Abstract
To examine the roles of epigenetic modulation on hair follicle regeneration, we generated mice with a K14Cre-mediated loss of DNA methyltransferase (DNMT) 1. The mutant shows an uneven epidermal thickness and alterations in hair follicle size. When formed, hair follicle architecture and differentiation appear normal. Hair subtypes exist but hair fibers are shorter and thinner. Hair numbers appear normal at birth but gradually decrease to fewer than 50% of control in 1 year old mice. Sections of old mutant skin shows follicles in prolonged telogen with hyperplastic sebaceous glands. Anagen follicles in mutants exhibit decreased proliferation and increased apoptosis in matrix transient amplifying cells. Although K15 positive stem cells in the mutant bulge are comparable in number to the control, their ability to proliferate and become activated to form a hair germ is reduced. As mice age, residual DNMT activity declines further and the probability of successful anagen reentry decreases, leading to progressive alopecia. Paradoxically, there is increased proliferation in the epidermis which also shows aberrant differentiation. These results highlight the importance of DNA methylation in maintaining stem cell homeostasis during the development and regeneration of ectodermal organs.
Keywords: stem cell, homeostasis, hair cycle, epigenetics, methylation
INTRODUCTION
The hair follicle, an organ with robust regenerative capabilities, undergoes episodic regenerative cycling in adults under normal physiological conditions. In adult animals, hair follicles cycle through phases of growth (anagen), regression (catagen), and quiescence (telogen) (Stenn et al., 2001; Schmidt-Ullrich et al., 2005; Cotsarelis et al., 2006). The architectural organization of hair follicles makes it easy to discern the location of hair stem cells, proliferating transient amplifying cells (TA) and differentiating cells. The length of a hair shaft is proportional to the duration of anagen. Furthermore, more than thirty thousand hair follicles grow on each individual, rendering them accessible to quantitative analyses (Plikus et al., 2008a; 2011). These characteristics make the hair an ideal model to study homeostasis among stem / TA / differentiated cells within the hair follicle (Blanpain and Fuchs, 2009).
Recently, epigenetic mechanisms involving modifications of histone tails or DNA have been shown to modulate the accessibility of genes to transcriptional machinery and thereby modulate gene activities without having to change the DNAgenomic sequence (Goldberg et al., 2007). We wondered what roles epigenetic processes may play in the development and regeneration of hair follicles.
DNMT1 function has been studied extensively. In the skin, DNA methylation plays a role in stem cell self-renewal and differentiation (Sen et al., 2010). These authors showed that DNMT1 is required to maintain epidermal lineage precursor cells. Upon differentiation the promoters of a number of genes involved in epithelial differentiation were demethylated. shRNA mediated suppression of DNMT1 reduced the progenitor pool as the cells differentiated prematurely.
However, the role of DNMT1 has not been studied in the regenerative cycling of hair follicles, nor in epidermal homeostasis in vivo. To assess the roles of DNMT1 in regulating hair filaments and hair follicles during the murine hair cycle, we crossed a K14-cre line that expresses Cre recombinase in epidermal basal cells with a floxed DNMT1 line to excise specific exons of the DNMT1 gene in the mouse epidermis. This cross created a conditional knock out of DNMT 1 in the K14 expressing epidermis. Here we report the skin pathology of these mice and the abnormal stem cell activity in epidermis and hairs.
RESULTS
DNMT1 is Expressed in Developing Skin and Cycling Hair Follicles
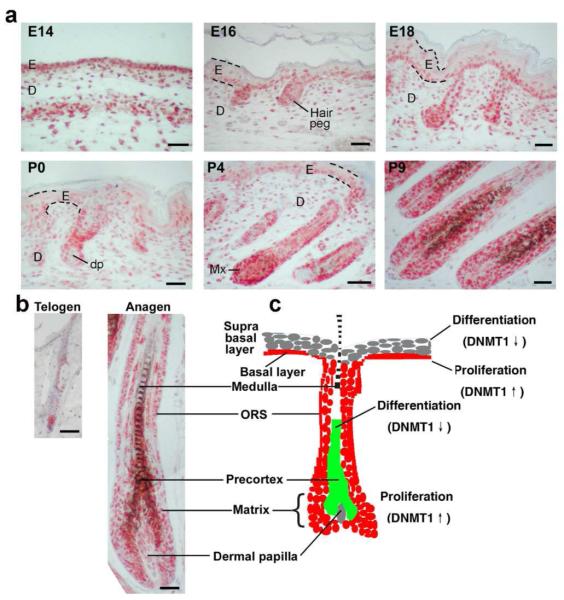
Hair placodes begin to form at embryonic day 14.5 (E14.5). DNMT1 is expressed widely in the epithelium at this stage. By E16–E18, the epidermis expands to become multi-layered and hair germs and hair pegs form. At these stages, DNMT1 is enriched in the basal epidermal layer and hair germs/pegs, but gradually disappears from the spinous, granular, and stratum corneum layers (Fig. 1A). After birth at postnatal day 0-9 (P0 – P9), DNMT1 is weakly expressed in the epidermal basal layer and more strongly in the hair matrix (Fig. 1A). In adult anagen hair follicles, DNMT1 is expressed in the outer root sheath (ORS), inner root sheath (IRS) and matrix. While in telogen hair follicles, DNMT1 is mostly expressed in the hair germ (Fig. 1B). A schematic summary of DNMT expression is shown (Fig. 1C).
