Abstract
Marijuana withdrawal contributes to the high relapse rates in individuals seeking treatment for marijuana-use disorders. Quetiapine, an atypical antipsychotic, reduces characteristic symptoms of marijuana withdrawal in a variety of psychiatric conditions including mood lability, sleep disruption, and anorexia. This human laboratory study investigated the effectiveness of quetiapine to decrease marijuana withdrawal and relapse to marijuana use in nontreatment seeking marijuana smokers. Volunteers were maintained on placebo or quetiapine (200 mg/day) in this double-blind, counter-balanced, within-subject study consisting of two 15-day medication phases, the last 8 days of which were inpatient. On the first inpatient day, active marijuana (6.2% delta (9)-tetrahydrocannabinol [THC]) was repeatedly smoked under controlled conditions. For the next 3 days, inactive marijuana (0.0% THC) was available for self-administration (withdrawal). On the subsequent 4 days, active marijuana (6.2% THC) was available for self-administration (relapse). Volunteers (n = 14) who smoked an average of 10 marijuana cigarettes/day, 7 days/week completed the study. Under placebo, withdrawal was marked by increased subjective ratings of negative mood, decreased sleep quality, decreased caloric intake, and weight loss. Compared to placebo, quetiapine improved sleep quality, increased caloric intake, and decreased weight loss. However, quetiapine increased marijuana craving and marijuana self-administration during the relapse phase. These data do not suggest that quetiapine shows promise as a potential treatment for marijuana dependence.
INTRODUCTION
The number of individuals with marijuana-use disorders is over twice that of any other illicit substance, with more people seeking treatment for marijuana dependence than for any other illegal drug in the United States (Substance Abuse and Mental Health Services Administration, 2010). The increasing trend in number of individuals diagnosed with marijuana dependence over the last decade has been attributed, in part, to the steady increase in marijuana potency (Compton et al., 2004), which has more than doubled since 1993 and continues to rise (Mehmedic et al., 2010), thus predicting that rates of marijuana dependence also will continue to grow. As marijuana dependence becomes a greater public health concern, identifying therapies that effectively help treatment-seekers achieve and maintain abstinence is critical. Currently, the few available treatments for marijuana dependence are primarily behavioral in nature and include contingency management, cognitive behavioral therapy, and motivation enhancement therapy. Though these therapies have shown some promise for decreasing marijuana use, maintaining abstinence proves to be a challenge (Stephens, Roffman, and Simpson, 1994; Copeland et al., 2001, Carroll et al., 2006; Stanger et al., 2009; Ramchand et al., 2011). In an effort to further expand and improve upon the available treatment options for marijuana dependence, recent studies have investigated potential candidate pharmacotherapies for their ability to decrease marijuana self-administration using a laboratory model of relapse (for review see Haney, 2009 and Vandrey and Haney, 2009). For these studies, medications are evaluated to determine if they change marijuana’s direct effects, abstinence-induced withdrawal symptoms, and marijuana self-administration during withdrawal following a period of abstinence. Candidate medications are chosen based upon preclinical and/or clinical evidence in support of their potential ability to modify cannabinoid-induced behavioral effects and withdrawal symptoms.
A marijuana withdrawal syndrome has been carefully documented under controlled laboratory conditions and outpatient studies in daily marijuana smokers. This syndrome has been shown to be time dependent and characterized by mood disturbances including increased irritability and restlessness, sleep disturbances, decreased appetite and food intake, and marijuana craving (Haney et al., 1999, 2004; Kouri and Pope, 2000; Budney et al., 2001, 2003; Hart et al., 2002; Allsop et al., 2011). These symptoms have been hypothesized to contribute to the high relapse rates in marijuana-dependent individuals (i.e., Budney et al., 2008; Levin et al., 2010). The only test medication as of yet to have decreased marijuana relapse significantly in a controlled human laboratory study is the alpha-2-adrenergic agonist lofexidine, which improved withdrawal-induced sleep disturbances. Withdrawal symptoms and relapse were further decreased when lofexidine was co-administered with oral delta (9)-tetrahydrocannabinol (THC; dronabinol) (Haney et al., 2008), a medication previously shown to attenuate marijuana craving, decreased food intake, physical symptoms, and mood disturbances associated with withdrawal (Haney et al., 2004). Though controlled human laboratory studies have demonstrated that pharmacologically attenuating withdrawal symptoms does not necessarily predict a decrease in relapse, they have provided insight into which symptoms seem to be most predictive of relapse. For instance, oral THC decreased certain withdrawal symptoms, but failed to decrease relapse when administered on its own (Haney et al., 2004, 2008). Additionally, test medications that attenuated specific withdrawal symptoms including marijuana craving (baclofen), and decreased food intake and disrupted sleep (mirtazapine), also failed to decrease relapse (Haney et al., 2010). Taken together, these findings suggest that medications that improve a constellation of marijuana withdrawal symptoms including sleep and mood disturbances, decreased appetite, and marijuana craving would provide the greatest decrease in withdrawal-associated relapse.
