Abstract
Elastin-like polypeptide (ELP) is a macromolecular carrier with thermally responsive properties that can passively accumulate in solid tumors and additionally aggregate in tumor tissue when exposed to hyperthermia. In this study, ELP was conjugated to the anticancer drug doxorubicin (DOXO) and three different cell-penetrating peptides (CPP) in order to inhibit tumor growth in mice compared to free doxorubicin. Fluorescence microscopy studies in MCF-7 breast carcinoma cells demonstrated that the three different CPP-ELP-DOXO conjugates delivered doxorubicin to the cell nucleus. All CPP-ELP-DOXO conjugates showed cytotoxicity with IC50 values in the range of 12-30 μM at 42 °C, but the ELP carrier with SynB1 as the cell-penetrating peptide had the lowest intrinsic cytotoxicity. Therefore, the antitumor efficacy of SynB1-ELP-DOXO was compared to doxorubicin under hyperthermic conditions. C57BL/6 female mice bearing syngeneic E0771 murine breast tumors were treated with either free doxorubicin or the SynB1-ELP-DOXO conjugate with or without focused hyperthermia on the tumor. Under hyperthermic conditions, tumor inhibition with SynB1-ELP-DOXO was 2-fold higher than under therapy with free doxorubicin at the equivalent dose, and is thus a promising lead candidate for optimizing thermally responsive drug polymer conjugates.
Keywords: ELP – elastin-like polypeptide; doxorubicin; (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH, INNO-206); acid-sensitive prodrugs; CPP - cell penetrating peptide; hyperthermia
1. Introduction
Doxorubicin (Dox) is an anthracycline used in the treatment of many types of cancer. It intercalates into the strands of DNA to inhibit the progression of topoisomerase II, which maintains essential DNA replication functions (Biersack H, 1996; Nelson WG, 1986). The side effects, however, are severe and include neutropenia, mucositis, alopecia, nausea, myelosuppression, and cardiotoxicity. The drug is active against proliferating tumor cells and, as a result, the treatment also affects normal cells that are dividing, such as cells in the bone marrow and the gastrointestinal tract lining. In order to improve the therapeutic index of doxorubicin, there is a need for efficient drug delivery systems to deliver doxorubicin to the tumor site while reducing side effects to normal tissues. For this purpose, a spectrum of prodrug approaches have been developed (Kratz et al., 2006).
Previous studies have demonstrated a macromolecular carrier that can provide targeted delivery with the aid of hyperthermia (Mackay and Chilkoti, 2008). Elastin-like polypeptide (ELP) is a thermally responsive biopolymer derived from the structural motif of mammalian Elastin and is composed of the pentapeptide repeat Val-Pro-Gly-Xaa-Gly, where Xaa is a guest residue and can be any amino acid except proline (Tatham and Shewry, 2000).
These biopolymer carriers are beneficial for delivering small drugs because they significantly increase the circulation time of bound drugs (Kawasaki et al., 1996) and thereby passively accumulate in the tumor due to the enhanced permeability and retention effect (Cassidy et al., 1989; Maeda et al., 2009; Takakura et al., 1990; Yamaoka et al., 1994). An advantage of using ELP over other biopolymers is that in addition to passive tumor targeting, it can aggregate and accumulate through induced hyperthermia in tumor tissue. It has been shown previously that ELPs will accumulate 2-fold more in a subcutaneously implanted murine tumor when the tumor is exposed to hyperthermia at 39 – 41 °C (Meyer et al., 2001).
A major problem with drug delivery is poor penetration due to the impermeability of the plasma membrane to macromolecules. To improve tumor accumulation and facilitate the entry into the cell, cell-penetrating peptides (CPP) are used as an effective approach of improving the cellular uptake of the macromolecule. In this study, the N-terminus of ELP was modified with one of three different CPPs – Bac, Tat, and SynB1. These peptides are derived from the bactenecin family of antimicrobial peptides (Sadler et al., 2002), a truncated version of the HIV-1 Tat protein (Vives et al., 1997), and antimicrobial peptides called protegrins found on porcine leukocytes (Aumelas et al., 1996). Previous applications of CPPs with ELP include drug delivery of a c-Myc inhibitory peptide (Bidwell et al., 2009; Bidwell and Raucher, 2005), a p21 peptide mimetic (Massodi et al., 2005; Massodi et al., 2010), a peptide inhibitor of splicing machinery (Bidwell et al., 2010), and paclitaxel (Moktan, 2010). These three CPPs were chosen out of other CPPs for their increased ability in uptake into the cell, ease in purification, and the nontoxicity in previous animal studies.
Subsequently, the (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO) was conjugated to a terminal cysteine residue on the C-terminus of ELP. In situ binding of maleimide-based prodrugs to endogenous albumin is meanwhile a pre-clinically and clinically validated technology of increasing the therapeutic index of anticancer agents. The (6-maleimidocaproyl)hydrazone derivative of doxorubicin (INNO-206, previously known as DOXO-EMCH) is an an acid-sensitive prodrug of doxorubicin that binds rapidly and selectively to the cysteine-34 position of circulating albumin (Kratz, 2007; Kratz et al., 2002) and is undergoing phase II studies in soft tissue sarcoma (see http://www.cytrx.com). The carboxylic hydrazone linker in DOXO-EMCH allows the drug to be cleaved at the acidic pH value of lysosomes (pH 4 - 5) and endosomes (pH 5.0 – 6.5) (Kratz F, 2007).
This study first compared the effectiveness of these three CPP-ELP carriers to deliver doxorubicin to breast cancer cells. The CPP-ELP-DOXO conjugates delivered comparable amounts of free dox according to confocal localization experiments, most likely due to DOXO being released. Secondly, the antitumor efficacy of the CPP-ELP-DOXO conjugate which demonstrated the best cytotoxicity and the lowest intrinsic toxicity of the carrier was compared to doxorubicin in a tumor-bearing breast cancer animal model and was superior to doxorubicin under hyperthermic conditions.
