Abstract
Background and Aims
Hepatocellular carcinoma (HCC) is frequently a lethal disease and one of the few malignancies that are still increasing in incidences around the world. Better animal models are highly desired to investigate the molecular basis of HCC and to develop novel therapeutic strategies. Alpha-fetoprotein (Afp) gene is expressed in fetal liver, silenced soon after birth, and highly re-expressed in hepatocellular carcinomas (HCC). We aimed to take advantage of the dramatic re-expression of Afp gene in HCC to develop a hepatocarcinogenesis reporter (HCR) mouse model for dual-modality, longitudinal in vivo imaging of liver tumor development and progression.
Methods
Knockin mice were established by placing a thymidine kinase (tk) - luciferase (luc) reporter gene cassette under the transcriptional control of the endogenous Afp promoter. DEN, a liver carcinogen, was used to induce liver tumors, which was monitored by both luc-based bioluminescent (BL) and tk-based positron emission tomography (PET) imaging.
Results
The expression profile of luc was identical to that of endogenous Afp gene during development. As early as two month after exposure to DEN, BLI revealed multifocal signals in the liver, long before the appearance of histologically apparent neoplastic lesions. By six months, BL and PET dual imaging showed strong signals in malignant HCC. By serendipity, strong BL signal was also detected in adult testes, a previously unknown site of Afp expression.
Conclusion
The HCR model enables longitudinal monitoring of liver tumor development and progression, providing a powerful tool in developing chemoprevention and therapeutic strategies for HCC.
Keywords: positron emission tomography, bioluminescence imaging, hepatocarcinogenesis, hepatocellular carcinoma, testes
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common type of human cancer and the third most common cause of cancer death worldwide. In North America, it is one of the few tumor types whose incidence is increasing, bucking the trend of continuing reduction for many other tumors. Typical HCC arises in a setting of chronic hepatitis with infection by hepatitis B and C viruses responsible for most HCC cases in humans [1, 2]. Although the pathogenic causes are well known, no effective treatment is available for most HCC patients except for surgical resection [3].
Historically, studying naturally occurring HCC in model organisms, particularly mice, have been instrumental in advancing our understanding of etiology of HCC. Genetically defined mouse models are increasingly used as powerful tools to evaluate genetic factors and therapeutic strategies for HCC [4-6]. However, because of the stochastic and non-visible nature of HCC occurrence, the detection and analysis of HCC from these models often require the termination of the animals, precluding the real time assessment of the behavior of individual HCC tumor under a natural microenvironment and/or over a defined period of time. In vivo imaging in small laboratory animals is an emerging and promising technology that can be used to facilitate noninvasive, longitudinal monitoring of naturally occurring tumors. In mice, two types of reporter-based imaging modalities, the luciferase (Luc) reporter-based in vivo bioluminescence imaging (BLI) and the thymidine kinase (TK) -based Positron Emission Tomography (PET) imaging, respectively, are particularly useful. BLI has been widely used in cancer imaging often with multiple xenograft tumor-based systems where tumor cells express Luc. It provides a reliable, sensitive, and rapid modality for noninvasively tracking of tumor status in mice [7-9]. On the other hand, PET is a highly quantitative detection method that also provides three-dimensional information for the imaging target in the context of its surrounding [10-12].
In order to monitor HCC in mice by BLI and PET imaging, we decided to create a mouse model in which Luc and TK are expressed specifically in HCC by placing the coding sequences of Luc and TK under the control of the promoter of Alpha-fetoprotein (Afp) encoding gene. Afp, a secreted polypeptide, is a serum tumor marker for HCC. It is highly expressed in fetal liver in both rodents and humans but is not expressed in normal adult liver. Interestingly, this protein is dramatically re-expressed in vast majority of HCCs [13]. Thus, it is a unique HCC tumor specific marker in both mice and humans. Here we took advantage of the dynamic regulation of Afp expression and generated a novel reporter mouse model that enables dual modality imaging of early and late stages of hepatocarcinogenesis in vivo.
Materials and Methods
Gene Targeting in Mouse Embryonic Stem (ES) Cells and Generation of Knock-in Mice
A targeting vector, pAfp-tv1, was constructed using the BAC modification system [14, 15] as described [16]. This vector was designed to introduce a fusion gene cassette, designated PuroTK-Luc, consisting of the coding sequences for both the fruit fly luciferase (Luc) and the PuroTK [19], linked by an internal ribosomal entry site (IRES) along with a loxP-PGKneo-loxP cassette [20] in between the A and T of the translation start codon of the Afp gene (Detail information for the plasmid construction will be made available upon request). This vector was electroporated into ES cells and targeted clones were identified by the standard mini-Southern approach. Two of these targeted clones were injected into blastocytes. Heterozygous mice carrying the targeted allele were obtained. Once heterozygous knock-in mice are obtained, they were mated with Zp3/cre transgenic mice which express the cre recombinase in the female oocytes and thus can facilitate the removal of PGKpuro-tk cassette flanked by a pair of LoxP sites [17]. This gave rise to mice in which the PuroTK-Luc cassette is placed right behind the Afp promoter. Such mice were identified by Southern blot analysis. Mice carrying this particular knock-in allele were identified by a PCR-based genotype using the following primers: 5’- ATG AAT TCG GCG CGC CGC CTG AAC TAC TGA AAC AAT-3’ and 5’ – GAA GAG TTC TTG CAG CTC GGT – 3’.