Figure 1. Expression of DNMT1 in Developing and Cycling Hair Follicles.
(A) Sections of mouse dorsal skin at E14, 16, and postnatal day 0, 4, 9 were subjected to immunohistochemistry. DNMT1 is mainly expressed in the basal layer of the epithelium and hair matrix. Some DNMT1 was seen in the dermis. (B) In the adult, DNMT1 was expressed in ORS and matrix region of anagen follicles, and in hair germ region in telogen. (C) Schematic highlighting the expression. DNMT1 is low in the differentiating suprabasal layer, and hair shaft. E, epidermis; D, dermis; dp, dermal papilla; Mx, matrix. Scale bar: 50 μm.
Generation and Characterization of K14-Cre DNMT1fl/fl Mice
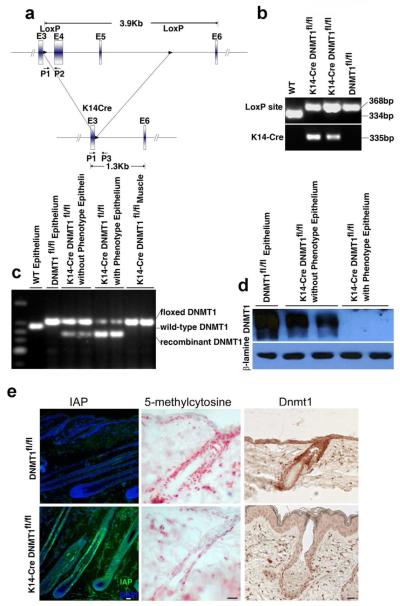
To investigate the role of DNMT1 in hair development and cycling, we generated K14-Cre DNMT1fl/fl mice by crossing K14-cre mice with DNMT1fl/fl mice (Fig. 2A). The genotype of all offspring demonstrates the presence of the LoxP element and K14-Cre (Fig. 2B). Cre mediated recombination is detected in dissected epidermis containing hair follicles from K14-Cre DNMT1fl/fl mice. Muscle serves as a negative control. Using primers P1 and P2 to amplify the floxed allele or P1 and P3 to amplify the recombined allele after excision, we find a K14-Cre mediated specific deletion of DNMT1 in skin epidermis but not in muscle (Fig. 2C). Many but not all genotypic DNMT1 floxed mice show obvious phenotypes. However, there is a good correlation between the level of DNMT1 deletion by recombination and the level of observed phenotype.
Figure 2. Genotyping and Conditional Deletion Efficiency of K14-Cre DNMT1fl/fl Mice.
(A) K14-Cre DNMT1fl/fl DNA schematic (Jackson-Grusby et al., 2001). (B) PCR amplification of LoxP and Cre. (C) PCR amplification of floxed vs Cre mediated excision of DNMT1. High DNMT1 excision levels are associated with a phenotype. Muscle is a negative control. (D) Western blot quantification of DNMT1. DNMT1 protein levels inversely correlated with a phenotype. (E) IAP, 5-methyl cytosine and DNMT1 expression were detected by IHC. IAP level was elevated while 5-methyl cytosine and DNMT1 were decreased in mutant mice compared with WT. Scale bar: 10 μm.
To further characterize DNMT1 loss in the skin of K14-cre DNMT1fl/fl mice, we performed Western blot analysis using anti-DNMT1 antibodies targeted downstream to the excision site. Mice with clear phenotypes show no detectable DNMT1 protein (Fig. 2D, right panel), while mice without obvious phenotypes show some DNMT1 expression, albeit less than wild type (Fig. 2D, left and middle panel). The DNMT1 deletion is further demonstrated by IHC, which shows a reduction, but not a complete loss of DNMT1 expression (Fig. 2E, right panel)
We also assessed activity of DNA methylation by measuring levels of intracisternal A particle (IAP). IAP is normally highly methylated and its expression is silenced. However, IAP expression can be reactivated upon DNA hypomethylation (Hutnick et al., 2010). We reasoned that if DNMT1 is effectively deleted in the epidermis, IAP might become expressed to detectable levels. Indeed, immunofluorescence shows IAP is absent in wild type skin but is highly expressed in the outer root sheath (ORS), with lower expression in the hair matrix of the mutant (Fig. 2E, left panel). Hair matrix cells are supposed to be derived from the ORS. The observation that matrix cells express IAP may be due to changes in methylation activity in the matrix or, by speculation, the expansion of epithelial cells whose DNMT is not completely inactivated. If DNMT is suppressed, we expect the methylation level by 5-methyl cytosine should also be reduced. IHC shows there were fewer 5-methyl cytosine positive cells (Fig. 2E, middle panel).
