Abstract
Artificially sweetened beverage consumption has been linked to obesity, and it has been hypothesized that considerable exposure to nonnutritive sweeteners may be associated with impaired energy regulation. The reward system plays an integral role in modulating energy intake, but little is known about whether habitual use of artificial sweetener (i.e., diet soda consumption) may be related to altered reward processing of sweet taste in the brain. To investigate this, we examined fMRI response after a 12-hour fast to sucrose (a nutritive sweetener) and saccharin (a nonnutritive sweetener) during hedonic evaluation in young adult diet soda drinkers and non-diet soda drinkers. Diet soda drinkers demonstrated greater activation to sweet taste in the dopaminergic midbrain (including ventral tegmental area) and right amygdala. Saccharin elicited a greater response in the right orbitofrontal cortex (Brodmann Area 47) relative to sucrose in non-diet soda drinkers. There was no difference in fMRI response to the nutritive or nonnutritive sweetener for diet soda drinkers. Within the diet soda drinkers, fMRI activation of the right caudate head in response to saccharin was negatively associated with the amount of diet sodas consumed per week; individuals who consumed a greater number of diet sodas had reduced caudate head activation. These findings suggest that there are alterations in reward processing of sweet taste in individuals who regularly consume diet soda, and this is associated with the degree of consumption. These findings may provide some insight into the link between diet soda consumption and obesity.
Sugar sweetened soft drinks have become extremely popular. In a cohort of 19–39 year olds, naturally sweetened soft drink intake more than doubled from 1977 to 2001 to account for approximately 10% of total daily energy consumption [1]. Increased incidence of obesity has accompanied the rising proportion of energy intake accounted for by nutritive sweeteners (NS), and in addition to increased body weight, sugar sweetened beverage intake is linked to increased prevalence of metabolic syndrome, diabetes mellitus, hypertension and cardiovascular disease [2].
Diet soda contains non-nutritive sweeteners (NNS), which provide the desired sweet taste without the calories. NNS afford individuals the experience of eating/drinking something sweet, presumably without the consequence of adding to total daily energy intake. Saccharin, an artificial sweetener, passes through the body without being metabolized in the digestive tract, thus releasing no energy to be stored as fat. Unfortunately, some research suggests that similar to naturally sweetened beverages, intake of beverages sweetened with NNS may also be linked to poor health outcomes [3]. Although this might suggest that the demographic most inclined to use NNS is already overweight or obese, intake of beverages sweetened with NNS has actually been shown to be predictive of future weight gain [4].
Multiple factors undoubtedly contribute to the link between consuming diet soda and weight gain. There may be an association between acute oral exposure to a non-energy containing palatable stimulus and augmented appetite [5–6]; however, critical reviews by Mattes and Popkin, and by Benton, indicate that the recent consensus is that appetite is unaffected by NNS when ingested with other energy sources [7–8]. Additionally, use of NNS may be associated with decreased homeostatic regulation ability, such as incomplete caloric compensation [7,9]. One explanation that has yet to be explored is the possibility that intake of beverages sweetened with NNS may be related to altered reward processing of sweet taste in the brain, which may result in changes in eating behavior. Sweet tastes stimulate several neurotransmitter systems (e.g., dopamine and endogenous opioids) involved in the reward response, which plays a role in the modulation of eating behavior.
Sweet foods may be preferentially sought out and selected due to activation of the reward system [10–11], or possibly consumed to excess due to compensation for a sluggish reward response [12–13]. Therefore, examination of activation of brain regions involved in taste and reward processing in response to sweet tastes with and without energy content may be an important indicator of how a natural sweet taste may differentially activate the reward system relative to an artificial sweetener that has no caloric value.
Previous research addressing this topic has generally reported greater activation in higher-order taste and reward processing regions to nutritive sweeteners (i.e., sucrose or glucose) compared to non-nutritive sweeteners such as sucralose or saccharin [14–16]. Specifically, the anterior cingulate and striatum are activated to a greater extent by a caloric sweet stimulus than an artifical sweetener [14], suggesting that the human brain can dissociate nutritive from NNS even if both taste similarly sweet.
One recent study reported greater activation of a beverage sweetened with artificial sweetener in several regions involved in taste and reward processing. Smeets and colleagues reported a main effect of energy content in the right amygdala and right lateral orbitofrontal cortex (OFC) in response to naturally and artificially sweetened orangeade. Specifically, the artificially sweetened beverage elicited a greater fMRI response in these regions compared to the naturally sweetened beverage [17].
The purpose of this study was to investigate the relationship between diet soda consumption and fMRI activation to a caloric sweet taste (sucrose dissolved in water) and a non-caloric sweet taste (saccharin dissolved in water). Individuals who drink diet soda have regular exposure to sweet tastes with no associated caloric value, and we hypothesize that this may impact the way their brain responds to sweet taste. Additionally, individuals who experience more pleasure from consuming artificially sweetened beverages may be individuals who consume them the most often. Therefore, we hypothesized greater activation to artificial sweetener in brain regions involved in processing food reward and hedonics in individuals who consume more NNS.
We used a hedonic evaluation task in order to elicit activation of brain regions involved in both taste processing and pleasantness evaluation. We hypothesized that both diet soda drinkers and non-drinkers would have widespread activation to sucrose in regions involved in taste (thalamus, anterior insula) and reward (orbitofrontal cortex, caudate nucleus, amygdala) processing. Based on previous neuroimaging research examining cortical responses to nutritive and nonnutritive sweet tastes, we hypothesized that there would be less activation to saccharin in the higher-order limbic and reward regions for non-diet soda drinkers, but similar activation patterns for sucrose and saccharin in diet soda drinkers. In other words, activation patterns produced by a non-nutritive sweetener would differ according to diet soda intake.
Methods
A detailed description of the protocol and the system for delivering taste stimuli in the fMRI environment used in the study are outlined in the Journal of Neuroscience Methods [18].
Participants
Twenty-four young adults ranging from 19 to 32 years of age (M = 24.0, SD = 3.3) were recruited from the San Diego community. Participants gave informed consent and received monetary compensation for their participation. The Institutional Review Boards at San Diego State University and the University of California, San Diego gave approval for the study. Each subject participated in two separate sessions detailed below.
Screening Session
During the first session, participants were screened for exclusionary criteria including ageuesia, anosmia, and upper respiratory infection or allergies within the prior two weeks. Taste thresholds for all participants were assessed using a forced choice procedure with a series of varying concentrations (.0032M to .36M) of sucrose solutions [19]. Odor threshold was assessed using a forced-choice procedure with varying concentrations of n-butyl alcohol presented monorhinically [19]. We have recently reported a link between adiposity and decreased brain activation in reward-related brain regions in young and older adults [12]. Therefore, we were careful to ensure that there were no differences in body mass index (BMI) between the two groups, which could potentially confound the results of the study. Body mass index was calculated by dividing each participant’s measured weight by the square of his or her measured height (Kg/cm2). Each participant also completed the Three-Factor Eating Questionnaire (TFEQ; [20]).