Animal Keeping, Liver Carcinogenesis and Tissue Analysis
Mice were cared for in accordance with guidelines set forth by the American Association for Accreditation of Laboratory Animal Care and the USPHS “Policy on Human Care and Use of Laboratory Animals,” and all studies were approved and supervised by The Case Western Reserve University Institutional Animal Care and Use Committee. Mice were generated on C57 BL/6j-129 background originally and backcrossed to C3H and FVB/N mice for 5 generations by standard genetic crosses, which were then bred with each other to generate cohorts of Afp-TK-ires-Luc positive or negative mice that were used in subsequent studies. Knockin mice and wild type littermates were subjected to a single i.p. injection of 20 μg/g body weight of diethylnitrosamine (DEN) (Sigma) 14 days after birth. Mice were sacrificed at indicated time point after injection. After imaging, the animals were euthanized by overdosing with pentobarbital and cut open for photography and tissue harvesting. Livers were excised, weighed, examined for macroscopic lesions and photographed. When indicated ex vivo imaging was performed. Individual liver lobes of were fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburgh, PA) or Z-FIX (ANATECH LTD, Battle Creek, MI). Paraffin-embedded sections were cut at 5 μm, and stained with H&E. In addition, a portion of liver or testis was embedded in Tissue Freezing Media (Triangle Biomedical Sciences, Durham, NC), and submerged in cold 2-methylbutane for cryosectioning and stored at −80°C.
Immunofluorescence
Frozen sections of liver and testis were fixed with 2% paraformaldehyde. After washing with PBS, the sections were blocked with 50 mmol/L NH4Cl and permeabilized with 0.3% NP40 for 10 minutes. The sections were then incubated with goat anti-Afp (Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 2 hours followed by detection with donkey anti-goat IgG-Red X (Jackson ImmunoResearch Laboratories, West Grove, PA) at room temperature for 30 minutes. Images were taken using Leica microscope.
In vivo Imaging with BLI & PET
BLI
For one-day-old pups, the animals were weighed precisely for calculating the amount of D-luciferin (Biosynth AG, Switzerland) to be carefully injected i.p. into the pups at 75 μg/kg body weight. Then, these one-day-old pups were placed in home made dark box with multiple light-absorbing cubicle chambers and subjected to imaging with a combined tri-imaging modality system developed by Thomas Jefferson National Lab (Newport News, VA) at either 3 or 6 minutes after injection of D-luciferin. For BLI of the adult mice, light signals were collected at 6 minutes after a single IP injection of 100μg/kg body weight D-luciferin using a Xenogen IVIS 200 cooled-CCD camera system (Palo Alto, CA). During the period of signal collection, the animals were sedated continuously via inhalation of isoflurane gas (1.5% mixed with oxygen) through a nose corn device (EZAnesthesia, Palmer, PA). Ex vivo bioluminescence imaging of isolated organs was performed immediately after euthanasia of the animals. Dissected organs were placed on a sheet of black background and imaged with IVIS. The BLI images were then pseudo-colored and overlaid on top of a conventional photograph of the animals.
PET
Animals were imaged pair-wise with a presumptive control and a testing animal for each scan using a microPET scanner R4 (Siemens/Concord, Knoxville, TN). A 20 minute transmission scan was conducted using a sealed point source in order to establish the attenuation map. During the period of image acquisition, the animals were sedated continuously via inhalation of isoflurane gas as in BLI. L-[F-18]-FMAU, the radiotracer used here was synthesized according to [18]. 250 uCi (9.25 MBq) of L-[F-18]-FMAU in 200 μL of saline solution was injected via tail vein into each animal. 40 minutes after injection, a 30 minutes emission scan was conducted in list mode acquisition. After the scan, the acquired data were binned into 5 minutes frames and reconstructed with attenuation correction using the ASIPRO package on the R4 scanner. The last frame of the emission images was colored in hot-metal for display.
RESULTS
Generation of the Afp-TK-ires-Luc Mouse Model
We took advantage of dramatic re-expression of Afp in liver cancer and generated mice for visualizing hepatocarcinogenesis processes in vivo. Toward this goal, we replaced Afp coding sequence with those of imaging reporters, thymidine kinase (TK) and luciferase (Luc); two enzymes that are commonly used for facilitating PET and BLI, respectively. Fig. 1 shows the targeting strategy used to generate mutant alleles of the Afp gene. The TK - Luc dual-reporter fusion gene cassette (designated as PuroTK-ires-Luc) was introduced into the Afp locus by the standard knock-in gene targeting experiment in the mouse ES cells, giving rise to new Afp.PGKneo.PuroTK-IRES-Luc allele (Fig. 1A.). Heterozygous mice carrying this allele were obtained. These mice were then mated with Zp3-Cre female mice to facilitate the removal of the PGKneo cassette from the original targeted allele, giving rise to a new modified Afp allele in which the PuroTK-IRES-Luc dual reporter cassette is placed right behind an endogenous Afp promoter as originally designed (Fig. 1). The imaging allele, designated as the hepatocarcinogenesis-reporter or HCR, is expected to produce the PuroTK fusion protein and luciferase with a profile identical to that of the unmodified endogenous Afp gene.
Figure 1. Gene targeting at the Afp locus.