2. Materials and Methods
2.1 ELP synthesis
pET25b+ expression vectors containing the constructs (CPP-ELP) were transformed into E. coli BLR (DE3) (Novagen, Madison, WI) for hyperexpression of the protein (Meyer and Chilkoti, 1999). The expression strains were used to inoculate TB Dry plus ampicillin (100 μg/mL) and grown at 37 °C, 220 rpm agitation for 24 hours. Cells were harvested by centrifugation (3,000 × g, 10 min), re-suspended in PBS, lysed by sonication (Fisher Scientific 550 Sonic Dismembrator), and centrifuged to remove cell debris (13,000 × g, 45 min). PEI (0.5% w/v) was added to the lysate to precipitate nucleic acids, which were then removed by centrifugation at 10 °C for 30 min. The phase transition of the polypeptide was induced by heating the lysate to 44 °C and increasing NaCl concentration to 2 M. The polypeptide was collected by centrifugation (11,000 × g, 10 min) and re-suspended in PBS. Purification of ELP was achieved by inverse transition cycling (Daniell et al., 1997; McPherson et al., 1996) which was repeated 3 to 5 times.
2.2 Synthesis of the CPP-ELP-DOXO conjugates (ELP-DOXO, Bac-ELP-DOXO, Tat-ELP-DOXO, and SynB1-ELP-DOXO)
The acid-cleavable (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH, 1) was synthesized as described previously by Kratz et al. (2002). Its uncleavable counterpart, the 6-maleimidocaproic acid amide derivative of doxorubicin (EMC-DOXO, 2), was synthesized by Kratz’s lab (Ryppa et al., 2008) in order to validate the significance of the acid-cleavable hydrazone bond for DOXO-EMCH potency. Both DOXO-EMCH and EMC-DOXO were then coupled to the free cysteine of ELP through a denaturing process. Briefly, to a 125 μM solution of the respective polypeptide in a 0.1 M sodium carbonate (pH 9) buffer, a 10-fold molar excess of tris(2-carboxyethyl)phosphine (TCEP) was added to prevent spontaneous formation of disulfide bonds for 20 min at room temperature in order to maximize efficiency and make the C-terminus cysteine site available for drug labeling; then a 6.9 mM solution of DOXO-EMCH was freshly prepared in a 10 mM sodium phosphate buffer (pH 5.8) and added to the protein and left to incubate for 45 min at 27 °C. EMC-DOXO was prepared by dissolving in 30 μL DMF then added to a solution of protein under the same circumstances. The inverse transition cycling procedure was repeated until very little to no free dox remained in the supernatant. The final protein-dox sample was collected in PBS, pH 7 and stored in aliquots at −20 °C. Dox levels were determined by spectrophotometry. The absorbance values at 495 and 280 nm were recorded, and the concentration of dox in the protein-drug sample was calculated by dividing the dox absorbance at 495 nm by the extinction coefficient of dox, 9250 M−1cm−1. Protein concentration was estimated as described in (Dreher et al., 2003) using the following equation:
Drug concentration relative to protein concentration gave the average drug to protein molar label ratio was 0.85 ± 0.02. This strategy has the potential of additionally delivering higher doses of doxorubicin by exploiting the aggregation properties of the thermally responsive biopolymer ELP in addition to its passive targeting through the EPR effect.
2.3 Characterization of the transition temperature
Each CPP-ELP-DOXO construct was heated at 1 °C/min in a multi-cell holder in a UV-Vis spectrophotometer (Cary 100, Varian Instruments). For comparison of all constructs, the Tt was determined at 20 μM ELP concentration in cell media containing 10 % serum. The temperature-induced aggregation was characterized by measuring the turbidity in the UV-Vis range of 350 nm as a function of temperature. A rise in ELP turbidity measured at 350 nm in response to an increase in temperature is a function of aggregate formation (Raucher and Chilkoti, 2001). The Tt was defined as the temperature at which the turbidity of the solution reached 50% of the maximum.
2.4 Cell culture
MCF-7 (ATCC, Manassas, VA) human breast carcinoma cells were grown as a monolayer in 75 cm2 tissue culture flasks and cultured in Minimal Essential Medium supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA), 1 mM sodium pyruvate, BME amino acids, 5 μg/mL insulin (Sigma, St. Louis, MO), 100 units/mL penicillin, and 100 μg/mL streptomycin. Cultures were maintained at 37 °C in a humidified atmosphere (5% CO2). For experiments, cell were removed from tissue culture flasks by treatment with 0.05% v/v trypsin-EDTA (Invitrogen) and counted using a Beckman Coulter counter Z series (Beckman Coulter, Inc). Cells were plated in 96 well plates (8,000 cells/well) for proliferation and in 6 well plates (100,000 cells/well) for flow cytometry. Cells were allowed to grow 24 h before treatment.
2.5 Flow cytometry analysis
MCF-7 cells were treated with 10 μM dox equivalent of CPP-ELP-DOXO or ELP-DOXO for 1 h at either 37 °C or 42 °C. After the treatment, cells were washed three times with PBS, harvested with a nonenzymatic cell dissociation buffer (Cellstripper; Cellgro, Manassas, VA), centrifuged for 3 min, and re-suspended in 0.5 mL PBS. Total uptake of the doxorubicin-labeled polypeptide was measured using a Cytomics FC 500 flow cytometer (Beckman Coulter, Fullerton, CA). Data was corrected for mean labeling efficiencies among the different polypeptides. The results shown are the average of at least 3 experiments (bars, SEM).
