Abstract
Objectives
We have previously shown that stromal-derived-factor-1 α(SDF-1α) is down-regulated within diabetic cutaneous wounds, and that direct application of recombinant SDF-1α increases wound closure rates, neovascularization and endothelial progenitor cell(s)(EPC) recruitment. However, increased wound levels of exogenous SDF-1α results in elevated systemic levels of this pro-angiogenic chemokine that raises concerns for tumorgenesis and inflammation. We now seek to test the efficacy of a novel, safer cell-based therapy (CBT) employing ex-vivo primed bone marrow stem cells (BMDSC) with SDF-1 α. We also elucidate the mechanism of action of this new approach for accelerating diabetic wound healing.
Methods
Unfractionated BMDSC from diabetic Leprdb/db mice were incubated for 20h with SDF-1α(100ng/mL) or BSA(control). Pre-treated-BMDSC (1×106) were injected subcutaneously into full-thickness skin wounds in Leprdb/db mice (n=8/group). Wound closure rates, capillary density and recruitment of EPC were assessed with serial photography, DiI-perfusion, confocal microscopy and immunohistochemistry. Expression of molecular targets, which may mediate pro-healing/pro-angiogenic effects of SDF-1α-primed-BMDSC was evaluated by PCRArray and immunoblotting assay. The biological function of a potential mediator was tested in a mouse wound healing model. Serum SDF-1α levels were measured with ELISA.
Results
SDF-1α-primed-BMDSC significantly promote wound healing (p<.0001), neovascularization (p=.0028) and EPC recruitment(p=.0059). Gene/ protein expression studies demonstrate up-regulation of EphRB4 and Plasminogen as downstream targets potentially mediating the pro-healing and pro-angiogenic responses. Ex-vivo BMDSC activation and subsequent inoculation of cells into wounds does not increase systemic SDF-1α levels.
Conclusion
We report a novel CBT that is highly effective in promoting healing and neovascularization in a murine model of Type 2 Diabetes. Furthermore, we identify new molecular targets that may be important for advancing the field of wound healing.
Introduction
The past decades have heralded significant advancements in our understanding of stem cell biology and how it relates to tissue regeneration and repair. Of late, this vital knowledge has been at the forefront of intense investigation seeking to employ the high therapeutic potential of these pluripotent stem cells to create cell based therapies (CBTs) that can target, and ultimately restore tissue/organ integrity.1,2 Delayed diabetic cutaneous wound healing is, perhaps, one of the most prevalent pathophysiologic processes standing to benefit from such developing CBTs.3 Given that convincing evidence has demonstrated there to be a diabetes associated reduction4–9 and dysfunction4,10,11 of circulating bone marrow-derived stem cells (BMDSC), it stands to reason that modulation of diabetic BMDSC to enhance their circulation, wound levels and/or function can have significant clinical implications.
Numerous stem cell based therapies are currently being tested in the pre-clinical and clinical setting that are utilizing distinct subpopulations of BMDSC [i.e., mesenchymal stem cells (MSC) and endothelial progenitor cells (EPC)], in combination with various growth factors and signaling molecules, for numerous disease processes including diabetic wound healing.12–17 These immature BMDSC subpopulations have been shown to promote neovascularization and healing by direct cell-cell interactions, whereby they differentiate in-situ into a variety of cell lineages, including fibroblasts, myoblasts, adipocytes and mature endothelial cells1,18, and they have also been shown to secrete numerous cytokines, growth factors and extracellular matrix proteins (ECM) that support the healing process in a paracrine manner.3,18–21 Autologous transplantation of stem cell subpopulations, however, can present some limitations: 1) the small percentage of pluripotent MSC and EPC, respectively, that can be isolated, which would require the processing of large volumes of blood or bone marrow for adequate yield; and 2) the limitation of repeated cell passages and expansion, as these cells may undergo unfavorable changes in phenotype with time.22 In line with these findings, the inherent reduction and dysfunction of diabetic BMDSC would only serve to intensify these limitations, thus making their clinical utilization more complicated. Nevertheless, BMDSC can be activated, or “primed”, with a variety of chemokines, cytokines and growth factors before their use to enhance their functional capabilities.23–25
Specifically, the pro-angiogenic chemokine, stromal-derived factor 1-α (SDF-1α), which becomes highly expressed by numerous cell types during hypoxia (i.e., after infarct, wounding), activates BMDSC by binding to the cell surface receptor CXCR4.26 Accordingly, SDF-1α has been studied extensively in the context of BMDSC recruitment and neovascularization for a variety of reparative and pathological disease processes27–29, which in addition to wound healing30–33, include tumorigenesis and mestastasis34,35, myocardial infarction36 and restoration of blood flow in critical limb ischemia.37,38 Thus, it is well established that SDF-1α plays a crucial role in BMDSC activation, mobilization and recruitment.
Our previous work has found that the local concentration of SDF-1α is markedly reduced in the diabetic wound. Moreover, we have demonstrated that by raising local tissue levels of SDF-1α with either exogenous administration of recombinant SDF-1α, or with virally transduced SDF-1α expressing bone marrow derived fibroblasts, significant improvement in wound closure rate, neovascularization, EPC engraftment and collagen deposition is achieved.30,31 As previously stated, however, elevated local tissue levels and/or increased systemic levels of SDF-1α have been implicated in tumorigenesis and metastasis.34,35 Indeed, mounting evidence continues to show that elevated systemic levels of SDF-1α, or increased expression of its receptor CXCR4, are strongly correlated with relapse, metastasis and a worse prognosis in numerous neoplastic processes, including breast, gastric, prostate and colon cancer.39–45 In addition to cancer, elevated systemic or local expression of SDF-1α has also been linked with several inflammatory disease processes, which include inflammatory liver disease46, lupus induced nephritis47,48, rheumatoid arthritis and osteoarthritis.49,50 Thus, such highly effective and promising therapeutic approaches that function by directly, or indirectly, increasing the local expression/concentration of SDF-1α bring about challenges that may limit their clinical application.
To develop an effective and safe protocol for the treatment of diabetic wound healing, we explore the efficacy of a novel CBT utilizing SDF-1α-activated autologous bone marrow to promote diabetic wound healing. We sought to judiciously exploit the pro-angiogenic capabilities of SDF-1α, via ex vivo incubation, to mitigate any potential systemic effects. We postulated that in vitro incubation of unfractionated autologous BMDSC would activate these cells, enhancing their pro-angiogenic and pro-healing capacity even after the removal of SDF-1α from the incubation media. Thus, we hypothesized that diabetic wounds treated with ex vivo SDF-1α primed BMDSC would have a more robust healing response in line with our previous findings, and those of other investigators.12,30,31,51 That is, SDF-1α activated BMDSC would promote better healing and increased neovascularity (i.e., vessel density, EPC engraftment) compared to control diabetic wounds treated with non-activated BMDSC. We then tested if ex vivo priming with SDF-1α increases endothelial cell (EC)-EPC interaction. Lastly, we explored the potential paracrine effect of plasminogen (Plg) that may partially account for the accelerated wound healing observed in wounds treated with SDF-1α primed BMDSC.
Methods
Mice
All animals were cared for in accordance with the guidelines set forth by the University of Miami Institutional Animal Care and Use Committee. Genetically diabetic male BKS.Cg--+ Leprdb/+ Leprdb/OlaHsd (Leprdb/db) mice were obtained from Harlan Laboratories (Indianapolis, IN). All mice were used experimentally between 12–13 weeks of age. A total of 6–8 mice per treatment group were used. Prior to all procedures, serum glucose was measured from the tail vein using a glucometer. Mean serum glucose levels in all diabetic mice were 391 mg/dl with a range of 302 to 503 mg/dl. Animals had free access to food and water before and after the operation.
Bone Marrow Cell Harvest and ex vivo Treatment
24 hours prior to wounding experiments, diabetic donor mice (n=4) were sacrificed for bone marrow (BM) cell harvest and fresh isolation of unfractionated BMDSC. The femurs and tibiae were carefully cleaned from surrounding soft tissue, and the distal epiphysis of each bone was removed using a sharp surgical micro scissor. The BM was harvested by inserting a syringe needle (27-gauge) into one end of the bone and flushing with Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco). Mononuclear cells were counted, divided equally into two groups (1. control (BSA) group; 2. SDF-1α treatment group) and plated on 10 mm cell culture dishes at a density of 1 × 107/ml cells per dish in DMEM containing 10% Bovine Serum Albumin (BSA; Sigma-Aldrich), 100 u/ml penicillin (Sigma-Aldrich) and 100 u/ml streptomycin (Sigma-Aldrich). Control cells were treated with phosphate buffered saline (PBS) + BSA (100ng/ml); SDF-1α primed cells were treated with murine SDF-1α (mSDF-1α, 100ng/ml). Cultures were maintained at 37°C in a humidified atmosphere containing 95% air and 5% CO2 for a period of 20 hours. Following the 20 hour treatment period, cells were harvested, washed with 10 ml PBS × 3 and re-suspended at a concentration of 1 × 107/ml in PBS for the in vivo wounding arm of the experiment.