Using a laboratory model of relapse, the current study investigated the ability of quetiapine, an atypical antipsychotic, to decrease marijuana withdrawal symptoms and relapse. Quetiapine has been reported to effectively improve sleep, and decrease anxiety, mood lability, and irritability in a variety of patient populations while producing few adverse effects (Buckley, 2001; Cohrs et al., 2004). Quetiapine is hypothesized to produce these effects by increasing neurotransmission of dopamine and serotonin by acting as a 5-HT2A and D2 antagonist, a partial agonist at the 5-HT1A receptor, and by inhibiting the norepinephrine transporter (for review see Pae et al., 2010). Like other atypical antipsychotics, quetiapine is associated with weight gain and increased appetite (Kulkarni and Kaur, 2001), which could reverse anorexia associated with marijuana withdrawal. Further support that quetiapine may show promise for marijuana-use disorders comes from the report that marijuana craving and use decreased in a small sample of marijuana-using patients maintained on quetiapine for schizophrenia (Potvin, Stip, and Roy, 2004). Based upon quetiapine’s clinical pharmacology, its potential as a short-term pharmacotherapy to attenuate marijuana withdrawal symptoms and facilitate abstinence was evaluated in this 2-phase inpatient, within-subject design. Marijuana’s direct effects, withdrawal symptoms, and marijuana self-administration were assessed under quetiapine (200 mg/day) and placebo maintenance conditions in healthy, daily marijuana smokers. Quetiapine was hypothesized to decrease marijuana withdrawal symptoms and relapse relative to placebo.
MATERIALS AND METHODS
Participants
Volunteers, 21–45 years of age were recruited through newspaper advertisements. Those meeting inclusion/exclusion criteria after an initial phone screen were invited to the laboratory for further screening. Participants were told that the objective of the study was to determine how an FDA-approved medication influences marijuana’s effects; they were told that they would be smoking two different strengths of marijuana, i.e., “Dose A” and “Dose B” but were not told anything about their relative potencies. Participants were accepted into the study if they were healthy, as determined by a physical examination, electrocardiogram, and urine and blood chemistries and not seeking treatment for their marijuana use. Marijuana use was determined by urine toxicology and self-report. To be eligible for participation, volunteers had to report current regular marijuana use. Repeated use of other drugs, with the exception of nicotine, alcohol, or caffeine as determined by urine toxicology and self-report, and/or current use of over-the-counter or prescription medication was exclusionary, as was alcohol dependence. Those who met DSM (Diagnostic and Statistical Manual of Mental Disorders), fourth edition revised criteria for current Axis I psychopathology were not eligible for study participation. Females were excluded if they were pregnant or nursing. Written informed consent was obtained for all aspects of the study. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute (NYSPI) and were in accord with the Declaration of Helsinki.
General Design
This within-subject, double-blind, placebo-controlled study consisted of two 8-day inpatient phases each preceded by a 7-day outpatient phase. The study took place in the residential laboratory at the NYSPI. Participants were maintained on quetiapine or placebo for 15 days; the dose order was randomized across participants and the medication dose was switched during the intermediate 7-day outpatient phase, such that each participant was exposed to both medication conditions. Capsules, packed with riboflavin (placebo) or riboflavin and quetiapine, were taken twice a day (1100 and 2300 hours) throughout the entire study. During the outpatient phases, participants came to the laboratory to receive capsules for that day (one taken during the visit and the other to be taken at 2300 hours) and to report medication side effects. Urine samples were also collected during these visits to verify abstinence from all illicit drugs except marijuana and to monitor medication compliance by measuring the presence of riboflavin in the urine using ultraviolet detection.
Active marijuana (6.2% THC, referred to as ‘Dose A’ throughout the study) and inactive marijuana (0.0% THC, referred to as ‘Dose B’ throughout the study) were ‘sampled’ using standardized described smoking procedures described below on consecutive days prior to both inpatient phases. Participants smoked 3 puffs during each sample session and were told that the strength of the marijuana would not change during the study. They were also advised to pay attention to how each dose made them feel because each dose would be available for self-administration on certain study days.
The residential laboratory is configured with four private rooms for participants, two single-occupancy bathrooms, two single-occupancy shower, a recreational area, and two vestibules used to exchange supplies between the participants and staff (see Haney et al., 1999). Participants were continuously observed (except when in a private dressing area and the bathroom) with video and audio monitoring equipment, the outputs from which terminated in an adjacent control room. Participant’s rooms were equipped with a desktop computer connected to a computer in a control room, facilitating communication and interaction between the staff and participants, but not between participants. Throughout each inpatient day, participants wore an Actiwatch Activity Monitoring System (Actiwatch: Respironics Company, Bend OR) that was the size of a standard wristwatch. These watches, which tracked gross motor activity and provided objective measures of sleep including onset of sleep, number of sleep bouts, percent total sleep time, and percent total wake time (Perez et al., 2008), were removed briefly each day to download the previous day’s data. During the inpatient phases, participants woke up at 0815 and completed a 7-item visual analog scale (VAS) sleep questionnaire (Haney et al., 2004), a 44-item VAS intended to measure marijuana-elicited affective and physical subjective effects and a task battery (described below). Marijuana smoking (experimenter- or self-administered) occurred at set times, 6 times a day (1000, 1130, 1300, 1430, 1600, and 2200 hours). The VAS and task battery were completed after each marijuana smoking time-point even if marijuana was not self-administered. At 1100 and 2300 hours, capsules were administered. At 2300, Actiwatches and $35 in faux paper money representing 50% of the day’s study earnings were also distributed to the participants. The money was used to purchase puffs of marijuana on self-administration days. Lights were turned off at 2400 hours, and sleeping was not allowed until 2330 hours.
