod2 can sense C. pneumoniae29, 30.Some studies report that Nalp3 is important for Ct-induced caspase-1 activation and IL-1β production29, 31.
We recently demonstrated that Ct infection of human trophoblast cells leads to the induction of IL-1β secretion11, which is consistent with studies in other cell types23, 29, 32,33. The trophoblast is also known to produce IL-1β via the inflammasome component, ASC in response to the Nalp3 agonist, monosodium urate (MSU)34. Therefore, the aim of this study was to understand the molecular basis for the secretion of the pro-inflammatory cytokine IL-1β after Ct infection of human trophoblast cells. The approach we took was to inhibit the expression or function of the key TLRs and NLRs that have been associated with Ct infection20–31, and to determine the effect on IL-1β production. In this study we report that Ct-induced IL-1β production in the trophoblast is independent of TLR2, TLR4, MyD88, Nalp3 and ASC. However, activation of Nod1 by Ct mediates IL-1β secretion by human trophoblast cells.
RESULTS
Chlamydia trachomatis infection of trophoblast cells induces IL-1β expression, processing, and secretion
For IL-1β to be secreted it must first be expressed as a precursor pro-IL-1β, which is then cleaved into its bioactive form; and typically IL-1β processing is mediated by active caspase-119. We, therefore, examined whether our previous observations of Ct-mediated IL-1β secretion by the trophoblast11 was associated with its induction and processing. For this we used two human first trimester trophoblast cell lines, HTR8 and Sw.71, which we had previously shown to be readily infected by Ct serovar D11. For this current study the trophoblast cells lines were either not infected (NI), or infected with a rifampin resistant strain of Ct serovar D at an MOI of 1, and after 24 hours, as we have previously published11, ~60% of the cells were infected (data not shown).
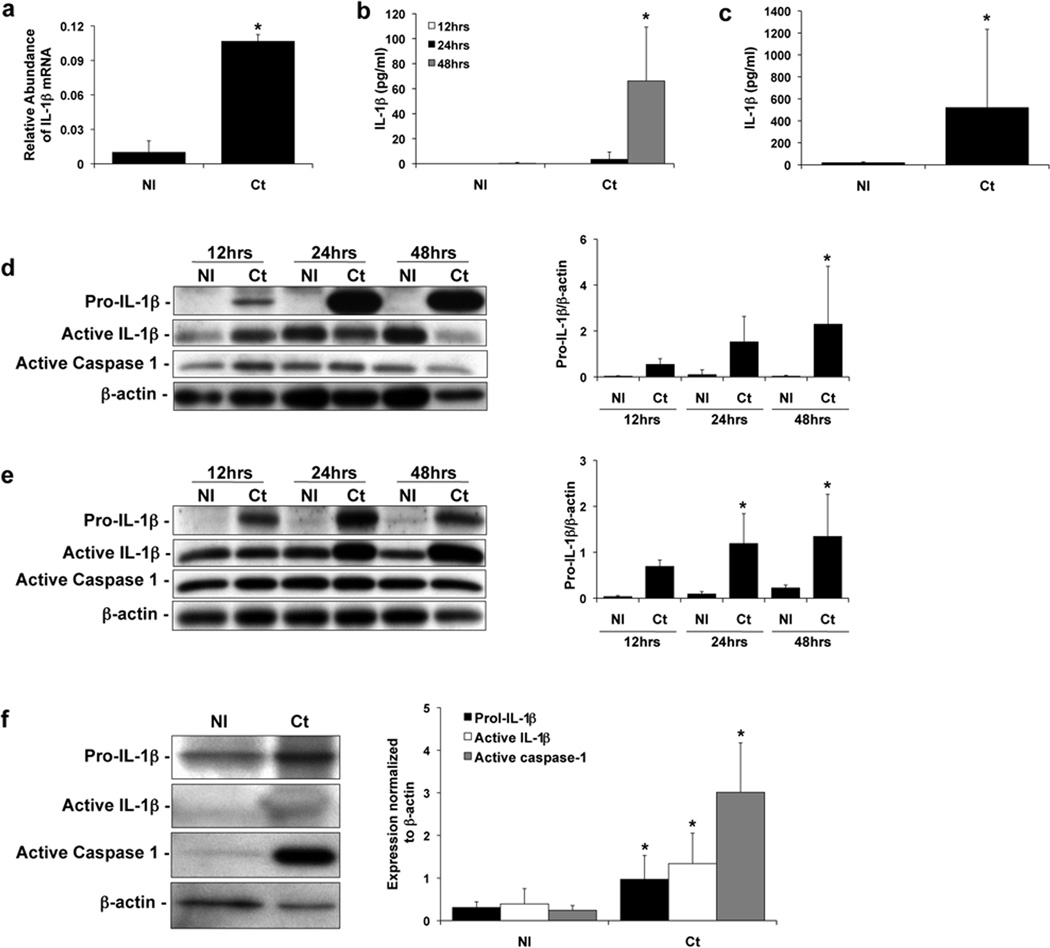
We found that after Ct infection, both mRNA, pro-IL-1β, and processed IL-1β were induced. After 24 hours, Ct infection of the HTR8 trophoblast cells significantly induced IL-1β mRNA expression (Figure 1a). Ct infection of the HTR8 and Sw.71 cells also significantly induced IL-1β secretion into the culture supernatants after 48 hours and 36 hours, respectively (Figure 1b & c). In addition to secreting IL-1β more rapidly, the Sw.71 cells secreted much higher levels of IL-1β than the HTR8 cells (Figure 1b & c)11. Next, we examined the levels of intracellular pro-IL-1β (31kDa), active-IL-1β (17kDa), and active-caspase-1 (20kDa) protein by Western blot analysis and densitometry. Uninfected HTR8 and Sw.71 trophoblast cells expressed no or minimal levels of pro-IL-1β, however, after Ct infection as early as 12 hours there was an induction of pro-IL-1β expression, and this continued to increase at 24 and 48 hours post infection (Figure 1d & e). Both the HTR8 and Sw.71 cells had detectable amounts of intracellular active IL-1β and active caspase-1, regardless of infection status, and levels were not significantly different between infected and uninfected cells (Figure 1d & e). Therefore, we examined IL-1β and caspase-1 protein expression in the culture supernatants by Western blot. Interestingly, in the uninfected the Sw.71 cell supernatants there were detectable levels of pro-IL-1β, but little if any active IL-1β or active caspase-1 (Figure 1f), unlike in the cell lysates (Figure 1e). In the Sw.71 cell supernatants 36 hours post-infection, however, there were significantly higher levels of pro-IL-1β, active-IL-1β and active caspase-1 compared to the non-infected cells (Figure 1f).