2.6 Cell proliferation
For comparison of the potency of each CPP-ELP, CPP-ELP-DOXO, and free dox, MCF-7 cells were plated in 96 well plates and allowed to grow for 24 h. The cells were treated with varying concentrations of each polypeptide conjugate, either dox equivalent concentration or ELP concentration for 1 h at 37 or 42 °C. After treatment, cells were washed and returned to the incubator with fresh media. 72 h later, cell proliferation was measured using MTS ([3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay (Promega, Madison, WI). Results represent the mean of at least 3 experiments (bars, SEM).
2.7 Fluorescence microscopy
In order to compare the intracellular localization of ELP-DOXO and the 3 CPP-ELP-delivered DOXO constructs with free dox, MCF-7 cells were plated on coverslips and grown to 50 % confluence. Cells were treated with 20 μM of each polypeptide at dox equivalent doses and free dox for 1 h at 37 °C or 42 °C. After treatment, cells were washed, and fresh media was added. 4 h after treatment, cells were rinsed with PBS and fixed with 4 % paraformaldehyde, stained with 0.25 μg / ml Hoechst, and visualized using a Nikon Eclipse E600 epifluorescent microscope with a 60× oil immersion objective. All images were captured with the Coolsnap fx digital camera (Roper Scientific, Tucson, AZ) and processed with MetaView (Universal Imaging Corporation, Downingtown, PA). PMT voltages were adjusted to maximize image resolution and intensity. Therefore, image intensity does not correlate with the amount of polypeptide present in the cells. Quantification was carried out as previously described (see 2.5 - flow cytometry).
2.8 Animal model
Female C57BL/6 mice with an average body weight of 20 g were purchased from NCI (Frederick, Maryland). Animals were housed in appropriate caging in a 12 h light-dark rhythm under standard temperature and humidity conditions. 1 × 106 E0771 murine breast cancer cells were implanted into the mammary fat pad and allowed to grow to a volume of approximately 150 mm3 by caliper measurement using the formula: Volume = (Length * Width2)/2. Once tumors reached this size, treatment began on Day 0 and continued on Days 2, 4, and 6 by injection into the femoral vein. Each group consisted of at least 5 mice and received 7 mg / kg dox equivalent dose in a 300 μl bolus. Mice were carefully monitored for weight, tumor size, and general welfare every day. During all treatments, animals were anesthetized using ~ 1.5% isoflurane. All animal experiments were performed in accordance with the University of Mississippi Medical Center’s Institutional Animal Care and Use Committee.
In order to actively target ELP to the tumor site, the tumor must be heated to ~ 41 °C while the rest of the body stays at normal physiological temperature (37 °C). To accomplish this, a Me 540L Laser Sys*Tim (Mettler Electronics) was placed 2-3 mm above the skin over the tumor while the surrounding area was shielded. Heating was carried out in cycles (Dreher et al., 2007) of 20 min heating, 10 min cooling for a total of 2 h, in which animals were kept under isoflurane anesthetic. After treatment, animals were allowed to wake and were monitored until Day 14 at which time they were euthanized with isoflurane overdose, and tumors were excised and weighed.
2.9 Statistical Analysis
Dose response curves were fitted to a sigmoidal equation using Microcal Origin 6.0 in order to determine the concentration which displayed 50% of maximum inhibitory effect (IC50 value). Statistical difference of a drug treatment and controls were analyzed using One-Way Anova for pair-wise comparisons of the groups.
3. Results and Discussion
3.1 Design and synthesis of CPP-ELP-DOXO conjugates
A primary mode of action for doxorubicin is the inhibition of topoisomerase II (Biersack H, 1996; Cummings J, 1991). Therefore, drug delivery systems that can deliver dox to the nucleus can lead to maximum inhibition of cell proliferation. Since a macromolecular carrier cannot enter the nucleus without a nuclear targeting signal, doxorubicin must be cleaved from the macromolecule. The acid-sensitive linker in the (6-maleimidocaproyl)hydrazone doxorubicin derivative (DOXO-EMCH, see Figure 1 A) ensures a rapid intracellular release of doxorubicin due to the cleavage of the carboxylic hydrazone bond in the acidic endosomal and lysosomal compartments of the cell following cellular uptake (Kratz et al., 2002). In order to enhance its potency, DOXO-EMCH was conjugated to ELP alone as well as to ELP bearing three different cell penetrating peptides (CPPs, i.e. Bac, Tat, and SynB1 – Table 1) to yield ELP-DOXO, Bac-ELP-DOXO, Tat-ELP-DOXO, and SynB1-ELP-DOXO. The incorporated CPPs were evaluated for their influence on cellular uptake, intracellular localization of doxorubicin, and their effect on cellular proliferation.
Figure 1.
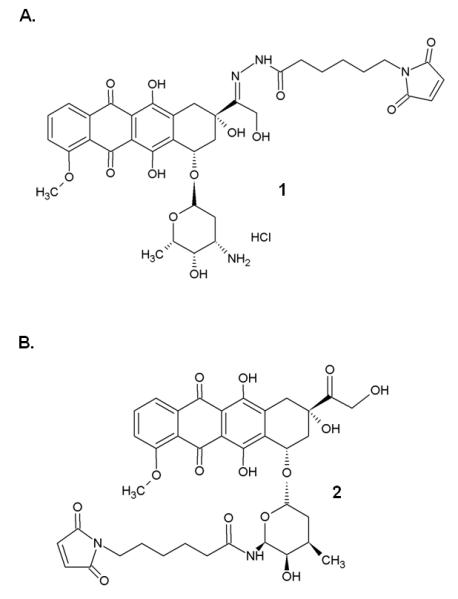
Chemical structure of 1 (6-maleimidocaproyl)hydrazone derivative of doxorubicin, DOXO-EMCH (re-named INNO-206) and 2 (6-maleimidocaproyl)amide derivative of doxorubicin, EMC-DOXO.