During the first inpatient day of each phase, participants smoked 3 puffs of experimenter-administered active marijuana (Dose A) 6 times during the day. The purpose of this day was to standardize marijuana exposure across participants prior to the withdrawal phase and to compare marijuana’s direct effects under quetiapine and placebo conditions. During the subsequent 3 days (days 2–4; withdrawal phase), participants had the option of self-administering up to 3 puffs of inactive marijuana (‘Dose B’) 6 times a day for a maximum of 18 puffs/day. On the remaining days (5–8; relapse phase), active marijuana (‘Dose A’) was available for self-administration. Puffs of marijuana (Dose A or B) were purchased using the money distributed to the participants each night. The first puff of the day cost $10, with each subsequent puff that day costing $3.
Drugs
Size 00 opaque capsules with lactose filler and riboflavin (50 mg) containing quetiapine or placebo were prepared by the New York State Psychiatric Institute Research Pharmacy. The dose of quetiapine was chosen based upon earlier reports demonstrating that a dose range of 150–400 mg/day is well-tolerated and effective in stabilizing mood in patient populations (Buckley, 2001). Although steady state plasma levels are attained within 2 days, doses were titrated up over the course of 5 days to improve tolerability (Days 1–2 0/50; Days 3–4, 25/75 mg; Day 5–15, 50/150 mg). Because sedation is an effect of the medication, a larger proportion of the daily dose was administered before bed. Following study termination, participants were instructed to continue taking capsules for 3 additional days so that dosing was not abruptly terminated (Day 1, 25/75 mg; Day 2, 0/50 mg; Day 3, 0/25 mg).
Marijuana cigarettes (0.0 or 6.2% THC; ca. 800 mg) were provided by the National Institute on Drug Abuse. Cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 hours prior to the administration. Marijuana was smoked according to a cued-puffing procedure: ‘inhale’ (5s), ‘hold smoke in lungs’ (10s), and ‘exhale,’ with a 40-second interval between each inhalation (Foltin et al., 1987).
Subjective-effect scales and performance tasks
Most subjective-effect ratings were measured using computerized visual analog scales (VAS), a series of lines labeled ‘not at all’ at one end (0 mm) and ‘extremely’ at the other end (100 mm). Participants were instructed to indicate how they felt at that particular moment.
Subjective Effect-Visual Analog Scale (SE-VAS)
Subjective ratings of mood and physical symptoms were assessed using a 44-item VAS intended to measure marijuana-elicited affective and physical subjective effects as described previously (Haney et al., 2010).
Task Battery
The task battery was designed to measure attention, psychomotor ability, learning, and memory (Foltin et al., 1993) and consisted of a 3-min repeated acquisition task, 10-min divided attention task (DAT), 3-min digit-symbol substitution task (DSST), and an immediate and delayed digit-recall task (DRT). Briefly, for the repeated acquisition task, four buttons corresponding to positions on the keypad were illuminated on the computer screen and participants were required to learn and then enter a 10-response sequence as quickly as possible in a given time limit. The DAT assessed attention and required participants to track a moving target on a computer screen using a mouse while signaling when a brief stimulus appeared in one of the four corners. Accurate tracking of the target increased its speed throughout the task. Psychomotor performance was tested using the DSST, which presented the participant with nine 3 × 3 matrices of blocks, with a single blackened square in each row; below each matrix was an identifying number (1–9). A number appeared on the screen indicating which pattern of highlighted boxes from the above matrices should be replicated using a 9-key keypad. Performance accuracy and speed were recorded. Lastly, delayed and immediate recall were evaluated using the DRT. For this task, the participant was required to enter an 8-digit sequence that appeared on the computer screen, and again when it disappeared (immediate recall). Participants were then asked to recall and recognize one of the sequences at the end of the task battery (delayed recall/recognition).
Food Intake
Food was freely available throughout the day except between 2330 and 0815 hours. Participants received a box of food each morning containing a wide variety of meal items including snacks and beverages to be consumed at any time during the day. Frozen foods and additional food items were also available upon request. Before eating or drinking any of the items, participants scanned a custom-designed barcode associated with the item, specifying the product and portion size.
Sleep
Subjective ratings of sleep quality were measured using a 7-item VAS sleep questionnaire (Haney et al., 2004), which consisted of a series of lines labeled ‘not at all’ at one end (0 mm) and ‘extremely’ at the other end (100 mm). The lines were labeled with ‘I slept well last night, ‘ ‘I woke up early this morning,’ ‘I fell asleep easily last night, ‘I feel clear-headed this morning,’ ‘I woke up often last night,’ ‘I am satisfied with my sleep last night.’ Participants were also asked to report how many hours they estimated sleeping the previous night. Objective measures of nighttime sleep and activity were provided by the Actiwatch Activity Monitoring System.
Tobacco Cigarette Smoking
Cigarettes and clean ashtrays were distributed to participants who smoked tobacco at 0815 and returned at the 2330 hours. Participants were not allowed to share cigarettes and were instructed to keep butts in their ashtrays rather than throwing them away. At the end of each day, the research staff counted the butts in the ashtray to measure daily cigarette smoking.
