Abstract
The link between aging and stress resistance is well established, but the nature of this relationship and which mechanisms are shared is still unknown. Mori et al. (2012) demonstrate that microRNA processing, specifically in adipose tissues, is a major component in aging and stress survival.
The next few decades will see a change in population demographics with increased numbers of elderly persons across the globe. Aging itself is the greatest risk factor for a range of diseases including diabetes, cancer, and cardiovascular disease. In anticipation of this population change and the associated health care challenges, research into diseases of aging and the biology of aging has intensified. One of the most robust interventions that delays aging and the onset of age-associated disease is caloric restriction (CR), the reduction of calorie intake without incurring malnutrition. CR studies have proven useful as a means to understand aging and age-associated disease vulnerability; changes that are causative in the aging process must necessarily be impeded by a regimen of delayed aging. In this issue, the study by Mori et al. (2012) demonstrates that attenuated microRNA processing in adipose tissues is a conserved feature of aging in mice, worms, and humans, and in the case of the former two species, the change is prevented by CR.
The importance of adipose tissue function in health and disease is revealed by the range of diseases previously associated with aging that are more prevalent among overweight and obese individuals. The traditional view of adipose tissues as biologically inert lipid storage depots has undergone a dramatic revision: it is now evident that adipose tissues are highly responsive endocrine organs that influence metabolic homeostasis and systemic inflammation (Kershaw and Flier, 2004). These signals are transmitted through adipose-derived signaling molecules including adipokines, lipokines, cytokines, and secreted enzymes (Sethi and Vidal-Puig, 2007). It has also become clear that adipose tissues are sensitive to aging and that the impact of age is depot specific (Cartwright et al., 2007). Fat distribution is altered with age and adipocytes from aged animals lose the ability to adequately store fat and respond appropriately to systemic signaling (Tchkonia et al., 2010). Similar defects are observed in adipocytes from obese individuals. CR induces an overt shift in adipose tissue mass, morphology, and function. Adipose depots are smaller with smaller adipocytes, transcripts of genes involved in metabolism are increased and transcripts of pro-inflammatory genes are reduced (Anderson and Weindruch, 2010). This CR-induced reprogramming of adipose tissue may be important in attenuating disease risk. In contrast to CR, aging and obesity are associated with enhanced disease incidence suggesting a potential role of adipose tissue dysfunction in creating disease vulnerability.
The discovery of an age-induced decline in microRNA processing specifically in adipose tissues adds a new piece to the puzzle. Mori et al. show that levels of the key microRNA regulatory protein dicer are reduced in adipose depots (subcutaneous and perigonadal) in young adult animals (six months of age) and a coordinated decline in levels of multiple microRNA species is observed. In CR animals, dicer levels do not decline and at eighteen months dicer is as abundant as very young control fed mice. Interestingly, the age-associated decline across all microRNAs is not identical in terms of timing and extent, hinting at the complexity of microRNA processing and maturation (Jinek and Doudna, 2009). CR preserves levels of microRNAs impacted by age, and in addition alters levels of a subset of microRNAs independent of age. This is a fascinating development that implicates microRNA processing in adipose tissue in the mechanisms of anti-aging by CR (Figure 1). The age associated decline in dicer, ancillary microRNA processing proteins, and microRNA species is also observed in isolated adipocytes from humans indicating that this is a highly conserved phenomenon that is directly relevant to human aging.
Figure 1. Aging and Stress converge on MicroRNAs.