Figure 1. Chlamydia infection of human trophoblast cell lines leads to induction of IL-1β expression and IL-1β processing.
Trophoblast cells were either non-infected (NI) or infected with Chlamydia (Ct), after which supernatants were collected and either RNA or protein extracted from cells. (a) Ct infection of HTR8 cells significantly induced IL-1β mRNA expression after 24hrs as determined by qRT-PCR; and (b) significantly induced IL-1β secretion after 48hrs as determined by ELISA. (c) Ct infection of Sw.71 cells significantly induced IL-1β secretion after 36hrs (n=3; *p<0.05 relative to the NI control). Cell lysates from (d) HTR8 and (e) Sw.71 cells after either NI or infection with Ct were evaluated for pro-IL-1β (31kDa), active IL-1β (17kDa) and active caspase-1 (20kDa) expression by Western blot (representative blots are shown). Barcharts show quantification of protein expression as determined by densitometry and normalized to β-actin (n=3; *p<0.05 relative to the NI control). (f) Cell supernatants from Sw.71 cells either NI or infected with Ct for 36hrs were evaluated for pro- and active IL-1β and active caspase-1 expression by Western blot. Barcharts show quantification of protein expression as determined by densitometry and normalized to β-actin (n=4; *p<0.05 relative to the NI control).
Chlamydia trachomatis-induced trophoblast IL-1β expression and secretion is not dependent upon TLR2, TLR4, or MyD88
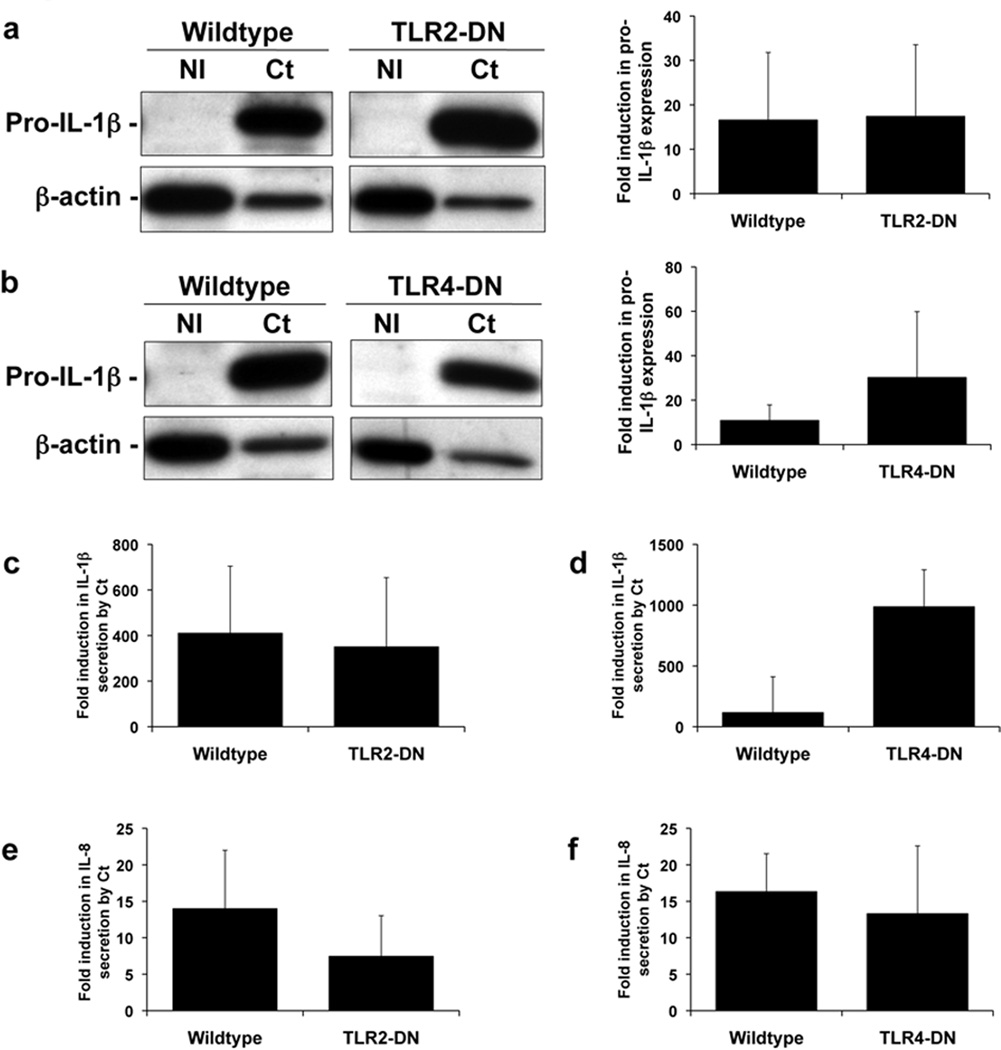
Having established that Ct infection of trophoblast cells led to the induction of IL-1β expression, processing, and secretion, we sought to determine which innate immune receptors activated by Ct were contributing to this response. PRRs that have been reported to be activated by chlamydia are TLR2, TLR4, Nod1, Nod2, and the Nalp3 inflammasome20–31. We first tested whether TLR2 and TLR4 were involved using trophoblast cell lines stably transfected to express a TLR2-dominant negative (DN) and TLR4-DN that we had previously created and characterized in the trophoblast cell line 3A and HTR8, respectively35–37. Using these cell lines we previously showed that TLR2 is involved in trophoblast cell apoptosis induced by gram-positive bacterial peptidoglycan37, because this was blocked in the TLR2-DN cells; and TLR4 mediates trophoblast anti-phospholipid antibody-induced inflammation36, because this was blocked in the TLR4-DN cells. There was no significant difference in the levels of Ct-induced intracellular pro-IL-1β in the TLR2-DN cells (Figure 2a) or TLR4-DN cells (Figure 2b) when compared to the wildtype cells. Similarly, there was no significant difference in the secreted levels of Ct-induced IL-1β or IL-8 in the TLR2-DN cells (Figure 2c & e) or TLR4-DN cells (Figure 2d & f) when compared to the wildtype cells.
Figure 2. Chlamydia-induced trophoblast pro-IL-1β expression and secretion of IL-1β and IL-8 is independent of TLR2 and TLR4.