Data Analysis
Repeated measures analysis of variance (ANOVA) with planned comparisons was used to assess the direct effects of active marijuana, marijuana withdrawal, and relapse as a function of quetiapine condition. Dependent variables included peak subjective ratings of drug effect, mood, physical symptoms, and drug craving, daily cigarette smoking, caloric intake (total daily intake and per eating occasion), eating patterns (number of eating occasions), body weight, performance on the task battery (refer to Foltin et al. 1993 and Haney et al. 1997), subjective and objective measures of sleep, and relapse (number of puffs purchased). Three planned comparisons were performed for most endpoints. The first comparison assessed if there was evidence of marijuana withdrawal by comparing data obtained when active marijuana was experimenter administered to the data obtained during the withdrawal phase only under the placebo quetiapine condition. The second comparison assessed quetiapine’s effects on marijuana withdrawal by comparing data obtained during the withdrawal phase under placebo and quetiapine conditions. For both these comparisons, data from day 1 of the withdrawal phase were not included because symptoms of withdrawal do not tend to emerge until 24 hours after cessation of marijuana smoking. Lastly, the effects of quetiapine on marijuana’s direct effects were determined by comparing data obtained when experimenter-administered marijuana was smoked under placebo and quetiapine conditions. Quetiapine’s effect on relapse was determined by comparing the number of puffs purchased of active marijuana (relapse phase) under placebo and quetiapine conditions. Latency to first relapse (measured in days) and percent of participants relapsing under each medication condition were also assessed. Quetiapine’s effect on placebo marijuana self-administration was determined by comparing the number of puffs purchased during the withdrawal phase under placebo and quetiapine conditions. Results were considered statistically significant when p values were equal to or less than 0.05 using the Huynh-Feldt correction. A between-group analysis was used to determine if a medication order effect contributed to differences observed between phases.
RESULTS
Demographic Characteristics
Table 1 describes the demographic information of the 14 volunteers who completed the study (12 males; 2 females). An additional 6 volunteers enrolled (5 males, 1 female), but did not complete the study. During the marijuana withdrawal phase, 4 participants dropped out of the study (1 maintained on placebo, and 3 on quetiapine). One participant (maintained on placebo) left on the 4th inpatient day because he reported feeling like he was being controlled and wasting his time, while the other 3 receiving quetiapine reported physical discomfort; 2 participants reported gastrointestinal discomfort (nausea, vomiting, stomach pain, constipation) and discontinued on the 1st and 3rd inpatient days. The 3rd participant complained of general physical discomfort and left on the 3rd inpatient day.
Table 1.
Demographic Characteristics of Study Participants.
| Age (years) | 26 ± 4 |
| Sex (M/F) | 14/2 |
| Race (B/W) | 10/8 |
|
| |
| Days/Week Marijuana Use | 6.6 ± 0.9 |
| Marijuana Cigarettes/Day | 10.0 ± 6.5 |
|
| |
| Cigarette Smokers | 10/14* |
| Cigarettes/Day | 5.7 ± 3.7 |
|
| |
| Weekly Alcohol drinkers | 7/14 |
| Days/Week Alcohol Use | 2.8 ± 2.2 |
Note: Data are presented as means (± SD) or as frequency. Sex is indicated as female (F) and male (M) and race is indicated as Black (B) and White (W).
One participant began smoking cigarettes under the quetiapine condition while inpatient.
Subjective-effects ratings and drug craving
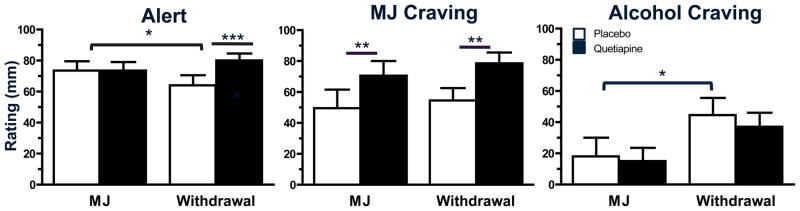
Figure 1 depicts subjective effect ratings of ‘Alert,’ and marijuana and alcohol craving when marijuana was smoked and during withdrawal. Under placebo conditions, ratings of ‘Alert’ decreased during withdrawal relative to when marijuana was smoked (p ≤ 0.05). Though quetiapine did not alter marijuana’s effects on these measures, it increased these ratings during the withdrawal phase relative to placebo (p ≤ 0.05). A similar pattern was observed for ratings of ‘Stimulated;’ however, differences in peak ratings for the item were due to a medication order effect (data not shown; p ≤ 0.05).
Figure 1.
Peak (mean ± SEM) subjective ratings of ‘Alert’ and marijuana and alcohol craving during experimenter-administered marijuana (MJ) smoking (6.2% THC) and marijuana withdrawal as a function of quetiapine dose. Participants rated each effect on a 100 mm line (0 mm = ‘not at all’; 100 mm = ‘extremely’). Data obtained from participants endorsing alcohol intake at least 1/week are portrayed for alcohol craving (N = 7). Under placebo conditions, significant differences between active marijuana administration and marijuana withdrawal are indicated by a bracket. A line above two adjacent bars indicates a significant difference between placebo and quetiapine conditions (* = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001).
Ratings of marijuana craving did not significantly change between the marijuana smoking and withdrawal phases when participants were maintained on placebo, yet quetiapine increased marijuana craving both when marijuana was smoked and during withdrawal (p ≤ 0.01). Ratings of alcohol craving increased during the withdrawal phase relative to when marijuana was smoked among the participants who reported drinking at least one alcoholic beverage per week (p ≤ 0.05). Quetiapine did not affect these ratings in either marijuana condition. No difference in ratings of cigarette craving among tobacco smokers was detected between the marijuana smoking and withdrawal phases under placebo quetiapine, yet participants smoked more cigarettes during the withdrawal phase when maintained on quetiapine compared to placebo (8.8 ± 0.9 vs 7.0 ± 0.8 cigarettes per day; p ≤ 0.05). One participant, a former cigarette smoker, resumed smoking cigarettes during the marijuana withdrawal phase while maintained on quetiapine; these data are not included in the analyses of cigarette smoking and craving.