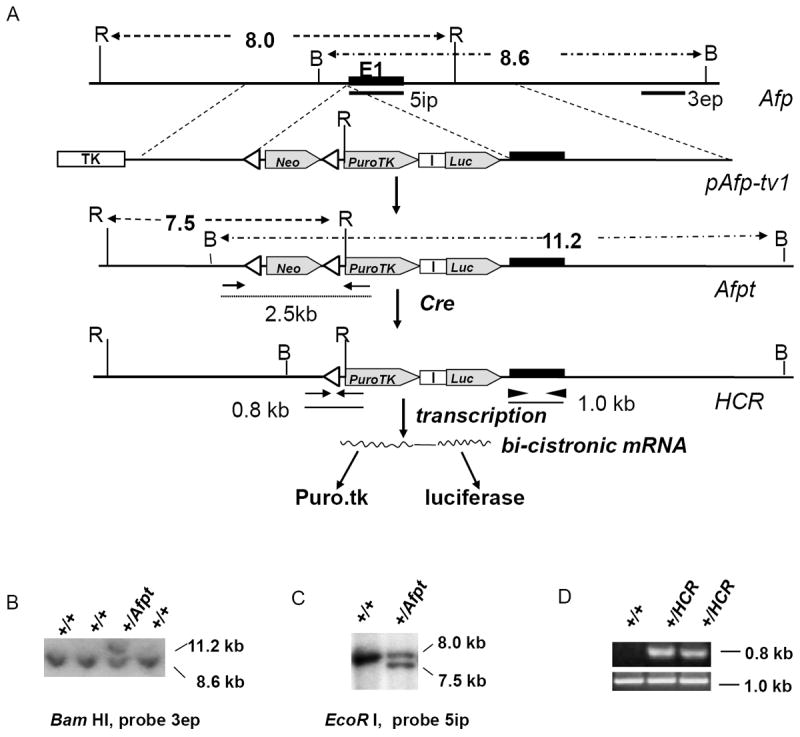
A) A schematic illustration of the gene targeting strategy. Targeting vector (pAfp-tv1) contains a LoxP-Neo-LoxP-PuroTK-ires-Luc cassette between the two arms of homology for gene targeting. By design, the cassette is inserted behind the A of the ATG translation start codon of the Afp gene, generating the primary knock-in allele (the Afpt allele). Cre-mediated deletion of the Neo selection marker then gave rise to the final knock-in allele (the HCR allele). Transcription from this allele, driven by the Afp promoter, is expected to produce a bi-cistronic mRNA with which Puro.TK and luciferase can be produced upon translation, providing two potential reporters for imaging. Targeted clones were identified by Southern blot by the presence of a novel 11.2 Kb Bam HI fragment in addition to the 8.6 Kb wild-type fragment using a 3’ external probe (3ep) and the presence of a 7.5 Kb Eco RI fragment in addition to the 8.0 wild-type fragment by 5’ internal probe (5ip). The HCR allele was detected by a PCR genotyping strategy using a pair of primer corresponding to the Afp promoter and the Puro.TK cassette, respectively. This PCR is designed to generate a 2.5 kb and 0.8 kb product from the afp-t and HCR allele respective (the 2.5 kb product is not amplified under our experimental condition). The sizes of the diagnostic Bam HI (B) and Eco RI (E) fragments of wild-type (Afp) and targeted (Afpt) allele, respectively, were indicated. The positions of the pair of primers used to identify the HCR allele are also shown. B) Initial screening for putative clones containing the afp-t allele by Southern blot. C) Identification of correctly targeted clones by Southern blot from the putative targeted clones identified in B. D) Identification of mice carrying the HCR allele by PCR genotyping. HCR mice were identified by detecting the 0.8 kb PCR fragment specific for the HCR allele (upper panel). As a control, another pair of primers was used to amplify a 1.0 kb fragment from the wild-type Afp gene (lower panel).
The Expression of the Luciferase Reporter in HCR Mice Recapitulates That of the Endogenous Afp Gene
Heterozygous HCR mice were fertile and did not display any phenotypic or histological abnormalities, showing that introduction of the TK-ires-Luc cassette into the Afp locus did not significantly affect either development or postnatal growth (Fig. 2 A and C). Mating among these heterozygous mutant mice gave rise to progeny of all expected genotypes at a ration consistent with Mendelian segregation. Homozygous HCR mice also were indistinguishable from their normal counterpart except the females were sterile, consistent with a previous report [19].
Figure 2. Detection of luciferase activity in HCR mice.