Wildtype trophoblast (3A and HTR8) and trophoblast cells transfected to express either a TLR2-dominant negative (DN) (in 3A cells) or a TLR4-DN (in HTR8 cells) were either NI or infected with Ct for 48hrs. (a & b) Cell lysates were evaluated for pro-IL-1β expression by Western blot (representative blots are shown). Barcharts show quantification of pro-IL-1β levels, as determined by densitometry and normalized to β-actin. Culture supernatants were analyzed for (c & d) IL-1β and (e & f) IL-8 levels by ELISA. Data are pooled from three independent experiments and no significance was observed between the response of wildtype and TLR2-DN or TLR4-DN cells to Ct infection.
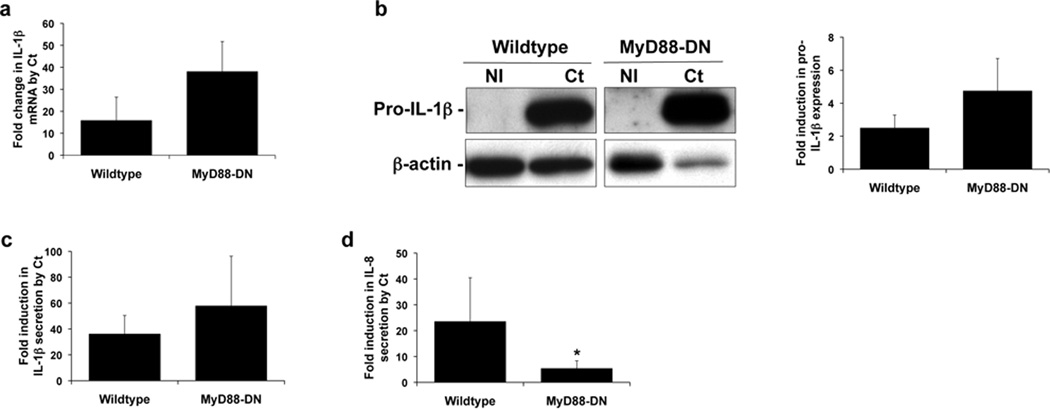
Since neither TLR2 nor TLR4 appeared to play a role in Ct-induced IL-1β production, we tested the potential role of other TLRs by inhibiting the function of the common adapter protein, MyD8817. For this we established a HTR8 trophoblast cell line, stably transfected to express a MyD88-DN, that we have previously characterized35, 36. The ability of Ct infection to induce trophoblast IL-1β RNA expression (Figure 3a) and intracellular pro-IL-1β protein expression (Figure 3b) was unaffected by the presence of the MyD88-DN. Similarly, the levels of Ct-induced IL-1β secretion was the same in the wildtype and MyD88-DN trophoblast cells (Figure 3c). However, the presence of the MyD88-DN significantly reduced the ability of Ct infection to induce trophoblast IL-8 secretion (Figure 3d).
Figure 3. Chlamydia-induced trophoblast IL-8 secretion, but not IL-1β expression or secretion is dependent on MyD88.
Wildtype HTR8 trophoblast and HTR8 cells transfected to express a MyD88-DN were either NI or infected with Ct for 48hrs. (a) RNA was analyzed for IL-1β mRNA levels by qRT-PCR; (b) cell lysates were analyzed for pro-IL-1β expression levels by Western blot (representative blots are shown) and densitometry; and culture supernatants were measured for (c) IL-1β and (d) IL-8 by ELISA. Data are pooled from three independent experiments; *p<0.05 relative to the wildtype cells.
Chlamydia trachomatis-induced trophoblast IL-1β secretion is not dependent upon ASC or Nalp3
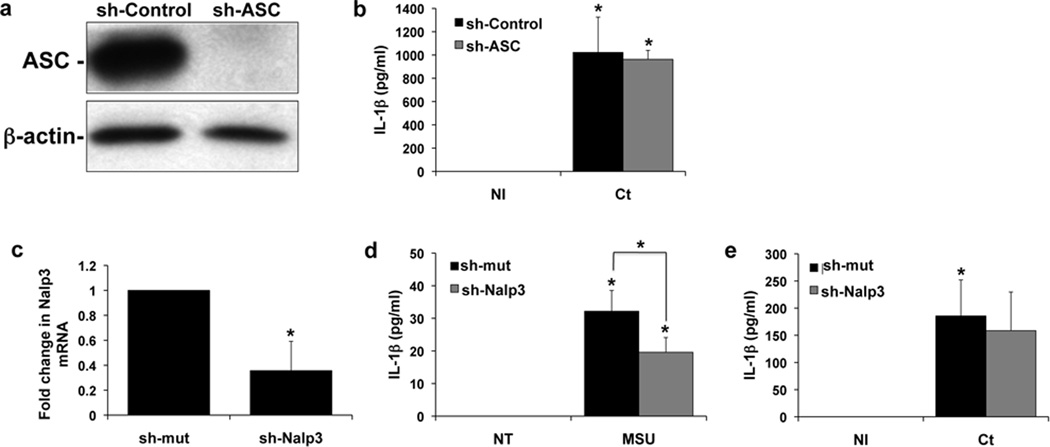
Having established that the TLR/MyD88 pathway was not involved in Ct-induced IL-1β production, we investigated the potential role of the Nalp3 inflammasome. We have previously reported that the Nalp3 agonist, MSU, can induce IL-1β production via ASC in the Sw.71 trophoblast cell line, suggesting that the Nalp3 inflammasome is functional in these cells34. Thus, for this current study we used the same stable trophoblast cells expressing either shRNA specific for ASC (sh-ASC) or a non-targeting control (sh-control), and by Western blot found good knockdown of the ASC protein (Figure 4a). Nonetheless, there was no significant difference in the levels of secreted IL-1β in the sh-ASC cells when compared to the sh-control cells after Ct infection (Figure 4b). In addition, the HTR8 cells, which secrete IL-1β in response to Ct infection (Figure 1b)11, lack ASC protein34. Stable cell lines expressing either shRNA specific for Nalp3 (sh-Nalp3) or a non-targeting control (sh-mut) were also established. We were unable to determine Nalp3 protein knockdown since, to our knowledge, there are no reliable commercially available antibodies to Nalp3. However, Nalp3 mRNA levels were significantly lower in the sh-Nalp3 cells when compared to the sh-mut cells (Figure 4c). While MSU-induced IL-1β secretion was significantly reduced in the sh-Nalp3 cells when compared to the sh-mut cells (Figure 4d), we could not detect an effect on Ct-induced IL-1β secretion (Figure 4e) in the cells containing Nalp3 shRNA.
Figure 4. Chlamydia-induced trophoblast IL-1β secretion is not dependent on ASC or Nalp3.