Participants were asked how many sodas containing artificial sweeteners they consumed per week. The “diet soda drinkers” (DSD) group included individuals who endorsed drinking at least one artificially sweetened soda (e.g., Diet Coke, Diet Sprite, etc.) per week. Individuals who were included in the group of “non diet soda drinkers” reported that they did not consume at least one diet soda per week. The diet soda drinkers reported consuming, on average, 8 diet sodas per week (SD = 7.64). Half of the diet soda drinkers reported consuming at least one diet soda per day.
To determine whether the non-diet soda drinkers were more sensitive to 6-n-propylthiouracil (PROP), PROP taster status (nontaster, medium taster, supertaster) was determined. Specifically, each participant rated the intensity of a solution of 0.0032 M PROP in distilled water using the generalized labeled magnitude scale [21]. Participants rinsed with distilled water, took a sip of the PROP solution, swished it around for a few seconds, and expectorated. They were asked to provide a rating of intensity prior to rinsing the mouth with distilled water. The taster groups were defined on the basis of the participants’ gLMS ratings. Nontasters provided intensity ratings of 17 or below, supertasters provided ratings of 80 or above, and medium tasters provided ratings between these values [22].
Neuroimaging Session
The neuroimaging session was conducted at the University of California, San Diego Center for Functional Magnetic Resonance Imaging (fMRI). Participants fasted for a minimum of 12 hours prior to the scan. Outside of the scanner, participants reported their perceived hunger and psychophysical ratings of pleasantness and intensity of the two taste stimuli (specified below) using modified versions of the General Labeled Magnitude Scale (gLMS; [21,23–24]).
Stimulus Delivery
The following stimuli were presented as aqueous solutions: sucrose (0.64M) and saccharin (0.014M). Participants lay supine in the scanner and were fitted with a bite bar to minimize head movement, including that associated with swallowing, and to allow the tubing for taste delivery to rest comfortably between the lips. The stimuli were individually filled in syringes and delivered to the tongue of the participant through 25-foot long tubing connected to programmable pumps located in the operator room. The pumps were computer-programmed to deliver 0.3 ml of solution was presented in 1 sec from each syringe at the appropriate time.
Two functional scans and one structural scan were collected. The purpose of running two functional scans was to increase the number of data points and increase power without reducing the number of slices collected in each brain volume. To minimize any movement in space, the two functional runs were only separated in time by collection of 3-dimensional field maps (described below). Each stimulus was delivered 8 separate times for each functional run, presented pseudo-randomly with a 10s ISI. Distilled water was presented twice after each stimulus, the first time as a rinse and the second as a baseline for data analysis. Therefore, a minimum of 30 seconds elapsed before the same stimulus was presented again (except for water delivery, no stimulus was presented twice in a row). This procedure was designed to minimize habituation and adaptation of the gustatory system.
During the functional runs, taste stimulation was paired with a hedonic evaluation task. Functional data were collected during the 10-second period coinciding with each taste (or water) presentation and the participant’s rating of the pleasantness of the stimulus. Specifically, 1 second was allowed for taste (or water) delivery, 2 seconds were allowed for swallowing (with a cue “please swallow” presented visually to participants on a screen), and 7 seconds were reserved for participants to provide a magnitude estimate of the pleasantness of the taste. To provide the pleasantness rating, the participant used a joystick to place a crosshair on a number corresponding to a general labeled magnitude scale (gLMS) for pleasantness. This whole process was completed with the use of an interactive computer interface displayed on a screen, visible to the participant via a mirror (see Haase et al. 2007 for more detail).
Image Acquisition
The fMRI scan was performed using a 3T GE Signa EXCITE Short-Bore research scanner. Structural images for anatomical localization of functional images were collected before the functional scans using a high-resolution T1-weighted whole-brain FSPGR sequence (Field of view (FOV) = 25.6cm, slice thickness = 1mm, resolution 1×1×1 mm3, echo time (TE) = 30ms, Locs per slab = 190, flip angle = 15). A whole brain gradient echo planer pulse sequence was used to acquire T2*-weighted functional images (32 axial slices, FOV = 19.2cm, matrix size = 64×64, spatial resolution = 3×3×3 mm3, flip angle = 90, echo time (TE) = 30ms, repetition time (TR) = 2000ms).
Image Analysis
Functional data were processed using Analysis of Functional NeuroImage (AFNI) software [25] and FMRIB Software Library (FSL; [26]). The data were first preprocessed through motion correction and alignment of the anatomical image and functional runs. An automated in vivo shimming method using 3-dimensional field maps was employed to correct for heterogeniety of the magnetic field and reduce signal loss using FSL [26]. Images were spatially smoothed to 4 full width at half maximum, automasked to clip voxels outside of the brain, and normalized to Talaraich space to control for individual structural differences. The two functional runs were rescaled to a baseline of 100 and concatenated for each participant.
A Deconvolution was run on each individual’s concatenated run using 3dDeconvolve within AFNI [27]. Deconvolution is a multiple regression analysis used for fMRI data with the purpose of fitting specific time points with distinct coefficients representing an estimate of the impulse response function for each voxel. Deconvolution was used to fit each voxel’s time series to an activation model (based on the specified input contrasts like sucrose minus water) and then test these models for significance. This estimate was given as an output statistic (for each voxel) called the fit coefficient.
At the group level, one-sample t-tests were then run on the fit coefficient at each voxel separately for the two groups (diet soda drinkers and non-drinkers) for two conditions: (1) sucrose minus water; and (2) saccharin minus water. Group statistical maps were thresholded at the cluster level using the AFNI program AlphaSim [25]. AlphaSim uses Monte Carlo simulation to compute the probability of the generation of a random field of noise and determines the cluster size necessary to control for false positives at an alpha of 0.05. Therefore, significant clusters met an individual voxel threshold of p=0.001 (a voxel was considered “activated” if its corresponding t statistic was associated with a p value of equal to or less than 0.001), and consisted of a minimum of 5 contiguous voxels.