Surgical Procedures for Wounding and in vivo BMDSC Wound Inoculation
For wounding experiments, diabetic mice (n=8/group) were anesthetized with an intraperitoneal injection of 80 mg/kg of ketamine and 20 mg/kg xylazine. Next, the dorsal surface was depilated. Under sterile conditions, one full-thickness excisional wound was created using an 8mm punch biopsy on the mid back of each mouse, approximately 1 cm to the right of the mid-line exposing the pannus carnosus. The designated control wounds (n=8) and SDF-1α wounds (n=8) were then inoculated intradermally with control (BSA) treated cells or SDF-1α primed cells (1 × 106 BMDSC cells/ wound; 100 µl cell suspension/wound), respectively. In a subsequent wounding experiment, full thickness wounds were created in a similar fashion. Mice were again separated equally into 2 groups (n=6): 1) SDF-1α primed cells (1 × 106 BMDSC cells/ wound) only, and 2) SDF-1α primed cells plus PAI-1 (1 × 106 BMDSC cells/ wound) plus 10 µg/ml (effective dose of 1 µg/100 µl per wound) of plasminogen activator inhibitor-1 (PAI-1). PAI-1 (USBiological, Swampscott, MA) was administered intradermally to dorsal wounds with SDF-1α primed BMDSC on POD 0 and thereafter through POD 5 (total dose 1 µg in 100 µl per wound per day).
Assessment of Wound Closure
Wounds were observed for 21 days, or until full closure, whichever came first. Digital photographs of the wounds were taken with a Canon digital camera at days 0, 3, 7, 10, 12, 16 and 20. Time to wound closure was defined as the time at which the wound bed was completely re-epithelialized. Standard calibration of all wound surface area measurements was facilitated by the inclusion of a ruler in the photographs. Wound area was quantified using ImageJ software (Imaging Processing and Analysis in Java, National Institutes of Health, MD), and the wound closure or recovery rate was expressed as a percentage of the initial wound using the following equation: [(original wound area minus daily wound area) / (original wound area)] × 100.
Wound Blood Vessel Perfusion and Laser Scanning Confocal Microscopy
Direct in vivo labeling of wound vasculature was achieved using a specifically formulated aqueous solution (7 mL/mouse) containing DiI (Sigma-Aldrich), as previously reported.52 Briefly, anesthetized mice were perfused with DiI solution via intracardiac injection bringing the DiI solution in direct contact with the endothelium. Next, 10ml of fixative (4% paraformaldehyde) was injected following DiI perfusion, and the skin around the wound was excised for confocal microscopy to analyze wound vessel density. The vascular network was visualized by scanning the entire wound tissue to a depth of 150–300 µm using laser scanning confocal microscopy (Leica MP/SP5/FCS/FLIM spectral multiphoton/confocal upright microscope, Leica Microsystems). Vessel density was quantified assessing total number of red DiI-labeled vessels normalized to the entire scanned wound area with ImageJ software.
Immunohistochemistry, Immunostaining and Immunoblotting
Immunostaining and immunohistochemistry (IHC), was performed using 5-µm paraffin sections from the wounds of the perfused mice (vessels labeled with DiI) for examination of EPC recruitment and capillary density. Paraffin-embedded sections first underwent standard de-paraffinization and rehydration procedures, and they were then probed with fluorescein isothiocyanate-anti-CD34 (Cell Signaling Technology, Danvers, MA) for overnight at 4°C. Next, sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA). The nuclei were then counterstained with 4',6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI). Negative controls for all antibodies were made by replacing the primary antibodies with non-immunogenic, isotype matched antibodies from the same manufacturer. Tissue sections were analyzed using fluorescence microscopy and ImageJ software to quantify fluorescent intensity. Hematoxylin and Eosin (H&E) staining was performed according to standard methods for assessment of epitheliazation.
Immunoblotting was performed as previously described.30 Briefly, unfractionated BMDSC were lysed, and protein concentrations were determined by DC protein assay (Bio-Rad). Equal amounts of protein were subjected to 4%–12% SDS gel electrophoresis under reducing conditions and transferred to PVDF membranes. The transferred PVDF membranes were subsequently probed with antibodies to Plasminogen (Abcam, Cambridge, MA), Eph Receptor B4 (Santa Cruz Biotechnology Inc., Santa Cruz, CA) or β-actin (AC-15, Abcam, Cambridge, MA). Membranes were then probed using HRP-conjugated secondary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and subjected to enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ). Membranes were stripped and re-blotted as required for each experiment.
Polymerase Chain Reaction (PCR) Array
Two quantitative PCR arrays were performed: 1. The Mouse Wound Healing RT2 Profiler polymerase chain reaction (PCR) Array, which quantitatively profiles the expression of 84 genes central to the wound healing process (Qiagen, Valencia, CA). For this array, unfractionated BMDSC were stimulated with recombinant mSDF-1α protein (100 ng/mL) versus BSA (100 ng/mL) for 20 hours; 2) The human adhesion molecules and extracellular matrix (ECM) RT2 Profiler PCR Array, which quantitatively profiles the expression of 84 genes of adhesion molecules and ECM (PA-011, SABiosciences, Frederick, MD) was performed as previously described31. All cells were harvested and total RNA was extracted using Trizol (Invitrogen). Synthesis of cDNA was then performed using RT2 First Strand Kits (Qiagen, Valencia, CA). PCR array was carried out in accordance with the manufacturer’s protocol. The threshold cycle values were used to plot a standard curve. All samples were normalized to the relative levels of β-actin, and the results were expressed as fold-increase. The threshold for increased gene expression was designated as ≥ 1 fold increase in mRNA for all experiments.
MTT Proliferation Assay
MTT Cell Proliferation Assay (Roche Applied Science, Indianapolis, IN) is an in vitro assay for the measurement of cell proliferation. MTT assay was performed in accordance with the manufacturer’s protocol on harvested unfractionated Lepr db/db BMDSC 20 hours after ex vivo incubation with BSA (100 ng/ml) or SDF-1α (100 ng/ml), respectively, to determine any alterations/changes in cell proliferation between control (BSA) and SDF-1α primed BMDSC.
Apoptosis Assay
Difference in cell apoptosis between BMDSC treated with BSA (100 ng/ml) versus SDF-1α (100 ng/ml) was determined using flow cytometry. After 20 hour incubation with either BSA or SDF-1α, apoptosis was determined using a fluorescein isothiocyanate (FITC)-conjugated annexin-V propidium iodide (annexin-V/PI) kit (Abcam Inc., Cambridge, MA) according to the manufacturer’s instructions.
Enzyme-Linked Immunosorbent Assay
Mouse serum SDF-1α concentration analysis was performed 24 hours post wounding procedure using Quantikine SDF-1α ELISA kit (DY460, R&D Systems), which specifically detects murine SDF-1α, based on the manufacturer’s protocol.
Data Processing and Statistics
In vivo histology data was analyzed using Image J software. Histological images were taken from multiple sections (at least 8 sections per sample) and the wound edges were determined using the position of the first hair follicle. P values were calculated for the indicated groups by using two tailed students t-test.
