Abstract
Objective
To examine psychological factors in relation to antral follicle count (AFC), a marker of ovarian reserve, in a multiethnic sample of 683 pre-menopausal women in the Ovarian Aging (OVA) Study.
Methods
In cross-sectional analyses, linear regression was performed to determine whether AFC decline across women varied over levels of depression as well as depression in combination with psychological stress. The total and subscale scores of the Center for Epidemiological Studies Depression Scale (CESD) were used to measure depression and the Perceived Stress Scale was used to measure psychological stress.
Results
Following covariate-adjustment, the 2-way interaction of age-x-positive affect and the 3-way interaction of age-x-positive affect-x-stress were related to AFC (b = 0.047, p = .036; b = 0.012, p = .099, respectively). In stratified analyses, stress was related to AFC in women with low positive affect (b = −.070, p = .021) but not in women with high positive affect (b = .018, p = .54). AFC decline across women was progressively higher in women with low positive affect who reported low (−0.747 follicles/year), mid (−0.920 follicles/year), and high (−1.112 follicles/year) levels of stress. Results examining the CESD total and remaining subscale scores were all non-significant (p’s > .05).
Conclusions
Cross-sectional evidence suggests that 1) women with low positive affect may experience accelerated AFC decline and 2) low positive affect may be a vulnerability factor, or, alternatively, high positive affect may be a protective factor, in moderating the negative effects of psychological stress on AFC decline.
Keywords: depression, psychological stress, reproductive aging, ovarian aging, ovarian reserve, antral follicle count (AFC)
Recent models of depression have proposed that major depressive disorder (MDD) may accelerate biological aging,1–2 citing associations between depression and markers of cellular aging.3–6 Whether depression may be linked to aspects of reproductive biology underlying ovarian aging in particular, however, is not known. At present, epidemiological support for a connection between depression and ovarian aging stems primarily from The Harvard Study of Moods and Cycles in which women with a history of MDD compared to women with no current or prior history of MDD experienced a 20% increased rate of entry into peri-menopause, independently of confounding factors (e.g., cigarette smoking).7 In addition, women with more severe depressive symptomatology (assessed by a score of 8+ on the Hamilton Rating Scale for Depression) and women with more severe depressive symptomatology who were also taking anti-depressant medications had 2 and 3 times, respectively, the risk of entering peri-menopause earlier than their never-depressed counterparts.
An abundant literature shows depression and psychological stress are strongly interrelated. In vulnerable individuals, psychosocial stressors can trigger the onset of depression and once depression has developed, influence its severity, course, and probability of recurrence.8–13 Moreover, being depressed may itself increase one’s sensitivity to stress making the ill effects of subsequent (even minor) stressors more potent.14–15 It has been hypothesized that the connection between stress and depression may partially explain the 2-fold increased risk of depression in women compared to men.16 Women are disproportionately exposed to stress and/or respond to stress more intensely17–18; women report higher levels of perceived stress19; and women are more likely to have experienced significant life stress prior to the onset of depression.20 The commonness of experiencing psychological stress highlights the importance of understanding its putative role in promoting and exacerbating depression outcomes as well as depression-related sequelae such as potential biological alterations underlying mechanisms of somatic aging.
To date, most investigations of depression and ovarian function have examined reproductive hormones as indicators of ovarian age (e.g.,21–23). Because such markers (e.g., follicular-phase levels of FSH) are widely variable in aging and do not appreciably change until a woman has already experienced a significant loss in ovarian function,24–25 their use remains limited. Alternatively, advances in transvaginal ultrasonography (TVUS) in infertile women have recently been applied in healthy women to evaluate antral follicle count (AFC), an indirect measure of the number of primordial follicles remaining in the ovary.26–28 Validity for the use of AFC as a marker of ovarian reserve is supported by studies showing AFC to covary with chronological age, correlate with ovarian response in treatments using Assisted Reproductive Technologies (ARTs), and, in statistical models, predict menopause onset.27,29–37 In contrast to hormonal indicators, AFC is stable across consecutive menstrual cycles and across follicular and luteal phases of the menstrual cycle.33,38–39 Taken together, evidence supports the value of utilizing AFC as a tool in studying ovarian follicle loss and the factors that may influence this process.
The goals of the current study were to examine depression as well as depression in combination with psychological stress in relation to ovarian aging in a multi-ethnic cross-sectional sample of 683 pre-menopausal women. In accordance with the present literature, we sought to determine whether 1) AFC decline across women would be greater among women reporting higher levels of depression; and 2) whether there would be an exacerbating effect of stress whereby AFC decline across women would be greater among women reporting higher levels depression and higher levels psychological stress. These hypotheses were tested via 2-(age-x-depression) and 3-way (age-x-depression-x-stress) interactions in relation to AFC. On an a priori basis, depression levels were evaluated by the total score of the Center for Epidemiological Studies Depression Scale (CESD) as well as its subscale scores which represent particular types of depressive symptoms (e.g., depressed affect); psychological stress was measured by the Perceived Stress Scale. All analyses included statistical adjustment for possible confounding factors (i.e., race/ethnicity, SES, menarcheal age, use of hormone-containing medication for birth control, parity, cigarette smoking, and BMI). For conceptual clarity, throughout the current report we use the term decline to describe AFC differences across women to reflect the age-related phenomenon that the number of ovarian antral follicles decreases with advancing chronological age; however, we emphasize that the current study is cross-sectional and these differences are reported across and not within individuals.
Methods
Participants
The current sample was comprised of participants in the Ovarian Aging (OVA) Study, an investigation of the correlates of reproductive aging, including women belonging to Kaiser Permanente (KP) of Northern California, a large, integrated health care delivery system that provides medical care to approximately one third of the population of Northern California. The KP membership compared to the population of Northern California is generally representative in its socio-demographic and health-related characteristics, especially when the comparison is limited to those with health insurance.40 Women were included in the OVA Study if they were between 25–45 years of age, had regular menses, had their uterus and both ovaries intact, self-identified as white, African-American, Latina, Chinese, or Filipina, and were able to speak/read English, Spanish, or Cantonese. Exclusions included the self-report of major medical illnesses, use of medications affecting the menstrual cycle in the 3 months prior to study enrollment, and current pregnancy or breastfeeding.