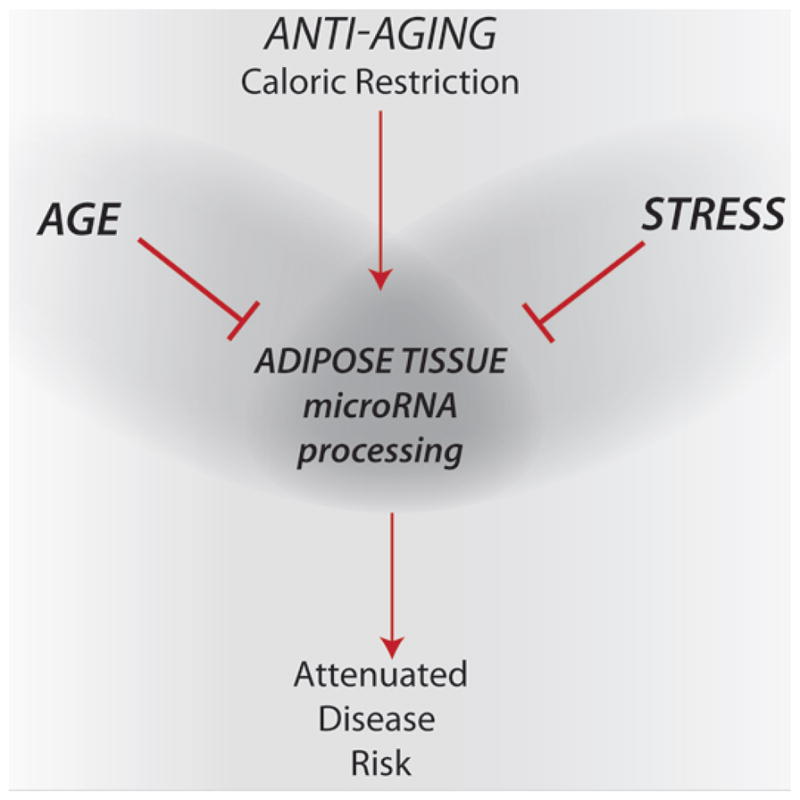
Adipose tissue microRNA processing links longevity and stress resistance and is implicated in the mechanisms of delayed aging by caloric restriction The age-associated decline in dicer and ancillary microRNA processing proteins is specific to adipose tissues. The same process is targeted by exogenous stressors and required for stress resistance indicating that preservation of microRNA processing is critical for survival and longevity. Caloric restriction regulates microRNA processing in adipose tissues, preserving integrity and possibly exerting an anti-aging program that attenuates disease risk.
It is widely accepted that there is a mechanistic link between longevity and stress resistance. Indeed stress survival screens have been successfully applied to identify genes that regulate lifespan in diverse species. As demonstrated in the Mori et al. study, oxidative and DNA damage stressors depress dicer expression in preadipocytes indicating that microRNA processing is targeted when cells are exposed to adverse conditions. Dicer knockout impairs the ability of preadipocytes to respond to stress and the adipose tissue-specific dicer knockout mouse is more vulnerable to oxidative stress. An in depth analysis identifies miR-125b, a microRNA that regulates the p53 pathway, as a major contributor to the stress phenotype of dicer knockout cells and shows that p53 that is also differentially regulated in dicer knockout mice. In a series of elegant genetic experiments in C. elegans, the role of dicer in longevity and stress survival is confirmed. Additional copies of dicer extend lifespan and promote survival in response to stress. Importantly, it is the microRNA processing function of dicer that is critical in conferring these phenotypes. A significant finding from these studies is that changes in microRNA processing in mouse adipose tissue or worm intestine (adipose tissue equivalent in worms) act in a cell non-autonomous manner. These studies confirm a role of microRNA processing in adipose tissues in the shared mechanisms of stress resistance and longevity (Figure 1).
MicroRNAs are regulators of gene expression, altering the ability of transcripts to generate new proteins. Their influence includes negative and positive feedback that can amplify responsiveness of the transcription apparatus or buffer the impact of multiple disparate regulatory inputs (Ebert and Sharp, 2012). Now that the importance of adipose tissue microRNA processing in aging has been established, a host of questions necessarily follow. Which transcripts and processes are regulated by aging and CR though changes in microRNA processing? The current study points to microRNA regulation of the p53 pathway as a key component. What other key microRNA species are involved? What is responsible for the cell non-autonomous aspect of adipose tissue function - adipokines, lipokines, secreted microRNA vesicles? Future directions aside, the findings of Mori et al study bring a new perspective to the biology of aging itself. Causative elements in the aging process necessarily occur in advance of the phenotypes of aging. The timing of dicer decline and its opposition by CR raise the intriguing possibility that the decline of microRNA processing in adipose tissues contributes causally to the aging process. This study opens exciting new avenues for exploration that will impact biology of aging research for years to come and there is nothing dicey about that.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21:134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol. 2007;42:463–471. doi: 10.1016/j.exger.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457:405–412. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Mori MA, Raghavan P, Thomou T, Boucher J, Robida--Stubbs S, Macotela Y, Russell SJ, Kirkland JL, Blackwell TK, Kahn CR. Role of microRNA Processing in Adipose Tissue in Stress Defense and Longevity. Cell Metabolism. 2012 doi: 10.1016/j.cmet.2012.07.017. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