(a–b) Sw.71 trophoblast cells were transfected to express either shRNA for ASC (sh-ASC) or a control sequence (sh-control) (a) Western blot of lysates from the Sw.71 trophoblast cells expressing either sh-control or sh-ASC for ASC expression. β-actin served as a loading control. (b) 36 hrs after either NI or Ct infection, levels of secreted IL-1β in the supernatants of sh-control and sh-ASC cells were measured by ELISA. n=3; *p<0.05 relative to the NI control. (c–d) Sw.71 trophoblast cells were transfected to express either shRNA for Nalp3 (sh-Nalp3) or a control sequence (sh-control). (c) RNA from the Sw.71 trophoblast cells expressing either sh-control or sh-NAlp3 was analyzed for Nalp3 mRNA expression by qRT-PCR. n=3; *p<0.05 relative to the sh-control. (d) 72hrs after either no treatment (NT) or treatment with MSU (100µg/ml) levels of secreted IL-1β in the supernatants of sh-control and sh-Nalp3 cells were measured by ELISA. n=3; *p<0.05 relative to the NT control unless otherwise indicated. (e) 36 hrs after either NI or Ct infection, levels of secreted IL-1β in the supernatants of sh-control and sh-Nalp3 cells were measured by ELISA. n=3; *p<0.05 relative to the NI control.
IL-1β secretion after trophoblast Chlamydia trachomatis infection is mediated by Nod1
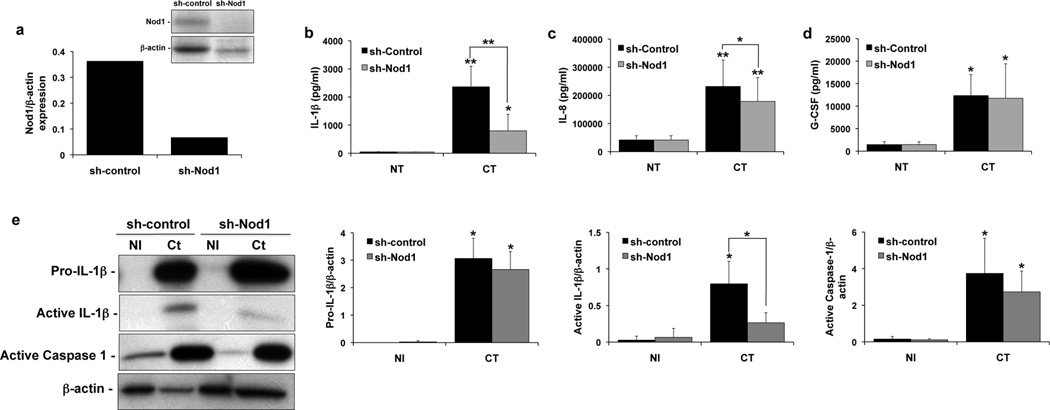
Both the Sw.71 and HTR8 trophoblast cell lines express Nod1, whereas the Sw.71 cells, but not the HTR8 cells, express Nod238. Having found that neither Nalp3 nor ASC were involved in the Ct-induced IL-1β response, we, therefore, turned our attention to determining if Ct stimulation of Nod1 could be inducing IL-1β secretion. Thus, a stable Sw.71 cell line expressing shRNA directed against Nod1 was established (sh-Nod1). As shown in Figure 5a, there was good reduction of Nod1 protein expression in the sh-Nod1 cells when compared to the sh-control cells. At 36 hours post-infection, secretion of IL-1β (Figure 5b) and IL-8 (Figure 5c) was significantly reduced in the sh-Nod1 cells when compared to the sh-control. In contrast, Ct-induced G-CSF secretion was unaffected by Nod1 knockdown (Figure 5d). As shown in Figure 5e, analysis of the supernatants by Western blot revealed that Nod1 knockdown significantly reduced the secreted levels of active IL-1β induced by Ct infection. However, there was no significant difference in the Ct-induction of secreted pro-IL-1β or active caspase-1 in the sh-Nod1 cells when compared to the sh-control cells. Therefore, signaling through the Nod1 receptor after Ct infection of human trophoblast cells is important for IL-1β secretion.
Figure 5. Chlamydia-induced trophoblast IL-1b processing and secretion is dependent on Nod1.
Sw.71 trophoblast cells were transfected to express either shRNA for Nod1 (sh-Nod1) or a control sequence (sh-control) and were either NI or infected with Ct for 36hrs. (a) Expression of Nod1 protein in the sh-control and sh-Nod1 cells was determined by Western blot analysis, and barchart shows densitometry with Nod1 expression normalized to β-actin levels. (b–d) Barcharts show levels of secreted IL-1β, IL-8 and G-CSF in the supernatants of the sh-control and sh-Nod1 cells. (e) Supernatants were evaluated for pro- and active IL-1β and active caspase-1 expression by Western blot. Barcharts show quantification of protein expression in the sh-control and sh-Nod1 cells as determined by densitometry and normalized to β-actin. n=4; *p<0.05; **p<0.001 relative to the NI control unless otherwise indicated.
DISCUSSION
The trophoblast develops from the blastocyst to form the placenta. These cells provide an interface between the fetus and the mother and invade deep into the maternal endometrium (decidua) to remodel the maternal vasculature39. They are distinctive cells that constitutively secrete chemokines, such as IL-8, MCP-1, and GRO-α, which are important for the normal recruitment of innate immune cells to the maternal-fetal interface, in the absence of a threat40. Like cells of the innate immune system, the trophoblast express many functional PRRs and are able to sense a wide variety of pathogens and infectious components, leading to a range of responses from pro-inflammatory and anti-microbial/viral, to pro-apoptotic15, 16. We previously demonstrated that Ct infection of human first trimester trophoblast cells induces secretion of the potent pro-inflammatory cytokine, IL-1β11. In this current study, we report that Ct-induced trophoblast IL-1β secretion is associated with the transcription of IL-1β mRNA, the translation and processing of pro-IL-1β, and the activation of caspase-1. In addition, we report, for the first time, the importance of Nod1 for mediating trophoblast IL-1β secretion in response to a Ct infection.
Using two human first trimester trophoblast cell lines (HTR8 and Sw.71) we found that Ct infection increases IL-1β mRNA expression and induces the expression of intracellular pro-IL1β protein. Interestingly, while non-infected trophoblast cells had little or no detectable levels of intracellular pro-IL-1β, we were able to detect intracellular active caspase-1 and active IL-1β. However, Ct infection had no effect on the intracellular expression levels of active caspase-1 and IL-1β. In contrast, active IL-1β and active caspase-1 were detected in trophoblast culture supernatants, but only following Ct infection. These findings suggest that there are intracellular stores of bioactive IL-1β in the trophoblast that are released soon after interaction with Ct; and that IL-1β secretion in response to Ct infection is further increased over time following the strong induction of pro-IL-1β expression, active caspase-1 and IL-1β processing. These observations in the trophoblast are supported by studies in monocytes showing that these cells exhibit constitutive caspase-1 activation, leading to the rapid release of active IL-1β after a single signal19, and that active caspase-1 can be externalized41.