Results
Ex vivo SDF-1α Treatment of Diabetic Bone Marrow Cells Enhances Proliferation Rate, but Does Not Alter Cell Viability
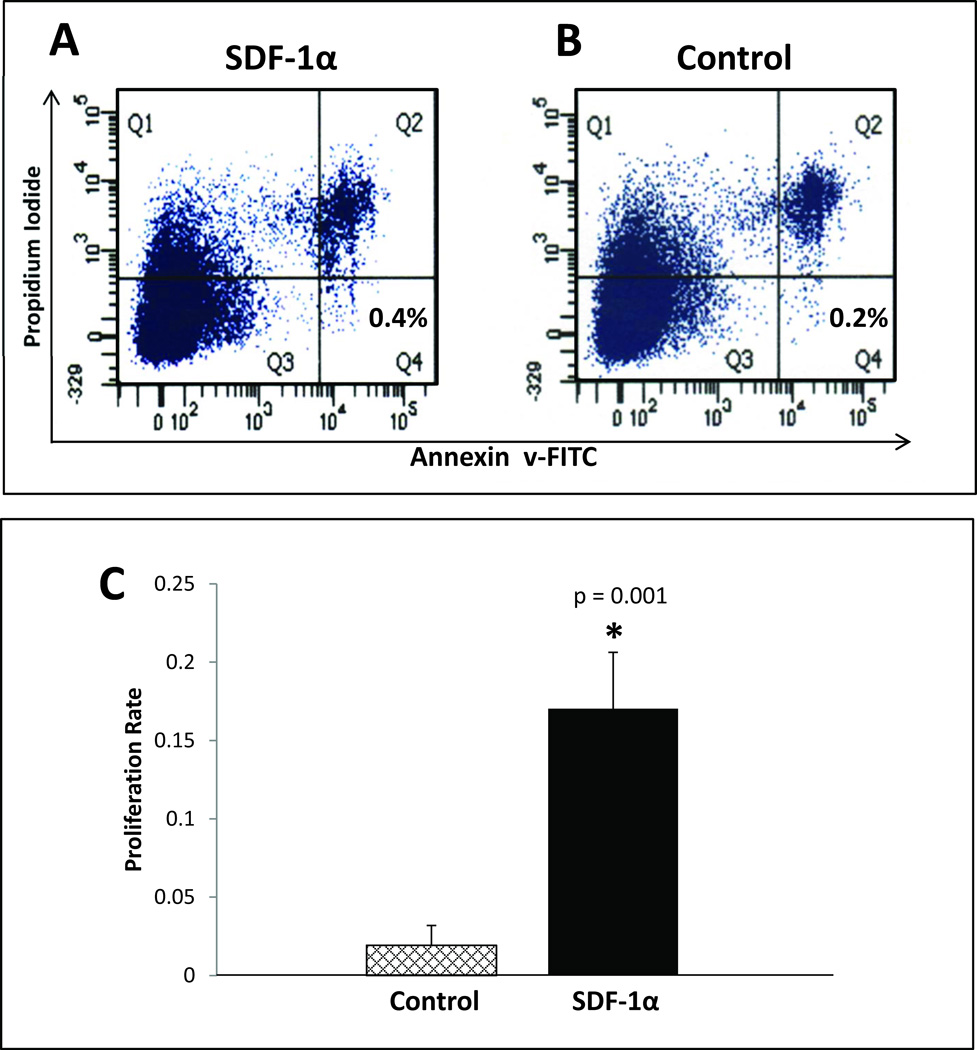
We have previously shown that direct inoculation of diabetic wounds with recombinant SDF-1α causes a transient increase in systemic levels of SDF-1α.30 We attempted to limit any potential systemic exposure to SDF-1α by treating the selected cell population ex vivo prior to diabetic wound treatment. Unfractionated, freshly isolated BMDSC harvested from Lepr db/db mice were treated with BSA versus SDF-1α (100 ng/mL) for 20 hours. The effects of SDF-1α on cell apoptosis and proliferation were examined using flow cytometry and MTT, respectively. Cells in the SDF-1α treatment group and the control (BSA) treatment group were equally viable after 20-hour treatment period (Figure 1A and 1B). In comparison, the proliferation rate of SDF-1α activated cells was enhanced 4 fold (p<.001) when compared to control cells (Figure 1C). Using an in vitro adhesion assay, we observed that ex vivo priming with SDF-1α significantly increases the direct interaction of mature endothelial cells (EC) with the EPC subpopulation of the primed BMDSC (EC-EPC adhesion data not shown).
FIGURE 1. Ex vivo treatment of BMDSC with SDF-1α enhances proliferation, but it does not provide cell survival advantage compared to control (Bovine Serum Albumin) treated BMDSC.
BMDSC were harvested from donor Leprdb/db and subjected to ex vivo treatment with either SDF-1α (100 ng/ml) or control (BSA, 100 ng/mL) in identical fashion performed for in vivo wounding experiments. After the 20 hour incubation period, flow cytometry quantification of apoptotic cells using FITC-conjugated annexin-V/PI was performed and expressed as a percentage of total cells (bottom right panel) for A: SDF-1α treated cells and B: Control treated cells. C: MTT proliferation assay comparing SDF-1α treated cells versus control treated cells.
Treatment of Diabetic Wounds with SDF-1α primed BMDSC Does Not Effect Systemic Levels of SDF-1α
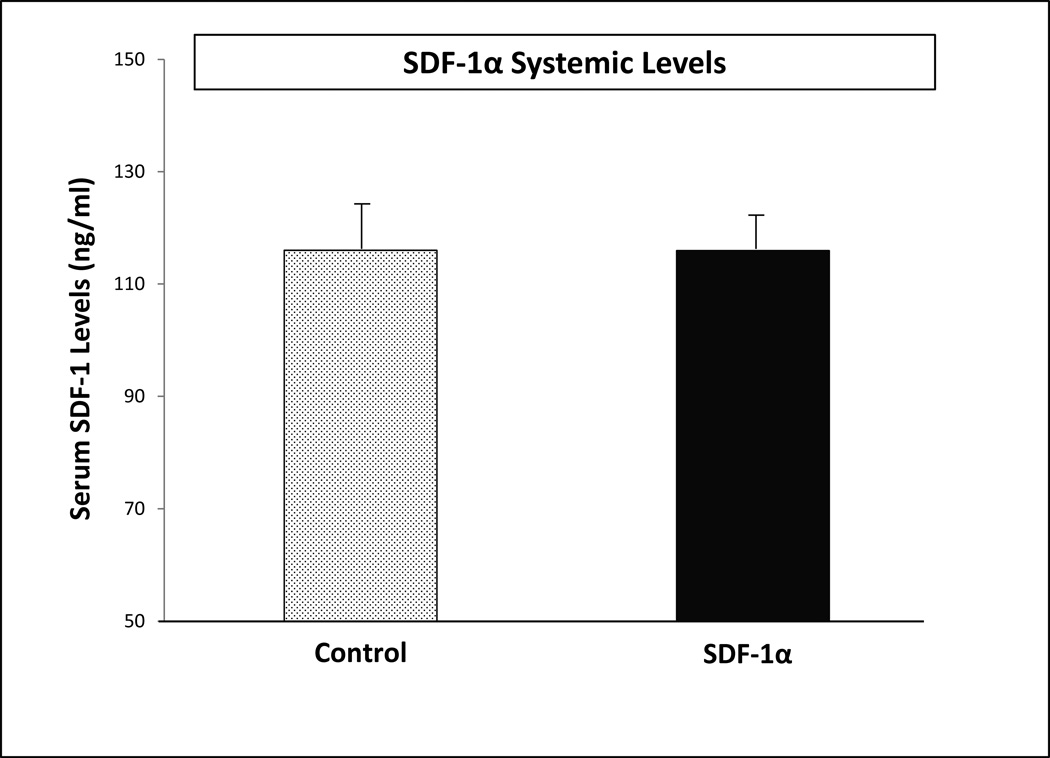
Unfractionated BMDSC isolated fresh and incubated ex vivo with SDF-1α versus BSA for 20-hours were injected into diabetic wounds created on the dorsal skin of Lepr db/db mice. To determine whether SDF-1α treated BMDSC exhibit any effect on endogenous SDF-1α levels after wound inoculation, serum was collected from mice in each treatment group 24 hours post-wounding plus cell inoculation procedure. Systemic SDF-1α levels were analyzed using ELISA. There was no significant difference in systemic levels of SDF-1α between the control group and the SDF-1α treatment group 24 hours post-inoculation into wounds (Figure 2). Thus, the CBT utilized for these experiments was successful in minimizing systemic exposure to recombinant SDF-1α.
FIGURE 2. Ex vivo activation of BMDSC with SDF-1α does not increase systemic SDF-1α levels.
Serum samples were collected 24 hours after cell inoculation into wounds and subjected to ELISA specific to murine SDF-1α.
