Abstract
OBJECTIVE
We studied whether lower calf muscle density and poorer upper and lower extremity strength are associated with higher mortality rates in men and women with PAD.
BACKGROUND
Men and women with lower extremity peripheral arterial disease (PAD) have lower calf muscle density and reduced lower extremity strength compared to individuals without PAD.
METHODS
At baseline, participants underwent measurement of calf muscle density with computed tomography in addition to knee extension power, and isometric knee extension, plantar flexion, and hand grip strength measures. Participants were followed annually for up to four years. Results are adjusted for age, sex, race, body mass index, the ankle brachial index (ABI), smoking, physical activity, and comorbidities.
RESULTS
Among 434 PAD participants, 103 (24%) died during a mean follow-up of 47.6 months. Lower calf muscle density was associated with higher all-cause mortality (lowest density tertile-hazard ratio (HR)=1.80 (95% Confidence Interval (CI)-1.07-3.03), 2nd tertile-HR=0.91 (95% CI-0.51-1.62); highest density tertile (HR=1.00), P trend=0.020) and higher cardiovascular disease mortality (lowest density tertile-HR=2.39 (95% CI-0.90-6.30), 2nd tertile-HR=0.85 (95% CI-0.27-2.71); highest density tertile (HR=1.00), P trend=0.047). Poorer plantar flexion strength (P trend=0.004), lower baseline leg power (P trend=0.046), and poorer handgrip (P trend=0.005) were associated with higher all-cause mortality.
CONCLUSION
These data demonstrate that lower calf muscle density and weaker plantar flexion strength, knee extension power, and hand grip are associated with increased mortality in participants with PAD, independently of the ABI and other confounders.
Keywords: Mortality, intermittent claudication, prognosis, Physical functioning
Lower extremity peripheral arterial disease (PAD) affects eight million men and women in the United States (1). The prevalence is expected to increase as the population lives longer with chronic disease. Men and women with PAD have greater lower extremity functional impairment and faster functional decline than people without PAD (2-3). Lower extremity skeletal muscle, particularly calf muscle, is the end-organ affected by obstructed lower extremity arteries in PAD. Consistent with this association, individuals with PAD have more adverse calf muscle characteristics and poorer lower extremity strength than people without PAD (4-5). Histopathologic data demonstrate that lower extremity ischemia is associated with calf muscle apoptosis, Type II muscle fiber atrophy, and impaired mitochondrial function (5-7). An improved understanding of the prognostic significance of lower extremity muscle pathophysiologic changes in PAD may help delineate mechanisms of adverse outcomes in PAD.
We studied associations of computed tomography-measured calf muscle density, calf muscle area, and calf muscle percent fat with mortality rates in men and women with PAD. We hypothesized that lower calf muscle density, lower calf muscle area, and higher calf muscle percent fat would be associated with higher all-cause and cardiovascular disease mortality. We also studied associations of knee extension power, knee extension isometric strength, and plantarflexion isometric strength with mortality in people with PAD. We hypothesized that poorer knee extension power, knee extension strength, and plantarflexion strength would be associated with higher all-cause and cardiovascular disease mortality. To determine whether associations of poorer strength with higher mortality are systemic in people with PAD, we studied associations of hand grip isometric strength with mortality.
METHODS
Study Overview
The institutional review boards of Northwestern University and Catholic Health Partners Hospital approved the protocol. Participants gave written informed consent. Participants were part of the Walking and Leg Circulation Study II (WALCS II), a prospective, observational study designed to identify associations of calf skeletal muscle characteristics and leg strength with functional performance and functional decline in people with and without PAD (4,8,9). Participants underwent baseline measures and returned annually for follow-up. Participants were also followed for the outcome of mortality.
Participant Identification
PAD participants in WALCS II included 238 participants with PAD attending their fourth annual follow-up visit in the original Walking and Leg Circulation Study (WALCS) (4,8) and 240 PAD participants newly identified for WALCS II.
PAD participants were identified from among consecutive patients diagnosed with PAD in three Chicago-area non-invasive vascular laboratories (4,8,9). A small number of PAD participants were identified from among consecutive patients in a large general internal medicine practice who were found to have a low ABI at their study visit and were included among PAD participants (4,8). PAD was defined as an ankle brachial index (ABI) < 0.90 at the baseline visit for WALCS II (4,8). All participants were age 59 and older at baseline.
Participation rates and exclusion criteria for the WALCS II cohort have been described (4,9). The following exclusion criteria were applied at the time of original study enrollment to participants in the original WALCS cohort and those newly identified: Patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because they have severely impaired functioning. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded.
Ankle Brachial Index Measurement
A hand-held Doppler probe (Nicolet Vascular Pocket Dop II; Nicolet Biomedical Inc, Golden, Colo) was used to obtain systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries (2,4,8). Each pressure was measured twice. The ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures in each leg by the mean of the 4 brachial pressures (2,4,8). Zero values for the dorsalis pedis and posterior tibial pulses were set to missing for the ABI calculation. Average brachial pressures in the arm with highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets and the 2 brachial pressures differed by 10 mm Hg or more in at least one measurement set, since in such cases subclavian stenosis was possible (10). The lowest leg ABI was used in analyses.