Table 2 describes subjective ratings of physiological symptoms, mood, drug effect as a function of quetiapine condition on the day that experimenter–administered marijuana was smoked and during the withdrawal phase. Under the placebo condition, a trend was observed for increased ratings of ‘Muscle Pain’ and ‘Upset Stomach’ during marijuana withdrawal relative to when active marijuana was smoked (p ≤ 0.10). Quetiapine had no effect on these ratings relative to placebo when active marijuana was smoked (left-hand column of Table 2). However, during the marijuana withdrawal phase, quetiapine significantly decreased ratings of ‘Muscle Pain,’ ‘Stomach Pain,’ ‘Upset Stomach,’ and ‘Nausea’ relative to placebo (right-hand column of Table 2; p ≤ 0.05).
Table 2.
Subjective ratings of physical symptoms, mood and drug effect
| Placebo | Quetiapine | ||||
|---|---|---|---|---|---|
| MJ | Withdrawal | MJ | Withdrawal | ||
| Physical Symptoms | Nauseated | 18.5 (9.4) | 24.1 (6.9) | 15.0 (7.1) | 9.9 (1.9)* |
| Stomach Pain | 17.6 (8.4) | 23.1 (6.5) | 8.7 (3.8) | 7.5 (1.4)** | |
| Upset Stomach | 18.4 (8.6) | 29.4 (7.6) | 9.4 (5.1) | 8.3 (2.3)*** | |
| Muscle Pain | 7.2 (5.4) | 19.8 (6.1) | 10.7 (5.5) | 6.3 (2.5)* | |
| Mood & Drug Effect | Good Effect | 64.1 (10.1) | 23.6 (6.6)### | 75.9 (6.6) | 19.8 (8.1) |
| High | 72.0 (7.3) | 15.5 (5.5)### | 81.7 (4.2) | 13.0 (8.1) | |
| Mellow | 65.9 (8.0) | 46.8 (6.6)# | 69.4 (6.0) | 53.5 (6.9) | |
Representative peak (mean ± SEM) subjective ratings of physical symptoms, mood, and drug effect during experimenter-administered marijuana (MJ) smoking (6.2% THC) and during withdrawal as a function of placebo and quetiapine administration. Participants rated each effect on a 100 mm line (0 mm = ‘not at all’; 100 mm = ‘extremely’). Significant difference between the marijuana and withdrawal conditions under placebo quetiapine indicated by
= p ≤ 0.05,
= p ≤ 0.01,
= p ≤ 0.001.
Significant differences between placebo and quetiapine indicated by
= p ≤ 0.05;
= p ≤ 0.01;
= p ≤ 0.001.
Subjective ratings of positive mood and drug effect decreased during the withdrawal phase relative to when marijuana was smoked when participants were maintained on placebo capsules (p ≤ 0.05; Table 2). Quetiapine did not alter the effects of marijuana or withdrawal on these ratings.
Sleep Measures
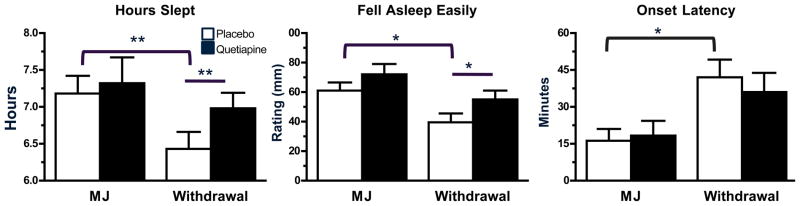
Quetiapine’s effects on the subjective and objective measures of sleep quality during marijuana smoking and withdrawal are portrayed in Figure 2. Under placebo quetiapine, withdrawal decreased sleep quality with lower ratings of ‘hours slept’ and ‘fell asleep easily’ relative to when marijuana was smoked (p ≤ 0.05). Though quetiapine did not alter these subjective ratings of sleep quality when marijuana was smoked, it increased these ratings during the withdrawal phase relative to placebo capsules (p ≤ 0.05). Objective sleep measures revealed that when maintained on placebo capsules, participants took longer to fall asleep during marijuana withdrawal compared to when marijuana was smoked (p ≤ 0.05). Quetiapine did not affect objective sleep measures when marijuana was smoked or during the withdrawal phase.
Figure 2.
Representative peak (mean ± SEM) sleep ratings and Actiwatch sleep measure during active marijuana administration (6.2% THC) and marijuana withdrawal as a function of quetiapine dose. See Figure 1 legend for details.