In order to examine the interaction between diet soda drinking and the effect of sweetener type on fMRI activation, a 2x2 ANOVA was run on fMRI activation with diet soda drinking group (drinkers v. non-drinkers) and tastant (saccharin v. sucrose) as the factors. Because our hypotheses centered around differential activation of brain regions involved in reward and hunger modulation, and some of these regions are relatively small (e.g., structures in the midbrain, the nucleus acumbens), we restricted the search to within mesial temporal lobe regions involved in the dopamine reward response, the orbitofrontal cortex, the basal ganglia, midbrain, and the insula. Because we had a priori hypotheses regarding which regions would respond differentially between groups, we used a more liberal individual voxel threshold of p = 0.01, and corrected for multiple comparisons using AlphaSim [25], which yielded a minimum cluster threshold of 8 voxels when searched within the previously defined volume.
A region of interest (ROI) analysis was performed to directly compare fMRI activation of the groups to saccharin and sucrose. Based on our hypotheses, we chose to extract the mean activation from Brodmann Area 47 of the orbitofrontal cortex (OFC), the amygdala, the nucleus accumbens, and an inferior region of the insular cortex. Anatomical boundaries for the ROIs were defined using the Talairach and Tournoux Atlas in AFNI. Using mean fit coefficients (averaged activation over each ROI) calculated separately for each stimulus, a repeated-measures analysis of variance (ANOVA) was run on mean activation using region, hemisphere, and taste as within-group factors, and diet soda drinking status as the between-group factor.
Last, to determine whether brain regions involved in processing reward value (i.e., orbitofrontal cortex, caudate head, body and tail, nucleus accumbens, and amygdala) respond differentially to sweet taste according to the amount of diet soda consumption, we ran zero-order correlations between the averages calculated from defined ROIs and number of diet sodas consumed per week within the DSD group.
Results
Demographics and Behavioral Data
One-Way ANOVAs were run to determine potential differences between the groups (diet soda drinkers and non-diet soda drinkers) in demographics and hunger ratings. There were no significant group differences in age, body mass index, (BMI), odor threshold, taste threshold, restraint on the Three Factor Eating Questionnaire, or hunger ratings post 12-hour fast. There were 5 males and 7 females in each group. See Table 1 for group means and standard deviations.
Table 1.
Demographics and Taste Psychophysics
| Mean (SD) | ||||
|---|---|---|---|---|
| Demographics | Non-Diet Soda Drinkers | Diet Soda Drinkers | F | Significance |
| Age (years) | 23.00 (2.3) | 23.9 (3.3) | .433 | p > .05 |
| BMI | 25.03 (5.6) | 27.13 (6.2) | .559 | p > .05 |
| TFEQ - Restraint | 7.63 (5.2) | 10.90 (3.9) | 2.38 | p > .05 |
| Odor threshold L | 7.88 (.84) | 6.90 (1.5) | 2.62 | p > .05 |
| Odor threshold R | 7.13 (.99) | 6.30 (1.9) | 1.18 | p > .05 |
| Taste threshold | .003 (.004) | .007 (.009) | 1.27 | p > .05 |
| Hunger | 31.25 (25.7) | 28.40 (17.4) | .079 | p > .05 |
To examine differences in intensity and pleasantness ratings of the stimuli, repeated measures ANOVAs were run on pleasantness and intensity ratings with taste as the within-subject factor and diet soda group as the between-group factor. For pleasantness ratings, there was no taste by group interaction, F(1, 22) = 2.85, p = 0.11, η2 = .12, or main effect of taste, F(1, 22) = 2.18, p = 0.15, η2 = 0.09, or group, F(1, 22) = 0.55, p = 0.47, η2 = 0.03. The mean pleasantness ratings of sucrose were 63.3(SD = 13.2) and 54.0(SD = 14.3) for diet soda drinkers and non-diet soda drinkers, respectively. The mean pleasantness ratings of saccharin were 53.3(SD = 20.1) and 54.6(SD = 11.0), for diet soda drinkers and non-diet soda drinkers, respectively.
For intensity ratings, there was no taste by group interaction, F(1, 22) = 2.80, p = 0.11, η2 = 0.11, main effect of taste, F(1, 22) = .23, p = 0.64, η2 = 0.01, or main effect of group, F(1, 22) = 0.35, p = 0.56, η2 = .02. The mean intensity ratings of sucrose were 32.6(SD = 20.0) and 33.3(SD = 12.3) for diet soda drinkers and non-diet soda drinkers, respectively. The mean intensity ratings of saccharin were 38.6(SD = 23.8) and 30.0(SD = 10.9), for diet soda drinkers and non-diet soda drinkers, respectively. Finally, there were similar numbers of PROP supertasters in each group (diet soda drinkers = 2; non-drinkers = 1), and the only 2 nontasters were in the non-diet soda drinkers group.
Independent Sample T-Tests
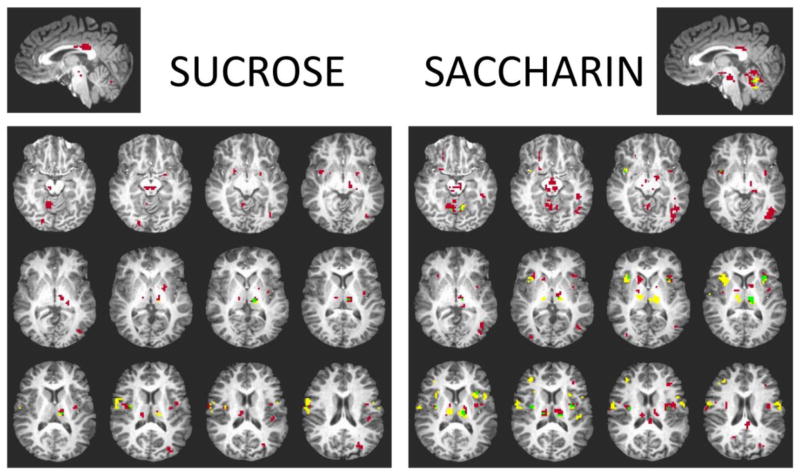
Independent sample t-tests were run separately for the two groups for the sucrose minus water and saccharin minus water conditions, independently. Figure 1 illustrates areas of activation to sucrose and saccharin in the diet soda drinkers group only, the non-diet soda drinkers group only, or overlapping activation in both groups. Significant activation to saccharin during pleasantness evaluation is displayed for diet soda drinkers and non-diet soda drinkers in Table 2. Activation to saccharin reached significance in overlapping areas in both groups, including the bilateral cerebellum, thalamus, precuneus, and insular cortex. In addition, activation was significant for both groups to saccharin in the left cingulate gyrus, left postcentral gyrus, and right precentral gyrus. The nonnutritive sweetener, saccharin, elicited more clusters of activation for participants who regularly drink diet soda. Specifically, this group demonstrated activation of the midbrain (including dopaminergic substantia nigra and ventral tegmental area), bilateral lentiform nucleus, caudate body, and right orbitofrontal cortex (Brodmann Area 47). See Table 2 for a complete list of regions and Talaraich atlas coordinates for both groups.