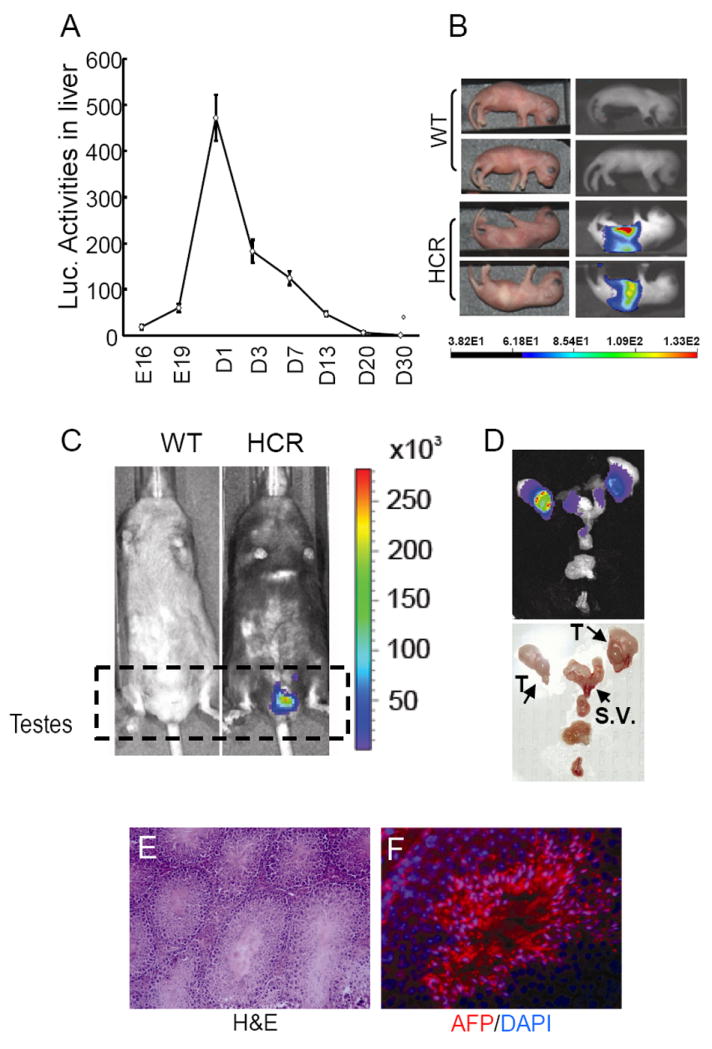
A) Detection of luciferase activity in the liver of HCR mice at various ages by chemoluminescent assay. B) Detection of luciferase activity in newborn HCR mice by bioluminescent imaging. C) Detection of luciferase activity in adult HCR mice. Note the pattern of luciferase activity in testes. D) BLI of a male reproductive tract. Abbreviations used are: T, testes; S.V., seminal vesicles. E) Photographic image of reproductive tract. F) Histology of testes (magnification 20 ×). G) Detection of Afp in testes by immunofluorescence (magnification 40 ×).
Afp is known to be expressed in early developing liver or during hepatocarcinogenesis, but is largely absent in normal adult liver. Thus, we first examined whether Luc expression followed similar developmental regulation in HCR mice. The livers of mice between E16 to P30 were injected with D-luciferin, and livers were harvested and subject to conventional biochemical enzymatic luciferase assay. We found that luciferase activity could be detected in the fetal liver from HCR mice but not those from the wild-type control. The activity could be detected as early as E16.5, and the highest level was detected at postnatal day 1 (Fig. 2A). It rapidly dissipated and became completely undetectable by three weeks. The pattern is very similar to that of the endogenous Afp gene [20]. BLI confirmed biochemical analyses and detected strong signal in neonatal liver (Fig. 2B), which disappeared from the adult liver (Fig. 2B,C).
Unexpectedly, strong BLI signals were consistently detected in adult male HCR mouse in areas around the testes (Fig. 2C). When the reproductive tract was dissected and imaged ex vivo (Fig. 2D and E), BLI signals were strongly detected in the testes and at lower levels in the seminal vesicles, suggesting that Afp promoter is active in male reproductive cells. To verify Afp expression in the testes, immunofluorescence staining with an anti-Afp antibody was performed, as the heterozygous HCR mice still expressed Afp from the remaining wild type allele. Fig. 2G shows that Afp protein is indeed highly expressed in spermatids from the HCR mice that still retain one Afp allele, as did the wild type mice. It is possible that the lower BLI signals detected in seminal vesicles might have come from sperms stored in seminal vesicles. Consistent with the normal fertility of HCR mice, histological analysis did not reveal any defects in testes (Fig. 2F). To our knowledge, this is the first demonstration of Afp expression in an adult tissue.
In sum, these data suggest that the expression of the PuroTK-IRES-Luc transcript indeed recapitulates that of the endogenous Afp gene in the HCR mice.
HCR Mice Allow in vivo Visualization of Early Preneoplastic Lesions Following DEN-Induced Hepatocarcinogenesis
We next asked whether the PuroTK-IRES-Luc dual reporter gene was specifically activated during hepatocarcinogenesis to allow longitudinal monitoring of hepatocarcinogenesis process by BLI, PET, or both. Liver tumors can be induced with DEN, a classical hepatocarcinogen, in young mice on susceptible genetic backgrounds. To facilitate induction of liver tumors, we initially transferred the HCR allele from the original liver tumor-resistant C57BL/6j-129Sv mixed genetic background into C3H genetic backgrounds that that is highly susceptible to DEN-induced hepatocarcinogenesis. After four rounds of backcrossing, two week-old C3H male mice were given a single intraperitoneal injection of DEN. Longitudinal imaging was commenced twice a month later. Remarkably, significant levels of BLI signals were detected in HCR mice but not the control mice as early as two months after the DEN injection (Fig. 3A). To confirm this observation, livers from a subgroup of mice were dissected and subject to BLI ex vivo. Fig. 3C shows that multiple Luc-positive foci were present on every lobe. However, gross inspection under dissection microscope and detailed histological examination failed to identify any clear neoplastic lesions at this early stage (Fig. 3B, D and E). Indeed not clear cell or basophilic foci were detected. PET imaging is known to be less sensitive than BLI imaging. As a result, PET imaging of same mice at this early stage did not detect any consistent signals. Because luciferase and thymidine kinase were expected to be translated from the same bicistronic transcript (Fig. 1), it is possible that PuroTK was expressed but at a level that was too low to be visualized by PET imaging.