Direct Inoculation of Diabetic Wounds with SDF-1α Primed BMDSC is an Effective Cell Based Therapy Promoting Diabetic Wound Healing and Neovascularization
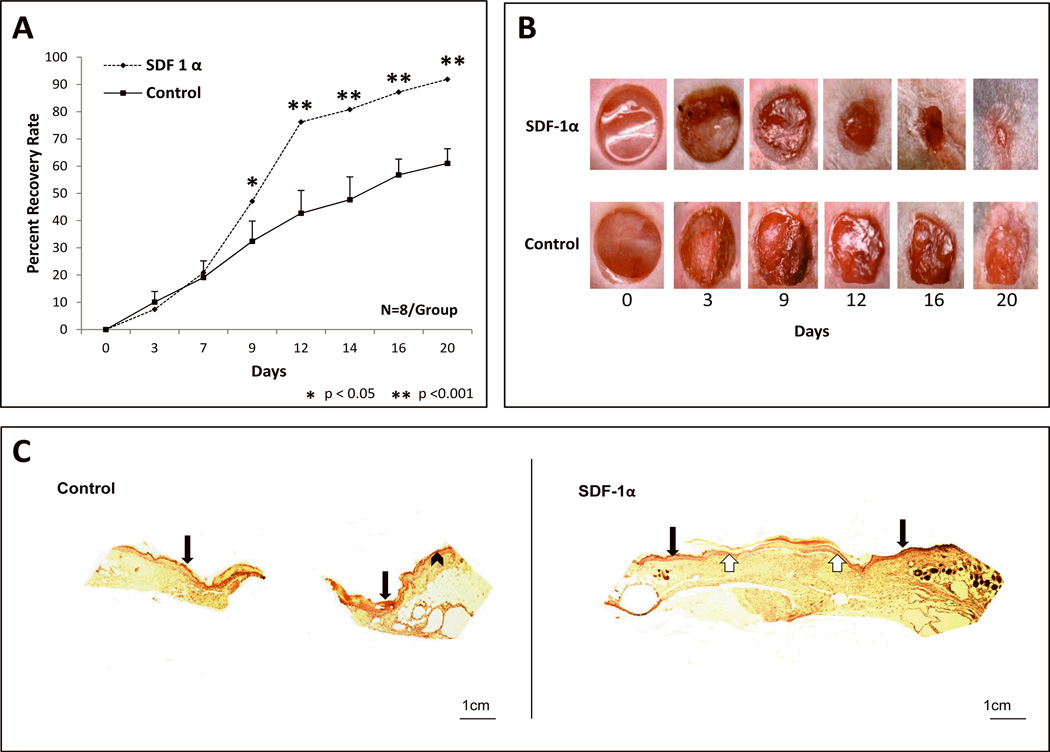
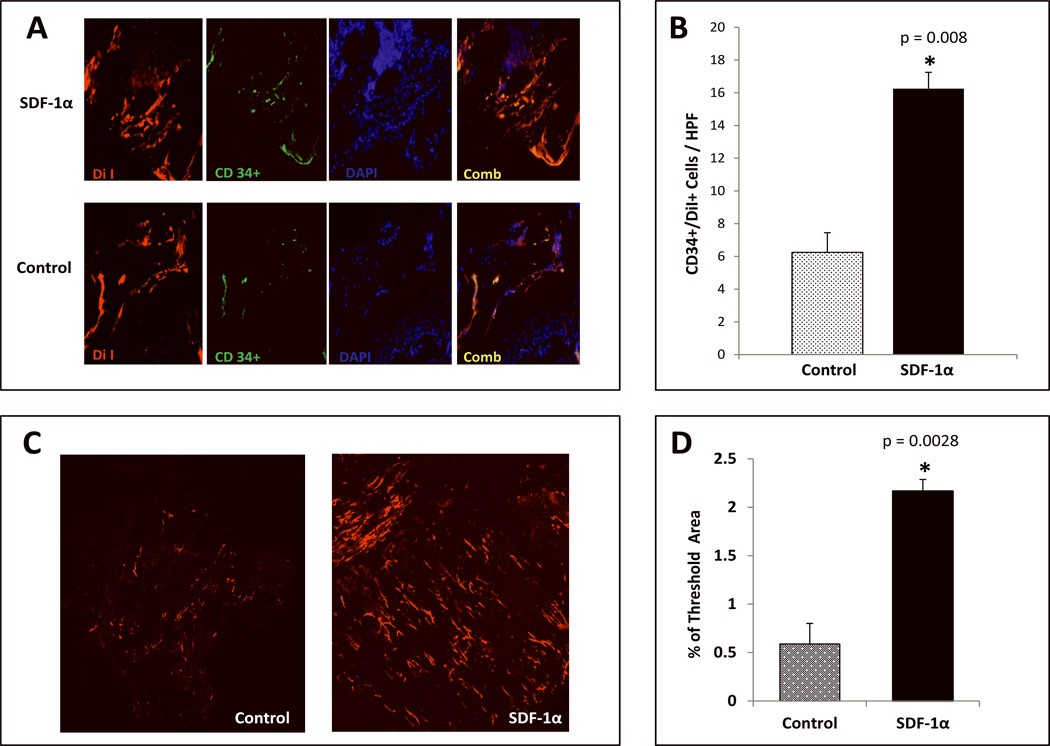
Our previous work has established the significant pro-healing effects of SDF-1α in the diabetic wound with respect to EPC mobilization and recruitment/adhesion to the wound bed.30 The purpose of the present study was to test the efficacy of a novel cell based therapy using autologous, unfractionated BMDSC activated ex vivo with SDF-1α in a genetic murine model of Type 2 Diabetes Mellitus. Diabetic cutaneous wounds treated with ex vivo SDF-1α activated BMDSC had a significantly faster healing rate when compared to wounds treated with control BMDSC (Fig. 3A). The most significant difference was observed during days 7–12 (1.8 fold difference in wound closure observed starting on day 9). Wounds were harvested and subjected to Hematoxylin & Eosin (H & E) to assess for epitheliazation. H & E staining showed incompletely healed wounds for the control group, which were inoculated with BSA-stimulated BMDSC cells compared to completely healed wounds that were inoculated with SDF-1α-stimulated BMDSC cells (Figure 3C). Wounds were harvested and subjected to IHC analysis after DiI in vivo perfusion to assess for the recruitment of EPC-like cells (CD34+/DiI+ cells) within the vascular network. EPC engraftment (Figure 4A–B) was significantly greater in wounds treated with SDF-1α activated BMDSC cells when compared to control wounds treated BSA BMDSC. In addition, wound vessel density was significantly greater (4 fold increase) in the diabetic wounds treated with SDF-1α activated BMDSC, as assessed by DiI in vivo perfusion and confocal microscopy at the time point examined (day 20) (Figure 4C–D). These data provide evidence corroborating the pro-healing and pro-angiogenic influence of BMDSC activated ex vivo with SDF-1α in a murine model of Type 2 Diabetes Mellitus.
FIGURE 3. SDF-1α activation of BMDSC promotes diabetic wound healing and re-epitheliazation.
A: Wound healing rate expressed as percent recovery. Two groups of Type 2 diabetic Leprdb/db mice were wounded and treated with SDF-1α Primed diabetic BMDSC versus control (BSA) diabetic BMDSC. Wounds were followed and analyzed using digital photography and Image J software. Diabetic wounds treated with SDF-1α activated cells had significantly faster wound closure rates starting from POD 9 when compared to diabetic wounds treated with control BMDSC. B: Representative images of wounds at different time points are shown for both groups. C: Representative hematoxylin/eosin-stained paraffin sections from 21-day wounds treated with control BMDSC versus SDF1-α activated BMDSC (5 × magnification). Black arrows indicate total wound area and point to the epithelial layer of normal skin immediately lateral to the wound edge. White arrows depict the area of complete re-epitheliazation in wound treated with SDF-1α Primed BMDSC.
FIGURE 4. SDF-1α activation significantly increases vessel density and promotes engraftment of BM derived EPC-like cells into diabetic wounds.
A: Presence of EPC-like cells within diabetic wound vessels determined by co-expression (yellow) of DiI dye (red) and CD34 (green) as detected by immunostaining. B: Quantification of EPC-like cells expressed as mean number of cells ±SD per high power field (20×): minimum of 8 sections were assessed per group. C: Wound blood vessel perfusion as detected by DiI dye. Red networks are representative of DiI-stained blood vessels within the diabetic wounds. Images shown were acquired using laser scanning confocal microscopy at day 21 post wounding. D: Quantification of vessel density in the wounds expressed by % threshold area, which covers all vessels detected as a percent of the entire wound area. Wounds treated with SDF-1α Primed BMDSC had a significantly higher vessel density compared to BSA control BMDSC. Data are presented as mean ±SD of 8 wounds in each group
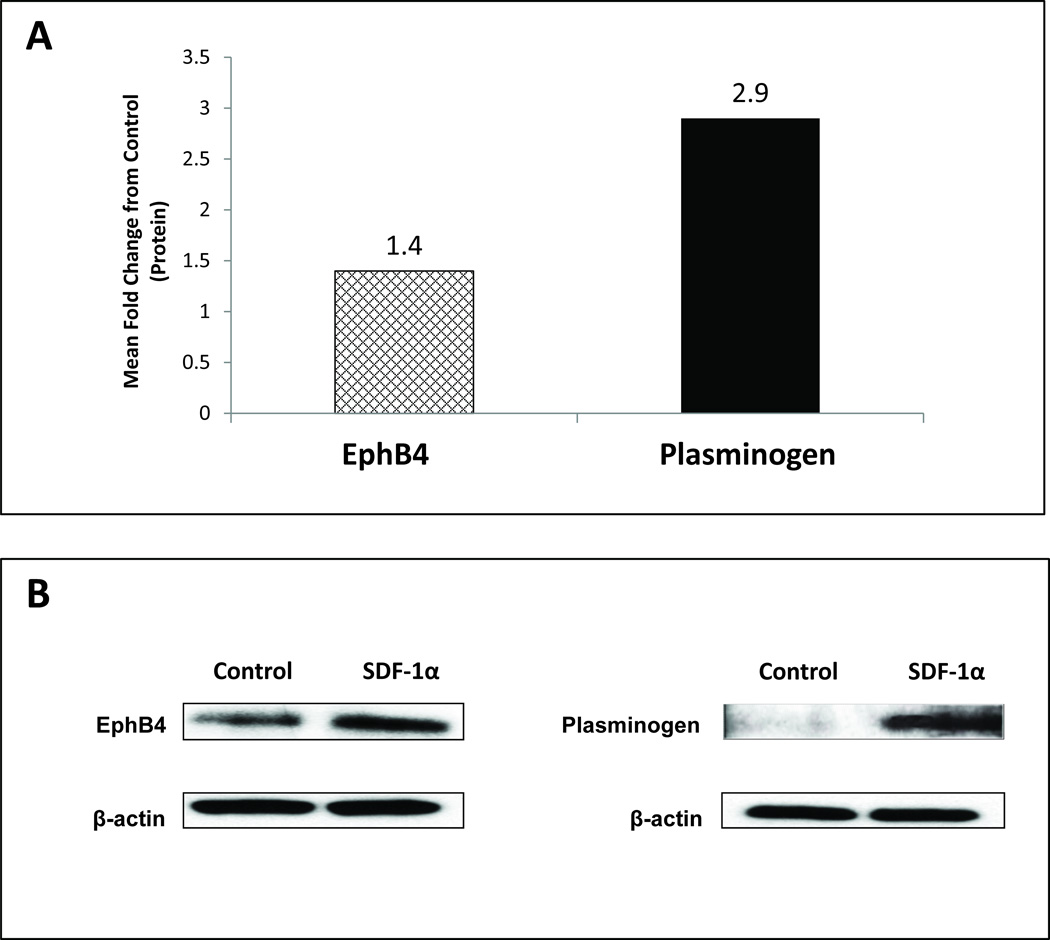
SDF-1α Up-regulates Expression of Plasminogen (and numerous adhesion molecule changes) in Diabetic Unfractionated BMDSC
To study potential downstream mechanisms whereby SDF-1α ex vivo priming potentiates the wound healing response of BMDSC, we performed RT2 PCR to analyze specific genes, up-regulated by SDF-1α, that are important to the wound healing response. In total, 84 genes involved in the 3 critical phases of wound healing were assessed. Of the 84 genes analyzed, there was up-regulation of 4 genes (>1.2 fold; Table I). We focused on two genes consistently up-regulated with SDF-1α stimulation in diabetic BMDSC, which are involved in the remodeling/angiogenesis phase of wound healing (Figure 5A): Plasminogen (Plg) and Epherin receptor B4 (EphB4). Further validation of the observed gene expression studies with western blot analysis confirmed a corresponding increased protein expression of Plg (2.9 fold increase) and EphB4 (1.4 fold increase) (Figure 5B). These experiments identified Plg as one potential clinically relevant target that may contribute to the pro-healing effects of SDF-1α activated BMDSC and we performed additional in vivo studies on this target. Gene expression studies also demonstrated numerous adhesion molecules strongly expressed in BMDSC with SDF-1α priming, including integrin α4 and integrin αV (data not shown).