Figure 1.
Brain activation in response to sucrose and saccharin. Red indicates activation only in diet soda drinkers, yellow indicates activation only in non-diet soda drinkers, and green indicates overlapping activation in both groups.
Table 2.
Significant clusters of activation in response to Saccharin in Diet Soda Drinkers and Non-Drinkers
| Region | Hemisphere | Talairach Atlas Coordinates | # Voxels | Max. Intensity | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
|
SACCHARIN-WATER
| ||||||
|
Diet Soda Drinkers
| ||||||
| Cerebellum | R | 26 | −47 | −19 | 400 | 1.18 |
| Cerebellum | L | −21 | −49 | −19 | ||
| Midbrain/VTA | R | 2 | −20 | −13 | 104 | 0.74 |
| Midbrain/Substantia Nigra | R | 11 | −18 | −7 | ||
| Inferior Temporal Gyrus/BA 37 | L | −46 | −68 | −1 | 71 | 0.79 |
| Insula | L | −41 | −5 | 15 | 71 | 0.7 |
| Precuneus | L | −20 | −71 | 35 | 60 | 0.68 |
| Thalamus | L | −11 | −17 | 12 | 41 | 0.83 |
| Precentral Gyrus | R | 56 | −5 | 27 | 35 | 0.69 |
| Middle Frontal Gyrus/BA 9 | R | 47 | 32 | 32 | 33 | 0.88 |
| Postcentral Gyrus | L | −47 | −20 | 48 | 27 | 1.08 |
| Insula | L | −32 | 23 | 6 | 26 | 0.69 |
| Inferior Parietal Lobule | R | 35 | −50 | 45 | 21 | 0.68 |
| Precuneus | L | −20 | −74 | 48 | 18 | 1.66 |
| Superior Parietal Lobule | L | −32 | −59 | 51 | 17 | 1.09 |
| Superior Parietal Lobule | R | 26 | −71 | 48 | 17 | 1.76 |
| Insula | R | 38 | −8 | 18 | 16 | 0.61 |
| Thalamus | R | 11 | −17 | 18 | 16 | 0.71 |
| Paracentral Lobule | L | −8 | −26 | 48 | 15 | 1.12 |
| Thalamus/Mammillary Body | L | −11 | −17 | 3 | 14 | 0.6 |
| Cingulate Gyrus | R | 2 | −32 | 30 | 14 | 1.54 |
| Cingulate Gyrus | L | −1 | −31 | 29 | ||
| BA 37 | L | −48 | −38 | −10 | 12 | 0.27 |
| Caudate Body | R | 14 | 11 | 9 | 12 | 0.54 |
| Posterior Cingulate | L | −2 | −38 | 24 | 12 | 0.85 |
| Precuneus | R | 26 | −65 | 42 | 12 | 0.88 |
| Inferior Frontal Gyrus/BA 47 | R | 23 | 29 | −10 | 10 | 0.66 |
| Insula | R | 35 | 20 | 6 | 9 | 0.7 |
| Precentral Gyrus | R | 59 | −7 | 23 | 9 | 0.68 |
| Caudate Body | R | 11 | −5 | 15 | 8 | 0.56 |
| Middle Frontal Gyrus | L | −34 | 35 | 18 | 8 | 0.42 |
| Insula | L | −37 | 5 | −4 | 7 | 0.66 |
| Lentiforn Nucleus/Putamen | R | 21 | 8 | −7 | 6 | 0.43 |
| Insula | R | 41 | 5 | −4 | 6 | 0.56 |
| Lentiform Nucleus/Medial Globus Pallidus | L | −13 | −4 | −4 | 6 | 0.93 |
| Caudate Body | L | −7 | 5 | 11 | 6 | 0.81 |
| Middle Frontal Gyrus | L | −31 | 11 | 32 | 6 | 0.5 |
| Inferior Parietal Lobule | L | −37 | −43 | 41 | 6 | 0.56 |
| Middle Occipital Gyrus | R | 35 | −82 | 5 | 5 | 0.69 |
|
| ||||||
| Non-Diet Soda Drinkers | ||||||
|
| ||||||
| Cerebellum | R | 32 | −40 | −22 | 101 | 1.07 |
| Postcentral Gyrus/BA 1 | L | −52 | −16 | 50 | 100 | 1.32 |
| Precuneus | R | 26 | −56 | 42 | 61 | 0.8 |
| Cerebellum | L | −35 | −44 | −19 | 57 | 1.19 |
| Precentral Gyrus | R | 44 | −14 | 36 | 54 | 1.07 |
| Thalamus/Medial Dorsal Nucleus | L | −8 | −20 | 12 | 50 | 0.85 |
| Insula | R | 38 | 14 | 9 | 44 | 0.83 |
| Thalamus | R | 8 | −17 | 15 | 30 | 0.8 |
| Postcentral Gyrus | R | 65 | −11 | 15 | 29 | 1.35 |
| Precentral Gyrus | L | −59 | −5 | 21 | 24 | 1.12 |
| BA 13 | L | −32 | 14 | 15 | 23 | 0.84 |
| Precuneus | L | −17 | −71 | 51 | 19 | 1.42 |
| Cingulate Gyrus | L | −1 | 11 | 41 | 13 | 0.6 |
| Inferior Frontal Gyrus | L | −48 | 14 | 14 | 9 | 0.7 |
| Insula | R | 35 | −11 | 18 | 8 | 0.53 |
| Middle Frontal Gyrus | L | −38 | 26 | 30 | 7 | 0.43 |
| Insula | R | 38 | 5 | −6 | 6 | 0.8 |
| Insula | L | −34 | −10 | 17 | 6 | 0.45 |
| Paracentral Lobule | L | −8 | −23 | 45 | 6 | 0.68 |
| Superior Parietal Lobule | L | −32 | −56 | 51 | 6 | 0.78 |
| Insula | L | −47 | −23 | 18 | 5 | 0.57 |
| Precuneus | R | 17 | −71 | 48 | 5 | 1.17 |
Regions listed demonstrated significant activation at p < .05, corrected for multiple comparisons. Clusters are reported in order of size (number of voxels activated) from largest to smallest. Maximum intensity refers to the fit (beta) coefficient of maximum intensity in each cluster. Some regions listed contain more than 1 cluster of activation, and are repeated in the Table so that the coordinates of each cluster can be displayed.