The OVA Study protocol required women to participate in an in-person interview and anthropometric assessment at the first study visit and a transvaginal ultrasound (TVUS) at the second study visit. In addition, a questionnaire packet of self-report measures was added to the OVA Study protocol 7 months following the initiation of the study. Participants were provided the questionnaire packet at the first study visit and asked to return it by regular mail. The average interval between questionnaire administration and completion was 2.6 (4.9) weeks with 68% of women completing the questionnaires within 2 weeks and the average interval between the completion of the questionnaires and the TVUS was 5.9 (6.7) weeks. The participants included in the current analysis were those women who had complete TVUS and questionnaire data. Of the 1019 women who completed the OVA Study, 879 women participated in the study in the timeframe in which the questionnaire packet was added to the study protocol. Of these women, 683 (77.7%) women had complete TVUS and questionnaire data. Missing data were because 39 women had missing TVUS data and 157 women did not return the questionnaire packet. Missing TVUS data were due to (1) the presence of ovarian cyst >30 mm on either ovary invalidating AFC measurement (n = 24), (2) the presence of other masses (e.g., fibroid) obstructing view of either ovary (n = 9), (3) obesity or anatomical anomalies preventing visualization of either ovary (n = 4), and (4) the loss of ultrasound images due to technical error (n = 2). The study protocol was approved by the University of California San Francisco Committee on Human Research as well as the Kaiser Permanente of Northern California Institutional Review Board. Informed, written consent was obtained from all study participants.
Measures
Ovarian Aging
In accordance with a standardized protocol,41 ovaries were imaged using trans-vaginal ultrasonography (TVUS) performed by one of two reproductive endocrinologists (M.I.C., M.P.R.) between menstrual cycle days 2–4. A Shimadzu SDU-450XL machine with a variable 4- to 8-mHz vaginal transducer was used to measure the transverse, longitudinal, and anteroposterior diameters of each ovary. Follicles (defined as all echo-free structures in the ovaries) with a mean diameter across two dimensions of 2–10 mm were counted by the reproductive endocrinologist. Each measurement was taken twice and averaged. The total number of follicles was then summed across the right and left ovary to determine AFC. In a sub-sample of 50 OVA Study participants, review of TVUS exams showed inter-rater reliability between the two reproductive endocrinologists was excellent (r = .92) as was test-re-retest reliability for each reproductive endocrinologist measured over two consecutive months (average r = .91).
Depressive Symptoms
Depressive symptoms were measured using the Center for Epidemiological Studies Depression Scale (CESD).42–43 The CESD is a 20-item, self-report questionnaire assessing depressive symptoms over the past week. Each item is scored on a 0–3 point scale. Response choices indicate the frequency with which each symptom (or item) is experienced, ranging from “rarely or none of the time (< 1 day)” scored 0 to “most or all of the time (5–7 days)” scored 3. Following the reversal of scores on 4 questions measuring positive affect, items are summed to produce a total score (ranging from 0 to 60) with higher values reflecting more depressive symptoms. In addition, 4 subscale scores are derived including (1) depressed affect, (2) positive affect, (3) somatic and retarded activity, and (4) interpersonal difficulties with higher values reflecting more depressed affect, more positive affect (utilizing non-reversed items), more somatic symptoms, and more interpersonal problems, respectively. Confirmatory factor analyses support use of the sub-scales, showing a second-order factor solution with first order factors representing the individual CESD subscales and a second order depressive symptoms factor.44 Internal reliability for the CESD is high in psychiatric (α = .90) and non-psychiatric samples (α = .85).45–46 Validity of the CESD is supported by its ability to distinguish patient from non-patient samples, to correlate with clinician ratings of depression, to fluctuate in accordance with treatment for depression, and to correlate with other measures of depression.43,46–50
The individual CESD items included in each subscale score are listed in the following. The depressed affect subscale included items: 1) I felt that I could not shake off the blues even with help from my family or friends; 2) I felt depressed; 3) I thought my life had been a failure; 4) I felt tearful; 5) I felt lonely; 6) I had crying spells; and 7) I felt sad. The positive affect subscale included items: 1) I felt that I was just as good as other people; 2) I felt hopeful about the future; 3) I was happy; and 4) I enjoyed life. The somatic and retarded activity subscale included items: 1) I was bothered by things that usually don’t bother me; 2) I did not feel like eating; my appetite was poor; 3) I had trouble keeping my mind on what I was doing; 4) I felt that everything that I did was an effort; 5) My sleep was restless; 6) I talked less than usual; and 7) I could not “get going”. The interpersonal difficulties subscale included items: 1) People were unfriendly; and 2) I felt that people disliked me.
In the present sample, internal reliability was high for the CESD total score (α = .88) as well as the depressed affect (α = .86) and positive affect (α = .76) subscales but was lower for the somatic and retarded activity (α = .66), and interpersonal difficulties (α = .55) subscales. Results of factor analysis with oblique rotation replicated the original CESD 4-factor solution. Factor loadings were as follows: depressed affect (.401 – .795); positive affect (.514 – .797); somatic and retarded activity (.338 – .697); and interpersonal difficulties (0.623 – 0.572). Pearson correlation coefficients among the subscales (range −.296 – .678) were all statistically significant at p <.001.
Psychological Stress
Psychological stress was evaluated using the 4-item Perceived Stress Scale (PSS), a self-report questionnaire assessing the extent to which individuals appraise their lives as being stressful over the past month.19,51 Two positively-worded items (reversed scored) and two negatively-worded items are scored on a 5-point scale (0=never, 1=almost never, 2=sometimes, 3=fairly often, 4=very often) and summed to produce a total score (ranging from 0–16). Higher scores indicate greater perceived stress. The PSS has been used widely and its internal reliability and construct validity are well-established.19,51–52 In the present sample, internal reliability was high for the PSS (α = .75).
Statistical Analyses
Using linear regression, 2-way interactions of age by depression in relation to AFC were assessed to determine whether AFC decline across women varied over levels of depression. Covariates, main effects (age, depression), and the 2-way interaction term (age-x-depression) were entered as explanatory variables in predicting AFC. In accordance with recommendations by Aiken and West,53 mean-centering was used for variables included in the interaction terms. Separate analyses (represented by Models 1–5 of Table 2) were performed to examine depression as measured by the CESD total score as well as each of four CESD subscale scores: CESD depressed affect subscale, CESD positive affect subscale, CESD somatic and retarded activity subscale, and CESD interpersonal subscale. Covariates included race, individual-level socioeconomic status (SES), menarcheal age (in years), use of hormone-containing medication for birth control (0=no history of use; 1=positive history of use), parity (0=no live births; 1=1+ live births), cigarette smoking (0=never smoked; 1=current/past smoking), and body mass index (BMI) (weight in kg/height in m2). Race/ethnicity was represented by four dummy variables with white as the reference group. Individual-level SES was computed by adding standardized education and household income variables. Education was coded 1=<HS/some HS; 2=HS grad/GED; 3=some college/AA/vocational school; 4=college graduate; 5=graduate school (PhD, MS); 6=professional degree (MD, JD, DDS, MBA). Household income was coded 1=<$5,000; 2=$5,000–$15,999; 3=$16,000–$24,999; 4=$25,000–$34,999; 5=$35,000–$49,999; 6=$50,000–$74,999; 7=$75,000–$99,999; 8=$100,000–$149,999; 9=$150,000–$199,999; 10=$200,000+ and divided by the number of individuals in the household who were dependent on the reported income. For two individuals who reported not knowing their household income, only education contributed to the individual-level SES composite. The BMI distribution was normalized using a logarithmic transformation.