Table I.
Up-regulated Genes in unfractionated BMDSC after ex vivo SDF-1α (Stromal Derived Factor-1α) Stimulation for 20 h.
| Fold Change in mRNA | |
|---|---|
| EphReceptorB4 | 1.4 |
| Plasminogen | 1.27 |
| Tissue Inhibitor of Metalloproteinase1 | 1.02 |
| Transmembrane Serine Protease 6 | 1.13 |
FIGURE 5. SDF-1α enhances protein expression of Plasminogen and Epherin Receptor B4 (EphB4) in diabetic unfractionated BMDSC.
Unfractionated BMDSC from diabetic mice were stimulated with SDF-1α versus BSA (control) for 20 hours and subsequently harvested for protein expression analysis of Plasminogen and EphB4 using Western blotting assay. β-actin was used as a loading control. A: Protein expression for Plasminogen and EphB4 expressed as mean fold change compared to control cells, respectively. B: Representative Western blot for both groups.
Plasmin Activator Inhibitor-1 Partially Abrogates the Pro-healing Effects of SDF-1α Primed BMDSC
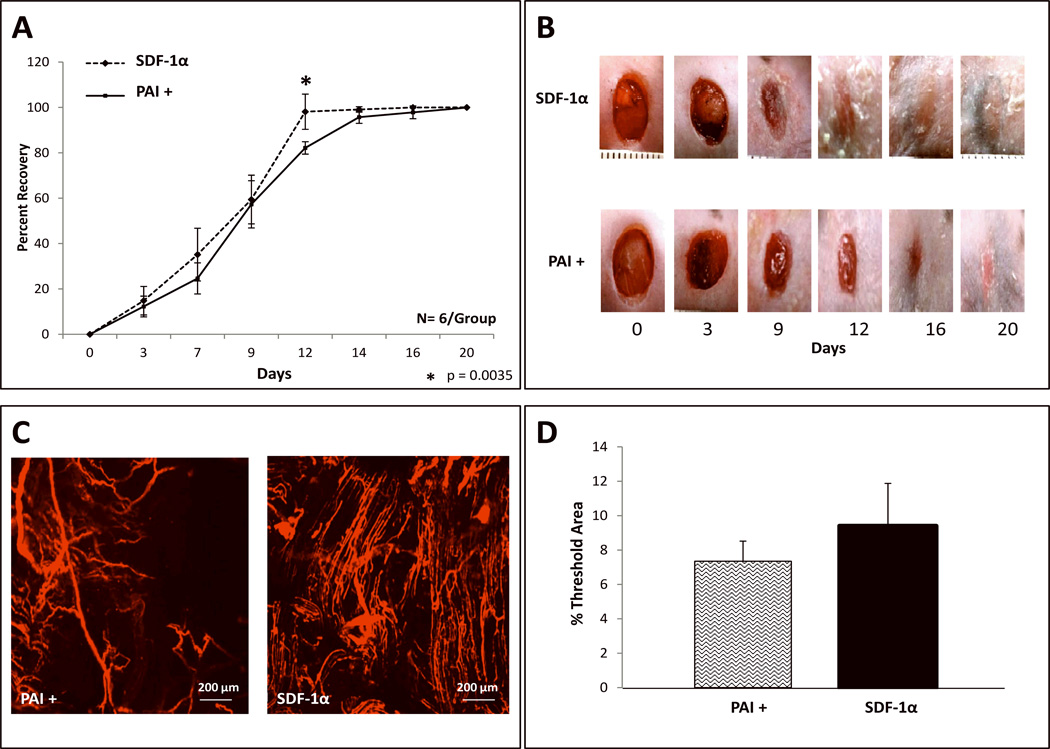
To investigate whether up-regulated levels of Plg in SDF-1α activated BMDSC is contributing to the enhanced wound healing rates and/or neovascularization observed in diabetic cutaneous wounds, we performed additional in vivo wounding experiments using the specific plasminogen/plasmin (Plg/Pm) antagonist, plasminogen activator inhibitor-1 (PAI-1). PAI-1 is the key regulator of Plg to plasmin (Pm) conversion; Pm is a fibrinolytic enzyme implicated in angiogenesis and tissue remodeling. Wounds concurrently treated with PAI-1 and SDF-1α activated BMDSC had significantly slower healing rates than those only treated with SDF-1α activated BMDSC at one time point, POD 12 (Figure 6A; p=.0035). However, similar percent healing rates were observed for all other time points between the 2 groups. Capillary density, as determined by DiI, showed a trend towards reduction after concurrent treatment with PAI-1 and SDF-1α activated BMDSC versus only SDF-1α activated BMDSC, but this trend did not reach a statistically significant difference at the time point examined, POD 20 (Figure 6C and Figure 6D; p=.092). EPC numbers were equivalent in both groups in correspondence with the similar vessel densities observed between groups (data not shown). These findings suggested that Plg inhibition with PAI-1 did not have a pronounced effect on the wound healing and pro-angiogenic effect of SDF-1α-Primed-BMDSC.
FIGURE 6. Inhibition of the Plasminogen/Pm axis by PAI-1 mildly reverses the wound healing and wound vessel density effects of SDF 1α-Primed-BMDSC.
A: Wound healing rate expressed as percent recovery. Two groups of Type 2 diabetic Leprdb/db mice were wounded and treated with [ex vivo SDF-1α activated diabetic BMDSC (labeled as SDF 1α)] versus [PAI-1 plus ex vivo SDF-1α activated diabetic BMDSC (labeled as PAI+)]. Wounds were followed and analyzed using digital photography and Image J software. Diabetic wounds in the PAI+ group had mildly prolonged wound closure rate compared to wounds treated only with SDF-1α activated BMDSC. A statistically significant delay in wound closure was noted between POD 9 through POD 12. B: Representative images of wounds at different time points are shown for both groups. C: Wound blood vessel perfusion as detected by DiI dye; images acquired using laser scanning confocal microscopy show DiI-stained blood vessels within the diabetic wounds, at day 21 post wounding. D: Quantification of vessel density in the wounds expressed as % threshold area, which covers all vessels detected as a percent of the entire wound area. SDF-1α treated wounds showed a trend towards less vessel density compared to PAI+ treated wounds, but the means did not reach statistical significance. Data are presented as mean ±SD of 6 wounds in each group.