The complete list of regions activated in response to sucrose in the DSDs and NSDs are listed in Table 3. The nutritive sucrose stimulus activated the bilateral cerebellum and postcentral gyrus, in addition to the left cingulate gyrus, left precentral gyrus, left thalamus, and right insular cortex. In addition to these regions, DSDs also had significant activation of the midbrain and bilateral lentiform nucleus.
Table 3.
Significant clusters of activation in response to Sucrose in Diet Soda Drinkers and Non-Drinkers
| Region | Hemisphere | Talairach Atlas Coordinates | # Voxels | Max. Intensity | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
|
SUCROSE-WATER
| ||||||
|
Diet Soda Drinkers
| ||||||
| Cerebellum | R | 41 | −64 | −22 | 225 | 1.25 |
| Cerebellum | L | −25 | −55 | −16 | 122 | 1.39 |
| Postcentral Gyrus/BA 43 | R | 65 | −10 | 20 | 65 | 1.42 |
| Thalamus | L | −10 | −16 | 11 | 41 | 0.96 |
| Precuneus | R | 23 | −73 | 41 | 30 | 0.98 |
| Cingulate Gyrus | R | 2 | −31 | 29 | 23 | 1.65 |
| Cingulate Gyrus | L | −1 | −31 | 29 | ||
| Precuneus | L | −27 | −74 | 21 | 22 | 0.65 |
| Midbrain/Red Nucleus | L | −3 | −21 | −7 | 16 | 0.55 |
| Midbrain/Substantia Nigra | L | −3 | −21 | −7 | ||
| Postcentral Gyrus | L | −46 | −19 | 44 | 14 | 1.18 |
| Insula | R | 38 | −7 | 17 | 12 | 0.58 |
| Thalamus | R | 14 | −19 | 17 | 11 | 0.61 |
| Insula | L | −40 | −28 | 20 | 11 | 0.7 |
| Cingulate Gyrus | R | 2 | −19 | 29 | 11 | 0.58 |
| Lingual Gyrus | R | 23 | −82 | −10 | 10 | 1.26 |
| Middle Occipital Gyrus | L | −43 | −73 | 2 | 10 | 0.59 |
| Superior Parietal Lobule/BA 7 | R | 26 | −70 | 47 | 10 | 1.35 |
| Insula | L | −34 | −10 | 17 | 8 | 0.58 |
| Lentiforn Nucleus/Putamen | L | −22 | −1 | −4 | 7 | 0.72 |
| Middle Occipital Gyrus | L | −31 | −82 | 17 | 6 | 0.87 |
| Precentral Gyrus | L | −49 | −7 | 29 | 6 | 1.18 |
| Lentiform Nucleus/Putamen | R | 23 | 2 | −4 | 5 | 0.8 |
| Insula | R | 41 | 2 | −1 | 5 | 0.63 |
|
| ||||||
| Non-Diet Soda Drinkers | ||||||
|
| ||||||
| Precentral Gyrus | R | 53 | −10 | 29 | 157 | 1.25 |
| Postcentral Gyrus | R | 54 | −16 | 32 | ||
| Postcentral Gyrus | L | −34 | −25 | 47 | 102 | 1.14 |
| Precentral Gyrus | L | −45 | −12 | 44 | ||
| Precuneus | L | −16 | −70 | 47 | 26 | 0.93 |
| Cerebellum | R | 14 | −61 | −13 | 19 | 0.81 |
| Thalamus | L | −7 | −19 | 11 | 17 | 0.89 |
| Cingulate Gyrus | L | −1 | −22 | 32 | 14 | 0.59 |
| Inferior Parietal Lobule | L | −31 | −52 | 47 | 8 | 0.66 |
| Precentral Gyrus | L | −61 | −7 | 29 | 7 | 0.94 |
| Paracentral Lobule | L | −10 | −25 | 44 | 7 | 0.64 |
| Cerebellum | L | −19 | −58 | −16 | 6 | 0.76 |
| Insula | R | 35 | −10 | 17 | 5 | 0.59 |
| Superior Parietal Lobule | R | 29 | −55 | 47 | 5 | 0.6 |
| Paracentral Lobule | L | −1 | −22 | 47 | 5 | 0.59 |
Regions listed demonstrated significant activation at p < .05, corrected for multiple comparisons. Clusters are reported in order of size (number of voxels activated) from largest to smallest.
Maximum intensity refers to the fit (beta) coefficient of maximum intensity in each cluster. Some regions listed contain more than 1 cluster of activation, and are repeated in the Table so that the coordinates of each cluster can be displayed.
Second Level ANOVA Analysis
A 2 factorial mixed-effects ANOVA was run on fMRI activation with soda drinking group and tastant as the two factors in order to investigate: (1) main effects of diet soda drinking, (2) main effect of caloric value on processing sweet taste in the brain, and (3) interactions between diet soda drinking and cortical activation to nutritive or nonnutritive sweet taste, examined through simple effects. First, there was a main effect of diet soda drinking in the midbrain F(1,22) = 11.06, p ≤ 0.01. Specifically, when collapsed over saccharin and sucrose, diet soda drinkers had a larger response in the dopaminergic ventral tegmental area of the midbrain. When separated by tastant, there was a significant effect of diet soda drinking on fMRI activation to saccharin F(1,22) = 11.22, p ≤ 0.01; greater activation was elicited in the midbrain (ventral tegmental area) of diet soda drinkers relative to non-drinkers. Additionally, two areas approached but did not reach the cluster threshold for statistical significance for greater activation in the diet soda drinkers: the right hypothalamus in response to saccharin (4 voxels activated), F(1,22) = 4.41, p ≤ 0.01; and the substantia nigra of the midbrain in response to sucrose (5 voxels activated), F(1,22) = 10.24, p ≤ 0.01. While activation did not reach the cluster threshold in these regions, both are relatively small in volume, and in an effort to maintain sensitivity, we chose to report these regions but note that the reader should interpret this with caution.
There was no main effect of tastant when explored within the predefined volumes of interest; in other words, when collapsed over group, saccharin did not produce differential activation relative to sucrose in any region of the search volume. However, there was a diet soda-drinking group by tastant interaction, where a region of the right orbitofrontal cortex (BA 47) was more activated in response to saccharin relative to sucrose in non-diet soda drinkers F(1,11) = 9.06, p ≤ 0.01. The diet soda drinkers did not differ in their response to nutritive sweetener relative to the nonnutritive sweetener; no regions reached significance in this condition.