Measuring Calf Skeletal Muscle Characteristics
We studied calf muscle characteristics because the superficial femoral artery is the most common site of lower extremity atherosclerosis (11,12) and calf musculature receives blood supply from the superficial femoral artery. Further, the calf muscles are those that classically are symptomatic in patients with PAD (13). Using a Computed Tomography (CT) scanner (LightSpeed, General Electric Medical Systems, Waukesha, WI, USA), 2.5 mm cross-sectional images of the calves were obtained at 66.7% of the distance from the distal to the proximal tibia without contrast (4,9). Cross-sectional calf muscle images were analyzed using BonAlyse (BonAlyse Oy, Jyvaskyla-Finland), a software for processing CT images that identifies muscle tissue, fat, and bone (4,9). The muscle outline was traced manually, excluding subcutaneous fat, and bone, by a study coordinator who was trained and certified in these measures prior to beginning analyses. When quantifying muscle area, the Bon Alyse software quantifies voxels within a range corresponding to muscle density (9 to 271 mg/cm3) and excludes voxels corresponding to fat density (-270 to 8 mg/cm3). Intra-muscular fat is quantified by summing voxels corresponding to fat tissue density within muscle tissue. Previous cadaver studies demonstrate that these methods provide an estimate of muscle cross-sectional area and fat content that are highly correlated with direct anatomic measures (14). Specifically, correlations between CT-measured skeletal muscle and lipid content with corresponding measures from cadavers were 0.97 and 0.96, respectively (14). Because larger individuals require greater muscle mass to support their larger frame, muscle area was adjusted for the square of individual tibia length (4,9). Muscle density is a measure of muscle quantity within a defined volume. Across the voxel range corresponding to muscle tissue (9 to 271 mg/cm3), individuals have varying quantities of muscle per cm3 (i.e. ranging from 9 to 271). Mean muscle density was calculated per individual, representing the quantity of muscle measured per cm3 (9).
Isometric Strength Measures
Knee extension isometric strength was measured in Newtons using a computer-linked strength chair (Good Strength Chair, Metitur Oy, Jyvasklya, Finland) (15). Transducers were placed for measurement of knee extension, plantarflexion, and hand grip strength. Data were collected electronically by computer over six seconds. Strength measurements using the Good Strength Chair have high test re-test reliability (pearson product moment correlations = 0.88-0.96) (15). Two trials were performed and the maximum strength was used in analyses (8,15).
Leg power
Validated methods developed by Bassey et al (8,16) were used to measure knee extension power in watts. The power rig uses a flywheel that is accelerated by pushing a footplate until the leg is extended (16). Power is derived from the final velocity of the flywheel measured with an optsoswitch attached to a microcomputer. Testing was stopped when at least five trials had been performed and the two highest measures were within five percent of each other. Up to nine trials were performed (8,16).
Mortality Assessment
We used the Social Security Administration death database to search for deaths through 11/12/10. At baseline, each participant provided names of three proxies to assist with ascertaining complete follow-up. Mortality information was also obtained from family members, proxies, and primary care physicians. Death certificates were obtained from the State of Illinois or from the patients’ medical record. Cardiovascular disease deaths were those with International Classification of Disease-10 codes in the range I01.0 through I99.9, including deaths due to coronary heart disease, stroke, peripheral vascular disease, and other cardiovascular disease.
Comorbidities
Comorbidities assessed were diabetes, angina, myocardial infarction, heart failure, cancer, chronic lung disease, and stroke. Disease-specific algorithms that combine data from patient report, medical record review, medications, laboratory values, and a questionnaire completed by the participant’s primary care physician were used to verify and document baseline comorbidities other than knee and hip arthritis, based on criteria previously developed (17).
Other Measures
Height and weight were measured at the study visit. Body mass index (BMI) was calculated as weight (kilograms)/(height (meters))2. Cigarette smoking history was determined with patient report. Physical activity was assessed using validated methods to ascertain patient report of the number of blocks walked during the past week (18).
Statistical Analyses
Baseline characteristics between men and women were compared using general linear models for continuous variables and chi-square tests for categorical variables. Among men and women with PAD, respectively, characteristics of decedents and survivors were compared using general linear models for continuous variables and chi-square tests for categorical variables.
We used proportional hazards analyses to compare all-cause and cardiovascular disease mortality rates across tertiles of each calf muscle characteristic (calf muscle area, calf muscle percent fat, and calf muscle density), adjusting for age, sex, and race. These analyses were designated “Model 1”. Tertiles of calf muscle characteristics were selected, rather than quartiles, because the number of deaths, particularly cardiovascular deaths, was relatively low. Next, these proportional hazards analyses were repeated with additional adjustment for comorbidities (diabetes, angina, myocardial infarction, heart failure, cancer, lung disease, and stroke), smoking (pack-years), BMI, physical activity, and ABI. These analyses were designated Model 2. Similar methods were used to evaluate associations of knee extension power, isometric knee extension strength, plantarflexion strength, and hand grip strength with all-cause and cardiovascular disease mortality. We used PROC PHREG in SAS to perform these analyses. P trend values were calculated to determine whether associations across tertiles of muscle measures represented linear trends.