Figure 3. Visualization of early preneoplastic lesions in HCR mice by BLI.
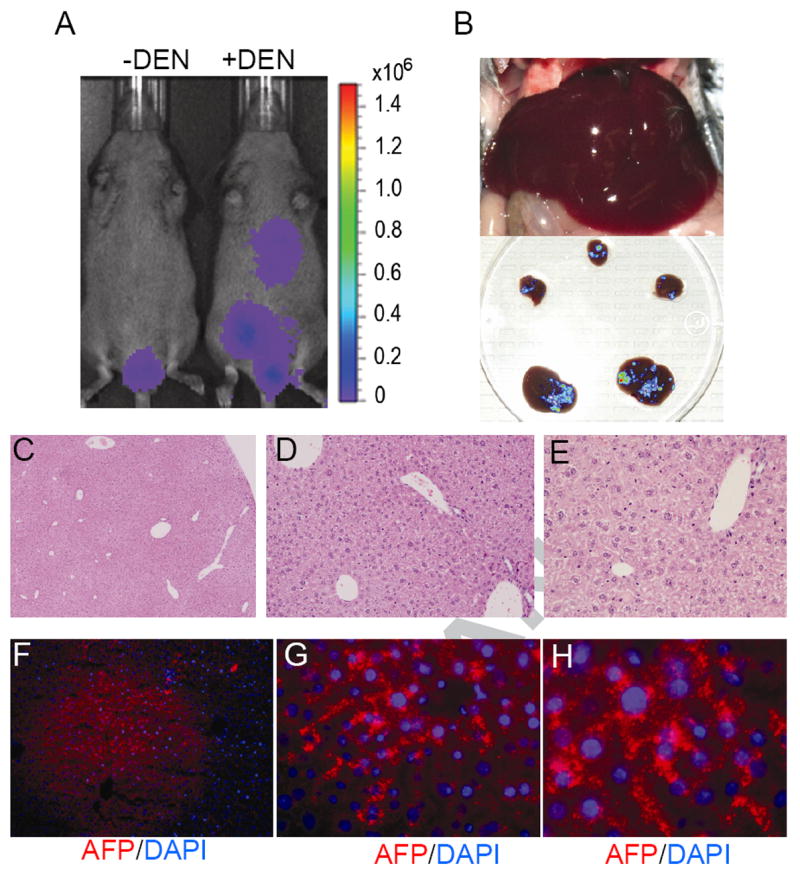
A) BLI on a pair of HCR mice. The animal on the right was injected with a single dose of DEN at two weeks of age and imaged two months later. The animal on the left was injected with vehicle control. Note the detection of hepatic BLI signal only in the DEN-injected animal. The testicular signals varied from animal to animal and were not correlated with DEN treatment (not shown). B) A photograph of the liver showing normal gross morphology. C) Ex vivo BLI of dissected liver lobes from DEN injected mouse superimposed on a digital photograph. D, E) Hemotoxylin and eosin staining of liver sections from DEN injected mouse (magnification, D, 5 ×; E, 20×). F, G). Expression of Afp in DEN-treated mouse liver detected by immunofluorescence (red, Afp. blue, DAPI. Magnification, F, 10 ×; G, 40 ×).
Since these heterozygous HCR mice also carry a normal copy of the endogenous Afp gene, we examined whether Afp was also expressed in these livers. Consistent with the multifocal BLI signals, numerous microscopic Afp-positive foci were readily detected across the liver sections (Fig. 3 F and G). Although the cellular origin of Afp-positive cells is yet to be defined, hepatocytes appeared to constitute a significant fraction where Afp displayed cytosolic staining pattern (Fig. 3H). These results indicate that Afp expression takes place during the early stages of hepatocarcinogenesis, before the appearance of histologically evident transformation. Importantly, these early events of hepatocarcinogenesis can be detected by BLI in HCR mice.
Longitudinal Monitoring of Liver Tumor Progression by Both BLI and PET Imaging
Next, we determined whether HCR mice will permit long-term real time monitoring of natural progression of liver tumors. Because DEN induces large number of fast-growing liver tumors that rapidly occupy most of liver parenchymal space on C3H genetic background, we backcrossed HCR mice onto FVB/NJ mice that are much less susceptible to DEN-induced hepatocarcinogenesis. Upon DEN exposure FVB mice develop a small number (less than 10) of discrete liver tumors, a fraction of which progress to malignant HCC. As such, FVB/NJ genetic background is more suitable for imaging later stage individual liver tumors. DEN-treated FVB/NJ mice also displayed BLI signal at early stage, albeit somewhat delayed compared with C3H mice. BLI signals became stronger over time. An example at six months after DEN treatment is shown in Fig. 4A, revealing strong BLI signals. Note the difference in signal intensities between Fig. 3A and Fig. 4A on the scale bars. The distribution patterns of Luc signals were suggestive of the presence of multiple tumors. However, the two-dimensional nature of BLI made it hard to ascertain whether they represent individual tumors.
Figure 4. BLI and micro-PET dual modality imaging of liver tumors mice six months after DEN treatment.