Table 2.
Covariate-adjusted linear regression analyses assessing age by depression effects on AFC decline across women.
| Linear Regression | Ba | 95% CI for Ba | Beta | p | R2 | |
|---|---|---|---|---|---|---|
| Model 1 | .268 | |||||
|
| ||||||
| Main Effects: | Age | −.861 | (−0.973 – −0.749) | −.499 | <.001 | |
| CESD (Total) | .047 | (−0.028 – 0.123) | .041 | .22 | ||
| 2-Way Interaction: | Age-X-CESD (Total) | −.006 | (−0.019 – 0.006) | −.032 | .33 | |
|
| ||||||
| Model 2 | .269 | |||||
|
| ||||||
| Main Effects: | Age | −.859 | (−0.971 – −0.747) | −.497 | <.001 | |
| CESD (Dep Affect) | .131 | (−0.041 – 0.303) | .049 | .14 | ||
| 2-Way Interaction: | Age-X-CESD (Dep Affect) | −.019 | (−0.048 – 0.010) | −.042 | .20 | |
|
| ||||||
| Model 3 | .273 | |||||
|
| ||||||
| Main Effects: | Age | −.876 | (−0.988 – −0.763) | −.508 | <.001 | |
| CESD (Pos Affect) | −.124 | (−0.371 – 0.123) | −.033 | .33 | ||
| 2-Way Interaction: | Age-X-CESD (Pos Affect) | .047 | (0.003 – 0.092) | .069 | .04 | |
|
| ||||||
| Model 4 | .265 | |||||
|
| ||||||
| Main Effects: | Age | −.867 | (−0.980 – −0.754) | −.502 | <.001 | |
| CESD (Somatic) | .033 | (−0.171 – 0.237) | .011 | .75 | ||
| 2-Way Interaction: | Age-X-CESD (Somatic) | .013 | (−0.022 – 0.049) | .024 | .46 | |
|
| ||||||
| Model 5 | .271 | |||||
|
| ||||||
| Main Effects: | Age | −.865 | (−0.979 – −0.752) | −.501 | <.001 | |
| CESD (Interpersonal) | .552 | (−0.068 – 1.171) | .059 | .08 | ||
| 2-Way Interaction: | Age-X-CESD (Interpersonal) | −.040 | (−0.143 – 0.062) | −.025 | .44 | |
unstandardized regression coefficient
Also using linear regression, 3-way interactions of age by depression by psychological stress in relation to AFC were assessed to determine whether AFC decline across women varied over levels of depression and psychological stress. Covariates, main effects (age, depression, stress), 2-way interaction terms (age-x-depression, age-x-stress, depression-x-stress), and the 3-way interaction term (age-x-depression-x-stress) were entered as explanatory variables in predicting AFC. Again, separate analyses (represented by Models 1–5 of Table 3) were performed to examine depression as measured by the CESD total score as well as each of four CESD subscale scores. The covariates were the same as those listed above.
Table 3.
Covariate-adjusted linear regression analyses assessing age by depression by stress effects on AFC decline across women.
| Linear Regression | Ba | 95% CI for Ba | Beta | p | R2 | |
|---|---|---|---|---|---|---|
| Model 1 | .277 | |||||
|
| ||||||
| Main Effects: | Age | −.831 | (−0.956 – −0.707) | −.482 | <.001 | |
| CESD (Total) | .005 | (−0.091 – 0.101) | .005 | .91 | ||
| Stress | .081 | (−0.197 – 0.359) | .023 | .57 | ||
| 2-Way Interactions: | Age-X-CESD (Total) | .005 | (−0.012 – 0.021) | .024 | .57 | |
| Age-X-Stress | −.033 | (−0.080 – 0.015) | −.053 | .18 | ||
| CESD (Total)-X-Stress | .016 | (−0.009 – 0.042) | .045 | .22 | ||
| 3-Way Interaction: | Age-X-Total-X-Stress | −.003 | (−0.007 – 0.001) | −.054 | .19 | |
|
| ||||||
| Model 2 | .277 | |||||
|
| ||||||
| Main Effects: | Age | −.844 | (−0.967 – −0.721) | −.488 | <.001 | |
| CESD (Dep Affect) | .039 | (−0.179 – 0.256) | .015 | .73 | ||
| Stress | .076 | (−0.193 – 0.345) | .022 | .58 | ||
| 2-Way Interactions: | Age-X-CESD (Dep Affect) | −.001 | (−0.038 – 0.036) | −.003 | .95 | |
| Age-X-Stress | −.028 | (−0.073 – 0.017) | −.046 | .22 | ||
| CESD (Dep Affect)-X-Stress | .039 | (−0.021 – 0.099) | .048 | .20 | ||
| 3-Way Interaction: | Age-X-Dep Affect-X-Stress | −.004 | (−0.014 – 0.005) | −.034 | .40 | |
|
| ||||||
| Model 3 | .280 | |||||
|
| ||||||
| Main Effects: | Age | −.828 | (−0.951 – −0.705) | −.480 | <.001 | |
| CESD (Pos Affect) | .007 | (−0.278 – 0.293) | .002 | .96 | ||
| Stress | .115 | (−0.145 – 0.375) | .033 | .39 | ||
| 2-Way Interactions: | Age-X-CESD (Pos Affect) | .016 | (−0.037 – 0.069) | .023 | .55 | |
| Age-X-Stress | −.024 | (−0.068 – 0.020) | −.039 | .29 | ||
| CESD (Pos Affect)-X-Stress | −.044 | (−0.123 – 0.035) | −.039 | .27 | ||
| 3-Way Interaction: | Age-X-Pos Affect-X-Stress | .012 | (−0.002 – 0.026) | .063 | .099 | |
|
| ||||||
| Model 4 | .276 | |||||
|
| ||||||
| Main Effects: | Age | −.861 | (−0.982 – −0.740) | −.498 | <.001 | |
| CESD (Somatic) | −.047 | (−0.279 – 0.184) | −.015 | .69 | ||
| Stress | .155 | (−0.104 – 0.415) | .044 | .24 | ||
| 2-Way Interactions: | Age-X-CESD (Somatic) | .038 | (−0.003 – 0.079) | .069 | .07 | |
| Age-X-Stress | −.050 | (−0.096 – −0.005) | −.083 | .03 | ||
| CESD (Somatic)-X-Stress | .010 | (−0.056 – 0.076) | .010 | .77 | ||
| 3-Way Interaction: | Age-X-Somatic-X-Stress | −.003 | (−0.014 – 0.008) | −.024 | .55 | |
|
| ||||||
| Model 5 | .279 | |||||
|
| ||||||
| Main Effects: | Age | −.842 | (−0.959 – −0.725) | −.487 | <.001 | |
| CESD (Interpersonal) | .252 | (−0.429 – 0.933) | .027 | .47 | ||