In order to elucidate the molecular mechanism for the production and secretion of IL-1β by the trophoblast after Ct infection of human trophoblast, we targeted PRRs known to be associated with cellular responses to chlamydia20–31. Although TLRs are normally located on the plasma membrane, TLR2 and MyD88 can localize to the inclusion membrane of Ct-infected cells and mediate cytokine production20. In a model of genital tract infection, TLR2 was shown to be important for Ct-induced cytokine production and oviduct pathology, without affecting the course of infection. In contrast, TLR4 knockout mice had normal pathology22. MyD88, which is part of the signaling pathways of most TLRs and the IL-1 receptor (IL-1R)17, has also been shown to be important for Ct-induced cytokine production, including IL-1β21, 29. However, in our system, using dominant-negative cells lines, previously characterized35–37, we found that the inhibition of TLR2, TLR4 or MyD88 function had no effect on the ability of Ct to induce intracellular pro-IL-1β expression or IL-1β secretion.
Since the TLR/MyD88 pathway was not involved in Ct-induced IL-1β production by the trophoblast, we turned our attention to the role of the Nalp3/ASC inflammasome. In other cell types, Ct-induced IL-1β transcription, processing, and secretion have been shown to be mediated by the Nalp3/ASC inflammasome29, 31, and Ct-induced caspase-1 activation and IL-1β processing is associated with pathology in vivo42. Similarly, C. pneumoniae infection of macrophages induces IL-1β via the Nalp3 inflammasome43, 44, but in contrast to the Ct model, caspase-1-mediated IL-1β secretion is critical for resolution of the infection and protection against pathology43, 44. Nonetheless, these studies have demonstrated a role for Nalp3 and ASC in chlamydia-induced IL-1β. By knocking down Nalp3 and ASC expression in the Sw.7 trophoblast cell line, we found that Ct-induced intracellular pro-IL-1β expression and IL-1β secretion were not dependent on either Nalp3 or ASC. This was further supported by our observation that the HTR8 cell line, which lacks ASC34, also produces IL-1β in response to Ct infection11. This lack of Nalp3 and ASC involvement in Ct-triggered IL-1β production by the Sw.71 cells is not due to the absence of inflammasome function in these cells. The Nalp3 agonist MSU was able to induce IL-1β secretion in Sw.71 cells, and this secretion was inhibited when Nalp3 and ASC expression were reduced34.
Another group of PRRs associated with chlamydia infection is the Nod proteins, Nod1 and Nod2, which recognize peptidoglycan peptides16. While peptidoglycan has not been detected biochemically in chlamydiae, both C. trachomatis and C. muridarum produce a rudimentary proteoglycan motif that is recognized by Nod1, leading to activation of NFκB and pro-inflammatory cytokines27, 28. In addition, C. pneumoniae has been shown to induce an inflammatory response via Nod1 and Nod230. However, none of these studies reported whether the Nod proteins were involved in chlamydia-induced IL-1β production. Since the HTR8 trophoblast cells produce IL-1β after Ct infection, but lack Nod238, we focused on the role of trophoblast Nod1 in Ct-induced IL-1β. While knockdown of Nod1 expression in the Sw.71 trophoblast cells inhibited Ct-induced IL-1β secretion, we did not observe a decrease in either pro-IL-1β expression or caspase-1 activation. Because the Nod1 protein has a CARD domain its activation can lead to activation of caspase-1 and IL-1β production45. This dichotomy could have several explanations. Firstly, there are other NLRs that activate caspase-1 in an ASC-independent manner, such as Nlrc4 (Ipaf) or NLRs that have a CARD but not a pyrin domain19. Thus, other NLRs, in addition to Nod1, may be triggered by Ct infection. Secondly, Ct-induced IL-1β processing in the trophoblast may be independent of caspase-1. Indeed, alternative mechanisms for Ct-induced IL-1β maturation include cleavage by serine proteases, typically produced by neutrophils and monocytes46, and matrix metalloproteinases (MMPs), such as MMP-9 and MMP-247, which are highly expressed by the trophoblast48.
How Nod1 is triggering trophoblast secretion of IL-1β following Ct infection still remains to be determined. Since IL-1β does not have a hydrophobic signal sequence required for entering the classical secretory pathway, hypothesized non-classical pathways of IL-1β release involve exocytosis of secretory lysosomes or exosomes, shedding of plasma membrane microvesicles, and export through the plasma membrane using specialized transporters49. Trophoblast cells are known to constitutively release microvesicles and exosomes under normal physiological conditions, and this shedding from the placenta is elevated in pathological pregnancies50. Moreover, these microparticles are known to contain bioactive proteins50,51. In addition, Ct infection of THP-1 macrophages induced non-classical secretion of macrophage migration inhibitory factor (MIF) by association with a golgi complex protein p11552. Therefore, Nod1-induced IL-1β release by Ct infected trophoblast cells may be mediated by non-classical export pathways.
Another interesting finding to come out of these studies was that Ct-induced IL-8 secretion by the trophoblast was independent of TLR2 and TLR4, but dependent upon MyD88 and Nod1. One possible explanation for this finding is that induction of IL-8 may proceed through an IL-1β autocrine loop, since the IL-1R signals through MyD8817. This has been reported in other systems. In macrophages the Nod2 agonist, MDP, induces IL-1β secretion which in turn triggers the production of other cytokines, including IL-853. Interestingly, although Nod1 mediated Ct-induced secretion of IL-1β and IL-8, Ct-induced G-CSF secretion occurred independently of Nod1, again highlighting that there may be multiple immune pathways activated upon Ct infection.
There is a well-established link between bacterial infections and adverse pregnancy outcomes, such as preterm birth54, and IL-1β is an important mediator of preterm labor55. Therefore, understanding the innate immune pathways activated by pathogens that lead to IL-1β production is important. Ct and C. pneumoniae have been detected in the decidual and placental tissues from complicated pregnancies6–9 and in mice, Ct infection triggers preterm delivery56. Therefore, the ability of chlamydia to induce IL-1β at the maternal-fetal interface could have a negative impact on pregnancy outcome and a woman’s reproductive potential. Our work establishes that Ct infection of the trophoblast activates the Nod1 pathway, leading to IL-1β secretion. Since functional Nod1 is expressed by the trophoblast across gestation57, it can be a target for pathogens throughout pregnancy. Indeed, administration of the Nod1 agonist, iE-DAP, to pregnant mice induces preterm delivery, reduces fetal weight, and induces an inflammatory response57. Thus, targeting the Nod1 pathway may be useful to diminish preterm labor associated with bacterial infections.