Table 1.
Peptide sequences and nomenclature used in this study.
| Polypeptide | Sequence |
|---|---|
| ELP | GPGVG-(VPGXG)150-WPGSGGC |
| Bac-ELP | RRIRPRPPRLPRGPGVG-(VPGXG)150-WPGSGGC |
| Tat-ELP | YGRKKRRQRRRGPGVG-(VPGXG)150-WPGSGGC |
| SynB1-ELP | RGGRLSYSRRRFSTSTGRGPVPG-(VPGXG)150-WPGSGGC |
ELP is a thermally sensitive biopolymer consisting of the pentapeptide sequence VPGXG, repeated 150 times, where X is any amino acid other than proline. Bac is a cell penetrating peptide derived from the bactenecin family of antimicrobial peptides. Tat is derived from the HIV-1 Tat protein. SynB1 is from protegrins found in porcine leukocytes.
3.2 Thermal targeting of CPP-ELP-DOXO conjugates
The 3 CPPs used in this study have been used previously (Bidwell et al., 2009; Massodi et al., 2009; Moktan et al., 2010), and they each localize to a specific location inside the cell. In order to determine which CPP is the most efficient for transporting doxorubicin into tumor cells, ELPs were engineered that incorporate a CPP at its N-terminus and DOXO-EMCH at its C-terminus. ELP is composed of a VPGXG motif repeated 150 times where X is any amino acid other than proline. The sequences of each CPP are shown in Table 1. Because the transition temperature (Tt) is affected by the ELP concentration, each construct’s Tt was determined and fell between the ideal range of 39 – 42 °C (Figure 2). The Tt is inversely dependent upon the concentration of the polypeptide. All sequences used in this study are water-soluble for all assays, and their Tt’s are achievable physiologically. Using a laser, mild hyperthermia can be effectively localized and contained within the tumor site in contrast to high temperature ablation therapy (Shinohara, 2004). Because of the defective vasculature and impaired lymphatic drainage of a tumor, the application of mild heat results in the retention and concentration of ELP at the tumor site. Typical clinical mild hyperthermia treatment consists of 1 h exposure of the tumor at 40 – 42 °C which is well tolerated by patients (Dewhirst et al., 1997; Lecornet et al., 2010; Maluta et al., 2007; Takahashi et al., 2002).
Figure 2.
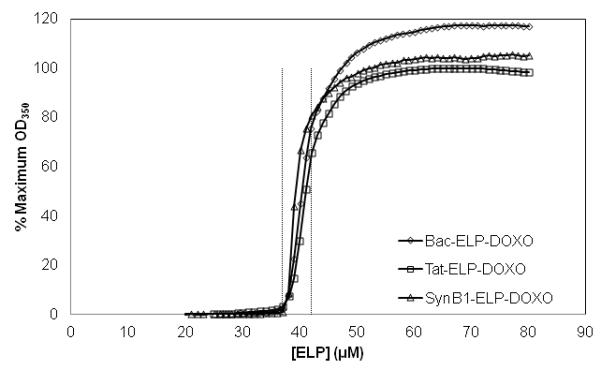
Turbidity profiles (OD350nm) of 20 μM Bac-ELP-DOXO (Δ), Tat-ELP-DOXO (○), and SynB1-ELP-DOXO (◻) were obtained at a heating rate of 1 °C/min. The Tt was defined as the temperature at 50% of the maximum turbidity.
Subsequently, CPP-ELP-DOXO polymers were studied regarding their cellular uptake properties, the intracellular distribution of doxorubicin and their cytotoxicity against MCF-7 breast cancer cells.
3.3 Evaluation of CPP-ELP-DOXO conjugates
For dox to reach the site of action intracellularly, the polypeptides must be internalized. To determine which CPP showed the most efficient cellular association to MCF-7 breast cancer cells, the association of dox fluorescence with MCF-7 cells was measured by flow cytometry. Cells were treated with each CPP-ELP-DOXO or ELP-DOXO as a control at 10 μM dox equivalent for 1 h at either 37 or 42 °C, then rinsed and collected. The fluorescence intensity was measured by flow cytometry. It has been previously shown (Raucher and Chilkoti, 2001) that cellular uptake of the thermally responsive ELP was enhanced by the thermal phase transition of the polypeptide. Figure 3 shows that the fluorescence intensity of cells harvested immediately after incubation at 42 °C with all 3 CPP-ELP-DOXO conjugates was higher compared to cells incubated at 37 °C due to the production of ELP aggregates subsequent to the binding of the CPP to the cell membrane. Further proving the feasibility of using a CPP, ELP-DOXO uptake was at least 3-fold less than any CPP-ELP-DOXO. However, as assessed by flow cytometry, there is no major difference in the cellular uptake among the three CPP-ELP-DOXO conjugates. Previous studies using confocal microscopy and flow cytometry with CPP-ELPs demonstrated that the polypeptide aggregates are internalized by endocytosis (Bidwell and Raucher, 2005; Massodi et al., 2005). Once internalized, the acidic environment of the endosomes and lysosomes causes cleavage of the hydrazone linker releasing doxorubicin that can diffuse into the nucleus. In this study, cells were incubated at either 37 or 42 °C for 1 h, then rinsed and allowed to incubate at 37 °C for 4 h at which time the cells were fixed, stained with Hoechst, and mounted. Figure 4 shows fluorescent images of MCF-7 cells treated with the three CPP-ELP-DOXO conjugates and ELP-DOXO. After 4 h, doxorubicin can be seen inside the nucleus in cells treated at both 37 and 42 °C. These results demonstrate uptake in the nucleus at 42 °C where the drug can exert its inhibitory effects. Fluorescence signal intensity was adjusted for the purposes of seeing the localization, therefore image intensity does not correlate with the amount of dox internalized. Again, nuclear localization of doxorubicin was comparable among the three CPP-ELP-DOXO conjugates.