Gross Phenotypes in DNMT1 Deleted Mice
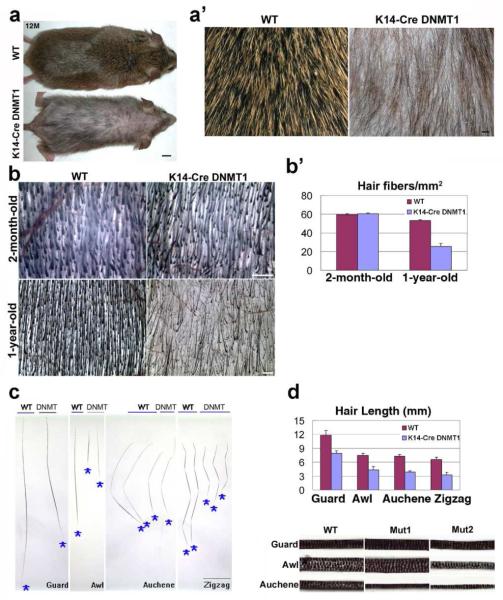
At P3, no major phenotype differences are observed (not shown). At P7 and P9, K14-Cre DNMT1fl/fl mice are smaller and exhibit slight delays in hair development. By 2 months, K14-Cre DNMT1fl/fl mice start to show alopecia phenotypes. Body hairs are sparse and short compared to controls. Hairless patches also appear on the trunk. By 1 year the K14-Cre DNMT1fl/fl mice have grown to equal the size of controls but still exhibit a sparse and ruffled hair coat (Fig. 3A, A’). For further quantification, dorsal skin was excised, inverted and hair density was measured under a dissection microscope (Fig. 4). At 2 months of age, no significant difference in hair density is observed (Fig. 3B, B’; WT: 59.6±0.9 hairs / mm2, K14-Cre DNMT1fl/fl 60.4±1.2 hairs / mm2, P>0.05). At one year, hair density is less than half that found in normal littermates (Fig. 3B, B’. WT: 53.2±1.0 hairs / mm2, K14-cre DNMT1fl/fl 25.6±3.0 hairs / mm2, P<0.05).
Figure 3. K14-Cre DNMT1fl/fl Mice Shows Reduced Hair Size in All Hair Types, and Reduction of Hair Density in Older Mice.
(A) 1-year-old control and mutant. Size bar: 0.5 cm. (A’) Enlargement. Mutant hair is thinner in size and reduced in number. Size bar: 1 mm. (B) Hair numbers gradually decrease from 2-months to 1 year. Scale bar: 50 μm. (B’) Density of hair fibers in mutant and WT mice in young and old mice. Shown as mean ±SD. (C) Comparison of guard, awl, auchene and zigzag hairs. Shapes are generally fine, but mutant hairs are shorter. Size bar: 3 mm (D) Both hair length and width are reduced. Medulla and width of different hair types. Mutant 2 is more affected than mutant 1. Scale bar: 0.2 mm. Bottom: Quantitative comparison of hair lengths by types (mean ±SD).
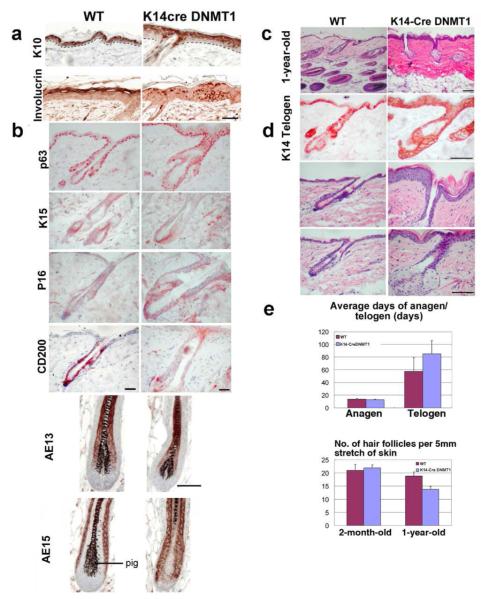
Figure 4. Molecular Characterization of K14-Cre DNMT1fl/fl Mouse Skin.
(A) Mutant shows unevenness in the thickness of epidermis and the size of hair follicles. Some follicles show larger diameter follicles and hair canals, but others are smaller. Otherwise the morphology of hair follicles appears normal. IHC of 3 month-old mutants show wide and patchy expression of involucrin. (B) IHC show s mutants have normal expression of p63, K15, AE13, and AE15, but reduced CD200 and increased of P16 expression. Scale bar: 50 μm. (C) 1-year-old mutant skin is mainly composed of telogen follicles. (D) First row, skin from old mutants shows most follicles in telogen, and many without club hairs. K14 IHC appear normal. Second row, uneven thickness of epidermis is obvious. Third row, enlarged sebaceous glands. Scale bar: 0.4 mm. (E) Anagen duration is about the same in 1-year old mutants, , but telogen duration increases significantly. Old mutants showed decreased hair follicle density. Bar diagram is shown as mean ±SD.Two-tailed unpaired Student’s t tests were used (*, p < 0.05).