Within-group Variance in Diet Soda Drinkers – ROI analysis
Correlations were run between activation to sucrose and saccharin in the amygdala, nucleus accumbens, and caudate head, body and tail and number of diet sodas per week in diet soda drinkers. To correct for multiple statistical tests, a Bonferroni-like correction was applied and a threshold of p < 0.008 was used for statistical significance. One outlier had a diet soda consumption level that was 4 SD greater than the mean of the rest of the group. Whether this one outlier was removed or retained, there was a statistically significant association between the activation to saccharin in the right caudate head and diet soda consumption. (See Figure 2). Specifically, with the outlier removed, right caudate head activation was negatively associated with the amount of diet soda consumed weekly, (r(11) = −0.86, p = 0.001). Individuals who consumed greater amounts of diet soda demonstrated less activation of the right caudate head in response to saccharin than individuals who consumed less diet soda. There was no relationship between caudate head activation to sucrose and weekly diet soda consumption (left caudate head: r(11) = 0.11, p = 0.76; right caudate head: r(11) = 0.05, p = 0.90).
Figure 2.
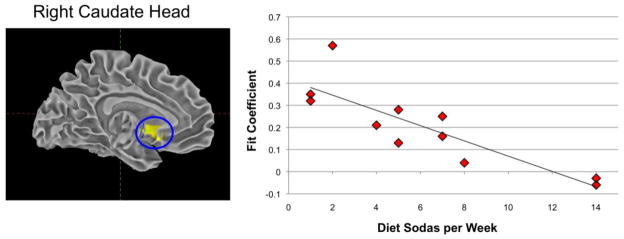
Association between diet sodas consumed weekly and right caudate head activation in response to saccharin (r(11) = −0.86, p = 0.001).
Direct Comparison of Reward Regions – ROI analysis
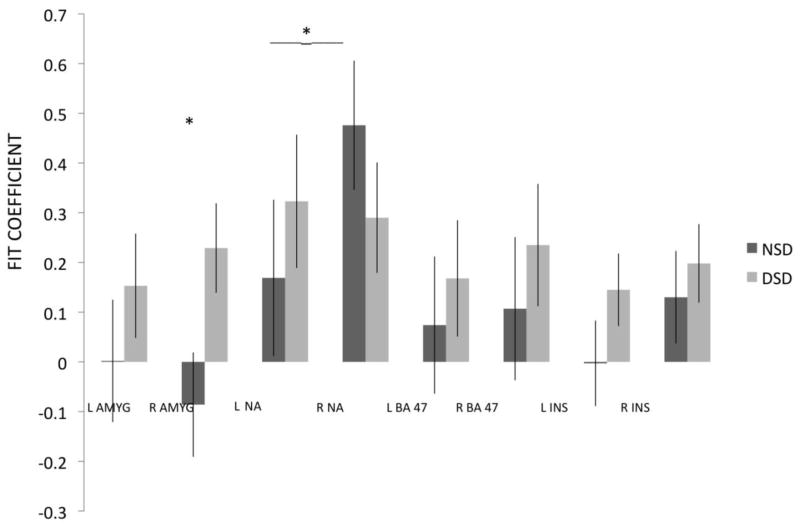
A repeated-measures ANOVA was run on mean activation (the fit coefficients) with three within-group factors: region (i.e., amygdala, nucleus accumbens, orbitofrontal cortex/BA 47, and inferior insula), hemisphere, and taste; and group as the between-group variable. Mauchly’s test indicated that the assumption of sphericity had been violated for the Region by Taste, (χ2(5) = 17.12, p = 0.004), Region by Hemisphere (χ2(5) = 12.7, p = .026), and Region by Hemisphere by Taste, within-subject effects, (χ2(5) = 17.01, p = .005). Therefore, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (εs = 0.60, 0.75, and 0.60, respectively). The analysis revealed a significant Region by Hemisphere by Group interaction, F(2,38) = 3.53, p = 0.034, partial η2 = .17, with no effect of taste stimulus. Newman Keuls post-hoc tests revealed that activation of the right amygdala (See Figure 3) in diet soda drinkers (M = 0.229; SE = 0.09) was significantly greater than activation in this region in non-drinkers (M = −0.086, SE = 0.11). Additionally, right nucleus accumbens activation to sweet taste in the non-drinkers group (M = 0.476; SE = 0.13) was significantly greater than left nucleus activation in this group (M = 0.169; SE = 0.16).
Figure 3.
Direct comparison of activation in diet soda drinkers and non-diet soda drinkers. NSD = Non-diet soda drinkers; DSD = Diet soda drinkers; L = Left Hemisphere; R = Right Hemisphere; AMYG = Amygdala; NA = Nucleus Accumbens; BA 47 = Orbitofrontal Cortex/Brodmann Area 47, INS = Inferior Insula. *Activation of the right amygdala was significantly greater in diet soda drinkers relative to non-drinkers. Right nucleus accumbens activation was significantly greater than left nucleus accumbens activation in the non-diet soda drinkers.
Discussion
There are neuroimaging data to suggest that the human brain can dissociate a sweet nutritive taste from a sweet nonnutritive taste [14–17]. However, to date, there is no human research investigating whether this phenomenon is altered in the brain of those who report regular consumption of NNS. In this study, we examined self-reported regular diet soda drinkers and non-diet soda drinkers to investigate whether regular consumption of NNS in soda beverages is associated with differential activation of taste and reward regions in the brain to caloric and non-caloric sweet tastes.
Inspection of the psychophysical ratings of intensity and pleasantness suggest that the saccharin and sucrose stimuli were matched on sweetness intensity and pleasantness. Additionally, the mean pleasantness ratings for sucrose and saccharin for both groups were in the “moderately pleasant” to “strongly pleasant” range on the general labeled magnitude scale for pleasantness. The imaging results suggest that, although there were no differences in ratings of perceived pleasantness or sweetness intensity between the groups, diet soda drinkers process sweet taste differently in the brain during pleasantness evaluation compared to non-diet soda drinkers. Specifically, diet soda drinkers demonstrated more widespread activation to both saccharin and sucrose in reward processing brain regions (orbitofrontal cortex, lentiform nucleus), and in direct comparisons, exhibited greater activation of the dopaminergic midbrain and right amygdala than non-diet soda drinkers did. Although, a region of the orbitofrontal cortex was differentially activated according to tastant in non-diet soda drinkers, the diet soda drinkers did not demonstrate discrepant responses to nutritive and nonnutritive sweeteners. Caudate head activation to saccharin was negatively associated with diet soda consumption; less caudate activation was associated with greater weekly diet soda consumption.
fMRI Response to Sucrose and Saccharin
We found that the task of tasting and evaluating the pleasantness of saccharin, elicited activation of a greater number of regions in both groups than the caloric sucrose stimulus. Averaging over pleasantness and intensity evaluation, our laboratory has previously reported activation in response to sucrose during the physiological state of hunger in the primary taste cortex, orbitofrontal cortex, midbrain, and several limbic regions including the amygdala. In contrast, saccharin elicited fewer areas of activation; specifically, only the right cuneus, lingual gyrus, and thalamus reached statistical significance [16].