In our WALCS cohort, we identified gender differences in associations of isometric knee extension strength with functional decline and mortality among participants with PAD (19,20). Therefore, we first assessed associations of each calf muscle characteristic and strength measure with mortality separately among men and women. When the association of a specific muscle measure with mortality was similar between men and women, we combined data from men and women. Associations of muscle measures with mortality were considered similar for men and women with the following criteria were met: 1) there was no interaction of sex on the association of the muscle measure with mortality and 2) the direction of the association between the third vs. the first tertile for each muscle measure with mortality was the same for men and women, adjusting for age, sex, and race. Based on these criteria, data for men and women were not combined for associations of calf muscle percent fat with all-cause mortality or for associations of isometric leg strength, hand grip, and plantar flexion strength with cardiovascular mortality.
Analyses were performed using SAS statistical software (version 9.1, SAS Institute Inc, Cary, NC).
RESULTS
A total of 434 participants with PAD underwent computed tomography imaging of calf skeletal muscle at baseline and are included in these analyses. Of these, 391 had knee extension power measured, 319 had isometric knee extension measured, 330 had isometric plantarflexion measured, and 329 had isometric hand grip measured at baseline. Mean follow-up was 47.6 months ± 15.8.
Table 1 shows characteristics of men and women participants with PAD. Women with PAD included a higher proportion of African-Americans and lower proportions of participants with diabetes mellitus, angina, and heart failure as compared to men with PAD (Table 1).
Table 1.
Characteristics of Men and Women with Peripheral Arterial Disease*
| Overall (N=434) | Female (N=201) | Male (N=233) | P value* | |
|---|---|---|---|---|
| Age (years) | 75 (8.2) | 75.9 (8.2) | 74.4 (8.1) | 0.056 |
| African-American (%) | 16.6 | 24.4 | 9.9 | <.0001 |
| Body mass index (Kg/M2) | 27.9 (5.1) | 27.4 (5.6) | 28.3 (4.6) | 0.057 |
| Ankle Brachial Index | 0.63 (0.2) | 0.62 (0.16) | 0.64 (0.15) | 0.368 |
| Current smoker (%) | 15.2 | 15.4 | 15.0 | 0.908 |
| Diabetes (%) | 32.3 | 26.9 | 36.9 | 0.026 |
| Cancer (%) | 20.3 | 22.4 | 18.5 | 0.310 |
| Hypertension (%) | 73.5 | 76.1 | 71.2 | 0.251 |
| Angina (%) | 35.8 | 28.1 | 42.2 | 0.002 |
| Myocardial Infarction (%) | 26.3 | 23.9 | 28.5 | 0.282 |
| Stroke (%) | 21.0 | 19.4 | 22.3 | 0.457 |
| Heart failure (%) | 29.3 | 24.4 | 33.5 | 0.038 |
| Pulmonary disease (%) | 44.2 | 46.8 | 42.1 | 0.325 |
| ACE inhibitors (%) | 29.5 | 26.4 | 32.2 | 0.185 |
| Statin therapy (%) | 55.3 | 52.7 | 57.5 | 0.319 |
| Beta-blocker therapy (%) | 42.6 | 42.3 | 42.9 | 0.895 |
Data shown are means (standard deviations). The P value shown represents the P value for the comparison of values between men and women.
Table 2 shows characteristics of survivors and decedents among men and women with PAD, respectively. Overall, there were 62 (26.6%) deaths among men and 41 (20.3%) deaths among women. Among the 52 male decedents with death certificates, 22 (42%) deaths were from cardiovascular disease and 12 (23%) were from cancer. Among the 34 female decedents with death certificates, 13 (38%) deaths were from cardiovascular disease and nine (26%) were from cancer. In both men and women, decedents were older than survivors (Table 2). Among men, decedents included a higher proportion of participants with pulmonary disease, compared to survivors. Among men and women, respectively, decedents had lower calf muscle area, lower knee extension power, and lower handgrip compared to survivors. Among men, decedents had lower calf muscle density and lower knee extension isometric strength compared to survivors (Table 2).
Table 2.