Discussion
In our present work we have demonstrated an innovative and efficacious CBT that safely harnesses the vital pro-angiogenic/pro-healing characteristics of SDF-1α essential for diabetic cutaneous wound healing. We show that diabetic wounds inoculated with autologous SDF-1α primed BMDSC have markedly improved wound closure rates, wound neovascularization, epitheliazation and significantly increased numbers of EPC compared to control diabetic wounds treated with control BMDSC. Notably, the donor mice used to obtain the BMDSC were genetically diabetic and hyperglycemic similar to the recipient mice. The experimental conditions were selected to maximize relevance to a potential autologous CBT clinical protocol. Furthermore, the present study investigated the possible role of the Plg/Pm cascade in SDF-1α mediated diabetic wound neovascularization and wound healing.
In this most current investigation, a genetic murine model of Type 2 diabetes (Leprdb/db) was used, and, like diabetic patients, Leprdb/db mice display impairments in wound healing, wound granulation tissue formation and angiogenesis.51,53,54 We found a nearly 2 fold increase in wound closure rate between the SDF-1α/BMDSC treated wounds and the control treated wounds starting on post- operative day (POD) 9, and wound closure rate remained significantly greater from POD 9 until the end of the experiment (POD 20). What is more, a significantly higher percent of SDF-1α treated wounds were completely healed at POD 20 compared to control wounds (93% vs. 61%; p<.001). Such a marked difference in healing rates was not expected. That is, since both wounds were being treated with unfractionated BMDSC, a less profound difference had been anticipated. This may point to the inherent decreased repair functions of diabetic BMDSC.
To assess whether the differences in wound closure rates observed could be accounted for by a disparity in cell viability with ex vivo cell manipulation, viability (apoptosis) and proliferation assays were performed to determine a potential SDF-1α mediated cell survival advantage. SDF-1α treatment did not elicit a specific cell survival advantage; however, the proliferation rate of SDF-1α treated BMDSC was approximately 4 fold greater compared to control treated cells. Thus, SDF-1α activated cells and control cells were equally viable at inoculation (after ex vivo priming), but the proliferation rate of cells primed with SDF-1α was markedly enhanced. The utilization of unfractionated BMDSC precluded us from determining which BM cell subtype(s) were induced to proliferate with SDF-1α treatment. Nevertheless, these findings lead us to speculate that SDF-1α-induced diabetic BMDSC proliferation enables a more immediate and more effective participation of these cells in the wound healing process (i.e., wound re-epitheliazation and neovascularization).
Impaired wound healing in diabetes may be a consequence of several factors, including deficient neovascularization.55–57 While problem diabetic wounds result, in part, from impaired neovascularization, the complexity of this process is still being defined. Our previous work has established a strong correlation between the diabetic phenotype and the significant down regulation of SDF-1α in diabetic cutaneous wounds resulting in deficient EPC recruitment, impaired neovascularization and delayed healing.30 Here, our novel cell based therapy demonstrates that wounds inoculated with diabetic-SDF-1α-Primed-BMDSC have significantly faster healing rates and enhanced neovascularization compared to wounds treated with diabetic-control-BMDSC.
Numerous studies demonstrate that BMDSC contribute to the repair and regeneration of various tissue types, including blood vessels27,58, myocardium 36, bone59, cartilage60 and skin.61 With respect to the skin in particular, these stem cells have been found in the healing wound directly contributing to different cell types, such as keratinocytes, epithelial cells and endothelial cells.61–64 In addition to their differentiation potential, BMDSC have also been shown to elaborate specific cytokines integral to skin repair and neovascularization.63,65 Thus, these cells have the capability of directly and/or indirectly (paracrine effect) participating in the healing response. Our findings strongly support the direct participation of BMDSC in wound healing, and indicate that SDF-1α-mediated cell activation is critically involved in contributing to diabetic wound neovascularization and healing, for we observed increased numbers of EPC-like cells incorporated into the neovascular network of wounds treated with SDF-1α-Primed-BMDSC.
EPC are a heterogeneous and unstable population of cells. To date, there is no standard set of surface markers that definitively identify the EPC. Markers used to identify these cells vary widely depending on the species under study (e.g., AC133 only pertains to human EPC whereas SCA-1 is a more reliable antigen for murine EPC). Currently, there is no consensus on the best combination of cell surface markers to describe the EPC. Instead, a combination of co-expressed markers is used to describe them. In many studies, one earlier marker (AC133, CD34, or Sca-1) plus one endothelial cell specific marker, such as VEGFR2/KDR, CD31, are widely used to define EPC. CD34, in particular, is selectively expressed within the human and murine hematopoietic systems on stem and progenitor cells,66–68 and it has been widely used to define EPC in a variety of studies.69–73 In our investigation, we used a combination of CD34 (an early marker mainly expressed on hematopoietic stem cells and endothelial progenitor cells) and uptake of fluorescent DiI labeled acetylatated-low density lipoprotein, which is known to be a specific characteristic of mature endothelial cells, to define CD34+/DiI+ cells as EPC-like cells that present within the wound neovascular network.
Indeed, our most current findings are congruent to our previous work demonstrating the critical role of SDF-1α mediated expression of endothelial cell (EC) expressed E-selectin, an adhesion molecule promoting EC-EPC interaction, in diabetic cutaneous wounds.31 Thus, we explored the SDF-1α mediated up-regulation of other adhesion molecules expressed within the EPC subpopulation that could shed new light on our findings. Gene expression studies outlined numerous EPC adhesion molecules (data not shown) strongly expressed in the presence of SDF-1α, including integrin α4 and integrin αV, which are intricately involved in angiogenesis, vasculogenesis and vascular remodeling.27,74–76 Findings from an in vitro assay of EC-EPC adhesion after SDF-1α ex vivo priming (data not shown) also implicates adhesion molecules as downstream effectors of the cell-cell mediated pro-healing and/or pro-angiogenic effects. Thus, we observed further evidence to suggest that SDF-1α promotes the engraftment of EPC into the neovasculature of diabetic wounds, potentially by facilitating EC-EPC interaction. However, EPC represent a small proportion of BMDSC (< 1% of all nucleated cells), and the dramatic enhancement of wound vessel density and re-epitheliazation in SDF-1α treated wounds likely supports the participation of additional cell subpopulations and co-existing paracrine mechanism(s). Thus, the role of adhesion molecules in this type of CBT is highly complex and will require a separate, future dedicated study.
With respect to a potential paracrine mechanism, we examined downstream targets influenced by SDF-1α activation that could potentially be influencing the pro-healing response observed in vivo. We first performed gene expression studies to isolate key downstream targets up-regulated by SDF-1α, which are integral to the wound healing process. Two of the key genes consistently up-regulated were EpherinB4 and Plasminogen (Plg). In this work, we focused our efforts on Plg based on prior evidence implicating the Plg/Plasmin (Pm) cascade in angiogenesis, and in disrupted wound healing.77–82
Plg is the inactive pro-enzyme form of Pm, a serine protease that is highly regulated and widely involved in extracellular matrix proteolytic activity.77 While the major physiological function of this system is fibrinolysis to maintain vascular patency, a number of studies have implicated Plg/Pm in facilitating cell migration via direct proteolysis of extracellular matrix proteins, or, indirectly, by activation of other matrix degrading pathways (e.g., metalloproteases). These proteolytic events are pivotal in wound healing, tissue remodeling and angiogenesis.77,83,84 The primary inhibitor of Plg to Pm conversion is plasminogen activator inhibitor-1 (PAI-1). Physiologic levels of PAI-1 serve to balance the degradative capability of Plg/Pm. Various growth factors mediate temporal changes in the expression of PAI-1 and its target, plasminogen activators (i.e., u-PA, t-PA), thereby influencing cell migration by barrier proteolysis and/or ECM adhesion modulation.85 Interestingly, low levels of PAI-1 expression, leading to increased Plg, has been associated with accelerated wound healing, angiogenesis, fibrinolysis and bleeding.81,86,87 Conversely, elevated PAI-1 expression, leading to decreased Plg, has been associated with obesity, atherosclerosis, hypercoagulability and, inconsistently, with delayed wound healing responses in diabetes.82,87–90
We performed in vivo wounding experiments to more precisely determine what specific role, if any, the up-regulation of Plg was having on the enhanced wound closure rates and neovascularization observed among the diabetic wounds treated with SDF-1α-Primed-BMDSC. We locally administered a supraphysiologic, anti-angiogenic dose of recombinant PAI-1 (total dose of 1 µg in 100 µl) 80 to diabetic wounds for 5 consecutive days to ensure adequate inhibition of the Plg/Pm enzymatic axis. Diabetic wounds inoculated with SDF-1α–Primed-BMDSC and blocked with concurrent PAI-1 injections (SDF-1α/PAI-1) did not appear grossly different from the wounds treated with only SDF-1α activated cells at any time point during the experiment. The only difference appreciated was a mildly prolonged wound closure rate in the SDF-1α/PAI-1 group that reached statistical significance during the POD 9 through POD 12 time points (the most critical time interval during which the largest difference in wound healing was observed in all in vivo wounding experiments). Nevertheless, nearly all mice in the SDF-1α/PAI-1 group achieved complete closure of wounds by POD 20 in similar fashion to mice treated with SDF-1α activated cells, albeit over a slightly more protracted time course. In correlation with the fairly similar wound closure rates appreciated between the two groups, wound vessel density and EPC-like cell numbers did not reach a statistically significant difference (vessel density p=0.092; EPC data not shown) at the time point examined (POD 20), although vessel density did appear to trend down in the SDF-1α/PAI-1 group. These modest inhibitory effects of PAI-1 point to other downstream mediators (in addition to Plg/Pm) as effectors of the SDF-1α-Primed-BMDSC pro-healing and pro-angiogenic effects, likely candidates being adhesion molecules, as discussed above. Alternatively, redundant pathways within the Plg/Pm enzymatic cascades may account for the observed modest inhibitory effects.