Frank and colleagues [15] reported less activation of reward areas in response to the non-caloric sucralose stimulus relative to the caloric sucrose, and while Chambers et al. [14] reported activation of the primary taste cortex, dorsolateral prefrontal cortex, striatum, and anterior cingulate in response to glucose (a caloric sweet taste), the saccharin solution only elicited activation of the primary taste cortex and left dorsolateral prefrontal cortex. Importantly, these two studies did not include hedonic evaluation of taste in their paradigms, which may contribute to the differences in our findings.
Diet Soda Drinkers v. Non-Diet Soda Drinkers
Both groups demonstrated activation to sweet tastes in prototypical taste regions including the insula, thalamus, and somatosensory areas. Differences in activation patterns were more pronounced within higher-order taste regions that are likely involved in processing hedonics and reward value. Diet soda drinkers demonstrated greater activation in the midbrain, lentiform nucleus, and caudate in response to saccharin. Activation of the dopaminergic ventral tegmental area of the midbrain, and right amygdala was greater in the diet soda drinkers.
Dopamine (DA) plays an important role in the mediation of reward value. The animal literature suggests that dopamine signaling via D1 and D2-like receptors is critical to reward-learning. Creating and strengthening flavor preferences based on taste or postingestive effects of stimuli, seems to be modulated by DA to a greater extent than the opioid system [27]. Animal research also suggests that DA release is modulated by both pleasant taste stimulation independent of postingestive consequences [28–29], and also carbohydrate metabolism in the absence of taste signaling [30].
While both seem to independently influence DA (31), orosensory information, and postingestive factors likely interact to modulate DA signaling in the brain. Orosensory stimuli are linked to postingestive nutritive effects of consumption [32–33]. In other words, sweet taste is a strong predictor of energy content and generally, the sweeter the taste, the higher its energy density [34]. In rats, there is evidence that weakening the association between sweet taste and energy content of foods using NNS results in increased caloric intake, increased body weight, and diminished caloric compensation [34]. Given the timing between rises in the consumption of artificially sweetened beverages, and dramatic increases in obesity rates, we hypothesize that this may be occurring in humans as well. Individuals who choose to use NNS are more regularly exposed to a sweet taste that is devoid of energy content. Therefore, the link between sweet taste and carbohydrate metabolism may be weakened, changing the way in which the reward system responds to sweet taste, and impairing energy regulation.
Differences in reward processing in diet soda drinkers and non-diet soda drinkers could have important implications for understanding links between diet soda consumption and obesity that haven’t yet been explained. Specifically, food reward is a strong factor driving eating behavior [13]. Exemplifying the importance of reward in influencing eating behavior is the change in reward value of the same stimulus (taste, odor, food picture) that can be demonstrated from hunger to satiety [35]. For example, sensory-specific satiety is a phenomenon demonstrated when an individual eats a certain food to satiety and the sensory properties of that food are no longer as rewarding, a mechanism which encourages dietary variety [36].
We speculate that individuals with more exposure to NNS may already experience greater reward in response to sweet tastes; this may be a factor in developing a preference towards drinking sweetened (naturally or artificially) beverages to begin with. Subsequently, individuals who are regularly exposed to sweet nonnutritive tastes may have “trained” themselves to enjoy artificially sweetened beverages as much as naturally sweetened beverages, and this may be related to the observation that diet soda drinkers have increased responses to saccharin in certain reward-related dopaminergic brain regions. In addition, a weakened association between sweet taste and energy value may have an impact on physiological signals and eating behaviors (e.g., eating beyond satiety), which could lead to weight gain.
Nutritive versus Nonnutritive Sweet Taste
Based on the literature, we expected to find differences between the nonnutritive and nutritive tastes in areas involved in hunger modulation (orbitofrontal cortex, hypothalamus) and the dopamine reward response (nucleus accumbens, amygdala, midbrain), with sucrose eliciting a greater response than saccharin, especially in non-diet soda drinkers.
There was a diet soda group by tastant interaction on fMRI activation in a lateral region of the orbitofrontal cortex. Specifically, the non diet soda drinkers demonstrated greater activation of this region of the orbitofrontal cortex in response to saccharin relative to sucrose. Similarly, Smeets and colleagues [17] also reported greater activation of the right amygdala and right lateral OFC in response to an artificially sweetened beverage.
There was no difference between the sucrose and saccharin tastes in the orbitofrontal cortex of the diet soda drinkers; in other words, they did not demonstrate differential brain responses to a natural versus an artificial sweetener. We might speculate that this could be related to the finding that regular consumption of NNS weakens the link between sweetness intensity and energy content, culminating in less efficient signaling of nutrient value [34]. This finding would predict that nutritive and nonnutritive sweeteners would elicit a similar reward response in the brain.
Association Between Brain Activation and Weekly Diet Soda Consumption
The caudate head, part of the dorsal striatum, was negatively associated with diet soda consumption in diet soda drinkers. It has been suggested that dopamine (DA) release in the dorsal striatum facilitates feeding [37–38] and opioid stimulation of the dorsal striatum can stimulate intake of palatable food [39]. Thus, the dorsal striatum is hypothesized to play a modulatory role in food motivation and reward.
Recent evidence suggests: (1) decreased caudate activation in food-reward neuroimaging paradigms is related to obesity [12–13,40]; (2) there is a positive association between obesity and artificial sweetener (diet soda) consumption [3–4]; and the present study suggests (3) decreased activation of the caudate head is associated with higher levels of artificially sweetened beverage consumption. These various associations among consumption of NNS, obesity, and decreased caudate activation represent an interesting phenomenon. We speculate that this may suggest that diet soda consumption could be negatively related to dopamine release in the caudate, which may in turn be related to weight gain.
To our knowledge, this is the first study to report differential brain activation to sweet tastes as a function of consumption of artificially sweetened beverages. Although we speculate about the potential for these findings to increase knowledge regarding the possible link between diet soda consumption and obesity, we were careful to ensure that our samples did not differ in BMI, an indirect measure of adiposity. The purpose of this was to focus on the relationship between artificial sweetener use and brain activation without the potential confound of differential levels of body fat in our samples. Future research investigating interactions between adiposity and exposure to artificial sweetener will be important to provide further insight into this potential association. Finally, we focused specifically on self-reported artificial sweetener use. The study suggests that further investigation into the relationship between brain activation to sweet tastes and a number of other variables, including consumption of natural sweeteners, patterns in artificially sweetened beverage use (e.g., during meals vs. in between meals), and other dietary factors (e.g., exposure to sweet, energy-dense foods; caffeine) is warranted.