Figure 3.
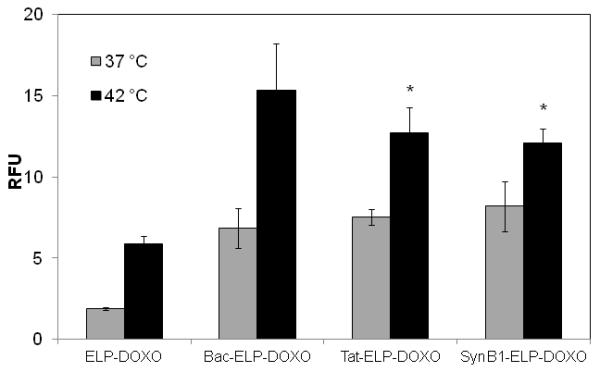
Uptake of doxorubcin in MCF-7 cells. MCF-7 cells were incubated with ELP-DOXO, Bac-ELP-DOXO, Tat-ELP-DOXO, and SynB1-ELP-DOXO (10 μM in cell culture media) for 1 h at either 37 °C or 42 °C. Dox fluorescence intensity was determined using flow cytometry and is expressed in relative fluorescence units (RFU). * indicates statistical significance between ELP-DOXO at 42 °C and both Tat- and SynB1-ELP-DOXO at 42 °C (p < 0.01). The data represents the mean of 3 experiments (error bars, SEM).
Figure 4.
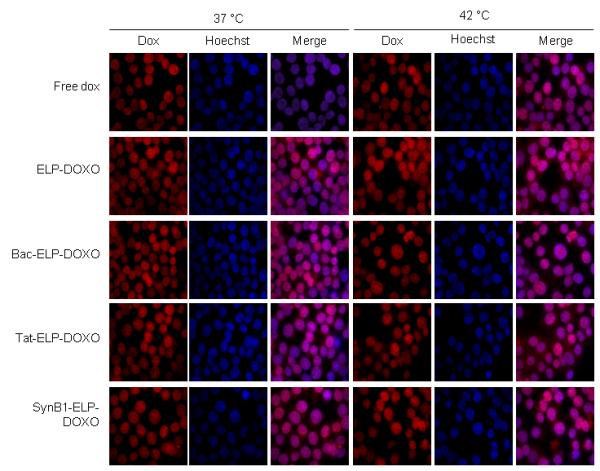
Intracellular Localization of doxorubicin in MCF-7 cells. Cells were plated on a 0.2 μM coverslip and treated the next day at 20 μM for 1 h at either 37 °C or 42 °C. After treatment, cells were washed, fresh media added, and allowed to incubate at 37 °C for 4 h. Coverslips, after staining with Hoechst to mark nuclei, were mounted on a slide and imaged with a Nikon epifluorescence microscope.
Previously, we have synthesized ELP-fused doxorubicin conjugates which showed modest anti-proliferative effects in vitro and were not superior to doxorubicin. This iteration used ELP fused to doxorubicin via a cathepsin cleavable GFLG linker. The Tat CPP was used, and doxorubicin was modified by addition of a maleimide through the linker at the 3′-amino group of the sugar ring of doxorubicin. In confocal fluorescence experiments, Bidwell et al. showed a membrane and cytoplasmic localization of dox with very little doxorubicin accumulation inside the nucleus in contrast to free dox (Bidwell et al., 2007).
3.4 Cytotoxicity of the CPP-ELP-DOXO conjugates
All CPP-ELP-DOXO conjugates were evaluated for their ability to inhibit MCF-7 cell proliferation. Cells were treated for 1 h at either 37 or 42 °C with 2.5 – 30 μM dox equivalents of Bac-ELP-DOXO, Tat-ELP-DOXO, and SynB1-ELP-DOXO as well as free doxorubicin. In addition, a control for each CPP-ELP without DOXO was tested to determine if the CPP itself was toxic to the cells. At 1 h, the compounds were removed and fresh media added, and cells were allowed to incubate at 37 °C for 72 h. The cell viability data of each conjugate was fitted to a sigmoidal dose response curve to determine the IC50 values at 37 and 42 °C. These IC50 values are listed in Table 2. Bac-ELP-DOXO and Tat-ELP-DOXO demonstrated the highest cytotoxicity against MCF-7 cells. However, Bac and Tat contributed a large amount of the toxicity (30 – 40%) as can be seen when analyzing the dose-response curves shown in Figure 5 A - D. In contrast, SynB1-ELP-DOXO inhibited tumor cell proliferation up to 65%, while SynB1-ELP alone showed very little toxicity (see Figure 5 E and F). The cleavage from ELP is vital for anti-proliferative effects. A version without the acid-sensitive hydrazone was conjugated to SynB1-ELP and showed no toxicity in MCF-7 cells (Figure 5 H). Due to the fact that SynB1-ELP had the lowest intrinsic cytotoxicity, this CPP-ELP-DOXO conjugate was chosen for further in vivo investigations.
Table 2.