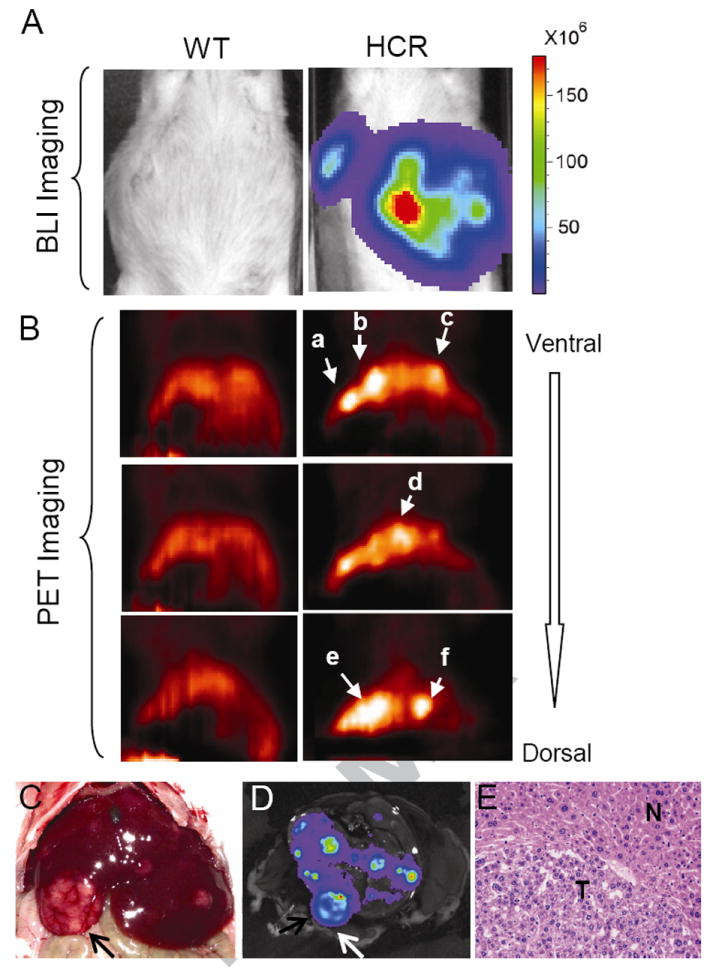
A) BLI of an HCR mouse (right) together with a wild type control (left). Mice were treated as described in Fig. 3 legend. Note the focal nature of BLI signals. B) Micro-PET imaging of the same mice. Serial coronal sections from ventral to dorsal were collected, and three sections are presented. Note distinct patterns of PET signals detected at different depths from the ventral surface of the liver. C) A photograph showing multiple visible liver tumors upon autopsy (top), most of which were Luc-positive based on ex vivo BLI as shown in D. Arrows point to a large tumor visible by both visual inspection and BLI. E) H&E analysis of a typical tumor. Most tumors were classified as benign hepatomas at this stage.
More importantly, at six months, the DEN-induced tumors could be readily detected by PET imaging based on Afp promoter-driven thymidine kinase activities (Fig. 4B). Interestingly, coronal ventral to dorsal serial imaging showed the presence of multiple PET-positive nodules at different depths, illustrating the power of three-D imaging by PET to detect distinct internal tumor masses. Indeed, autopsy and histological analyses revealed that the livers of these animals harbored multiple liver tumors (Fig. 4C and E). Ex vivo BLI of the liver was performed with the excised liver (Fig. 4D), which was correlated with visible surface tumor (arrows in Fig. 4C,D), although the internal tumors revealed by BLI and PET imaging could not be seen by visual inspection.
Effective Dual Modality Imaging of Malignant HCC
Most liver tumors induced by DEN at six month were benign hepatomas characterized by uniform nuclear morphology and clear tumor margins (Fig. 4E). To determine if malignant HCC can be effectively imaged by BLI and PET, tumors were allowed to progress for three more months and the mice were imaged again. In one of these mice, dramatically strong signals were detected by both BLI and PET imaging (Fig. 5A, B). Upon autopsy, a single large tumor was found that showed strong BLI signal by ex vivo imaging (Fig. 5C, D). Histological analyses revealed the tumor was a malignant HCC that had invaded into surrounding normal liver parenchyma (Fig. 5E, F). Similar observations were made in two other malignant mice, suggesting that HCR model may allow effective imaging of early pre-neoplastic lesion, hepatomas, as well as late stage malignant HCCs.
Figure 5. BLI and micro-Pet imaging of malignant HCC.