K14-Cre DNMT1fl/fl mouse vibrissae are curly and short (not shown). Their pelage consists of four hair types: guard, awl, auchene and zigzag; each with a distinctive morphology (Fig. 3C). We wondered if certain hair types develop abnormally or are lost preferentially due to reduced DNA methylation. Examination shows that the ratio of different hair types are similar. However, we observe a reduction of hair size in both diameter and length. This is seen in all hair types (Fig. 3D, P<0.05). Secondary hairs from K14-Cre DNMT1fl/fl have a reduced diameter and appear to be only half the length of WT. In general, the K14-Cre DNMT1fl/fl mouse hairs are thinner than corresponding wild-type hairs. In many of the awl hairs, the normal 3-4 columns of medulla cells are reduced to 1 to 2 columns (Fig. 3D, lower panel).
Histopathology and Molecular Characterization of DNMT1 Deleted Skin
DNMT1 deleted skin exhibits an unevenness in the thickness of the skin and follicle size of hairs. Some hair follicles have a wider hair canal (Fig. 4B, D). However, the general architecture of hair follicles and filaments which do form show no obvious abnormalities.
We used immunohistochemistry to explore whether abnormal hair differentiation occurred in the skin of adult K14-Cre DNMT1fl/fl mice (Fig. 4A, B). As in controls, K10 is expressed in the differentiated skin keratinocytes. However, in contrast to control skin, involucrin IHC showed precocious expression in some basal layer cells and patchy staining in suprabasal cells of the mutant.
In telogen follicles, p63, is present in the basal epithelium of follicular and inter-follicular skin. K15 is present in the hair germ of telogen follicles. K14 antibody stains the basal layer of the skin and outer root sheath layer of the follicle (Fig. 4 D). CD 34 IHC staining does not show differences between mutant and wild type hair follicles (not shown). On the other hand, P16, a cell cycle suppressor, is increased in the ORS of mutant follicles. CD200, which is expressed in the hair germ in the bulge area, is decreased in mutant hair follicles.
In anagen follicles, the monoclonal antibody AE15 stains the inner root sheath and medulla of the hair shaft. AE13 stains the precortex and the cortex of the hair shaft (Lynch et al., 1986). There are no distinct differences in expression patterns of hair differentiation markers between WT and mutant mice.
Progressive Changes during Aging of DNMT1 Deleted Mouse Skin
We wondered whether this loss of hair fibers reflects a comparable loss of hair follicles in the skin of K14-Cre DNMT1fl/fl mice. H&E stained sections of 1-year-old mouse skin show hair follicle density is reduced, only by about 25% in K14-Cre DNMT1fl/fl mice compared to their control littermates (Fig. 4C, D). Another dramatic difference is that mutant skin shows a large percentage of telogen follicles, while the WT skin contains patches of anagen and telogen hairs (Fig. 4D).
We tried to observe the progression of a regenerative hair wave (Plikus et al., 2008a, 2008b, 2011). In the mutant, the skin remains static and many follicles fail to reenter anagen even after 80 days (not shown). Telogen duration is longer in the mutant than in the WT, while the length of anagen is similar between mutant and WT hair cycles (Fig, 4E). Many telogen follicles in mutant skin are empty without club hairs and are surrounded by hyperplastic sebaceous glands (Fig. 4D). These results suggest either that the prolonged telogen enables follicles to lose their club hairs or that there are defects in retaining club hairs.
DNMT1 Deleted Mice Show Reduced Proliferation in Anagen Hair Matrix
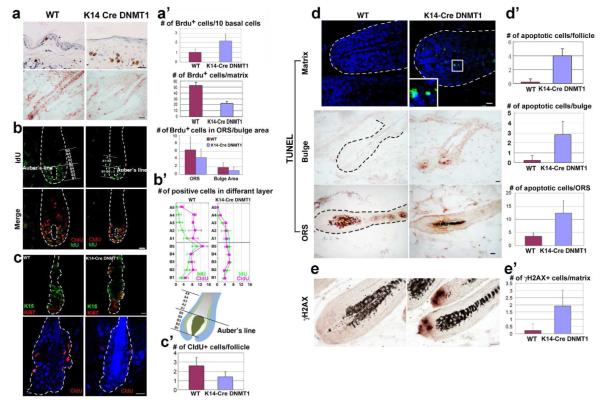
In anagen, TA cells in the matrix proliferate and then move upward and differentiate into the hair shaft (Zhang et al., 2009). We analyzed the dynamics of cell proliferation. First, BrdU was used to label proliferating cells. Three-month-old mice at day 6 in anagen were labeled with BrdU for 1h. We can see there are fewer labeled cells in the mutant’s matrix, ORS and bulge area (Fig. 5A). The number of BrdU positive cells in the hair matrix, ORS and bulge area was quantified (WT = 52.17 +/− 5.42, 6.31 +/− 3.22, 1.83 +/−1.17, respectively; K14-Cre DNMT1fl/fl = 22 +/− 3.03, 4.38 +/− 2.13, 1 +/− 0.89, respectively). We also calculated the mitotic index and found there is a reduction of BrdU positive cells / total cell number from 58 % to 40 % (Fig. 5A’; P<0.05,).
Figure 5. K14-Cre DNMT1 fl/fl Hair Follicles Show Reduced Proliferation and Increased Apoptosis in Hair Follicles.