Characteristics of Survivors and Decedents among Men and Women with Peripheral Arterial Disease*
| MEN | WOMEN | |||||
|---|---|---|---|---|---|---|
| Survival | Decedents | P value | Survival | Decedents | P value | |
| N | 171 | 62 | 160 | 41 | ||
| Age (years) | 73.6 (7.8) | 76.3 (8.5) | 0.027 | 75.1 (8.2) | 78.7 (7.5) | 0.014 |
| BMI | 28.4 (4.6) | 28.1 (4.8) | 0.706 | 27.8 (5.6) | 25.8 (5.6) | 0.04 |
| ABI | 0.65 (0.15) | 0.61 (0.16) | 0.138 | 0.62 (0.15) | 0.64 (0.19) | 0.479 |
| African American (%) |
8.8 | 13.0 | 0.350 | 26.3 | 17.1 | 0.222 |
| Current smoker (%) |
13.5 | 19.4 | 0.265 | 13.1 | 24.4 | 0.075 |
| Diabetes (%) | 34.5 | 43.6 | 0.206 | 26.3 | 29.3 | 0.697 |
| Cancer (%) | 17.5 | 21.0 | 0.552 | 23.1 | 19.5 | 0.621 |
| Hypertension (%) |
72.5 | 67.7 | 0.477 | 76.3 | 75.6 | 0.932 |
| Stroke (%) | 21.6 | 24.2 | 0.679 | 18.1 | 24.4 | 0.365 |
| Heart Failure (%) |
29.8 | 43.6 | 0.050 | 21.9 | 34.2 | 0.103 |
| Pulmonary disease (%) |
35.1 | 61.3 | <0.001 | 46.3 | 48.8 | 0.772 |
| Angina (%) | 39.4 | 50.0 | 0.149 | 26.3 | 35.0 | 0.274 |
| Myocardial Infarction (%) |
27.1 | 32.3 | 0.437 | 22.5 | 29.3 | 0.365 |
| Calf muscle area (mm2) |
6307 (1330) | 5673 (1327) | 0.002 | 4692 (1054) | 4261 (1012) | 0.020 |
| Muscle percent fat (%) |
10.0 (11.7) | 13.0 (11.5) | 0.096 | 11.9 (14.0) | 13.0 (15.3) | 0.665 |
| Calf muscle density (gm/cm3) |
33.5 (4.2) | 31.5 (4.0) | 0.002 | 32.3 (3.9) | 31.1 (4.1) | 0.0812 |
| Knee extension power (Watts) |
123.9 (52.1) | 94.49 (51.0) | <0.001 | 67.65 (33.7) | 53.84 (30.7) | 0.0351 |
| Isometric knee extension strength (Newtons) |
335.2 (113.2) | 290.9 (90.3) | 0.015 | 212.1 (72.9) | 182.4 (61.3) | 0.0653 |
| Isometric hand grip (Newtons) |
354.0 (99.2) | 300.14 (99.8) | 0.001 | 190.86 (62.2) | 160.70 (59.8) | 0.031 |
| Isometric plantarflexion (Newtons) |
391.3 (182.3) | 274.0 (136.4) | <0.001 | 296.0 (159.8) | 255.8 (117.9) | 0.245 |
Data shown are means (standard deviation).
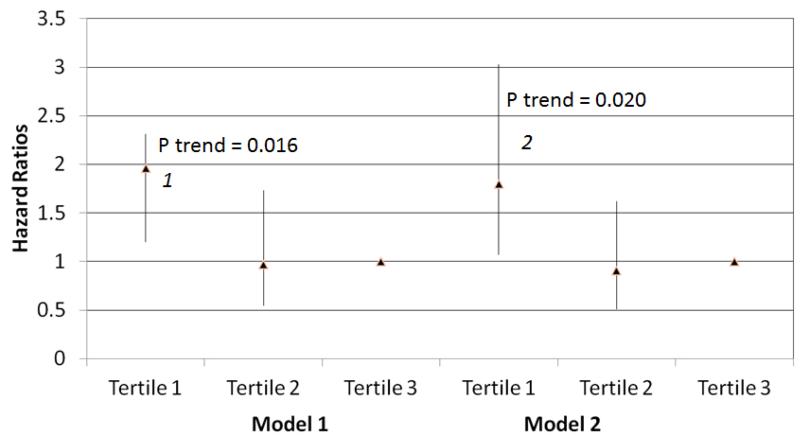
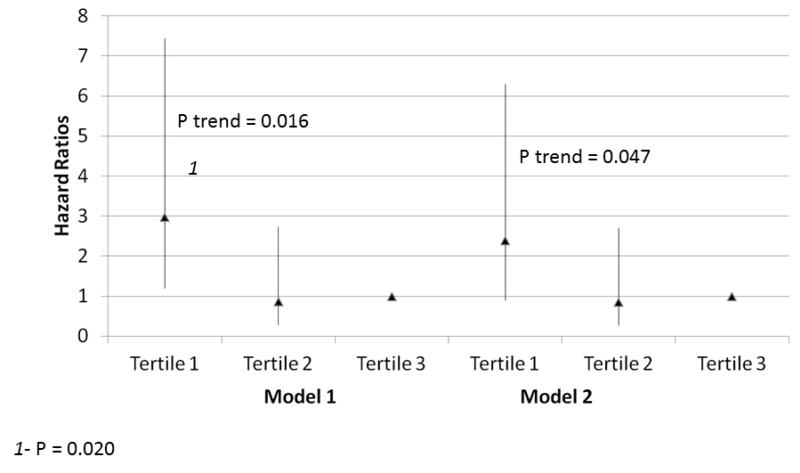
Within the entire cohort, lower calf muscle density was associated with higher all-cause mortality, adjusting for age, sex, and race in Model 1 (Figure 1) (p=0.016). This association was not substantially changed after additional adjustment for comorbidities, smoking, BMI, physical activity, and the ABI in Model 2 (Figure 1) (p=0.020). Similarly, lower calf muscle density was associated with higher cardiovascular disease mortality, adjusting for age, sex, and race in Model 1 (Figure 2) (p=0.012). This association remained statistically significant after additional statistical adjustment for comorbidities, smoking, BMI, physical activity, and the ABI in Model 2 (Figure 2) (p=0.047). Within the entire cohort we identified no significant associations of calf muscle area with all-cause mortality or cardiovascular disease mortality in either Model 1 or Model 2 (Table 3).