| Stress | .094 | (−0.148 – 0.336) | .027 | .45 | ||
| 2-Way Interactions: | Age-X-CESD (Interpersonal) | .031 | (−0.085 – 0.146) | .019 | .60 | |
| Age-X-Stress | −.029 | (−0.071 – 0.013) | −.048 | .17 | ||
| CESD (Interpersonal)-X-Stress | .123 | (−0.089 – 0.336) | .041 | .26 | ||
| 3-Way Interaction: | Age-X-Interpersonal-X-Stress | −.025 | (−0.059 – 0.008) | −.057 | .14 | |
unstandardized regression coefficient
Results
Sample Characteristics
Information related to the sociodemographic, reproductive, general health, and psychological characteristics of the current sample (N = 683) as well as women excluded due to not having returned the questionnaire packet (n = 157) are reported in Table 1. Women were ethnically diverse (24.3% white, 22.5% African-American, 20.9% Latina, 27.5% Chinese, 4.7% Filipina) and ranged in age from 25 to 45, M = 35.1 [5.6]). Sixty percent had a college degree or greater and 57.0% reported an annual household income of $50,000 or greater. The majority of women (67.3%) had a history of hormone-containing medication use for birth control and 41.9% had one or more live births. A minority of women reported current (6.6%) and past (14.5%) cigarette smoking while the sample on average was overweight (BMI: M = 26.53 [6.6]). Compared to the 683 participants, the 157 women who did not complete the questionnaires differed on race/ethnicity (χ2 = 64.02, p < .001), had lower levels of education (t = 5.44, p < .001) and income (t = 4.64, p < .001), and had higher BMIs (t = 4.60, p < .001).
Table 1.
Sociodemographic, reproductive, general health, and psychological factors in the current sample and in women excluded because they did not return the self-report questionnaire packet.
| Participants (N = 683) | Non-Participants (N = 157) | |||
|---|---|---|---|---|
|
| ||||
| Mean (SD) or % | Mean (SD) or % | Test Statistic | p | |
| Sociodemographics: | ||||
| Age | 35.05 (5.6) | 34.94 (5.7) | t = 0.22 | .83 |
| Race/Ethnicity | - | - | χ2 = 64.02 | <.001 |
| White | 24.3% | 5.1% | - | - |
| AA | 22.6% | 29.9% | - | - |
| Latina | 20.9% | 45.9% | - | - |
| Chinese | 27.5% | 16.6% | - | - |
| Filipina | 4.7% | 2.5% | - | - |
| Educational Levela | 3.61 (1.2) | 3.01 (1.3) | t = 5.44 | <.001 |
| Income/Dependentsb | 3.38 (2.0) | 2.54 (1.9) | t = 4.64 | <.001 |
| Reproductive Factors: | ||||
| AFC | 15.18 (9.7) | 14.62 (9.8) | t = 0.66 | .51 |
| Menarcheal Age | 12.55 (1.6) | 12.71 (1.7) | t = 1.17 | .24 |
| BC (% w hx of BC use) | 67.3% | 71.3% | χ2 = 0.93 | .33 |
| Parity (%1+ live births) | 41.9% | 59.2% | χ2 = 15.54 | <.001 |
| General Health: | ||||
| Smoking (% curr/past) | 21.1% | 17.8% | χ2 = 0.83 | .36 |
| BMI | 26.53 (6.6) | 29.29 (7.7) | t = 4.60 | <.001 |
| Psychological Factors: | ||||
| CESD (Total Score) | 11.38 (8.3) | - | - | - |
| CESD (Dep Affect) | 3.39 (3.7) | - | - | - |
| CESD (Pos Affect) | 9.16 (2.6) | - | - | - |
| CESD (Somatic) | 4.45 (3.1) | - | - | - |
| CESD (Interpersonal) | 0.72 (1.0) | - | - | - |
| PSS (Total Score) | 4.27 (2.8) | - | - | - |
Education was coded 1=<HS/some HS; 2=HS grad/GED; 3=some college/AA/vocational school; 4=college graduate; 5=graduate school (PhD, MS); 6=professional degree (MD, JD, DDS, MBA).
Household income was coded 1=<$5,000; 2=$5,000-$15,999; 3=$16,000-$24,999; 4=$25,000-$34,999; 5=$35,000-$49,999; 6=$50,000-$74,999; 7=$75,000-$99,999; 8=$100,000-$149,999; 9=$150,000-$199,999; 10=$200,000+ and divided by the number of individuals in the household who were dependent on the reported income.
Linear Regression: Is AFC decline across women greater among women with higher levels of depression?
Results of linear regression analyses performed to determine whether AFC decline across women varied over levels of depression are reported in Table 2. In Model 3 in which the CESD positive affect subscale was examined, explanatory variables accounted for 27.3% of the variance in AFC following backward elimination of independent variables with p-values >.20. Among the covariates, having a history of using hormone-containing medication for birth control was related significantly to lower AFC (b = − 1.631, p = .02). The main effect of age on AFC was statistically significant with greater chronological age related to lower AFC (b = − 0.876, p < .001); in this model, the effect of age on AFC was estimated at the sample mean of positive affect. In addition, the 2-way interaction term (age-x-positive affect) was related to AFC significantly (b = 0.047, p = .036), demonstrating that the relation between age and AFC varied over levels of positive affect. Results from the remaining Models 1, 2, 4, and 5 examining the CESD total score and the CESD depressed affect, somatic, and interpersonal subscale scores, respectively, showed the 2-way interaction terms were all non-significant (p’s > .05).