Previous data suggest that the matrix metalloproteinase (MMP) family of enzymes can perform the necessary proteolytic functions essential for wound healing and angiogenesis when the Plg/Pm cascade is deficient, and that inhibition of both proteolytic pathways, in turn, severely impairs wound healing.84 Thus, there exists functional overlap between tissue degrading proteases, which are involved in re-epitheliazation, ECM deposition/ECM turnover and neovascularization. Such functional overlap could relate to the similarity in wound healing and neovascularization observed between the SDF-1α and SDF-1α/PAI-1 groups. Other possibilities to explain the similar findings could relate to the time points during which PAI-1 was administered, or to the in vivo half-life of PAI-1, or to the distribution of the protein within the wound tissue. Lastly, the chosen time point during which wounds were harvested may have diluted the biologic effect because the effects of Plg inhibition may have diminished by day 20. Nevertheless, the fact that a significant difference in wound healing was observed between groups from POD 9 through POD 12 suggests that Plg is, indeed, partially involved in the healing response mediated by SDF-1α activated BMDSC. Yet, the local and compensatory up-regulation of other ECM degrading proteases, and the potential concurrent or collaborating role of adhesion molecules involved within diabetic wounds treated with SDF-1α activated BMDSC, will require further verification.
Conclusion
Our data provide strong preclinical evidence for the potential utility, safety and efficacy of ex vivo SDF-1α activation of BMDSC for the ever increasing unsolved problem of delayed diabetic cutaneous wound healing. Furthermore, we provide insight into the biologic enhancement of diabetic wound neoangiogenesis and re-epitheliazation that may be mediated, in part, by SDF-1α up-regulation of the Plg/Pm cascade within the BMDSC population. We also shed light on other potential targets within the BMDSC subpopulations that may be enhancing the engraftment of EPC within diabetic wounds inoculated with SDF-1α-Primed-BMDSC (i.e. some specific adhesion molecules). Most importantly, however, the ex vivo priming of BMDSC with SDF-1α may offer the opportunity to enhance the efficacy of numerous cell based therapies seeking to employ the tissue regenerating capabilities of BMDSC for the unsolved clinical problem of delayed diabetic cutaneous wound healing. Indeed, this novel CBT could easily be incorporated into new translational platforms for future clinical trials, the clinical implications of which could be far reaching.
Acknowledgments
Supported by grants from the National Institutes of Health (R01DK-071084 & R01GM081570), and a grant from the Wallace H. Coulter Center for Translational Research, University of Miami. Equipment used in this study belongs to the Surgery Department, and core facilities of University of Miami, Miller School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work is preclinical research on the efficacy and mechanism of action of an autologous cell based therapy for diabetic wound healing utilizing SDF-1α to prime diabetic unfractionated bone marrow stem cells ex-vivo.
Authors have no financial disclosures.
No pharmaceutical medications were used in this study
References
- 1.Lau K, Paus R, Tiede S, et al. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. 2009;18:921–933. doi: 10.1111/j.1600-0625.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 2.Brower J, Blumberg S, Carroll E, et al. Mesenchymal stem cell therapy and delivery systems in nonhealing wounds. Adv Skin Wound Care. 2011;24:524–532. doi: 10.1097/01.ASW.0000407648.89961.a6. quiz 533-4. [DOI] [PubMed] [Google Scholar]
- 3.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, et al. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 4.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 5.Turan RG, Turan CH, Bozdag-Turan I, et al. Impaired mobilization of CD133(+) bone marrow-derived circulating progenitor cells with an increased number of diseased coronary arteries in ischemic heart disease patients with diabetes. Circ J. 2011;75:2635–2641. doi: 10.1253/circj.cj-10-1284. [DOI] [PubMed] [Google Scholar]
- 6.Kang L, Chen Q, Wang L, et al. Decreased mobilization of endothelial progenitor cells contributes to impaired neovascularization in diabetes. Clin Exp Pharmacol Physiol. 2009;36:e47–e56. doi: 10.1111/j.1440-1681.2009.05219.x. [DOI] [PubMed] [Google Scholar]
- 7.Kahn MB, Yuldasheva NY, Cubbon RM, et al. Insulin resistance impairs circulating angiogenic progenitor cell function and delays endothelial regeneration. Diabetes. 2011;60:1295–1303. doi: 10.2337/db10-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadini GP, Boscaro E, de Kreutzenberg S, et al. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care. 2010;33:1097–1102. doi: 10.2337/dc09-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albiero M, Menegazzo L, Boscaro E, et al. Defective recruitment, survival and proliferation of bone marrow-derived progenitor cells at sites of delayed diabetic wound healing in mice. Diabetologia. 2011;54:945–953. doi: 10.1007/s00125-010-2007-2. [DOI] [PubMed] [Google Scholar]
- 10.Saito H, Yamamoto Y, Yamamoto H. Diabetes alters subsets of endothelial progenitor cells that reside in blood, bone marrow, and spleen. Am J Physiol Cell Physiol. 2012;302:C892–C901. doi: 10.1152/ajpcell.00380.2011. [DOI] [PubMed] [Google Scholar]
- 11.Kim KA, Shin YJ, Kim JH, et al. Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Arch Pharm Res. 2012;35:223–234. doi: 10.1007/s12272-012-0203-y. [DOI] [PubMed] [Google Scholar]
- 12.Badillo AT, Chung S, Zhang L, et al. Lentiviral gene transfer of SDF-1alpha to wounds improves diabetic wound healing. J Surg Res. 2007;143:35–42. doi: 10.1016/j.jss.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 13.Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25:73–78. doi: 10.1016/j.clindermatol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 15.Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Seeger FH, Zeiher AM, Dimmeler S. Cell-enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S110–S113. doi: 10.1038/ncpcardio0734. [DOI] [PubMed] [Google Scholar]
- 17.Jain P, Perakath B, Jesudason MR, et al. The effect of autologous bone marrow-derived cells on healing chronic lower extremity wounds: results of a randomized controlled study. Ostomy Wound Manage. 2011;57:38–44. [PubMed] [Google Scholar]
- 18.Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–991. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 19.Kado M, Lee JK, Hidaka K, et al. Paracrine factors of vascular endothelial cells facilitate cardiomyocyte differentiation of mouse embryonic stem cells. Biochem Biophys Res Commun. 2008;377:413–418. doi: 10.1016/j.bbrc.2008.09.160. [DOI] [PubMed] [Google Scholar]
- 20.Kofidis T, de Bruin JL, Yamane T, et al. Stimulation of paracrine pathways with growth factors enhances embryonic stem cell engraftment and host-specific differentiation in the heart after ischemic myocardial injury. Circulation. 2005;111:2486–2493. doi: 10.1161/01.CIR.0000165063.09283.A8. [DOI] [PubMed] [Google Scholar]
- 21.Nomura T, Ashihara E, Tateishi K, et al. Therapeutic potential of stem/progenitor cells in human skeletal muscle for cardiovascular regeneration. Curr Stem Cell Res Ther. 2007;2:293–300. doi: 10.2174/157488807782793808. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28:905–915. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 24.Zemani F, Silvestre JS, Fauvel-Lafeve F, et al. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644–650. doi: 10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 25.Lei Y, Haider HK, Shujia J, et al. Therapeutic angiogenesis. Devising new strategies based on past experiences. Basic Res Cardiol. 2004;99:121–132. doi: 10.1007/s00395-004-0447-x. [DOI] [PubMed] [Google Scholar]
- 26.Vagima Y, Lapid K, Kollet O, et al. Pathways implicated in stem cell migration: the SDF-1/CXCR4 axis. Methods Mol Biol. 2011;750:277–289. doi: 10.1007/978-1-61779-145-1_19. [DOI] [PubMed] [Google Scholar]
- 27.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 28.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 29.Wang JF, Park IW, Groopman JE. Stromal cell-derived factor-1alpha stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood. 2000;95:2505–2513. [PubMed] [Google Scholar]
- 30.Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu ZJ, Tian R, An W, et al. Identification of E-selectin as a novel target for the regulation of postnatal neovascularization: implications for diabetic wound healing. Ann Surg. 2010;252:625–634. doi: 10.1097/SLA.0b013e3181f5a079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landry Y, Le O, Mace KA, et al. Secretion of SDF-1alpha by bone marrow-derived stromal cells enhances skin wound healing of C57BL/6 mice exposed to ionizing radiation. J Cell Mol Med. 2010;14:1594–1604. doi: 10.1111/j.1582-4934.2009.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Restivo TE, Mace KA, Harken AH, et al. Application of the chemokine CXCL12 expression plasmid restores wound healing to near normal in a diabetic mouse model. J Trauma. 2010;69:392–398. doi: 10.1097/TA.0b013e3181e772b0. [DOI] [PubMed] [Google Scholar]