Figure 1. Adjusted Association of baseline calf muscle density with all-cause mortality in participants with peripheral arterial disease (N=434).
Model 1. Adjusted for age, sex, and race.
Model 2. Adjusted for covariates in Model 1 and the ankle brachial index, body mass index, comorbidities, physical activity, smoking history and study cohort (WALCS vs. WALCS II).
1P=0.008 for the comparison between the highest and lowest tertiles.
2 P=0.028 for the comparison between the highest and lowest tertiles.
Figure 2. Adjusted Associations of baseline calf muscle density with cardiovascular disease mortality in participants with peripheral arterial disease (N=434).
Model 1. Adjusted for age, sex, and race.
Model 2. Adjusted for covariates in Model 1 and the ankle brachial index, body mass index, comorbidities, physical activity, smoking history and study cohort (WALCS vs. WALCS II).
1P=0.020 for the comparison between the highest and lowest tertiles.
Table 3.
Associations of Lower Extremity Muscle Measures with All-Cause Mortality in Men and Women with Peripheral Arterial Disease.*
| Tertile 1 (poorest muscle measure at baseline) |
Tertile 2 | Tertile 3 (best muscle measure at baseline) |
Trend P value | |
|---|---|---|---|---|
| Calf Muscle Area and All-Cause Mortality | ||||
| Model 1 | 1.62 (0.92-2.85) | 1.35 (0.77-2.37) | 1.00 (reference) | 0.050 |
| Model 2 | 1.43 (0.77-2.32) | 1.45 (0.81-2.61) | 1.00 (reference) | 0.137 |
| Hand Grip and All-Cause Mortality | ||||
| Model 1 | 1.81 (0.94-3.48) | 0.95 (0.48-1.87) | 1.00 (reference) | 0.006 |
| Model 2 | 1.71 (0.89-3.32) | 0.86 (0.42-1.74) | 1.00 (reference) | 0.005 |
| Knee Extension Isometric Strength and All-Cause Mortality | ||||
| Model 1 | 2.44 (1.18-5.02) | 3.00 (1.46-6.16) | 1.00 (reference) | 0.038 |
| Model 2 | 1.96 (0.90-4.26) | 2.70 (1.27-5.70) | 1.00 (reference) | 0.185 |
| Calf Muscle Area and Cardiovascular Mortality | ||||
| Model 1 | 2.84 (0.89-9.05) | 2.50 (0.79-7.89) | 1.00 (reference) | 0.305 |
| Model 2 | 2.56 (0.74-8.84) | 2.63 (0.81-8.59) | 1.00 (reference) | 0.584 |
| Calf Muscle Percent Fat and Cardiovascular Mortality | ||||
| Model 1 | 0.63 (0.25-1.58) | 0.91 (0.42-1.95) | 1.00 (reference) | 0.305 |
| Model 2 | 2.56 (0.74-8.84) | 2.63 (0.81-8.59) | 1.00 (reference) | 0.584 |
| Knee Extension Power and Cardiovascular Mortality | ||||
| Model 1 | 2.27 (1.26 - 4.08) | 1.13 (0.61 - 2.07) | 1.00 (reference) | 0.004 |
| Model 2 | 1.90( 1.04 - 3.49) | 1.02( 0.55 - 1.90) | 1.00 (reference) | 0.046 |
Data shown are hazard ratios and 95% Confidence Intervals. Model 1 adjusts for age, sex, race. Model 1 for the calf muscle area measure also adjusts for tibia length. Model 2 adjusts for variables in Model 1 and smoking, body mass index, ankle brachial index, physical activity, comorbidities, and study cohort (WALCS vs. WALCS II). The best tertile at baseline (Tertile 3) serves as the reference group against which the worst tertile at baseline (Tertile 1) and Tertile 2 are compared. Thus, the hazard ratio group for the reference is 1.0 and hazard ratios for Tertiles 1 and 2 are relative to this reference.