IC50 values for ELP constructs.
| Construct | IC50 (37 °C) (μM ± SEM) | IC50 (42 °C) (μM ± SEM) |
|---|---|---|
| Bac-ELP | 62.464 ± 4.975 | 28.292 ± 6.570 |
| Tat-ELP | * | 35.123 ± 9.952 |
| SynB1-ELP | * | * |
| Bac-ELP-DOXO | 25.766 ± 5.664 | 12.113 ± 3.126 |
| Tat-ELP-DOXO | 18.358 ± 3.765 | 12.946 ± 1.145 |
| SynB1-ELP-DOXO | 62.916 ± 2.539 | 32.961 ± 4.092 |
| Free doxorubicin | 18.321 ± 2.463 | 9.792 ± 0.608 |
IC50 values were obtained for CPP-ELP, CPP-ELP-DOXO, and free dox in MCF-7 cells. Curves were fit using a sigmoidal equation, and IC50 values shown represent the mean ± SEM of at least three experiments with four replicates per drug concentration.
indicates an IC50 value beyond maximum concentrations tested.
Figure 5.
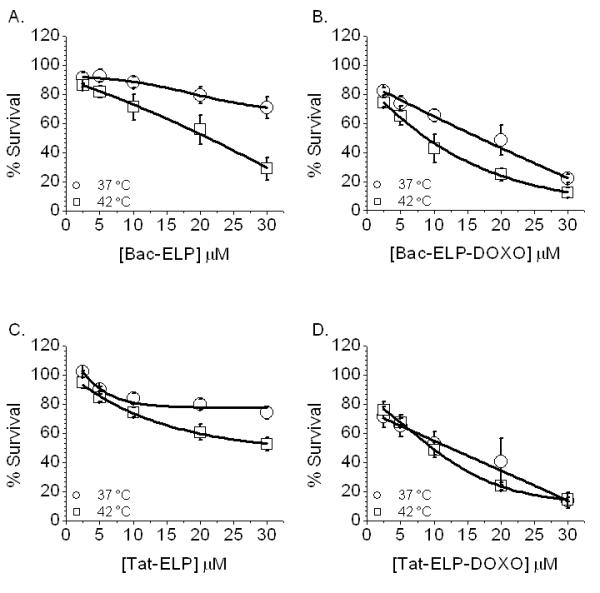
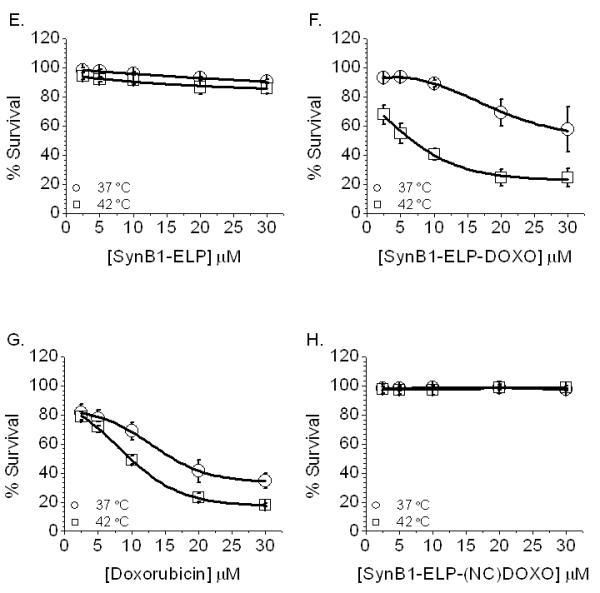
Cell Proliferation in MCF-7 cells. Cells were treated with the 3 CPP-ELP-DOXO constructs for 1 h, either at 37 or 42 °C. After treatment, cells were washed and fresh media was added. 72 h later, cell survival was measured by the MTS assay. Data was normalized to untreated cells at 37 °C and fit to a sigmoidal curve. Error bars are the result of at least 3 experiments, SEM.
3.5 Antitumor efficacy of SynB1-ELP-DOXO and doxorubicin under hyperthermic and non-hyperthermic conditions in a breast cancer model
In order to evaluate the antitumor efficacy of SynB1-ELP-DOXO in vivo, the E0771 murine breast carcinoma line was used. This cell line is useful because it is syngeneic and can therefore be used in a mouse model with an intact immune system (Ewens et al., 2005). C57BL/6 mice (NCI) (at least 5 per group) were implanted subcutaneously in the mammary fat pad with 1 × 106 E0771 cells. When the tumors reached ~ 150 mm3 by caliper measurement, treatment began on Day 0. Mice were administered by intravenous femoral injection with either saline, free dox at 7 mg / kg, or SynB1-ELP-DOXO at 7 mg / kg dox equivalent on Day 0, Day 2, Day 4, and Day 6 under isoflurane anesthesia. This dose of dox was chosen based on the maximum tolerated dose of SynB1-ELP, which was determined to be 800 mg / kg. Beyond this dose, heating of the tumor, which was close to the femoral artery, led to accrual of ELP and blockade of the femoral artery. This caused swelling and eventually necrosis of the limb (data not shown). Since the molar label ratio of SynB1-ELP to DOXO is 0.85, the dose of Dox equivalent to 800 mg / kg of SynB1-ELP in SynB1-ELP-DOXO was approximately 7 mg / kg. Free dox and SynB1-ELP-DOXO groups were further split into heated and non-heated groups. Heating consisted of a thermal cycling protocol previously shown to be effective (Dreher et al., 2007) and optimized by our lab (20 min heating, 10 min cooling × 4). Previously, this protocol was used to focus an infrared light to generate hyperthermia in the breast tissue (Bidwell et al., 2012) and in the cerebellum (Hearst et al., 2011). Using the heat cycling protocol, the tumor temperature repeatedly cycled in and out of the desired hyperthermia window (39 – 41 °C), which has been shown to be more effective than prolonged heating for ELP thermal targeting (Dreher et al., 2007). Immediately after each injection of polypeptide or dox, an infrared probe was placed just above the skin of the tumor while the skin around the tumor was blocked from heat in order to target the tumor. After treatment, animals were allowed to recover then placed back into the animal facilities. Animals were monitored daily for body weight and caliper measurement of the tumor. On Day 14, animals were sacrificed and tumors harvested and weighed.