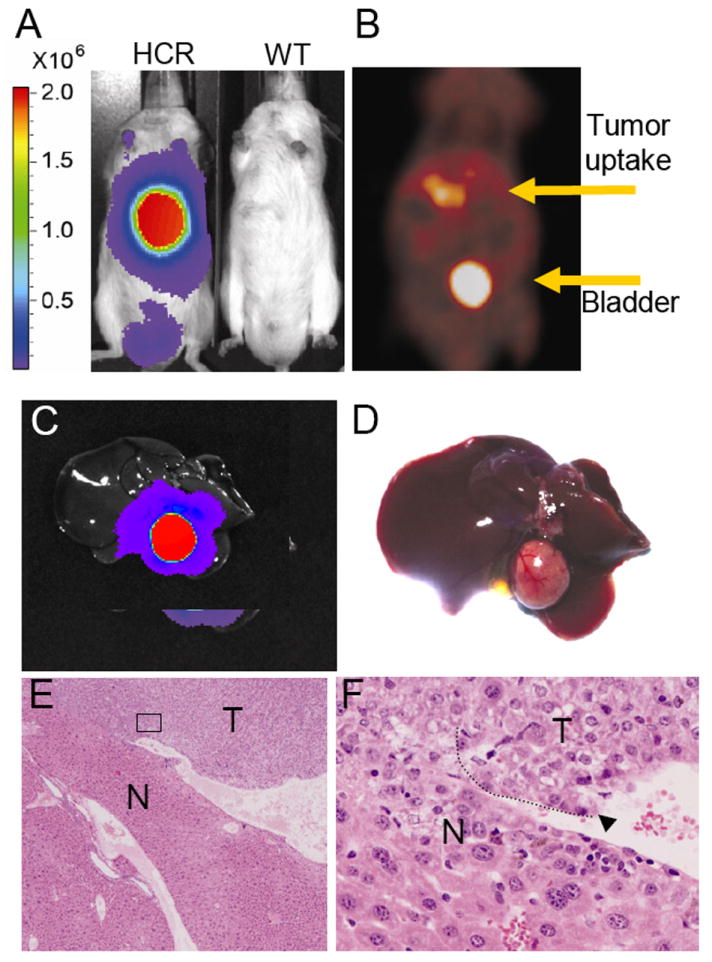
Mice were treated with DEN and imaged nine months later to allow sufficient time for tumor progression. A) BLI of wild type and HCR mice in vivo as described in Fig. 3A. The HCR mouse was also subject to micro-PET imaging (B). A single tumor was identified upon autopsy that was highly positive by ex vivo BLI (C, D). E, F) H&E staining of tumor sections. Note highly heterogeneous nuclear morphology and extensive invasion of tumor cells into surrounding normal liver tissues.
DISCUSSION
In this study, we developed a novel hepatocarcinogenesis reporter (HCR) mouse model that allows non-invasive and longitudinal imaging of the entire natural process of liver tumor development and malignant progression. Our results demonstrate that the HCR model faithfully recapitulated dynamic regulation of endogenous Afp gene expression. More importantly, the HCR mouse enabled simple BLI detection of early stage neoplastic lesions before they became histologically apparent, while the later stages of tumor progression can be monitored by dual BLI and PET imaging. Unexpectedly, the HCR model also led to the characterization of testes as the only organ that strongly express Afp proteins in normal adult mice.
Research on liver cancer has been facilitated by mouse model systems, which share morphologic, histological and molecular features with human HCC [21, 22]. These include chemically-induced liver cancers using several carcinogens and genetically engineered mouse models. The advent of HCR model described in this paper will significantly increase the power and utility of the mouse HCC models. Our data have demonstrated the successful application of HCR mice to monitor the DEN-induced liver tumor development and progression. By simply breeding the HCR mice to other genetically engineered mouse models for HCC, the natural process of hepatocarcinogenesis by the defined molecular pathways can be imaged in vivo. Remarkably, as early as two months after DEN treatment, BL imaging detected significant Afp promoter-driven luciferase expression in the liver, long before the appearance of clear cell and basophilic foci that are characteristic of early neoplastic lesions. Thus HCR mice may enable much earlier detection of cellular changes associated with carcinogenic insults. Immunofluorescence analysis confirmed the multifocal expression of Afp in the liver, in what appeared to be hepatocytes. A significant body of evidence exists that hepatocarcinogen exposure leads to rapid proliferation of oval cells and transitory hepatocyte-like cells, both of which express Afp (Sell, 2008, and Abelev and Eraiser, 1999). Whether or not the Afp-positive cells constitute oval cells, transitory hepatocyte-like cells or mature hepatocytes, or a mixture of all these cell types is yet to be determined.
It should be noted that not all HCC developed in mice are Afp positive (Jalanko and Ruoslahti, 1979). A similar situation also exists in human HCC as well as HCC progenitor/stem cells. Intriguingly, AFP-positive, but not AFP-negative human HCC exhibit features of hepatoblasts or mature hepatocytes (Yamashita et al. 2008; 2009; Mishra etal. 2009), suggesting that AFP positive HCCs are derived from hepatocyte lineages. Thus, our model could be incorporated into the studies that model these specific types of AFP positive HCCs.
Between the dual imaging modalities, BLI provides a sensitive approach for detecting early neoplastic lesions, which represents an unprecedented tool in studying chemoprevention at a very early stage. On the other hand, both BLI and micro-PET could be used to detect larger hepatomas and malignant HCC at later stages during hepatocarcinogenesis. Although PET is less sensitive in detecting liver tumors at early stages, it does provide a unique opportunity for acquiring quantitative and 3-D information regarding both individual tumors as well as its relationship with the surrounding hepatic tissues.
Serendipitously, we have found that in adult mice, the knockin reporter was highly expressed in the male reproductive tract, independent of their expression in the HCC. The function of Afp in testis is still not clear, although Afp is known to be elevated in testicular cancer [23]. Nevertheless, the testicular BLI signal provides an appealing and convenient internal control for BLI signal quantification.
In addition to the basic studies on the molecular basis of hepatocarcinogenesis, the HCR model will prove valuable in development and evaluation of chemopreventive and therapeutic strategies for HCC. Moreover, recent studies have indicated that human AFP represents a marker for hepatoblasts and its immediate derivatives as well as the stem/progenitor-like cells of HCC[24, 25]. Thus, the HCR mice reported here may also be used to monitor the activity of hepatocyte progenitor cells, particularly during the course of hepatocarcinogenesis.
