Abstract
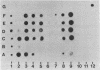
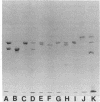
Spectrin, fodrin, and TW-260/240 form a group of structurally and functionally similar but not identical high molecular weight actin-binding proteins from chicken erythrocytes, brain tissue, or intestinal epithelial brush borders. Immunological data and one-dimensional peptide maps of the separated subunits suggest that a common (Mr 240,000) and a variant (Mr 220,000, 235,000, or 260,000) subunit account for the three different heterodimers. These results are in line with the related but distinct morphology of the three proteins observed in micrographs of rotary-shadowed molecules and the finding that the common (Mr 240,000) subunit seems to account for the calcium-dependent calmodulin-binding activity displayed by the three proteins. The possible functions of spectrin-like molecules in nonerythroid cells are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Davis J. Erythrocyte ankyrin: immunoreactive analogues are associated with mitotic structures in cultured cells and with microtubules in brain. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7550–7554. doi: 10.1073/pnas.78.12.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Calvert R., Bennett P., Gratzer W. Properties and structural role of the subunits of human spectrin. Eur J Biochem. 1980 Jun;107(2):355–361. doi: 10.1111/j.1432-1033.1980.tb06036.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Donnelly M. S., Weinberg D. S., Skarin A. T., Levine H. D. Sick sinus syndrome with seroconstrictive pericarditis in malignant lymphoma involving the heart: a case report. Med Pediatr Oncol. 1981;9(3):273–277. doi: 10.1002/mpo.2950090311. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Osborn M., Weber K. An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell. 1982 Apr;28(4):843–854. doi: 10.1016/0092-8674(82)90063-0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Weber K. Calmodulin-binding proteins of the microfilaments present in isolated brush borders and microvilli of intestinal epithelial cells. J Biol Chem. 1980 Nov 25;255(22):10551–10554. [PubMed] [Google Scholar]
- Goodman S. R., Zagon I. S., Kulikowski R. R. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7570–7574. doi: 10.1073/pnas.78.12.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Sternweis P. C., Northup J. K., Dromerick A. W., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties of the turkey erythrocyte protein. J Biol Chem. 1981 Dec 25;256(24):12911–12919. [PubMed] [Google Scholar]
- Hiller G., Weber K. Spectrin is absent in various tissue culture cells. Nature. 1977 Mar 10;266(5598):181–183. doi: 10.1038/266181a0. [DOI] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman D., Hsu D. J., Marchesi V. T. Evidence that spectrin binds to macromolecular complexes on the inner surface of the red cell membrane. J Cell Sci. 1980 Apr;42:1–22. doi: 10.1242/jcs.42.1.1. [DOI] [PubMed] [Google Scholar]
- Lux S. E. Dissecting the red cell membrane skeleton. Nature. 1979 Oct 11;281(5731):426–429. doi: 10.1038/281426a0. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Speicher D. W., Knowles W. J., Hsu C. J., Marchesi V. T. Identification of functional domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6592–6596. doi: 10.1073/pnas.77.11.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. G., Sheetz M., Singer S. J. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1359–1363. doi: 10.1073/pnas.72.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Calmodulin-binding protein of erythrocyte cytoskeleton. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1063–1070. doi: 10.1016/0006-291x(81)91931-8. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Willard M., Wiseman M., Levine J., Skene P. Axonal transport of actin in rabbit retinal ganglion cells. J Cell Biol. 1979 Jun;81(3):581–591. doi: 10.1083/jcb.81.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]