(A) One hour BrdU labeling of 3-month-old mice. In the epidermis, BrdU labeling is increased, but reduced in the hair matrix and ORS. Size bar: 20 μm. (A’). Numbers were quantified (A’, mean ± SD). (B) Cells were labeled with CldU (red) for 11.5 hr, then followed by IdU (green) for 30 mins. CldU cells moved above Auber’s line in WT, but not in mutant cells. Size bar: 50 μm. (B’) Upper: Numbers of CldU or IdU positive cells along the proximal-distal axis of hair follicle were quantified. (C) Double staining with K15 (green) and Ki67 (red) in telogen follicles and long term label retention of CldU. LRCs number was lower in mutants. Scale bar: 10 m. (C’) Quantification. Student’s t-test (*, P<0.05). (D) TUNEL positive cells (green fluorescent or brown IHC) in the matrix, bulge and ORS of mutants. DAPI stains nucleus (blue). (D’) Average number of TUNEL positive cells per follicle, bulge or ORS (mean ±SD). (E) γH2AX, indicators of DNA damage, is increased in hair bulbs (brown in IHC). (E’) Numbers of γH2AX-positive cells in follicle matrix were quantified (mean ± SD). Size bar: 20 μm.
Then, we estimated the upward movement of TA cells in the hair matrix using double labeling with CldU and IdU followed by a chase period. In the hair follicle, most cell proliferation occurs in the matrix below Auber’s line, which traverses the largest diameter of the dermal papilla (Peters et al., 2002). We expect that cells labeled earlier should move more toward the distal bulb, while newly labeled cells should be close to the base, or beneath Auber’s line.
We labeled mice with CldU for 11.5 hr followed by a short IdU labeling period of 0.5 hr. At the end of the 0.5 hr, mice are sacrificed and the distribution of both CldU (red) and IdU (green) positive cells are examined. In the WT follicles, we observe many CldU positive cells and roughly half are above Auber’s line. In mutant follicles, there are fewer CldU positive cells, consistent with the decrease of cell proliferation (Fig. 5A). Further, only about one third of CldU labeled cells are above Auber’s line, implying a reduced rate of upward movement (Fig. 5B, B’, red color). IdU positive cells have entered S phase within the most recent 0.5 hr. Most of them lay beneath Auber’s line (Fig. 5B, B’, green color). Thus TA cells in WT follicles proliferate and progress to hair filament differentiation, extending much further into the distal follicle than TA cells in K14-Cre DNMT1fl/fl hair follicles.
DNMT1 Deleted Mice Show Decreased Numbers of LRCs in the Stem Cell Region
To gauge the ability of DNMT1 to maintain progenitor stem cells, we measured the population size of long term label retaining cells. We injected CldU into newborn pups from post-natal day 3 to 5 and chased them for 8 weeks (n=3). Long term label retention cells (LRC) were analyzed by CldU staining (Fig. 5C lower). The number of CldU positive cells per follicle was lower in the mutant than in the WT (3 follicles per mouse. Fig. 5C’. P<0.05). We stained follicles in very early anagen with K15 and Ki67 antibodies (Fig. 5C upper). It seems hair stem cells in the K14-Cre DNMT1fl/fl mice can be activated properly during early anagen.
DNMT1 Deleted Mice Show Increased Apoptosis in Anagen Hair Follicles
We examined levels of apoptosis using the TUNEL assay. There are significantly more TUNEL positive cells in the matrix, ORS and bulge area of K14-Cre DNMT1fl/fl mice (Fig. 5D, D’. P<0.05). Since DNMT1 has been shown to participate in DNA repair processes (Mortusewicz et al., 2005), we also examined the expression of γH2AX, a marker for DNA damage. We observed many more γH2AX positive cells in K14-Cre DNMT1fl/fl mice compared to WT (Fig. 5E, E’, P<0.05).
Delayed Activation of Hair Stem Cells after Hair Plucking in K14-Cre DNMT1fl/fl Mice
We wondered whether hair stem cells could respond to activation signals properly. We used wax stripping to test the response. A 1cm2 region of dorsal skin is stripped of hairs. By 11 days after plucking, hairs have regenerated in controls but not in the K14-Cre DNMT1fl/fl mice (Fig. 6A, middle panel). The regeneration of hairs from mutant stem cells is significantly delayed but occurs at day 21 (Fig. 6A, right panel).
Figure 6. K14-Cre DNMT1 fl/fl Hair Follicles Show delay regeneration after plucking and Summary Diagram.