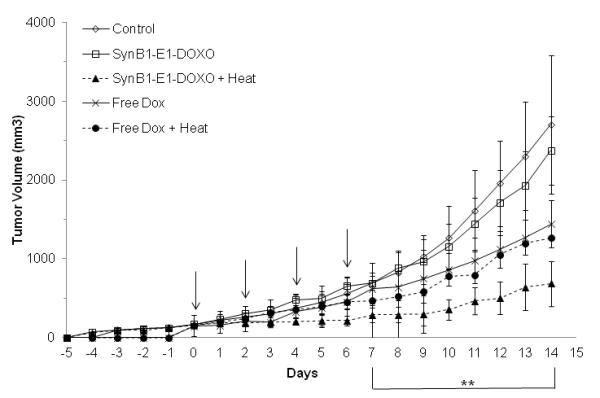
Figure 6 A shows the daily growth of the tumors. The SynB1-ELP-DOXO heated group has a statistically significant decrease in tumor size starting on Day 8 compared to both saline-treated and the SynB1-ELP-DOXO group that was left unheated (p < 0.0001). The average tumor size on Day 14 was 688 mm3 in the SynB1-ELP-DOXO group exposed to hyperthermia compared to 1267 mm3 of the dox group that was exposed to hyperthermia. This difference in tumor size was statistically significant (p < 0.001) (Figure 6 A). On Day 14, consistent with previous volume measurements, the harvested tumor weight was significantly smaller with an average weight of 0.76 g for the SynB1-ELP-DOXO group exposed to hyperthermia compared to 1.11 g for the dox groups (p < 0.009), both the hyperthermic group and normothermic group (Figure 6 B). A common side effect in chemotherapy treatments is weight loss. By conjugating DOXO to ELP, we hope to combat this effect, and in the SynB1-ELP-DOXO heated group, animals weighed an average of 20 g on Day 14 compared to the initial weight of 20.8 g. While they did lose weight during the course of treatment, they were able to regain most of it. In contrast, in the free dox heated group, animals initially weighed 20 g, and on Day 14, had an average weight of 18 g. Thus, animals treated with SynB1-ELP-DOXO and heat had an advantage over animals receiving free dox treatments (Figure 6 C). Taken together, the in vivo data show that SynB1-ELP-DOXO is a promising agent for the treatment of breast carcinomas using hyperthermia.
Figure 6.
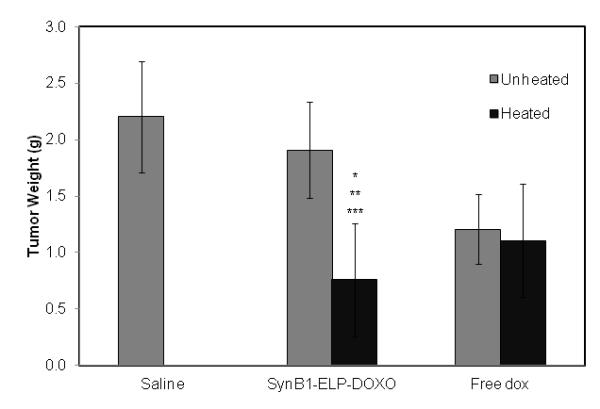
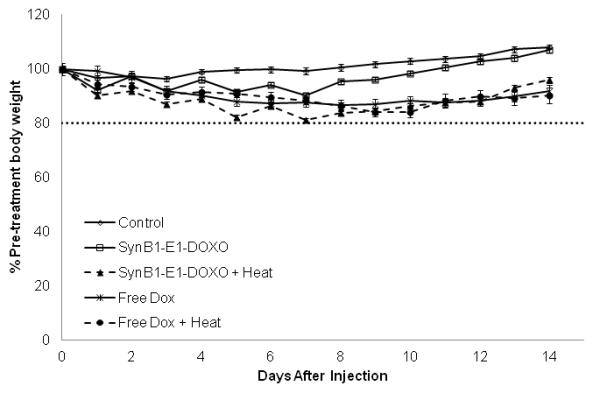
In vivo tumor reduction in E0771 breast cancer model. E0771 cells were injected subcutaneously in the mammary fat pad of C57BL/6 mice. Once tumors reached a volume of ~150 mm3, treatment with saline, free dox, or SynB1-ELP-DOXO was started on Day 0. (A) Arrows indicate treatment days on Day 0, 2, 4, and 6. Tumor volume was monitored daily by caliper measurement. ** indicates statistical difference between SynB1-ELP-DOXO hyperthermia group and saline, SynB1-ELP-DOXO no hyperthermia, and free dox no hyperthermia was observed on Day 7 through Day 14 with p < 0.0001, p < 0.0001, and p < 0.001 respectively. (B) On Day 14, animals were sacrificed, and tumors were removed and weighed. SynB1-ELP-DOXO heated tumors were smaller than in any other group. Statistical significance was determined by One-way Anova. * indicates statistical difference between SynB1-ELP-DOXO heat vs saline, p < 0.0001; ** indicates statistical difference between SynB1-ELP-DOXO heat vs SynB1-ELP-DOXO no heat, p < 0.0001; and *** indicates statistical difference between SynB1-ELP-DOXO heat vs free dox no heat, p < 0.01. (C) Animals were weighed every day beginning with Day 0 and continuing until Day 14. Free dox animals (X) lost weight after treatment and stayed at that level. SynB1-ELP-DOXO animals (Δ) initially lost weight but continued to regain their body weight.