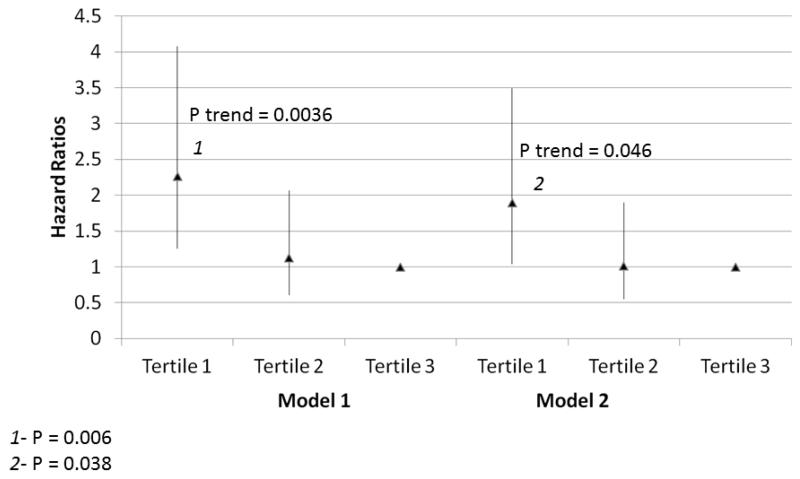
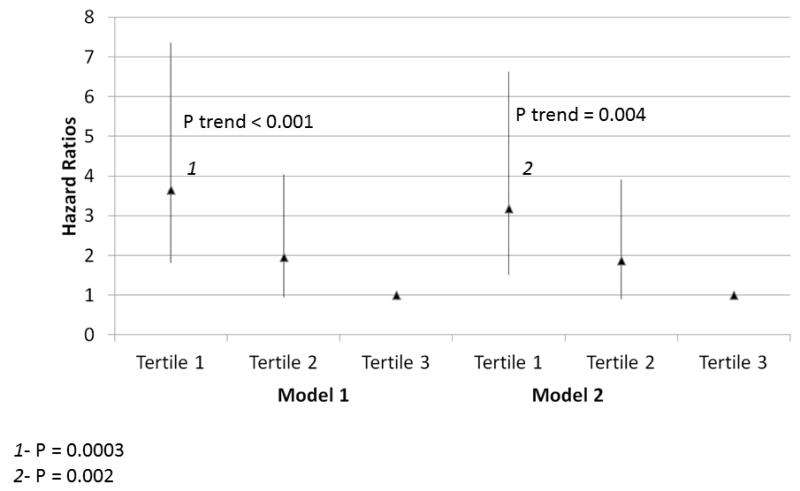
Within the entire cohort, lower knee extension power was associated with higher all-cause mortality, adjusting for age, sex, and race in Model 1 (Figure 3) (P trend=0.004). This association was maintained after additional statistical adjustment for comorbidities, BMI, ABI, physical activity, and smoking in Model 2 (Figure 3, P trend=0.046). Lower plantarflexion strength was associated with higher all-cause mortality, adjusting for age, sex, and race in Model 1 (Figure 4) (P trend<0.001). This association remained statistically significant even after additional adjustment for BMI, ABI, physical activity, comorbidities, and smoking in Model 2 (Figure 4, P trend=0.004). Lower hand grip strength was associated with higher all-cause mortality, adjusting for age, sex, and race in Model 1 (Table 3) (P trend=0.006). This association remained statistically significant even after additional adjustment for BMI, ABI, physical activity, and smoking in Model 2 (Table 3, P trend=0.005). Poorer knee extension strength was associated with higher all-cause mortality, adjusting for age, sex, and race in Model 1 (Table 3, P trend=0.038). However, this association was not statistically significant after additional adjustment for comorbidities, ABI, BMI, physical activity, and smoking in Model 2 (Table 3, P trend=0.185).
Figure 3. Adjusted Associations of baseline knee extension power with all-cause mortality in participants with peripheral arterial disease (N=391).
Model 1. Adjusted for age, sex, and race.
Model 2. Adjusted for covariates in Model 1 and the ankle brachial index, body mass index, comorbidities, physical activity, smoking history and study cohort (WALCS vs. WALCS II).
1P=0.006 for the comparison between the highest and lowest tertiles.
2 P=0.038 for the comparison between the highest and lowest tertiles.
Figure 4. Adjusted Associations of baseline plantar flexion strength with all-cause mortality in participants with peripheral arterial disease (N=330).
Model 1. Adjusted for age, sex, and race.
Model 2. Adjusted for covariates in Model 1 and the ankle brachial index, body mass index, comorbidities, physical activity, smoking history and study cohort (WALCS vs. WALCS II).
1P=0.0003 for the comparison between the highest and lowest tertiles.
2 P=0.002 for the comparison between the highest and lowest tertiles.
Associations of calf muscle percent fat with all-cause mortality and associations of isometric knee extension strength, hand grip, and plantar flexion strength with cardiovascular mortality differed between men and women, according to our criteria. Therefore, these associations are reported separately for men and women. Among men and women with PAD, respectively, there were no significant associations of calf muscle percent fat with all-cause mortality adjusting for age, sex, race, ABI, BMI, smoking, and comorbidities (P trend=0.427 for men and P=0.282 for women).
Because of relatively few cardiovascular deaths among women with baseline strength measures (N=7), results were unstable. Therefore, data are presented only for men. Because of the small number of cardiovascular deaths among men with baseline isometric strength measures (N=18), results are presented according to whether the baseline isometric strength measures were above vs. below the median value. Among men, poorer plantar flexion strength (P trend=0.006) and poorer hand grip (P trend=0.002) were associated with higher cardiovascular disease mortality, adjusting for age, sex, and race, BMI, physical activity, and smoking (Table 4, Model 2). We did not identify significant associations of knee extension isometric strength with cardiovascular disease mortality among men (Table 4).
Table 4.