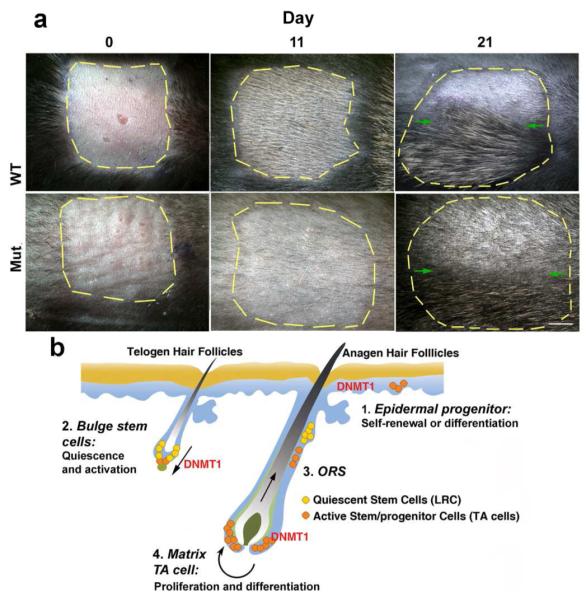
A) Hairs in a 1 cm square are striped with wax. At day 11 after waxing, new appear in WT but not in mutants. At day 21, both hairs have entered telogen. Upper half of the plucked region was shaved to see if they are still in anagen (above green arrows). Scale bar: 2 mm. B) Schematic summary showing the roles of DNMT1 in skin morphogenesis. DNMT1 is involved in regulating epidermal progenitors and also hair follicle homeostasis. In epidermis, mutant epidermis becomes thicker, In hair follicles, DNMT1 is involved in regulating the proliferation and apoptosis of bulge LRC stem cells, ORS, and matrix TA cells. When DNMT 1 is reduced, the probability of successful stem cell activation progressively decreases, leading to disrupted homeostasis in epidermis, hair follicle cycling, and response to plucking. Reduction of hair fibers and follicles lead to the progressive alopecia phenotype.
DISCUSSION
Here we first discuss the epidermal and hair phenotype, and then the implications for DNMT activity on the homeostasis of stem, TA and differentiated cells.
Epidermis Phenotype
In the skin, DNMT1 is expressed in the basal layer of the epidermis. A recent study focused on its function in human epidermis differentiation in vitro and found that DNMT1 was expressed in undifferentiated cells and is required for self-renewal of epidermal progenitor cells. shRNA mediated suppression of DNMT1 led to decreased capabilities of self-renewal and precocious epidermal differentiation (Sen et al., 2010). Our in vivo study on mice with genetic changes is distinct from this published work. Interestingly, we observed that the thickness of mutant epidermis is uneven. Regions with thickened epidermis have increased proliferation compared to wild type. This finding may imply a compensatory mechanism. Also, differentiation markers such as involucrin appear patchy: expressed in the basal and suprabasal layer yet not in all suprabasal cells. Histone methyl transferase, EZH, expression has been disrupted in the skin (Ezhkova et al., 2011). EZH 1, 2 null hair follicles degenerate due to defective proliferation and increased apoptosis. Paradoxically, the mice also show hyper-proliferation in the epidermis. Thus the epidermis in our mutant can be thicker and individual cells appear larger. and this pathology becomes more pronounced in the older mutants ( Fig. 2E and 4A). More work will be required to study the molecular differences.
Hair Phenotype
While the hair number appears normal in newborn mice, the number of hair fibers is progressively lost as mutant mice mature to adulthood. The number of hair fibers lost (>50%) is much greater than the reduction in hair follicles (20%), as many follicles stay in telogen in aging mice. Mutant mice show high variability in the size of hair follicles. Some follicles are smaller than normal, while some follicles are much larger (twice the diameter) with an enlarged hair canal (almost 5 times wider; Fig. 4B). While many molecules are suggested to regulate hair development, regeneration and cycling (Mikkola et al., 2007; Botchkarev et al., 2003), few have been implicated in regulating hair size. Sharov et al (Sharov et al., 2006) found epithelial Noggin can modulate hair follicle size and hair fiber thickness. Hair size and diameter can be regulated by β-catenin expressed within the dermal papilla (Enshell et al., 2010). The loss of uniform hair follicle size in our DNMT1 deleted mice implies that stem cell homeostasis is lost to varied degrees across the mouse skin. We speculate that it is possible that DNMT1 activity may be used to modulate hair follicle size in different body regions.
Hair fibers which form on the mutant skin do not show apparent architectural abnormalities or defective differentiation. All hair subtypes do exist. Unlike the uneven size of follicles, the hair fibers are consistently shorter in length and thinner in diameter.
Roles of DNMT1 in Homoeostasis Maintenance in Hair Follicles
Mutant hair follicles exhibit decreased TA cell proliferation in the ORS. They also demonstrate reduced upward migration. There is increased apoptosis in the hair matrix, ORS and bulge area and increased DNA damage in the hair matrix. We found that γH2AX is increased significantly in the DNMT1 defective hair matrix cells. DNMT1-deficient HeLa and HCT116 cells attenuate the cellular response to DNA damage by 5-aza cytodine and block expression of γH2AX (Palii et al., 2008). Accumulation of this damage in time may lead to degeneration and loss of hair follicles, and eventually the alopecia phenotype in aged mice.
However, K14 is expressed in the ORS, not in the matrix. Whether the effect on hair matrix is directly mediated by DNMT or results from disrupted homeostasis of cell populations within the hair follicle remains to be studied. This line of research may be approached in the future by mating hair matrix specific cre with floxed DNMT1 mice to drive the expression of deleted DNMT1 to the matrix.