Food Intake
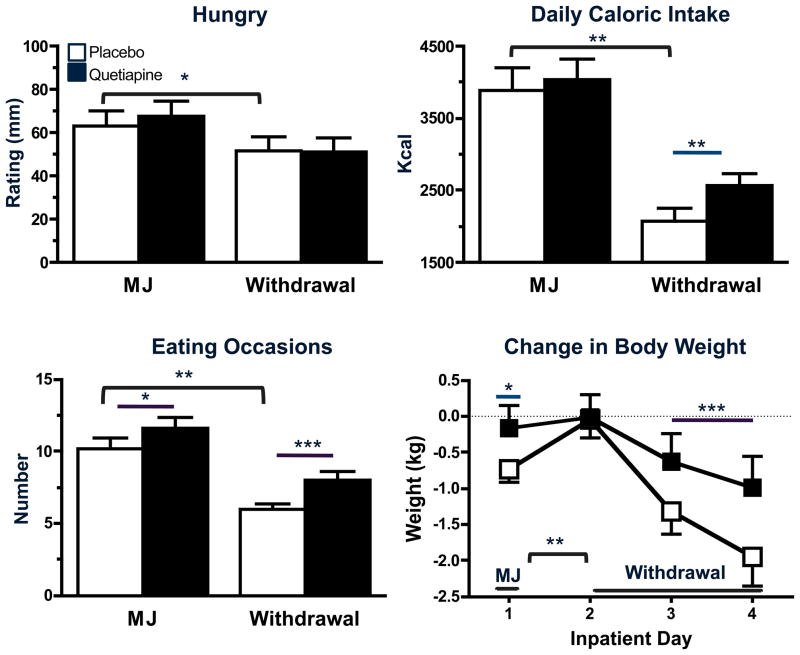
Quetiapine’s effects on appetite, caloric intake and weight gain when marijuana was smoked and during the withdrawal phase are portrayed in Figure 3. When participants were maintained on placebo capsules, subjective ratings of ‘Hungry,’ daily total caloric intake, and number of daily eating occasions were lower during the withdrawal phase relative to when marijuana was smoked (p ≤ 0.01); body weight also decreased during the withdrawal phase (p ≤ 0.01). When marijuana was smoked, quetiapine increased the number of daily eating occasions (p ≤ 0.05) and body weight (p ≤ 0.05), but did not affect subjective ratings of ‘Hungry’ or the number of daily calories consumed relative to placebo capsules. However, during the withdrawal phase, quetiapine increased daily caloric intake (p ≤ 0.01), number of eating occasions (p ≤ 0.01), and body weight (p ≤ 0.01) relative to placebo capsules.
Figure 3.
Peak (mean ± SEM) subjective ratings of ‘Hungry,’ daily kcal intake (mean ± SEM), number of daily eating occasions (mean ± SEM), and change in body weight from baseline (mean ± SEM) during experimenter- administered marijuana (MJ) smoking (6.2% THC) and marijuana withdrawal as a function of quetiapine dose. See Figure 1 legend for details.
Performance Effects
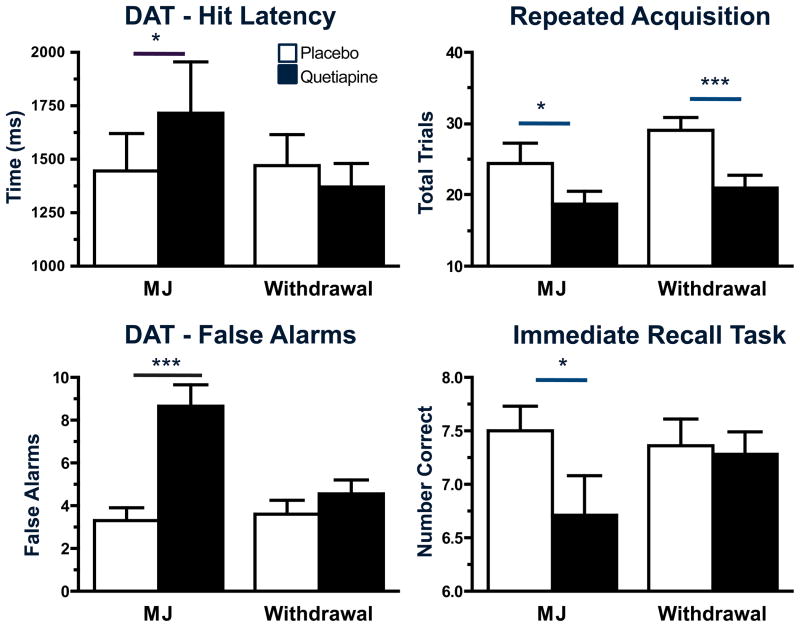
Quetiapine’s effects on attention, psychomotor function, and recall during marijuana smoking and withdrawal are portrayed in Figure 4. Task performance did not vary between marijuana smoking and the withdrawal phase when participants were maintained on placebo capsules. However, quetiapine decreased performance on tasks when marijuana was smoked relative to placebo capsules; hit latency and the number of false hits increased on the DAT, fewer trials were completed on the repeated acquisition task, psychomotor accuracy on the DSST decreased with fewer correct entries recorded (data not shown), and immediate recall was impaired with fewer correct numbers entered. Quetiapine’s negative effects on the repeated acquisition task were also observed during withdrawal with fewer trials completed during this phase relative to when participants were maintained on placebo capsules (p ≤ 0.001).
Figure 4.
Representative peak (mean± SEM) performance on tasks during experimenter-administered marijuana (MJ) smoking (6.2% THC) and marijuana withdrawal as a function of quetiapine dose. See Figure 1 legend for details.