MATERIALS AND METHODS
Reagents and antibodies
Monosodium urate (MSU) was purchased from InvivoGen (San Diego, CA). Rabbit polyclonal antibody to IL-1β (#2022), which recognizes the full-length pro and the processed active forms, was purchased from Cell Signaling Inc. (Danvers, MA). Rabbit polyclonal antibody to active caspase-1 #06–503) was purchased from Upstate (Lake Placid, NY). The rabbit anti-ASC polyclonal antibody was obtained from Calbiochem (Gibbstown, NJ). The rabbit polyclonal antibody recognizing Nod1 was purchased from (Imgenex, San Diego, CA). The rabbit polyclonal antibody for human β-actin was purchased from Sigma (St Louis, MO).
Trophoblast cell lines
Three human first trimester trophoblast cell lines were used in these studies: the SVneo-transformed HTR8 cells58; the SVneo-transformed 3A cells37; and telomerase-transformed Sw.71 cells59. HTR8 and 3A cells were cultured in RPMI and Sw.71 cells were cultured in DMEM (Gibco-Invitrogen; Grand Island, NY). Both media were supplemented with 10% fetal bovine serum (Hyclone, South Logan, UT), 10mM Hepes, 0.1mM MEM non-essential amino acids, 1mM sodium pyruvate and 100nm penicillin/streptomycin (Gibco-Invitrogen). Cells were maintained at 37°C/5% CO2. In previous studies we have shown that the HTR8 cells constitutively lack Nod2 and ASC expression34, 38.
Stably transfected trophoblast cell lines
HTR8 cells were stably transfected with either the pDeNy plasmid containing the human MyD88 dominant negative (MyD88-DN), or the pZERO plasmid containing the human TLR4-DN (Invivogen), as previously described35, 36. The 3A cells were stably transfected with the pZERO plasmid containing the human TLR2-DN (Invivogen) as previously described37. The Sw.71 cells were stably transfected with the pLKO.1 expression plasmids containing the ASC-shRNA construct, NM_013258.3-718s1c1 (sh-ASC), or a non-target shRNA control, SHC002 (sh-control) from Sigma Aldrich (St Louis, MO), as previously described34. The Sw.71 cells were also transfected with specific shRNA Nalp3-shRNA (sh-Nalp3) or a mutated targeting sequence as a negative control (sh-mut), which were both a kind gift from Dr Jenny Ting (Lineberger Comprehensive Cancer Center, Chapel Hill, NC)60. Knockdown of Nod1 (sh-Nod1) was performed using a specific shRNA targeting sequence, NOD1 NM_006092.1 from Sigma.
Infection of trophoblast cells with Chlamydia trachomatis
Chlamydia trachomatis (Ct) serovar D (rifampin-resistant) was a gift from Dr. Robert DeMars (University of Wisconsin, Madison, WI). Ct was propagated in HeLa cells grown in antibiotic-free DMEM (GIBCO-Invitrogen) and stock cultures generated as previously described11. Trophoblast cells (1 × 105) were seeded into wells of a 6-well plate and allowed to attach overnight. The next day, the cells were washed with 10ml of PBS and infected at a multiplicity of infection (MOI) of 1 in 2 ml of SPG by spinning at 350 × g at 8–10°C for 40 min in an Eppendorf centrifuge with a plate holder. This MOI was calculated based on an assay performed using HeLa cells11. The trophoblast cells were then washed with PBS to remove any unattached bacteria. Fresh serum-free OptiMEM (Gibco-Invitrogen) was then added to the plates, and the cells were cultured at 37°C for 12 – 48 hours, after which cell-free supernatants were collected and cells lysed for either RNA or protein isolation. Infection rates were determined by staining the cells with an anti-LPS antibody to Ct, and analysis performed by flow cytometry as previously described11.
Quantitative real-time RT-PCR
Total RNA was isolated from cells using the RNeasy Mini Prep kit from Qiagen following the manufacturer’s protocol. Reverse transcription of the RNA into cDNA was done on 5µg of total RNA using the Sprint RT Complete Oligo (dT) 18 kit from Clontech (Mountain View, CA) following the manufacturer’s protocol. Quantitative real-time RT-PCR (qRT-PCR) was performed using commercially available Applied Biosystems (Carlsbad, CA) probes for IL-1β (Hs00174097_m1), Nalp3 (Hs00918082_m1), and the control GAPDH (Hs99999905_m1), Taqman Universal Mastermix, and a Thermal Cycler (iQ5; Bio-Rad Laboratories, Hercules, CA). Samples were analyzed in duplicate and the target gene expression was normalized to GAPDH.
Western blot analysis
For analysis of proteins by Western blot, proteins diluted to 20µg with gel loading buffer were boiled for 5 minutes and resolved under reducing conditions on 12% SDS-PAGE gels and then transferred onto PVDF paper (PerkinElmer, Boston, MA). Membranes were blocked with 5% fat-free powdered milk (FFPM) in PBS/0.05% Tween-20 (PBS-T). Following washes with PBS-T, membranes were incubated overnight at 4°C with primary antibody in PBS-T/1% FFPM. Following this incubation, membranes were washed as before and then incubated with the goat anti-rabbit IgG secondary antibody conjugated to peroxidase (Vector Labs; Burlingame, CA) in PBS-T/1% FFPM. Following washes with PBS-T and then with distilled water, the peroxidase-conjugated antibody was detected by enhanced chemiluminescence (PerkinElmer). β-actin was used as internal control, in addition to Ponseau Red, to validate the amount of protein loaded onto the gels. Images were recorded and semi-quantitative densitometry performed using the Gel Logic 100 and Kodak MI software (Eastman Kodak, Rochester, NY).
Cytokine studies
Trophoblast culture supernatants were analyzed for IL-1β and IL-8 using commercial ELISA kits from R&D Systems (Minneapolis, MN) and Enzo Life Sciences (Farmingdale, NY), respectively. IL-1β, IL-8 and G-CSF were also measured using the Human BioPLex assay (BioRad) with detection and analysis performed using the Luminex 100 IS system (Millipore, Billerica, MA).
Statistical analysis
Data are expressed as mean ± SD. All experiments were performed at least three times and post-analysis data was pooled. Statistical significance (p<0.05) was determined using either Student’s t tests or, for multiple comparisons, one-way ANOVA followed by Bonferroni’s post-test.