Since mutant mouse hairs still undergo cycling and can respond to plucking, bulge stem cells can be activated. However, this ability gradually decreases as mice age, as evidenced by the increased telogen period in old mutants. The inability to activate stem cells for anagen re-entry could be due to depletion of hair stem cells or over-quiescent stem cells that fail to respond to activation signals. We found the mutant and wild type bulge cells express approximately similar levels of K15, implying the number of stem cells is not exhausted. Thus with a defect in DNMT, hair bulge stem cells do seem to maintain a reasonable population size, and are capable of being activated to become hair germs and form hairs. However, the activation process takes longer and occurs with lesser efficiency; and the ability for self-renewal is also compromised. Thus, over time, the number of successfully formed hair filaments reduces and the some follicles degenerate. The detailed molecular mechanism in the DNMT deficient bulge remains to be investigated. This is consistent with the idea that the activation of hair stem cells is a stochastic event (Plikus et al., 2011), we think the observed progressive alopecia phenotype is due to a decreasing probability of successful anagen re-entry. Higher expression of P16 is consistent with this thought.
Progressive loss of DNMT1 protein or enzyme activity has been reported in aging human fibroblasts (Casillas et al., 2003), suggesting that DNMT1 loss in the epithelium may be part of the aging process of the skin. Interestingly, K15 positive stem cells remaining in the bald scalp of patients with human androgenetic alopecia cannot be activated to become proliferative hair germs (Garza et al., 2011). Future work will identify the molecular targets of DNMT in these stem cells and to find out the relationship of mouse epidermal DNMT defects and human androgenetic alopecia.
MATERIALS AND METHODS
Generation and Analysis of Tissue Specific K14-cre DNMT1fl/fl Mice
Homozygous mice carrying the DNMT1fl/fl allele (Jackson-Grusby et al., 2001; Fan et al., 2001) were crossed with mice carrying the K14-Cre transgene (Andl et al., 2004; Hosokawa et al., 2009) and bred to homozygosity. Heterozygous mice did not show a phenotype. Cre excision resulted in an out of frame deletion of exons 4 and 5 producing a non-functional DNMT1 allelle. Usage of transgenic mice is approved by the USC Institutional Animal Care and Use Committee. Mice were genotyped according to Jackson-Grusby et al (2001). Briefly, genomic DNA was amplified by PCR. Primers for the DNMT1 5′ lox site, P1 (5′–GGGCCAGTTGTGTGACTTGG–3′) and P2 (5′–CTTGGGCCTGGATCTTGGGGA–3′), amplify a 334-bp WT and 368-bp DNMT1fl/fl fragment. Cre primers were Cre-F (5′–TTGCCCCTGTTTCACTATCCAG–3′) and Cre-R (5′–ATGGATTTCCGTCTCTGGTG–3′). Cre-recombination efficiency was assessed by PCR and Western Blot from genomic DNA and nuclear protein procured from epithelium at multiple ages. Primers P1 and P2 amplified the floxed allele and P1 and P3 (5′–ATGCATAGGAACAGATGTGTGC–3′) amplified the recombinant allele. The deletion efficiency was determined as the ratios of the recombinant allele to floxed allele.
Measurement of Hair Number, Length, Types
For hair number measurements, anagen stage dorsal skin was fixed in 4% paraformaldehyde and dehydrated through an ethanol series and counted with skin inverted (n=10 mice). The type and length of each fiber was determined under a dissection microscope (n=10 mice).
Immunochemical Procedure
Section immunohistochemistry was performed on mouse dorsal skin samples following the procedure of Jiang et al., 1998. Primary antibodies: rabbit anti-DNMT1 (1:200, Abcam Inc., Cambridge, MA), mouse anti-K14 (1:200, Thermo Fisher Scientific Inc., Fremont, CA), mouse anti-K10 (1:200, Thermo Fisher Scientific Inc., Fremont, CA), mouse anti-AE13 (1:200, Abcam Inc., Cambridge, MA), mouse anti-AE15 (1:200, Santa Cruz Biotechnology Inc., Santa Cruz, CA), mouse anti-involucrin (1:200, Thermo Fisher Scientific Inc., Fremont, CA), rabbit antiphospho-H2AX (1:100; Cell Signaling Technology, Inc., Danvers, MA), rabbit anti-Lef1 (1:100, Cell Signaling Technology, Inc., Danvers, MA), rabbit anti-IAP (from Dr. Fan G) (Hutnick et al., 2010), Rabbit anti- K15 and rabbit anti-Ki67 (1:500, Thermo Fisher Scientific, Inc., Fremont, CA). Western blotting was described.(Jiang et al., 2011).
BrdU, CldU and IdU Labeling
For LRC labeling, neonatal mice were subcutaneously injected with CldU (50 mg per kg body weight) twice daily for 3 d (n=3), from the third day after birth. After chasing for 8 wk, dorsal skin tissues were excised. For the detection of proliferating cells, mice with hair follicles at anagen day 6 were given intraperitoneal injections of BrdU (100mg BrdU / kg body weight; Sigma-Aldrich, St. Louis, MO) and sacrificed 1 hour after injection. Alternatively, mice were given CldU (100mg CldU /kg body weight; Sigma-Aldrich, St. Louis, MO) for 11.5 hr and IdU for 0.5h, then sacrifice. Tissues were fixed and sectioned as described above and stained with mouse anti-BrdU (Millipore, MAB3424), rat anti-CldU (Abcam, Ab6326-250) and mouse anti-IdU (BD, 347580) antibodies. Secondary antibody conjugated with Alexa Fluor 594 (Invitrogen Corporation., Carlsbad, CA).