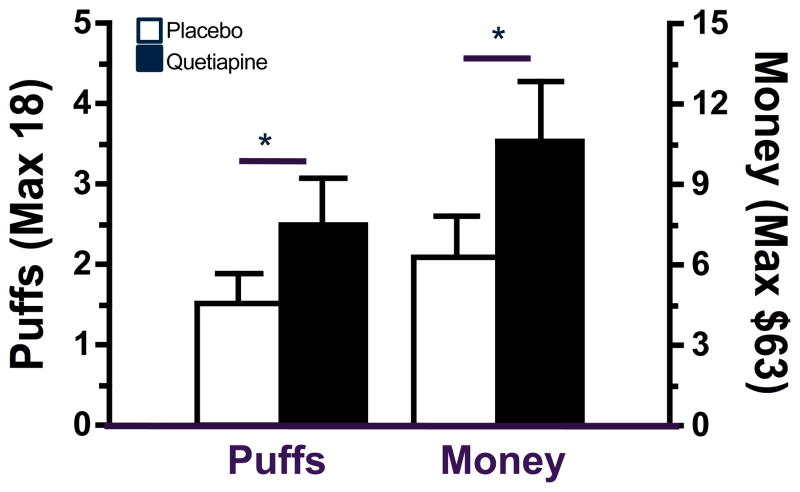
Relapse
Figure 5 illustrates the effect of quetiapine on relapse to marijuana self-administration when participants were given a choice to smoke active marijuana after 3 days of abstinence. Compared to placebo capsules, quetiapine increased the amount of money spent and number of active marijuana puffs purchased during the relapse phase (p ≤ 0.05). Though quetiapine increased marijuana self-administration, there was no difference in latency to first relapse between the active medication condition and placebo; the average number of days to first self-administer marijuana during the relapse phase for placebo capsules was 1.7 ± 0.3 days and for quetiapine was 1.2 ± 0.2 days. No difference was observed in the percentage of participants relapsing under the placebo and quetiapine conditions (42.9% versus 50.0%, respectively). During the withdrawal phase, when inactive marijuana was available for self-administration, the average number of inactive marijuana puffs purchased did not differ between quetiapine and placebo conditions (placebo = 3.6 ± 1.4 puffs versus quetiapine = 2.7 ± 1.2 puffs).
Figure 5.
Number of puffs (mean ± SEM) and dollars spent (mean ± SEM) on active marijuana when participants were maintained on placebo or quetiapine. Significant differences between placebo and quetiapine condition indicated by * = p ≤ 0.05.
DISCUSSION
The current study explored the effects of the atypical antipsychotic quetiapine on marijuana’s acute effects, symptoms of marijuana withdrawal, and marijuana relapse. Quetiapine was indeed effective in alleviating the sleep disruptions and anorexia that were associated with marijuana withdrawal. Additionally, when experimenter-administered marijuana was smoked, quetiapine did not increase the positive subjective effects associated with its abuse liability, an essential feature of a potential pharmacotherapy for marijuana use disorders. Though quetiapine alleviated some symptoms of marijuana withdrawal and did not alter marijuana’s direct effects, the medication increased both marijuana craving and relapse, suggesting that it does not show promise as a potential candidate pharmacotherapy for marijuana-use disorders.
In the current study, marijuana withdrawal disrupted sleep, and decreased appetite, caloric intake, and body weight, effects that are commonly reported during marijuana abstinence (Haney et al., 1999, 2004; Budney et al., 2001, 2003, 2008; Hart et al., 2002; Allsop et al., 2011). There was a tendency for withdrawal to affect a select few subjective ratings of physical symptoms, yet no changes in mood typical of marijuana withdrawal (increased irritability and anxiety) or craving were observed. These subjective effects were also not observed in a previous study using a similar study schedule assessing withdrawal symptoms over a 2-day period, 36–72 hours after active marijuana was last administered (Haney et al., 2010). Previous studies have demonstrated that the timecourse of marijuana withdrawal varies across symptoms, with some symptoms emerging earlier than others. For example, disruptions in caloric intake and eating behavior were observed on the first day of withdrawal, whereas subjective ratings of ‘Anxiety’ did not emerge until the 3rd day of withdrawal, and it wasn’t until the 4th day of withdrawal that ratings of ‘Irritability’ and subjective ratings of physical symptoms (‘Stomach Pain’) increased above baseline values (Haney et al., 1999). Differences in the onset and duration of marijuana withdrawal symptoms were also observed in an outpatient study (Budney et al., 2003). Thus, these effects might have been observed with a longer abstinence period. Despite the fact that the not all of the symptoms typical of marijuana withdrawal were observed under the current shortened abstinence condition, the features of withdrawal that were observed (sleep disturbances and decreased caloric intake) were attenuated by quetiapine administration.
Though quetiapine decreased some withdrawal symptoms, it increased marijuana craving independent of marijuana condition and relapse, defined as the amount of active marijuana self-administrated after a period of abstinence. The current study adds to those previously discussed in the Introduction because it demonstrates that although marijuana withdrawal symptoms can be attenuated pharmacologically, decreasing relapse to marijuana use proves to be a greater challenge. Like quetiapine, lofexidine and mirtazapine improved withdrawal-associated sleep disturbances, yet only lofexidine decreased relapse (Haney et al., 2008, 2010). The pharmacological actions of these three medication are distinct; lofexidine is hypothesized to decrease norepinephrine transmission, mirtazapine increases synaptic norepinephrine, and quetiapine has a wide-range of effects on multiple neurotransmitters due to its affinity for multiple receptors including 5-HT1A, 5-HT2A, and D2 receptors (for review see Pae et al., 2010). Therefore, the neurobiological mechanism by which these drugs improve withdrawal-induced symptoms are likely different, possibly contributing to their varying effects on relapse.