Associations of Calf Muscle Density and Isometric Strength Measures with Cardiovascular Mortality among Men with Peripheral Arterial Disease
| Below the Baseline Median for Strength Measure |
Above the Baseline Median for Strength Measure (reference) |
P Value | |
|---|---|---|---|
| Hand Grip and Cardiovascular Mortality | |||
| Model 1 | 1.70 (0.55-5.23) | 1.00 (reference) | 0.013 |
| Model 2 | 1.63 (0.47-5.68) | 1.00 (reference) | 0.002 |
| Knee Extension Isometric Strength and Cardiovascular Mortality | |||
| Model 1 | 1.89 (0.63-5.63) | 1.00 (reference) | 0.162 |
| Model 2 | 1.49 (0.44-5.03) | 1.00 (reference) | 0.170 |
| Plantarflexion Isometric Strength and Cardiovascular Mortality | |||
| Model 1 | 2.26 (0.77 - 6.66) | 1.00 (reference) | 0.012 |
| Model 2 | 3.89 (1.07 - 14.19) | 1.00 (reference) | 0.006 |
Data shown are hazard ratios and 95% Confidence Intervals. Model 1 adjusts for age, sex, race. Model 2 adjusts for age, sex, race, smoking, body mass index, ankle brachial index, physical activity, comorbidities, and study cohort. Men with baseline values above the median (i.e. best baseline muscle measure) served as the reference group against which men with baseline values below the median (i.e. worst baseline muscle measure) were compared. Thus, the hazard ratio group for men with baseline values above the median is 1.0 and hazard ratios for men with baseline values below the median are relative to this reference.
DISCUSSION
Results presented here show that lower calf muscle density is associated with higher all-cause and cardiovascular disease mortality among participants with PAD. Lower knee extension power, poorer plantar flexion strength, and poorer hand grip strength were associated with higher all-cause mortality in participants with PAD. Poorer plantar flexion strength and poorer hand grip strength were associated with higher cardiovascular disease mortality in men with PAD. These associations remained statistically significant even after adjustment for age, sex, comorbidities, physical activity, the ABI, and other covariates. To our knowledge, no prior studies have described associations of CT-measured calf muscle characteristics, knee extension power, hand grip isometric strength, or plantar-flexion isometric strength with all-cause or cardiovascular mortality in individuals with PAD.
Adverse lower extremity muscle characteristics may be associated with higher mortality in people with PAD if muscle characteristics reflect the extent of lower extremity atherosclerosis and/or the overall health of an individual. Previous studies demonstrate that adverse calf muscle characteristics and poorer lower extremity strength are associated with poorer functional performance among individuals with PAD (4,5,8). Poorer functional performance is associated with higher all-cause and cardiovascular mortality rates in PAD (21). This previously established association of poorer functional performance with higher rates of all-cause and cardiovascular disease mortality in PAD (21) may reflect the association of impaired lower extremity strength with higher mortality rates reported here.
Our study design does not allow us to discern why some, but not all, measures of calf muscle characteristics and strength were associated significantly and independently with mortality. However, some muscle measures may better represent atherosclerotic disease burden and overall health than other measures. For example, as compared to isometric measures of lower extremity strength, knee extension power is a more sophisticated measure of muscle function, which incorporates measures of strength, peripheral nervous system function, and central nervous system function. Our finding that poorer grip strength is associated with higher all-cause mortality may reflect that grip strength is a measure of overall health and frailty.
Hazard ratios for associations of muscle measures were typically greater for cardiovascular disease than for all-cause mortality. Our finding that muscle measures were associated with all-cause mortality reflects in part the strong association of muscle measures with cardiovascular disease mortality and may also reflect that most PAD participants who died from cancer were likely to have had simultaneous cardiovascular disease that may also have contributed to death.
A prior study from the InCHIANTI cohort, reported no significant associations of calf muscle density, calf muscle area, or calf muscle percent fat with mortality among individuals without PAD (22). The InCHIANTI Study included 934 community dwelling individuals age 65 and older without PAD living in two communities in Italy who were followed for a mean of 5.1 years. Although lower calf muscle density, lower calf muscle area, and higher calf muscle percent fat were each associated with higher mortality in unadjusted analyses, none of these associations remained statistically significant after additional adjustment for age and sex. A separate study from Health ABC that included 2292 men and women without PAD who were age 70-79 reported that weaker quadriceps isometric strength and weaker hand grip isometric strength were associated with higher mortality, adjusting for covariates including age, sex, race, and comorbidities (23). However, investigators found no significant associations of thigh muscle area with mortality. Together, these prior studies and results reported here suggest that associations of lower extremity muscle measures with mortality may differ between individuals with vs. without PAD.
We previously reported that poorer isometric knee extension strength was associated with higher all-cause mortality in 264 men with PAD in the original WALCS cohort, independently of age, sex, race, comorbidities, the ABI, and other confounders (19). However, this prior study showed no significant association of weaker isometric knee extension strength with higher cardiovascular disease mortality among men and no significant associations of weaker isometric knee extension strength with all-cause or cardiovascular disease mortality in 146 women with PAD (19). The current study identified no significant associations of knee extension isometric strength with mortality in men or women with PAD. As compared to our original WALCS study, the WALCS II study used a different method for measuring knee extension strength and included a shorter follow-up period. These differences may account for the slightly different results in the current study as compared to the prior study.