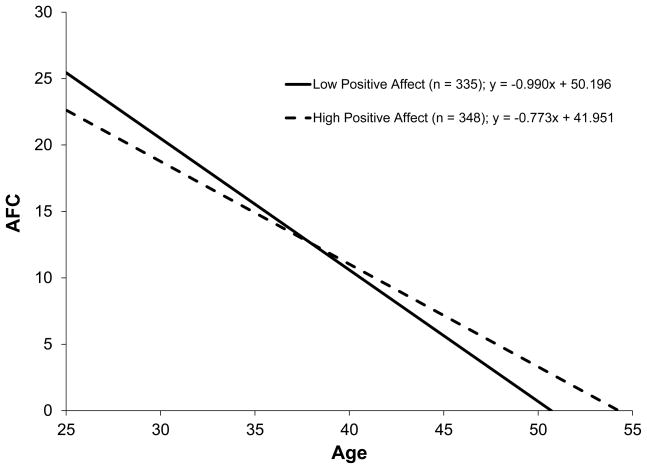
To illustrate the significant age-x-positive affect interaction term, the distribution of the positive affect subscale was divided at the median and separate regression equations were performed examining the relation between age and AFC in the women with low positive affect (n = 335) and in the women with high positive affect (n = 348). The slopes (unstandardized coefficients), plotted in Figure 1, showed AFC decline across women was higher (− 0.990 follicles/year) in the women with low positive affect than in the women with high positive affect (− 0.773 follicles/year). That is, AFC decline was 28% faster in the women reporting low versus high positive affect. The x-intercepts reflecting the ages at which AFC is projected to reach zero (or the estimated ages at menopause) were 50.7 for women with low positive affect and 54.3 for women with high positive affect.
Figure 1.
AFC decline across women (ages 25–45) reporting low versus high positive affect (median split) on the positive affect subscale of the CESD.
Linear Regression: Is AFC decline across women greater among women with higher levels of depression and higher levels of psychological stress?
Results of linear regression analyses performed to determine whether AFC decline across women varied over levels of depression and psychological stress are reported in Table 3. In Model 3 in which the CESD positive affect subscale was examined, explanatory variables accounted for 28.0% of the variance in AFC following backward elimination of independent variables with p-values >.20. Among the covariates, having a history of using hormone-containing medication for birth control was related significantly to lower AFC (b = − 1.624, p = .02). The main effect of age on AFC was statistically significant with greater chronological age related to lower AFC (b = − 0.828, p < .001); in this model, the effect of age on AFC was estimated at the sample means of positive affect and psychological stress. In addition, albeit a statistical trend, the 3-way interaction term (age-x-positive affect-x-stress) was also related to AFC (b = 0.012, p = .099), demonstrating that the relation between age and AFC varied over levels of positive affect and psychological stress. Results from the remaining Models 1, 2, 4, and 5 examining the CESD total score and the CESD depressed affect, somatic, and interpersonal subscale scores, respectively, showed the 3-way interaction terms were all non-significant (p’s > .05).
In stratified analyses, additional linear regression models were evaluated in the low (n = 335) versus high (n = 348) positive affect women (defined by dividing the distribution of positive affect at the median) in which AFC decline across women was examined over levels of psychological stress to determine whether women with low positive affect were more vulnerable to the effects of stress. First, among women with low positive affect (n = 335), results from analyses in which covariates, main effects (age, stress), and the 2-way interaction term (age-X-stress) were entered, explanatory variables accounted for 31.4% of the variance in AFC following backward elimination of independent variables with p-values >.20. The main effect of age on AFC was statistically significant with greater chronological age related to lower AFC (b = − 0.898, p < .001) and the main effect of stress on AFC was statistically significant with greater stress related to higher AFC (b = 0.340, p = .044); in this model, the effect of age on AFC was estimated at the sample mean of stress and the effect of stress on AFC was estimated at the sample mean of age. In addition, the 2-way age-x-stress interaction term (b = −.070, p = .021) was related to AFC significantly, demonstrating a moderating effect of psychological stress on AFC decline among women with low positive affect. Next, among women with high positive affect (n = 348), results from analyses using the same analytical strategy for variable entry showed the explanatory variables to account for 23.3% of the variance in AFC. Here, however, the 2-way age-x-stress interaction term was non-significant (b = .018, p = .54), demonstrating there to be no moderating effect of psychological stress on AFC decline among women with high positive affect.
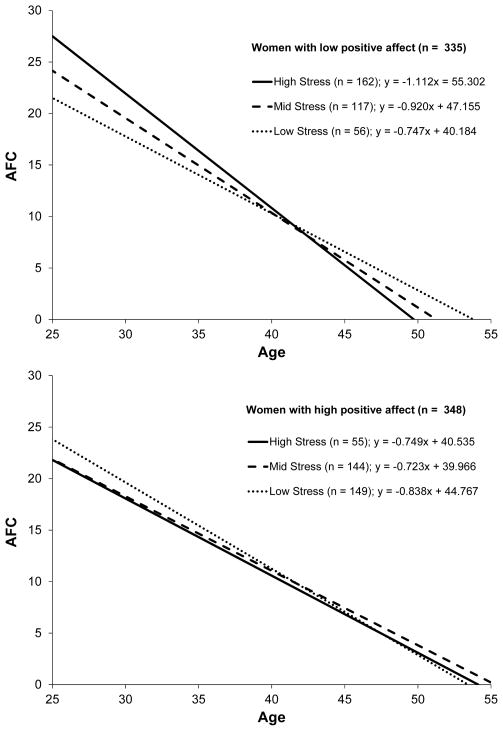
To illustrate the significant 2-way age-x-stress interaction term among the women with low positive affect, separate regression equations were performed to assess the relation between age and AFC in the women with low positive affect who reported low (n = 56), mid (n = 117), and high (n = 162) levels of stress defined by dividing the distribution of stress in the full sample into tertiles. The slopes (unstandardized coefficients), plotted in Figure 2, showed AFC decline across women was progressively higher in the women with low positive affect who reported low (− 0.747 follicles/year), mid (− 0.920 follicles/year), and high (− 1.112 follicles/year) levels of stress. That is, AFC decline was 23% faster in the women with low positive affect/mid stress versus low positive affect/low stress and 49% faster in the women with low positive affect/high stress versus low positive affect/low stress. The x-intercepts reflecting the ages at which AFC is projected to reach zero (or the estimated ages at menopause) were 53.8, 51.3, and 49.7 for women with low positive affect who reported low, mid, and high levels of stress, respectively. For comparison purposes, the x-intercepts were 53.4, 55.3, and 54.1 for women with high positive affect who reported low, mid and high levels of stress, respectively.
Figure 2.
AFC decline across women (ages 25–45) reporting low, mid, and high levels of stress among women with low (n = 335) versus high (n = 348) positive affect.