There are limitations to the study. We operationally defined “diet soda drinkers” as the participants in the study who endorsed regular drinking of diet soda (≥1 per week) and “non diet soda drinkers” as the participants who reported that they do not regularly consume diet soda (<1 per week). We did not investigate the use of other types of sweeteners and thus cannot make inferences as to whether or not the groups differed in the total amount of sweetened beverages they regularly consume. In addition, we chose to use saccharin as the non-nutritive sweet taste stimulus. Different types of NNS are used in various combinations in sodas sold commercially in the United States and in other countries, and individuals may vary in their degree of exposure to saccharin. Finally, we assumed that the individuals who regularly consume diet soda have a greater exposure to NNS than non diet soda drinkers, but future studies should consider the consumption of NNS in other foods and beverages to further investigate how the degree of exposure to NNS may relate to various physiological processes subserving hunger, satiety, and flavor perception.
In summary, we administered one nutritive (sucrose) and one nonnutritive (saccharin) taste to a group of individuals who do not drink diet soda and a group of individuals who do regularly consume diet soda. We found differences in activation patterns during a hedonic evaluation task, suggesting higher-order reward regions are activated in response to both sucrose and saccharin to a greater extent in the diet soda drinkers relative to the non-drinkers. Additionally, diet soda drinkers had greater activation of the dopaminergic midbrain and amygdala relative to the non-drinkers and did not respond differentially to the nutritive sweet taste compared to the nonnutritive sweet taste. Finally, within the diet soda drinkers, caudate head activation to saccharin was highly associated with the number of diet sodas consumed per week, suggesting that individuals who consume greater amounts of artificial sweetener have a decreased response in the caudate head to artificial sweetener. Taken together, these results suggest that regular consumption of diet soda may be related to alterations in the reward experienced from both nutritive and nonnutritive sweet tastes. We speculate that this may provide some insight into the link between diet soda consumption and obesity.
Highlights.
We examined fMRI response to sweet tastes in diet soda drinkers and non drinkers
Diet soda drinkers had greater activation in the dopaminergic midbrain and amygdala.
Diet soda drinkers did not have differential responses according to sweetener type.
Diet soda drinkers and non-drinkers had differential reward processing of sweet taste.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. American Journal of Preventive Medicine. 2004;27:205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 4.Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity. 2008;16:1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 5.Black R, Leiter L, Anderson G. Consuming aspartame with and without taste: Differential effects on appetite and food intake of young adult males. Physiology & Behavior. 1993;53:459–466. doi: 10.1016/0031-9384(93)90139-7. [DOI] [PubMed] [Google Scholar]
- 6.Blundell JE, Hill AJ. Paradoxical effects of an intense sweetener (aspartame) on appetite. Lancet. 1985;1(8489):1092–1093. doi: 10.1016/s0140-6736(86)91352-8. [DOI] [PubMed] [Google Scholar]
- 7.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. The American Journal of Clinical Nutrition. 2009;89:1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benton D. Can artificial sweeteners help control body weight and prevent obesity? Nutrition Research Reviews. 2005;18:63–76. doi: 10.1079/NRR200494. [DOI] [PubMed] [Google Scholar]
- 9.Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiology & Behavior. 2010;100:55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48:12–19. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Wang GJ, Fowler JS, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 12.Green E, Jacobson A, Haase L, Murphy C. Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Research. 2011;1386:109–117. doi: 10.1016/j.brainres.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 14.Chambers ES, Bridge MW, Jones DA. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. The Journal of Physiology. 2009;587:1779–1794. doi: 10.1113/jphysiol.2008.164285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank GK, Oberndorfer TA, Simmons AN, et al. Sucrose activates human taste pathways differently from artificial sweetener. NeuroImage. 2008;39:1559–1569. doi: 10.1016/j.neuroimage.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 16.Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. NeuroImage. 2009;44:1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smeets PA, Weijzen P, de Graaf C, Viergever MA. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. NeuroImage. 2010;54:1367–1374. doi: 10.1016/j.neuroimage.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 18.Haase L, Cerf-Ducastel B, Buracas G, Murphy C. On-line psychophysical data acquisition and event-related fMRI protocol optimized for the investigation of brain activation in response to gustatory stimuli. Journal of Neuroscience Methods. 2009;159:98–107. doi: 10.1016/j.jneumeth.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Murphy C, Gilmore M, Seery C, Salmon D, Lasker B. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiology of Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- 20.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 21.Bartoshuk LM, Duffy VB, Green BG, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiology & Behavior. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Bartoshuk LM. Comparing sensory experiences across individuals: Recent psychophysical advances illuminate genetic variation in taste perception. Chemical Senses. 2000:447–460. doi: 10.1093/chemse/25.4.447. [DOI] [PubMed] [Google Scholar]
- 23.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the “Labeled Magnitude Scale” for Measuring Sensations of Taste and Smell. Chemical Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 24.Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chemical Senses. 1993;18:683–702. [Google Scholar]
- 25.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23 (Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Touzani K, Bodnar R, Sclafani A. Neuropharmacology of learned flavor preferences. Pharmacology, Biochemistry and Behavior. 2010;97:55–62. doi: 10.1016/j.pbb.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise R. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xenakis S, Sclafani A. The effects of pimozide on the consumption of palatable saccharin-glucose solution in the rat. Pharmacol Biochem Behav. 1981;15:435–442. doi: 10.1016/0091-3057(81)90274-4. [DOI] [PubMed] [Google Scholar]
- 30.de Araujo I, Oliveira-Maia A, Sotnikova T, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 31.de Araujo I, Ren X, Ferreira JG. Metabolic sensing in brain dopamine systems. Results Probl Cell Differ. 2010;52:69–86. doi: 10.1007/978-3-642-14426-4_7. [DOI] [PubMed] [Google Scholar]
- 32.Sclafani A. Learned controls of ingestive behavior. Appetite. 1997;29:153–158. doi: 10.1006/appe.1997.0120. [DOI] [PubMed] [Google Scholar]
- 33.Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36:79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- 34.Swithers SE, Davidson TL. A Role for Sweet Taste: Calorie Predictive Relations in Energy Regulation by Rats. Behavioral Neuroscience. 2008;122:161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- 35.Cabanac M. Physiological Role of Pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- 36.Rolls B, Rolls E, Rowe E, Sweeney K. Sensory specific satiety in man. Physiology & Behavior. 1981;27:137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- 37.Small D, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 38.Szczypka MS, Kwok K, Brot MD, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 40.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]