- 34.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 35.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 36.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schatteman GC, Hanlon HD, Jiao C, et al. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000;106:571–578. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzau VJ, Gnecchi M, Pachori AS, et al. Therapeutic Potential of Endothelial Progenitor Cells in Cardiovascular Diseases. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 39.Ottaiano A, Franco R, Aiello Talamanca A, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II–III colorectal cancer patients. Clin Cancer Res. 2006;12:2795–2803. doi: 10.1158/1078-0432.CCR-05-2142. [DOI] [PubMed] [Google Scholar]
- 40.Rhodes LV, Short SP, Neel NF, et al. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer Res. 2011;71:603–613. doi: 10.1158/0008-5472.CAN-10-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furusato B, Mohamed A, Uhlen M, et al. CXCR4 and cancer. Pathol Int. 2010;60:497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 42.Ramos EA, Grochoski M, Braun-Prado K, et al. Epigenetic changes of CXCR4 and its ligand CXCL12 as prognostic factors for sporadic breast cancer. PLoS One. 2011;6:e29461. doi: 10.1371/journal.pone.0029461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wendel C, Hemping-Bovenkerk A, Krasnyanska J, et al. CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model. PLoS One. 2012;7:e30046. doi: 10.1371/journal.pone.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Diao X, Sun J, et al. Stromal cell-derived factor-1 and vascular endothelial growth factor as biomarkers for lymph node metastasis and poor cancer-specific survival in prostate cancer patients after radical prostatectomy. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Iwasa S, Yanagawa T, Fan J, et al. Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Res. 2009;29:4751–4758. [PubMed] [Google Scholar]
- 46.Terada R, Yamamoto K, Hakoda T, et al. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: implications for inflammatory liver diseases. Lab Invest. 2003;83:665–672. doi: 10.1097/01.lab.0000067498.89585.06. [DOI] [PubMed] [Google Scholar]
- 47.Balabanian K, Couderc J, Bouchet-Delbos L, et al. Role of the chemokine stromal cell-derived factor 1 in autoantibody production and nephritis in murine lupus. J Immunol. 2003;170:3392–3400. doi: 10.4049/jimmunol.170.6.3392. [DOI] [PubMed] [Google Scholar]
- 48.Warchol T, Lianeri M, Lacki JK, et al. SDF1-3' G801A polymorphisms in Polish patients with systemic lupus erythematosus. Mol Biol Rep. 2010;37:3121–3125. doi: 10.1007/s11033-009-9890-y. [DOI] [PubMed] [Google Scholar]
- 49.Chen HT, Tsou HK, Hsu CJ, et al. Stromal cell-derived factor-1/CXCR4 promotes IL-6 production in human synovial fibroblasts. J Cell Biochem. 2011;112:1219–1227. doi: 10.1002/jcb.23043. [DOI] [PubMed] [Google Scholar]
- 50.Kim KW, Cho ML, Kim HR, et al. Up-regulation of stromal cell-derived factor 1 (CXCL12) production in rheumatoid synovial fibroblasts through interactions with T lymphocytes: role of interleukin-17 and CD40L-CD40 interaction. Arthritis Rheum. 2007;56:1076–1086. doi: 10.1002/art.22439. [DOI] [PubMed] [Google Scholar]
- 51.Nishimura Y, Ii M, Qin G, et al. CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Invest Dermatol. 2012;132:711–720. doi: 10.1038/jid.2011.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Song Y, Zhao L, et al. Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat Protoc. 2008;3:1703–1708. doi: 10.1038/nprot.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenhalgh DG, Sprugel KH, Murray MJ, et al. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 54.Stepanovic V, Awad O, Jiao C, et al. Leprdb diabetic mouse bone marrow cells inhibit skin wound vascularization but promote wound healing. Circ Res. 2003;92:1247–1253. doi: 10.1161/01.RES.0000074906.98021.55. [DOI] [PubMed] [Google Scholar]
- 55.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A. 2009;106:13505–13510. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamarat R, Silvestre JS, Le Ricousse-Roussanne S, et al. Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol. 2004;164:457–466. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 59.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krampera M, Pizzolo G, Aprili G, et al. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39:678–683. doi: 10.1016/j.bone.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 62.Fathke C, Wilson L, Hutter J, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 64.Javazon EH, Keswani SG, Badillo AT, et al. Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen. 2007;15:350–359. doi: 10.1111/j.1524-475X.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrews RG, Singer JW, Bernstein ID. Monoclonal antibody 12-8 recognizes a 115-kd molecule present on both unipotent and multipotent hematopoietic colony-forming cells and their precursors. Blood. 1986;67:842–845. [PubMed] [Google Scholar]
- 67.Baumheter S, Singer MS, Henzel W, et al. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993;262:436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- 68.Wood HB, May G, Healy L, et al. CD34 expression patterns during early mouse development are related to modes of blood vessel formation and reveal additional sites of hematopoiesis. Blood. 1997;90:2300–2311. [PubMed] [Google Scholar]
- 69.Yang J, Li M, Kamei N, et al. CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PLoS One. 2011;6:e20219. doi: 10.1371/journal.pone.0020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 71.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 72.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex-vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 74.Eliceiri BP, Cheresh DA. The role of alpha v integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Falco E, Porcelli D, Torella AR, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 76.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 77.Romer J, Bugge TH, Pyke C, et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996;2:287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 78.Pepper MS, Vassalli JD, Orci L, et al. Proteolytic balance and capillary morphogenesis in vitro. EXS. 1992;61:137–145. doi: 10.1007/978-3-0348-7001-6_22. [DOI] [PubMed] [Google Scholar]
- 79.Carmeliet P, Moons L, Dewerchin M, et al. Receptor-independent role of urokinase-type plasminogen activator in pericellular plasmin and matrix metalloproteinase proteolysis during vascular wound healing in mice. J Cell Biol. 1998;140:233–245. doi: 10.1083/jcb.140.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Devy L, Blacher S, Grignet-Debrus C, et al. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J. 2002;16:147–154. doi: 10.1096/fj.01-0552com. [DOI] [PubMed] [Google Scholar]
- 81.Chan JC, Duszczyszyn DA, Castellino FJ, et al. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am J Pathol. 2001;159:1681–1688. doi: 10.1016/S0002-9440(10)63015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verkleij CJ, Roelofs JJ, Havik SR, et al. The role of thrombin-activatable fibrinolysis inhibitor in diabetic wound healing. Thromb Res. 2010;126:442–446. doi: 10.1016/j.thromres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 83.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 84.Lund LR, Romer J, Bugge TH, et al. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 1999;18:4645–4656. doi: 10.1093/emboj/18.17.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kutz SM, Hordines J, McKeown-Longo PJ, et al. TGF-beta1-induced PAI-1 gene expression requires MEK activity and cell-to-substrate adhesion. J Cell Sci. 2001;114:3905–3914. doi: 10.1242/jcs.114.21.3905. [DOI] [PubMed] [Google Scholar]
- 86.Iwaki T, Tanaka A, Miyawaki Y, et al. Life-threatening hemorrhage and prolonged wound healing are remarkable phenotypes manifested by complete plasminogen activator inhibitor-1 deficiency in humans. J Thromb Haemost. 2011;9:1200–1206. doi: 10.1111/j.1538-7836.2011.04288.x. [DOI] [PubMed] [Google Scholar]
- 87.Stefansson S, McMahon GA, Petitclerc E, et al. Plasminogen activator inhibitor-1 in tumor growth, angiogenesis and vascular remodeling. Curr Pharm Des. 2003;9:1545–1564. doi: 10.2174/1381612033454621. [DOI] [PubMed] [Google Scholar]
- 88.McGill JB, Schneider DJ, Arfken CL, et al. Factors responsible for impaired fibrinolysis in obese subjects and NIDDM patients. Diabetes. 1994;43:104–109. doi: 10.2337/diab.43.1.104. [DOI] [PubMed] [Google Scholar]
- 89.De Taeye B, Smith LH, Vaughan DE. Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr Opin Pharmacol. 2005;5:149–154. doi: 10.1016/j.coph.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 90.Juhan-Vague I, Alessi MC, Morange PE. Hypofibrinolysis and increased PAI-1 are linked to atherothrombosis via insulin resistance and obesity. Ann Med. 2000;321(Suppl):78–84. [PubMed] [Google Scholar]