Discussion
The goals of the current study were to examine depression as well as depression in combination with psychological stress in relation to ovarian aging as indexed by AFC, a validated marker of ovarian reserve. In cross-sectional analyses of 683 pre-menopausal women, results indicated that independently of covariates, AFC decline across women was greater in women with lower positive affect as measured by the positive affect subscale of the CESD. The average follicle decline across women was − 0.990 follicles per year in women with low positive affect compared to − 0.773 follicles per year in women with high positive affect. In addition, among women with low positive affect, AFC decline across women was greater in women reporting higher levels of psychological stress. That is, the average follicle decline across women was − 1.112 follicles per year in women with low positive affect who reported high levels of stress compared to − 0.920 follicles per year and − 0.747 follicles per year in women with low positive affect who reported mid and low levels of stress, respectively. Also, there was a significant main effect of greater stress on higher AFC estimated at the mean age of the sample. In contrast, among women with high positive affect, there was no effect of psychological stress on AFC decline. In summary, cross-sectional evidence suggests that 1) women with low positive affect may experience accelerated AFC decline and 2) low positive affect may be a vulnerability factor, or, alternatively, high positive affect may be a protective factor, in moderating the negative effects of psychological stress on AFC decline.
Among the CESD-derived variables, only low positive affect emerged as a potential psychological risk factor for accelerated ovarian aging. Depressive symptomatology more generally (as represented by the total score of the CESD) as well as other depressive symptom types (i.e., depressed affect, somatic, interpersonal difficulties) were unrelated to AFC decline. A prior literature suggests that low positive affect or the lack of positive emotion is a prominent symptom in depression. In a recent review, positive affect, even compared to DSM-IV symptoms of depression, showed the strongest correlation with depression diagnoses compared to other types of psychopathology.54 In addition, outside the depression literature, dimensional variability in positive affect has been shown to predict a variety of physical health outcomes (for a review see Pressman & Cohen, 2005).55 For example, among studies that have used the CESD positive affect subscale, low positive affect has been related prospectively to all-cause mortality,56 incident stroke,57 reduced survival in AIDS patients,58 and poorer physical functioning following a major health event (e.g., myocardial infarction).59 Although these studies varied methodologically, results generally showed positive affect but not the other CESD subscales predicted the indicated health outcome, supporting the contribution of positive affect uniquely. Lastly, the current findings are also consistent with models suggesting that individuals with low positive affect may be especially vulnerable to psychological stress and concomitant physiological alterations (e.g., autonomic nervous system activation; hypothalamic-pituitary-adrenal axis activation) commonly implicated in explaining stress effects on health and disease.55,60–61 How similar stress-related mechanisms may be operative in the context of ovarian aging, however, is not known.
Secondarily, results also indicated that among the covariates, a history of using hormone-containing medication for birth control was related independently to lower AFC. Findings from previous studies examining oral contraceptive (OC) use in relation to menopausal timing have been mixed, showing OC use related to later age at menopause62–63 or showing no relation at all64–65. The suggestion in the current study that hormone-containing birth control may actually have an inhibitory effect on antral follicle count was described in a previous report also from the OVA Study66; however, no other study to our knowledge has reported such a link. Future studies are necessary to determine whether this association is reproducible in an independent sample as well as whether OC use is related to AFC decline over time. Future studies are also necessary to further evaluate the significant main effect found in the current study in which greater stress was related to higher AFC in the context of a significant age-x-stress interaction among women with low positive affect. Interestingly, the possibility that stress may relate to the enhancement of fertility marked by having a higher AFC is not inconsistent with life history models which have proposed that adverse environments may promote biological preparation for current versus longer term reproduction to avert risks associated with a delay in reproduction.67–68
A primary weakness of the current study was the single assessment of depression, psychological stress, and AFC, limiting analyses to the cross-sectional examination of these psychological factors in relation to AFC decline across women. Whether depression and psychological stress relate to intra-individual change in AFC over time remains untested. The cross-sectional nature of the analyses also precluded characterization of the temporal relationship between depression and psychological stress. This may be particularly problematic in light of prior research suggesting the relationship between psychological stress and depression may change over time.14–15 A second primary weakness of the current study concerned the measurement of depression and psychological stress which was limited to assessments of current, self-reported symptoms of depression and perceptions of stress. Greater detail regarding depression diagnoses and depression history as well as exposures to specific psychosocial stressors, their timing, and severity is necessary to begin to more fully characterize the role of psychological factors in ovarian aging. Finally, the current findings should be considered preliminary due the relatively large number of analyses performed from which only results related to positive affect were statistically significant; and due to limitations in the generalizability of results as evidenced by significant differences on race/ethnicity, socioeconomic status, and body size between women who completed the self-report measures and those who did not return the questionnaire packet.
A primary strength of the current study was its novel focus on depression in relation to ovarian aging. Although prior research has suggested MDD may accelerate biological aging, there has been a paucity of research investigating the effects of depression on ovarian aging in particular. In addition, the current study makes an important contribution by assessing depression as well as psychological stress in connection with ovarian aging in order to begin to more fully characterize how these commonly inter-related psychological dimensions may act synergistically to impact AFC decline. Other notable strengths of the current study were its emphasis on the pre-menopausal period; its assessment of AFC; and its recruitment of a relatively large and diverse sample. The study’s emphasis on examining risk factors for ovarian aging among young, pre-menopausal women is important in potentially generating novel intervention opportunities to prolong ovarian function in women at-risk for accelerated ovarian aging and early menopause. The study’s use of AFC as a marker of ovarian reserve is superior to conventional methods of staging reproductive age which rely on menstrual cycle and hormonal characteristics which do not appreciably change until reproductive aging is more advanced.69 Lastly, the current sample was relatively large in size, well-characterized in terms of its reproductive and general health, and unique in its representation of five, approximately equal, race/ethnic groups.
Conclusions
The current study provides preliminary evidence that psychological factors may play a role in promoting ovarian aging during pre-menopause, showing AFC decline across women to be highest among women in whom positive affect was low and psychological stress was high. The emergence of low positive affect as a potential psychological risk factor for ovarian aging is consistent with a large literature supporting its prominence in depression diagnoses54 and broader relation to risk for poor physical health outcomes.55–59 Findings also extend previous literatures in which MDD was related to markers of accelerated biological aging1–5 and earlier onset peri-menopause7 by highlighting the potential of role of normative (i.e., sub-clinical) variability in depressive symptomatology in understanding ovarian aging. Future studies should address the weaknesses of the current study by implementing study designs that are longitudinal and by utilizing assessments of depression and psychological stress that capture women’s clinical diagnoses of depression and exposures to psychosocial stressors over the life course. In addition, potential mechanisms (e.g., health behaviors) explaining links between psychological factors and ovarian aging need to be investigated to begin to understand how potential stress-related effects on the body may impact the apparent disproportionate loss of ovarian follicles among women experiencing higher levels of depression and psychological stress.