ACKNOWLEDGMENTS
This work was supported by NIH/NIAID grants R01AI049571 (to PBK) and NIH/NICHD grants RO1HD049446 and PO1HD054713 (to VMA).
Footnotes
The authors have no financial conflicts of interest.
REFERENCES
- 1.Mylonas I. Female genital Chlamydia trachomatis infection: where are we heading? Arch Gynecol Obstet. 2012 Feb 19; doi: 10.1007/s00404-012-2240-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Wiesenfeld HC, Hillier SL, Krohn MA, Amortegui AJ, Heine RP, Landers DV, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100(3):456–463. doi: 10.1016/s0029-7844(02)02118-x. [DOI] [PubMed] [Google Scholar]
- 3.Baud D, Regan L, Greub G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr Opin Infect Dis. 2008;21(1):70–76. doi: 10.1097/QCO.0b013e3282f3e6a5. [DOI] [PubMed] [Google Scholar]
- 4.Howie SE, Horner PJ, Horne AW. Chlamydia trachomatis infection during pregnancy: known unknowns. Discov Med. 2011;12(62):57–64. [PubMed] [Google Scholar]
- 5.Dong ZW, Li Y, Zhang LY, Liu RM. Detection of Chlamydia trachomatis intrauterine infection using polymerase chain reaction on chorionic villi. Int J Gynaecol Obstet. 1998;61(1):29–32. doi: 10.1016/s0020-7292(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 6.Magon T, Kluz S, Chrusciel A, Obrzut B, Skret A. The PCR assessed prevalence of Chlamydia trachomatis in aborted tissues. Med Wieku Rozwoj. 2005;9(1):43–48. [PubMed] [Google Scholar]
- 7.Gomez LM, Parry S. Trophoblast infection with Chlamydia pneumoniae and adverse pregnancy outcomes associated with placental dysfunction. Am J Obstet Gynecol. 2009;200(5):526, e521–e527. doi: 10.1016/j.ajog.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baud D, Goy G, Jaton K, Osterheld MC, Blumer S, Borel N, et al. Role of Chlamydia trachomatis in miscarriage. Emerg Infect Dis. 2011;17(9):1630–1635. doi: 10.3201/eid1709.100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rours GI, de Krijger RR, Ott A, Willemse HF, de Groot R, Zimmermann LJ, et al. Chlamydia trachomatis and placental inflammation in early preterm delivery. Eur J Epidemiol. 2011;26(5):421–428. doi: 10.1007/s10654-011-9569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azenabor AA, Kennedy P, Balistreri S. Chlamydia trachomatis infection of human trophoblast alters estrogen and progesterone biosynthesis: an insight into role of infection in pregnancy sequelae. Int J Med Sci. 2007;4(4):223–231. doi: 10.7150/ijms.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Torre E, Mulla MJ, Yu AG, Lee SJ, Kavathas PB, Abrahams VM. Chlamydia trachomatis infection modulates trophoblast cytokine / chemokine production. J Immunol. 2009;182(6):3735–3745. doi: 10.4049/jimmunol.0800764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salaun B, Romero P, Lebecque S. Toll-like receptors' two-edged sword: when immunity meets apoptosis. Eur J Immunol. 2007;37(12):3311–3318. doi: 10.1002/eji.200737744. [DOI] [PubMed] [Google Scholar]
- 13.Bortoluci KR, Medzhitov R. Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell Mol Life Sci. 2010;67(10):1643–1651. doi: 10.1007/s00018-010-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 15.Abrahams VM. Pattern recognition at the maternal-fetal interface. Immunol Invest. 2008;37(5):427–447. doi: 10.1080/08820130802191599. [DOI] [PubMed] [Google Scholar]
- 16.Abrahams VM. The role of the Nod-like receptor family in trophoblast innate immune responses. J Reprod Immunol. 2011;88(2):112–117. doi: 10.1016/j.jri.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Brikos C, O'Neill LA. Signalling of toll-like receptors. Handb Exp Pharmacol. 2008;(183):21–50. doi: 10.1007/978-3-540-72167-3_2. [DOI] [PubMed] [Google Scholar]
- 18.Philpott DJ, Girardin SE. Nod-like receptors: sentinels at host membranes. Curr Opin Immunol. 2010;22(4):428–434. doi: 10.1016/j.coi.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6(2):e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connell CM, Ionova IA, Quayle AJ, Visintin A, Ingalls RR. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J Biol Chem. 2006;281(3):1652–1659. doi: 10.1074/jbc.M510182200. [DOI] [PubMed] [Google Scholar]
- 21.Nagarajan UM, Ojcius DM, Stahl L, Rank RG, Darville T. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J Immunol. 2005;175(1):450–460. doi: 10.4049/jimmunol.175.1.450. [DOI] [PubMed] [Google Scholar]
- 22.Darville T, O'Neill JM, Andrews CW, Jr, Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171(11):6187–6197. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 23.Bas S, Neff L, Vuillet M, Spenato U, Seya T, Matsumoto M, et al. The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. J Immunol. 2008;180(2):1158–1168. doi: 10.4049/jimmunol.180.2.1158. [DOI] [PubMed] [Google Scholar]
- 24.Shaw JL, Wills GS, Lee KF, Horner PJ, McClure MO, Abrahams VM, et al. Chlamydia trachomatis infection increases fallopian tube PROKR2 via TLR2 and NFkappaB activation resulting in a microenvironment predisposed to ectopic pregnancy. Am J Pathol. 2011;178(1):253–260. doi: 10.1016/j.ajpath.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackern-Oberti JP, Maccioni M, Cuffini C, Gatti G, Rivero VE. Susceptibility of prostate epithelial cells to Chlamydia muridarum infection and their role in innate immunity by recruitment of intracellular Toll-like receptors 4 and 2 and MyD88 to the inclusion. Infect Immun. 2006;74(12):6973–6981. doi: 10.1128/IAI.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romano Carratelli C, Mazzola N, Paolillo R, Sorrentino S, Rizzo A. Toll-like receptor-4 (TLR4) mediates human beta-defensin-2 (HBD-2) induction in response to Chlamydia pneumoniae in mononuclear cells. FEMS Immunol Med Microbiol. 2009;57(2):116–124. doi: 10.1111/j.1574-695X.2009.00586.x. [DOI] [PubMed] [Google Scholar]
- 27.Buchholz KR, Stephens RS. The cytosolic pattern recognition receptor NOD1 induces inflammatory interleukin-8 during Chlamydia trachomatis infection. Infect Immun. 2008;76(7):3150–3155. doi: 10.1128/IAI.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welter-Stahl L, Ojcius DM, Viala J, Girardin S, Liu W, Delarbre C, et al. Stimulation of the cytosolic receptor for peptidoglycan, Nod1, by infection with Chlamydia trachomatis or Chlamydia muridarum. Cell Microbiol. 2006;8(6):1047–1057. doi: 10.1111/j.1462-5822.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 29.Abdul-Sater AA, Said-Sadier N, Padilla EV, Ojcius DM. Chlamydial infection of monocytes stimulates IL-1beta secretion through activation of the NLRP3 inflammasome. Microbes Infect. 2010;12(8–9):652–661. doi: 10.1016/j.micinf.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opitz B, Forster S, Hocke AC, Maass M, Schmeck B, Hippenstiel S, et al. Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ Res. 2005;96(3):319–326. doi: 10.1161/01.RES.0000155721.83594.2c. [DOI] [PubMed] [Google Scholar]