In contrast to the prior report by Singh et al (19), the current study includes data on associations of CT-measured calf muscle density, calf muscle percent fat, and calf muscle area in addition to isometric hand grip, plantar flexion strength, and knee extension power with mortality among participants with PAD. Thus, the current report includes substantially new data as compared to previously reported measures of muscle and mortality among participants with PAD.
Our study has limitations. First, data are observational. Causal associations cannot be construed. Second, the number of cardiovascular deaths was low, particularly among women. The small number of cardiovascular deaths limited the statistical power to test the associations of muscle measures with cardiovascular outcomes and resulted in relatively wide confidence intervals of the point estimates for cardiovascular deaths. Third, we did not have data on PAD location. Location of lower extremity atherosclerosis may have influenced some of the associations studied here. Fourth, our results may have been influenced by residual confounding of age on the association of muscle characteristics with mortality. Fifth, although prior histopathologic data demonstrate that PAD is associated with calf muscle apoptosis, Type II muscle fiber atrophy, and impaired mitochondrial function (5-7), the WALCS II cohort did not include these measures and therefore we could not relate these histopathologic findings to mortality. Sixth, although prior studies have demonstrated that reduced leg strength and power are associated with gait abnormalities in individuals with PAD and claudication (24), the current study did not include data on gait abnormalities. Seventh, not all study participants had data for all strength measures.
In conclusion, poorer calf muscle density, plantar flexion strength, knee extension power, and hand grip are associated with higher mortality in people with PAD. Further study is needed to determine whether interventions, such as exercise, can reverse the adverse pathophysiologic lower extremity muscle findings in individuals with PAD and whether these interventions improve survival in men and women with PAD.
Acknowledgments
Funding Sources: Supported by grants #R01-HL58099, R01-HL64739, R01-HL071223, R01-HL076298, and R01-HL083064 from the National Heart Lung and Blood Institute and by grant #RR-00048 from the National Center for Research Resources, NIH. Supported in part by the Intramural Research Program, National Institute on Aging, NIH.
ABBREVIATIONS
- ABI
Ankle brachial index
- BMI
Body mass index
- CT
Computed tomography HR-Hazard ratio
- PAD
Peripheral arterial disease
- WALCS
Walking and leg circulation study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Hoff F, Ferrucci L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–6. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regensteiner JG, Wolfel EE, Brass EP, et al. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation. 1993;87:413–421. doi: 10.1161/01.cir.87.2.413. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell RB, Duscha BD, Robbins JL, et al. Increased levels of apoptosis in gastrocnemius muscle in patients with peripheral arterial disease. Vascular Medicine. 2007;12:285–290. doi: 10.1177/1358863X07084858. [DOI] [PubMed] [Google Scholar]
- 7.Pipinos II, Sharov VG, Shepard AD, et al. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg. 2003;38:827–832. doi: 10.1016/s0741-5214(03)00602-5. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Tian L, Ferrucci L, et al. Associations between lower extremity ischemia, upper and lower extremity strength, and functional impairment with peripheral arterial disease. J Am Geriatr Soc. 2008;56:724–729. doi: 10.1111/j.1532-5415.2008.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott MM, Ferrucci L, Guralnik JM, et al. Pathophysiological changes in calf muscle predict mobility loss at two-year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–1055. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: Prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Lindbom A. Arteriosclerosis and arterial thrombosis in the lower limb: A roentgenological study. Acta Radiol Suppl. 1950;80:1–80. [PubMed] [Google Scholar]
- 12.Hyvarinen S. Arteriographic findings of claudication patients. Ann Clin Res. 1984;16:1–45. [PubMed] [Google Scholar]
- 13.Rose GA. The diagnosis of ischemic heart pain and intermittent claudication in field surveys. Bull Wld Hlth Org. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and Computerized Tomography. J Appl Physiol. 1999;86(3):1097–8. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 15.Curb JD, Ceria-Ulep CD, Rodriguez BL, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54:737–42. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 16.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability, and validity. Eur J Appl Physiol Occup Physiol. 1990;60(5):385–390. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and social characteristics of older women with disability. National Institute on Aging; Bethesda, MD: 1995. NIH Pub. No. 95-4009. [Google Scholar]
- 18.Garg PK, Tian L, Criqui MH, et al. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh N, Liu K, Tian L, et al. Leg strength predicts mortality in men but not in women with peripheral arterial disease. J Vasc Surg. 2010;52:624–631. doi: 10.1016/j.jvs.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermann S, Liu K, Tian L, et al. Baseline lower extremity strength and subsequent decline in functional performance at six-year follow-up in persons with peripheral arterial disease. J Am Geriatr Soc. 2009;57:2246–2252. doi: 10.1111/j.1532-5415.2009.02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott MM, Tian L, Liu K, et al. Prognostic value of functional performance for mortality in peripheral artery disease. J Am Coll Cardiol. 2008;151:1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesari M, Pahor M, Lauretani F, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64A(3):377–384. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study cohort. J Gerontol A Biol Sci Med Sci. 2006;61A:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 24.Koutakis P, Johanning JM, Haynatzki GR, et al. Abnormal joint powers before and after the onset of claudication symptoms. J Vasc Surg. 2010;52:340–7. doi: 10.1016/j.jvs.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]