Acknowledgments
Funding: Preparation of this manuscript and the research described here were supported by NIH/NICHD and NIH/NIA (R01 HD044876); NIH/NIA (K08 AG03575); NIH/UCSF-CTSI (UL1 RR024131); Brain and Behavior Research Foundation; and RWJF (045820).
Footnotes
No author has a conflict of interest to declare.
References
- 1.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: Do stress and depression accelerate cell aging? Depress Anxiety. 2010;27(4):327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- 2.Wolkowitz OM, Epel ES, Mellon S. When blue turns to grey: Do stress and depression accelerate cell aging? World J Biol Psychiatry. 2008;9(1):2–5. doi: 10.1080/15622970701875601. [DOI] [PubMed] [Google Scholar]
- 3.Wolkowitz OM, Mellon SH, Epel ES, et al. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress - preliminary findings. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon NM, Smoller JW, McNamara KL, et al. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Hoen PW, de Jonge P, Na BY, et al. Depression and leukocyte telomere length in patients with coronary heart disease: Data from the Heart and Soul Study. Psychosom Med. 2011;73(7):541–547. doi: 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety. 2010;27(12):1111–1116. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- 7.Harlow BL, Wise LA, Otto MW, Soares CN, Cohen LS. Depression and its influence on reproductive endocrine and menstrual cycle markers associated with perimenopause: the Harvard Study of Moods and Cycles. Arch Gen Psychiatry. 2003;60(1):29–36. doi: 10.1001/archpsyc.60.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Daley SE, Hammen C, Rao U. Predictors of first onset and recurrence of major depression in young women during the 5 years following high school graduation. J Abnorm Psychol. 2000;109(3):525–533. [PubMed] [Google Scholar]
- 9.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 10.Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: Differential processes of psychosocial risk. J Abnorm Psychol. 1999;108(3):483–489. doi: 10.1037//0021-843x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 11.Hammen C, Davila J, Brown G, Ellicott A, Gitlin M. Psychiatric history and stress -predictors of severity of unipolar depression. J Abnorm Psychol. 1992;101(1):45–52. doi: 10.1037//0021-843x.101.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Hammen C. Annual Review of Clinical Psychology. Palo Alto: Annual Reviews; 2005. Stress and depression; pp. 293–319. [DOI] [PubMed] [Google Scholar]
- 13.Kendler KS, Walters EE, Kessler RC. The prediction of length of major depressive episodes: Results from an epidemiological sample of female twins. Psychol Med. 1997;27(1):107–117. doi: 10.1017/s0033291796003893. [DOI] [PubMed] [Google Scholar]
- 14.Slavich GM, Monroe SM, Gotlib IH. Early parental loss and depression history: Associations with recent life stress in major depressive disorder. J Psychiatr Res. 2011;45(9):1146–1152. doi: 10.1016/j.jpsychires.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monroe SM, Harkness KL. Life stress, the “Kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychol Rev. 2005;112(2):417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- 16.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 17.Almeida DM, Kessler RC. Everyday stressors and gender differences in daily distress. J Pers Soc Psychol. 1998;75(3):670–680. doi: 10.1037//0022-3514.75.3.670. [DOI] [PubMed] [Google Scholar]
- 18.Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression - A theoretical model. Arch Gen Psychiatry. 2000;57(1):21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- 19.Cohen S, Williamson G. ISSSO. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: Sage; 1988. Perceived stress in a probability sample of the United States. [Google Scholar]
- 20.Harkness KL, Alavi N, Monroe SM, Slavich GM, Gotlib IH, Bagby RM. Gender differences in life events prior to onset of major depressive disorder: The moderating effect of age. J Abnorm Psychol. 2010;119(4):791–803. doi: 10.1037/a0020629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan J, Burger HG, Szoeke C, et al. A prospective study of the association between endogenous hormones and depressive symptoms in postmenopausal women. Menopause-J N Am Menopause Soc. 2009;16(3):509–517. doi: 10.1097/gme.0b013e31818d635f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt PJ, Murphy JH, Haq N, Danaceau MA, St Clair LS. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology. 2002;27(8):907–920. doi: 10.1016/s0306-4530(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 23.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 24.Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating-hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54(2):297–302. doi: 10.1016/s0015-0282(16)53707-8. [DOI] [PubMed] [Google Scholar]
- 25.Martin JSB, Nisker JA, Tummon IS, Daniel SAJ, Auckland JL, Feyles V. Future in vitro fertilization pregnancy potential of women with variably elevated day 3 follicle-stimulating hormone levels. Fertil Steril. 1996;65(6):1238–1240. doi: 10.1016/s0015-0282(16)58347-2. [DOI] [PubMed] [Google Scholar]
- 26.Hansen K, Massey J, Craig L. Antral follicle counts obtained by transvaginal ultrasound and histological examination are correlated with ovarian non-growing follicle number. Fertil Steril. 2007;88:S79–S80. [Google Scholar]
- 27.Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: The decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23(3):699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- 28.Morris J, Thyer A, Soules M, Klein N. Antral follicle count by transvaginal ultrasound is reflective of the actual primordial follicle pool. Fertil Steril. 2002;78(S3) [Google Scholar]
- 29.Reuss ML, Kline J, Santos R, Levin B, Timor-Tritsch I. Age and the ovarian follicle pool assessed with transvaginal ultrasonography. Am J Obstet Gynecol. 1996;174:624–627. doi: 10.1016/s0002-9378(96)70439-8. [DOI] [PubMed] [Google Scholar]
- 30.Reuss ML, Kolton S, Tharakan T. Transvaginal ultrasonography in gynecologic office practice: Assessment in 663 premenopausal women. Am J Obstet Gynecol. 1996;175(5):1189–1194. doi: 10.1016/s0002-9378(96)70026-1. [DOI] [PubMed] [Google Scholar]
- 31.Rosen MP, Sternfeld B, Schuh-Huerta SM, Reijo-Pera RA, McCulloch CE, Cedars MI. Antral follicle count: Absence of significant midlife decline. Fertil Steril. 2010;94(6):2182–2185. doi: 10.1016/j.fertnstert.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Prediction of high ovarian response to controlled ovarian hyperstimulation: Anti-Mullerian hormone versus small antral follicle count (2–6 mm) J Assist Reprod Genet. 2009;26(6):319–325. doi: 10.1007/s10815-009-9319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheffer GJ, Broekmans FJM, Dorland M, Habbema JDF, Looman CWN, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72(5):845–851. doi: 10.1016/s0015-0282(99)00396-9. [DOI] [PubMed] [Google Scholar]