Acknowledgments
This project is supported in part by NIH grants R01 DK077876, R01 CA92259, R01 CA152371 to B. Wang and by NIH grant R24 CA110943 to J. Duerk. G. Luo is supported by NIH/NCI grant R01 CA88939. Z. Lee is supported by NIH/NCI grant R01 CA095307.
Abbreviations
- Afp
Alpha-fetoprotein
- HCC
hepatocellular carcinomas
- HCR
hepatocarcinogenesis reporter
- TK
thymidine kinase
- LUC
luciferase
- DEN
diethylnitrosamine
- PET
positron emission tomography
- BLI
bioluminescence imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002 Aug;31(4):339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 2.Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003 Aug 11;22(33):5093–5107. doi: 10.1038/sj.onc.1206557. [DOI] [PubMed] [Google Scholar]
- 3.Varela M, Sanchez W, Bruix J, Gores GJ. Hepatocellular carcinoma in the setting of liver transplantation. Liver Transpl. 2006 Jul;12(7):1028–1036. doi: 10.1002/lt.20833. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad I, Sansom OJ, Leung HY. Advances in mouse models of prostate cancer 3. Expert Rev Mol Med. 2008;10:e16. doi: 10.1017/S1462399408000689. [DOI] [PubMed] [Google Scholar]
- 5.Kim CF, Jackson EL, Kirsch DG, Grimm J, Shaw AT, Lane K, et al. Mouse models of human non-small-cell lung cancer: raising the bar. Cold Spring Harb Symp Quant Biol. 2005;70:241–250. doi: 10.1101/sqb.2005.70.037. [DOI] [PubMed] [Google Scholar]
- 6.Teoh NC, Dan YY, Swisshelm K, Lehman S, Wright JH, Haque J, et al. Defective DNA strand break repair causes chromosomal instability and accelerates liver carcinogenesis in mice. Hepatology. 2008 Jun;47(6):2078–2088. doi: 10.1002/hep.22194. [DOI] [PubMed] [Google Scholar]
- 7.Adams JY, Johnson M, Sato M, Berger F, Gambhir SS, Carey M, et al. Visualization of advanced human prostate cancer lesions in living mice by a targeted gene transfer vector and optical imaging 3. Nat Med. 2002 Aug;8(8):891–897. doi: 10.1038/nm743. [DOI] [PubMed] [Google Scholar]
- 8.Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, et al. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia. 2000 Nov;2(6):491–495. doi: 10.1038/sj.neo.7900121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vooijs M, Jonkers J, Lyons S, Berns A. Noninvasive imaging of spontaneous retinoblastoma pathway-dependent tumors in mice. Cancer Res. 2002 Mar 15;62(6):1862–1867. [PubMed] [Google Scholar]
- 10.Blasberg RG. In vivo molecular-genetic imaging: multi-modality nuclear and optical combinations. Nucl Med Biol. 2003 Nov;30(8):879–888. doi: 10.1016/s0969-8051(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 11.Blasberg RG. Molecular Imaging and Cancer. Mol Cancer Ther. 2003 Mar 1;2(3):335–343. [PubMed] [Google Scholar]
- 12.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002 Sep;2(9):683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 13.Barlow JF. Alpha fetoprotein (AFP) S D J Med. 1978 Sep;31(9):33. [PubMed] [Google Scholar]
- 14.Copeland NG, Jenkins NA, Court D. Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet. 2001 Oct;2(10):769–779. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- 15.Lee EC, Yu D, Martinez d V, Tessarollo L, Swing DA, Court DL, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001 Apr 1;73(1):56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Lu X, Barnes E, Yan M, Lou H, Luo G. Recql5 and Blm RecQ DNA Helicases Have Nonredundant Roles in Suppressing Crossovers. Mol Cell Biol. 2005 May 1;25(9):3431–3442. doi: 10.1128/MCB.25.9.3431-3442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997 Feb 1;7(2):148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay U, Pal A, Gelovani JG, Bornmann W, Alauddin MM. Radiosynthesis of 2’-deoxy-2’-[18F]-fluoro-5-methyl-1-[beta]-l-arabinofuranosyluracil ([18F]-l-FMAU) for PET. Applied Radiation and Isotopes. 2007 Aug;65(8):941–946. doi: 10.1016/j.apradiso.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Gabant P, Forrester L, Nichols J, Van Reeth T, De Mees C, Pajack B, et al. Alpha-fetoprotein, the major fetal serum protein, is not essential for embryonic development but is required for female fertility. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):12865–12870. doi: 10.1073/pnas.202215399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamiya A, Kinoshita T, Ito Y, Matsui T, Morikawa Y, Senba E, et al. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999 Apr 15;18(8):2127–2136. doi: 10.1093/emboj/18.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, et al. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004 Dec;36(12):1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 22.Sell S. Mouse models to study the interaction of risk factors for human liver cancer. Cancer Res. 2003 Nov 15;63(22):7553–7562. [PubMed] [Google Scholar]
- 23.Emerson RE, Ulbright TM. The use of immunohistochemistry in the differential diagnosis of tumors of the testis and paratestis. Semin Diagn Pathol. 2005 Feb;22(1):33–50. doi: 10.1053/j.semdp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009 Mar;136(3):1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Theise N, Chua M, Reid LM. The stem cell niche of human livers: symmetry between development and regeneration 4. Hepatology. 2008 Nov;48(5):1598–1607. doi: 10.1002/hep.22516. [DOI] [PubMed] [Google Scholar]