- 31.Abdul-Sater AA, Koo E, Hacker G, Ojcius DM. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. J Biol Chem. 2009;284:26789–26796. doi: 10.1074/jbc.M109.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Westreenen M, Pronk A, Diepersloot RJ, de Groot PG, Leguit P. Chlamydia trachomatis infection of human mesothelial cells alters proinflammatory, procoagulant, and fibrinolytic responses. Infect Immun. 1998;66(5):2352–2355. doi: 10.1128/iai.66.5.2352-2355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gervassi A, Alderson MR, Suchland R, Maisonneuve JF, Grabstein KH, Probst P. Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect Immun. 2004;72(12):7231–7239. doi: 10.1128/IAI.72.12.7231-7239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, et al. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol. 2011;65(6):542–548. doi: 10.1111/j.1600-0897.2010.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll TY, Mulla MJ, Han CS, Brosens JJ, Chamley LW, Giles I, et al. Modulation of trophoblast angiogenic factor secretion by antiphospholipid antibodies is not reversed by heparin. Am J Reprod Immunol. 2011;66(4):286–296. doi: 10.1111/j.1600-0897.2011.01007.x. [DOI] [PubMed] [Google Scholar]
- 36.Mulla MJ, Brosens JJ, Chamley LW, Giles I, Pericleous C, Rahman A, et al. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod Immunol. 2009;62(2):96–111. doi: 10.1111/j.1600-0897.2009.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abrahams VM, Aldo PB, Murphy SP, Visintin I, Koga K, Wilson G, et al. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180(9):6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costello MJ, Joyce SK, Abrahams VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57(1):67–80. doi: 10.1111/j.1600-0897.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 39.Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140(6):803–813. doi: 10.1530/REP-10-0294. [DOI] [PubMed] [Google Scholar]
- 40.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25(5):375–388. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 41.Laliberte RE, Eggler J, Gabel CA. ATP treatment of human monocytes promotes caspase-1 maturation and externalization. J Biol Chem. 1999;274(52):36944–36951. doi: 10.1074/jbc.274.52.36944. [DOI] [PubMed] [Google Scholar]
- 42.Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun. 2008;76(2):515–522. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan VK, et al. Caspase-1 dependent IL-1beta secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS One. 2011;6(6):e21477. doi: 10.1371/journal.pone.0021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3 / ASC inflammasome. J Immunol. 2010;184(10):5743–5754. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoo NJ, Park WS, Kim SY, Reed JC, Son SG, Lee JY, et al. Nod1, a CARD protein, enhances pro-interleukin-1beta processing through the interaction with procaspase-1. Biochem Biophys Res Commun. 2002;299(4):652–658. doi: 10.1016/s0006-291x(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 46.Prantner D, Darville T, Sikes JD, Andrews CW, Jr, Brade H, Rank RG, et al. Critical role for interleukin-1beta (IL-1beta) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1beta in mouse macrophages. Infect Immun. 2009;77(12):5334–5346. doi: 10.1128/IAI.00883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russo R, Siviglia E, Gliozzi M, Amantea D, Paoletti A, Berliocchi L, et al. Evidence implicating matrix metalloproteinases in the mechanism underlying accumulation of IL-1beta and neuronal apoptosis in the neocortex of HIV/gp120-exposed rats. Int Rev Neurobiol. 2007;82:407–421. doi: 10.1016/S0074-7742(07)82023-X. [DOI] [PubMed] [Google Scholar]
- 48.Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13(2):121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 49.Eder C. Mechanisms of interleukin-1beta release. Immunobiology. 2009;214(7):543–553. doi: 10.1016/j.imbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Holder BS, Tower CL, Forbes K, Mulla MJ, Aplin JD, Abrahams VM. Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology. 2012 doi: 10.1111/j.1365-2567.2012.03568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63(6):520–533. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 52.Merk M, Baugh J, Zierow S, Leng L, Pal U, Lee SJ, et al. The Golgi-associated protein p115 mediates the secretion of macrophage migration inhibitory factor. J Immunol. 2009;182(11):6896–6906. doi: 10.4049/jimmunol.0803710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hedl M, Abraham C. Distinct roles for Nod2 protein and autocrine interleukin-1beta in muramyl dipeptide-induced mitogen-activated protein kinase activation and cytokine secretion in human macrophages. J Biol Chem. 2011;286(30):26440–26449. doi: 10.1074/jbc.M111.237495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamont RF. The role of infection in preterm labour and birth. Hosp Med. 2003;64(11):644–647. doi: 10.12968/hosp.2003.64.11.2343. [DOI] [PubMed] [Google Scholar]
- 55.Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci. 2010;17(7):619–628. doi: 10.1177/1933719110373148. [DOI] [PubMed] [Google Scholar]
- 56.Pal S, Peterson EM, De La Maza LM. A murine model for the study of Chlamydia trachomatis genital infections during pregnancy. Infect Immun. 1999;67(5):2607–2610. doi: 10.1128/iai.67.5.2607-2610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardenas I, Mulla MJ, Myrtolli K, Sfakianaki AK, Norwitz ER, Tadesse S, et al. Nod1 Activation by Bacterial iE-DAP Induces Maternal-Fetal Inflammation and Preterm Labor. J Immunol. 2011;187(2):980–986. doi: 10.4049/jimmunol.1100578. [DOI] [PubMed] [Google Scholar]
- 58.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206(2):204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 59.Straszewski-Chavez SL, Abrahams VM, Alvero AB, Aldo PB, Ma Y, Guller S, et al. Isolation and Characterization of a Novel Telomerase Immortalized First Trimester Trophoblast Cell Line, Swan 71. Placenta. 2009;30:939–948. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]