One limitation of this study and other controlled laboratory studies of this kind is that medication effects on relapse are assessed in non-treatment seekers, rather than a population that is personally (rather than just financially) motivated to maintain abstinence. However, the relapse procedure used in the current laboratory study was designed to model the choices that treatment-seekers would have to make in the real world with the high ‘price’ of the initial choice to smoke marijuana. Recently, the predictive validity of the laboratory relapse model was supported with a clinical trial examining dronabinol for treating marijuana dependence (Levin et al., 2011). As the laboratory results predicted (Haney et al., 2004 and 2008), patients randomized to the active medication condition reported fewer withdrawal symptoms relative to the patients randomized to placebo but the two groups did not differ in their marijuana use (Levin et al., 2011).
A second potential limitation of the current study is that only a single dose of quetiapine was tested. Given the short medication phase, the dose was chosen in order to achieve therapeutic behavioral effects while avoiding adverse effects more commonly observed with longer administration, such as extrapyramidal symptoms. While the dose administered was effective at attenuating symptoms of marijuana withdrawal, it elicited decrements in cognitive performance. Therefore, exploring the effects of a higher quetiapine dose on relapse would likely not afford much potential therapeutic value. Though attenuation of withdrawal symptoms may be retained with a lower quetiapine dose, the possibility that it would have an opposite effect on relapse than the dose currently assessed seems unlikely.
Conclusions
Quetiapine effectively decreased a subset of symptoms associated with marijuana abstinence. However, the medication increased marijuana craving and importantly, relapse to marijuana use. Given that the clinical effectiveness of a medication to treat substance-use disorders is best predicted by its ability to decrease self-administration in the laboratory (Comer et al., 2008; Haney and Spealman, 2008) the current findings suggest that quetiapine does not show promise for the treatment of marijuana dependence.
Acknowledgments
The U.S. National Institute on Drug Abuse (NIDA) supported this research (DA09236). We also thank NIDA for providing study marijuana, and Dr. Frances Levin for the idea to test quetiapine. We are grateful to Meredith Petty, Laura Rolfe, Mathew Pecht, Danielle Lion, and Elyssa Berg for their superb assistance in data collection and Dr. Gillinder Bedi for assistance with marijuana administration.
References
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The cannabis withdrawal scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119(1–2):123–9. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Buckley PF. Broad therapeutic uses of atypical antipsychotic medications. Biol Psychiatry. 2001;50(11):912–24. doi: 10.1016/s0006-3223(01)01256-2. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58(10):917–24. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35(4):362–8. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J Consult Clin Psychol. 2006;74(5):955–66. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs S, Rodenbeck A, Guan Z, Pohlmann K, Jordan W, Meier A, Rüther E. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology (Berl) 2004;174(3):421–9. doi: 10.1007/s00213-003-1759-5. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat. 2001;21(2):55–64. doi: 10.1016/s0740-5472(01)00179-9. discussion 65–6. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Brady JV, Fischman MW, Emurian CS, Dominitz J. Effects of smoked marijuana on social interaction in small groups. Drug Alcohol Depend. 1987;20:87–93. doi: 10.1016/0376-8716(87)90079-2. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pippen PA, Kelly TH. Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug Alcohol Depend. 1993;32(2):93–106. doi: 10.1016/0376-8716(93)80001-u. [DOI] [PubMed] [Google Scholar]
- Haney M. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol. 2009;14(1):9–21. doi: 10.1111/j.1369-1600.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–19. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8:101–112. [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD, Foltin RW. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2010;211(2):233–44. doi: 10.1007/s00213-010-1888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008;197(1):157–68. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002;67:301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Pope HG., Jr Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8(4):483–92. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Kaur G. Pharmacodynamics of drug-induced weight gain. Drugs Today (Barc) 2001;37(8):559–571. doi: 10.1358/dot.2001.37.8.844171. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116(1–3):142–50. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, Gorelick DA. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111(1–2):120–7. doi: 10.1016/j.drugalcdep.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55(5):1209–17. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Pae CU, Sohi MS, Seo HJ, Serretti A, Patkar AA, Steffens DC, Masand PS. Quetiapine XR: current status for the treatment of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1165–73. doi: 10.1016/j.pnpbp.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Perez AY, Kirkpatrick MG, Gunderson EW, Marrone G, Silver R, Foltin RW, Hart CL. Residual effects of intranasal methamphetamine on sleep, mood, and performance. Drug Alcohol Depend. 2007;94(1–3):258–62. doi: 10.1016/j.drugalcdep.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stip E, Roy JY. The effect of quetiapine on cannabis use in 8 psychosis patients with drug dependency. Can J Psychiatry. 2004;49(10):711. doi: 10.1177/070674370404901020. [DOI] [PubMed] [Google Scholar]
- Ramchand R, Griffin BA, Suttorp M, Harris KM, Morral A. Using a cross-study design to assess the efficacy of motivational enhancement therapy-cognitive behavioral therapy 5 (MET/CBT5) in treating adolescents with cannabis-related disorders. J Stud Alcohol Drugs. 2011;72(3):380–9. doi: 10.15288/jsad.2011.72.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 2009;105(3):240–7. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: a test of the relapse prevention model. J Consult Clin Psychol. 1994;62(1):92–9. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-41, HHS Publication No (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Vandrey, Haney Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23 (7):543–53. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]