TUNEL Assay
In situ cell death detection kit (Roche) was used.
Hair plucking and Regeneration
We plucked pelage hairs from a 1cm2 area of the 6-month-old mice with wax. Pictures were taken every 2 days until the hair coat regenerated.
ACKNOWLEDGMENTS
This research was supported by the NIAMS through grants AR 42177, 47364 (to CMC), 60306 (to CMC and TXJ), and NS051411 (GF). Ji Li is supported by the National Natural Science Foundation of China. We thank Dr. Chi-Chiang Chen and Dr. Sung-Jan Lin for help and discussion, and Dr. Juehua Yu for performing IAP immunostaining. Confocal microscopy was performed by the Cell and Tissue Imaging Core of the USC Research Center for Liver Diseases, NIH grant No. P30 DK048522.
Footnotes
CONFLICT OF INTEREST The authors state no conflicts of interest.
REFERENCES
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–68. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Paus R. Molecular biology of hair morphogenesis: development and cycling. J Exp Zool B Mol Dev Evol. 2003;298:164–80. doi: 10.1002/jez.b.33. [DOI] [PubMed] [Google Scholar]
- Casillas MA, Jr, Lopatina N, Andrews LG, Tollefsbol TO. Transcriptional control of the DNA methyltransferases is altered in aging and neoplastically-transformed human fibroblasts. Mol Cell Biochem. 2003;252:33–43. doi: 10.1023/a:1025548623524. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–68. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA, Enshell-Seijffers D, Lindon C, et al. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–42. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–98. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Beard C, Chen RZ, Csankovszki G, Sun Y, Siniaia M, et al. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J Neurosci. 2001;21:788–97. doi: 10.1523/JNEUROSCI.21-03-00788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza LA, Yang CC, Zhao T, Blatt HB, Lee M, He H, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121:613–22. doi: 10.1172/JCI44478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Hosokawa R, Deng X, Takamori K, Xu X, Urata M, Bringas P, Jr, et al. Epithelialspecific requirement of FGFR2 signaling during tooth and palate development. J Exp Zool B Mol Dev Evol. 2009;312B:343–50. doi: 10.1002/jez.b.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutnick LK, Huang X, Loo TC, Ma Z, Fan G. Repression of retrotransposal elements in mouse embryonic stem cells is primarily mediated by a DNA methylation-independent mechanism. J Biol Chem. 2010;285:21082–91. doi: 10.1074/jbc.M110.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, et al. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet. 2001;27:31–9. doi: 10.1038/83730. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Stott NS, Widelitz RB, Chuong CM. Current methods in the study of avian skin appendages. In: Chuong CM, editor. Molecular basis of epithelial appendage morphogenesis. Austin, Texas, USA: 1998. pp. 359–408. [Google Scholar]
- Jiang TX, Tuan TL, Wu P, Widelitz RB, Chuong CM. From buds to follicles: Matrix metalloproteinases in developmental tissue remodeling during feather morphogenesis. Differentiation. 2011;81:307–14. doi: 10.1016/j.diff.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MH, O’Guin WM, Hardy C, Mak L, Sun TT. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. J Cell Biol. 1986;103:2593–606. doi: 10.1083/jcb.103.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola ML. Genetic basis of skin appendage development. Semin Cell Dev Biol. 2007;18:225–36. doi: 10.1016/j.semcdb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Mortusewicz O, Schermelleh L, Walter J, Cardoso MC, Leonhardt H. Recruitment of DNA methyltransferase I to DNA repair sites. Proc Natl Acad Sci U S A. 2005;102:8905–9. doi: 10.1073/pnas.0501034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. DNA methylation inhibitor 5-Aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. 2008;28:752–71. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EM, Tobin DJ, Botchkareva N, Maurer M, Paus R. Migration of melanoblasts into the developing murine hair follicle is accompanied by transient c-Kit expression. J Histochem Cytochem. 2002;50:751–66. doi: 10.1177/002215540205000602. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Chuong CM. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J Invest Dermatol. 2008a;128:1071–80. doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008b;451:340–4. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Baker RE, Chen CC, Fare C, de la Cruz D, Andl T, Maini PK, Millar SE, Widelitz R, Chuong CM. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–89. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–61. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–7. doi: 10.1038/nature08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Sharova TY, Mardaryev AN, Tommasi di Vignano A, Atoyan R, Weiner L, et al. Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl Acad Sci U S A. 2006;103:18166–71. doi: 10.1073/pnas.0608899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Terstappen LW, Huang S, Safford M, Lansdorp PM, Loken MR. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38− progenitor cells. Blood. 1991;77:1218–27. [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–78. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]