- 34.Frattarelli JL, Levi AJ, Miller BT, Segars JH. A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil Steril. 2003;80(2):350–355. doi: 10.1016/s0015-0282(03)00664-2. [DOI] [PubMed] [Google Scholar]
- 35.Klinkert ER, Broekmans FJ, Looman CW, Habbema JD, te Velde ER. The antral follicle count is a better marker than basal follicle-stimulating hormone for the selection of older patients with acceptable pregnancy prospects after in vitro fertilization. Fertil Steril. 2005;83(3):811–814. doi: 10.1016/j.fertnstert.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Broekmans FJ, Faddy MJ, Scheffer G, te Velde ER. Antral follicle counts are related to age at natural fertility loss and age at menopause. Menopause. 2004;11(6):607–614. doi: 10.1097/01.gme.0000123643.76105.27. [DOI] [PubMed] [Google Scholar]
- 37.Giacobbe M, Mendes Pinto-Neto A, Simoes Costa-Paiva LH, Martinez EZ. The usefulness of ovarian volume, antral follicle count and age as predictors of menopausal status. Climacteric. 2004;7(3):255–260. doi: 10.1080/13697130410001713715. [DOI] [PubMed] [Google Scholar]
- 38.Pache TD, Wladimiroff JW, de Jong FH, Hop WC, Fauser BC. Growth patterns of nondominant ovarian follicles during the normal menstrual cycle. Fertil Steril. 1990;54(4):638–642. doi: 10.1016/s0015-0282(16)53821-7. [DOI] [PubMed] [Google Scholar]
- 39.Scheffer GJ, Broekmans FJM, Bancsi LF, Habbema JDF, Looman CWN, Te Velde ER. Quantitative transvaginal two- and three-dimensional sonography of the ovaries: reproducibility of antral follicle counts. Ultrasound Obstet Gynecol. 2002;20(3):270–275. doi: 10.1046/j.1469-0705.2002.00787.x. [DOI] [PubMed] [Google Scholar]
- 40.Gordon N. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? Oakland, CA: Kaiser Permanente Division of Research; 2006. [Google Scholar]
- 41.Broekmans F, de Ziegler D, Howles C, Gougeon A, Trew G, Olivennes F. The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94(3):1044–1051. doi: 10.1016/j.fertnstert.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 42.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977 Sum;1(3):385–401. [Google Scholar]
- 43.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: A validation study. Am J Epidemiol. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 44.Hales DP, Dishman RK, Motl RW, Addy CL, Pfeiffer KA, Pate RR. Factorial validity and invariance of the center for epidemiologic studies depression (CES-D) scale in a sample of black and white adolescent girls. Ethn Dis. 2006;16(1):1–8. [PubMed] [Google Scholar]
- 45.Nunnally JC. Psychometric theory. 1967. Psychometric theory: (1967) [Google Scholar]
- 46.Radloff LS, Teri L. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clinical Gerontologist. 1986 Jun;5(1–2):119–136. [Google Scholar]
- 47.Blazer DG, Landerman LR, Hays JC, Simonsick EM, Saunders WB. Symptoms of depression among community-dwelling elderly African-American and White older adults. Psychol Med. 1998;28(6):1311–1320. doi: 10.1017/s0033291798007648. [DOI] [PubMed] [Google Scholar]
- 48.Clark VA, Aneshensel CS, Frerichs RR, Morgan TM. Analysis of effects of sex and age in response to items on the CES-D scale. Psychiatry Research. 1981;5(2):171–181. doi: 10.1016/0165-1781(81)90047-0. [DOI] [PubMed] [Google Scholar]
- 49.Hertzog C, Van Alstine J, Usala P, Hultsch D, Dixon R. Measurement properties of the Center for Epidemiological Studies Depression Scale (CES-D) in older populations. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1990;2(1):64–72. [Google Scholar]
- 50.Knight RG, Williams S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a sample of women in middle life. Behav Res Ther. 1997;35(4):373–380. doi: 10.1016/s0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- 51.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 52.Cohen S, Janicki-Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006 and 2009. J Appl Soc Psychol. 2011 [Google Scholar]
- 53.Aiken L, West S. Interactions in Multiple Regression. Lawrence Erlbaum; 1991. [Google Scholar]
- 54.Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: Evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clinical Psychology Review. 2010;30(7):839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131(6):925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 56.Ostir GV, Markides KS, Black SA, Goodwin JS. Emotional well-being predicts subsequent functional independence and survival. J Am Geriatr Soc. 2000;48(5):473–478. doi: 10.1111/j.1532-5415.2000.tb04991.x. [DOI] [PubMed] [Google Scholar]
- 57.Ostir GV, Markides KS, Peek MK, Goodwin JS. The association between emotional well-being and the incidence of stroke in older adults. Psychosom Med. 2001;63(2):210–215. doi: 10.1097/00006842-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Moskowitz JT. Positive affect predicts lower risk of AIDS mortality. Psychosom Med. 2003;65(4):620–626. doi: 10.1097/01.psy.0000073873.74829.23. [DOI] [PubMed] [Google Scholar]
- 59.Ostir GV, Goodwin JS, Markides KS, Ottenbacher KJ, Balfour J, Guralnik JM. Differential effects of premorbid physical and emotional health on recovery from acute events. J Am Geriatr Soc. 2002;50(4):713–718. doi: 10.1046/j.1532-5415.2002.50167.x. [DOI] [PubMed] [Google Scholar]
- 60.Salovey P, Rothman AJ, Detweiler JB, Steward WT. Emotional states and physical health. Am Psychol. 2000;55(1):110–121. doi: 10.1037//0003-066x.55.1.110. [DOI] [PubMed] [Google Scholar]
- 61.Fredrickson BL. The role of positive emotions in positive psychology - The broaden-and-build theory of positive emotions. Am Psychol. 2001;56(3):218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 63.vanNoord PAH, Dubas JS, Dorland M, Boersma H, teVelde E. Age at natural menopause in a population-based screening cohort: The role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68(1):95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]
- 64.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145(2):124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 65.Hardy R, Kuh D. Reproductive characteristics and the age at inception of the perimenopause in a British National Cohort. Am J Epidemiol. 1999;149(7):612–620. doi: 10.1093/oxfordjournals.aje.a009861. [DOI] [PubMed] [Google Scholar]
- 66.Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Pera RAR. Genetic variants and environmental factors associated with hormonal markers of ovarian reserve in Caucasian and African American women. Hum Reprod. 2012;27(2):594–608. doi: 10.1093/humrep/der391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy - an evolutionary theory of socialization. Child Dev. 1991;62(4):647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 68.Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychol Bull. 2004;130(6):920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- 69.Soules MR, Sherman S, Parrott E, et al. Stages of Reproductive Aging Workshop (STRAW) J Wom Health Gend Base Med. 2001;10(9):843–848. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]