Another approach by Chilkoti et al. has used ELP fused to multiple molecules of doxorubicin in the absence of a CPP. In this application, ELP-Dox conjugates with 8 Dox moieties conjugated to the macromolecule form micelle-like nanoparticles with Dox at the core surrounded by ELP. Tumor reduction in the C26 murine colon carcinoma cell line was enhanced by these micelles (MacKay et al., 2009). However, this nanoparticle was not thermally targeted to the tumor, so tumor targeting relied on the EPR effect alone. In contrast, the system described in this report has only one Dox per ELP, and it is actively targeted to the tumor by applied hyperthermia. Additionally, when evaluated by analytical ultracentrifugation at physiological concentrations, ELP sediments as a monomer, proving that SynB1-ELP-DOXO does not assemble into a nanoparticle (unpublished data).
4. Conclusions
In summary, this study demonstrates that ELP can be used as a thermally targeted drug vector for delivering doxorubicin to the tumor site. Comparing the CPPs, all constructs showed phase transition change in a clinically applicable temperature range, and internalization was clearly enhanced by the addition of a CPP. SynB1-ELP-DOXO, when combined with hyperthermia, reduced the size of the tumors in a syngeneic mouse breast cancer model more than two-fold compared to free doxorubicin. In systemic drug delivery, the designed polypeptide drug carrier will have several advantages over free chemotherapeutic agents. First, as a macromolecular drug carrier, it will preferentially accumulate in the tumors due to the enhanced permeability and retention (EPR) effect. Second, EPR-based passive targeting is further enhanced by an active stimuli response accumulation of ELP by locally applied hyperthermia. Finally, addition of cell penetrating peptide efficiently increases the ELP carrier’s tumor and cellular uptake. As a result, drugs delivered by this designed macromolecular carrier will exhibit significantly lower systemic toxicity compared to free drug. Therefore, we believe that the described thermally responsive ELP drug carrier has a great promise in the delivery of doxorubicin, its derivatives, and chemotherapeutics in general.
Acknowledgements
We would like to acknowledge Dr. F.M. Sirotnak for supplying the E0771 cell line. This work was supported by NSF Award #CBET-0931041 and the National Cancer Institute R21 CA113813-01A to D.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aumelas A, Mangoni M, Roumestand C, Chiche L, Despaux E, Grassy G, Calas B, Chavanieu A. Synthesis and solution structure of the antimicrobial peptide protegrin-1. Eur J Biochem. 1996;237:575–583. doi: 10.1111/j.1432-1033.1996.0575p.x. [DOI] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Davis AN, Raucher D. Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides. J Control Release. 2009;135:2–10. doi: 10.1016/j.jconrel.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Fokt I, Priebe W, Raucher D. Development of elastin-like polypeptide for thermally targeted delivery of doxorubicin. Biochem Pharmacol. 2007;73:620–631. doi: 10.1016/j.bcp.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Perkins E, Raucher D. A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth. Cancer Lett. 2012 doi: 10.1016/j.canlet.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4:1076–1085. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Whittom AA, Thomas E, Lyons D, Hebert MD, Raucher D. A thermally targeted peptide inhibitor of symmetrical dimethylation inhibits cancer-cell proliferation. Peptides. 2010;31:834–841. doi: 10.1016/j.peptides.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biersack H JS, Gromova I, Nielsen I, Westergard O, Andersen A. Active heterodimers are formed from human DNA topoisomerase II alpha and II beta isoforms. Proc Natl Acad Sci U S A. 1996;93:8288–8293. doi: 10.1073/pnas.93.16.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy J, Duncan R, Morrison GJ, Strohalm J, Plocova D, Kopecek J, Kaye SB. Activity of N-(2-hydroxypropyl)methacrylamide copolymers containing daunomycin against a rat tumour model. Biochem. Pharmacol. 1989;38:875–879. doi: 10.1016/0006-2952(89)90274-8. [DOI] [PubMed] [Google Scholar]
- Cummings J AL, Willmott N, Smyth JF. The molecular pharmacology of doxorubicin in vivo. Eur J Cancer. 1991;27:532–535. doi: 10.1016/0277-5379(91)90209-v. [DOI] [PubMed] [Google Scholar]
- Daniell H, Guda C, McPherson DT, Zhang X, Xu J, Urry DW. Hyperexpression of a synthetic protein-based polymer gene. Methods Mol Biol. 1997;63:359–371. doi: 10.1385/0-89603-481-X:359. [DOI] [PubMed] [Google Scholar]
- Dewhirst MW, Prosnitz L, Thrall D, Prescott D, Clegg S, Charles C, MacFall J, Rosner G, Samulski T, Gillette E, LaRue S. Hyperthermic treatment of malignant diseases: current status and a view toward the future. Semin Oncol. 1997;24:616–625. [PubMed] [Google Scholar]
- Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67:4418–4424. doi: 10.1158/0008-5472.CAN-06-4444. [DOI] [PubMed] [Google Scholar]
- Dreher MR, Raucher D, Balu N, Colvin O. Michael, Ludeman SM, Chilkoti A. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Control Release. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- Ewens A, Mihich E, Ehrke MJ. Distant metastasis from subcutaneously grown E0771 medullary breast adenocarcinoma. Anticancer Res. 2005;25:3905–3915. [PubMed] [Google Scholar]
- Hearst SM, Walker LR, Shao Q, Lopez M, Raucher D, Vig PJ. The design and delivery of a thermally responsive peptide to inhibit S100B-mediated neurodegeneration. Neuroscience. 2011;197:369–380. doi: 10.1016/j.neuroscience.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K, Maeda M, Inoue S, Yamashiro Y, Kaneda Y, Mu Y, Tsutsumi Y, Nakagawa S, Mayumi T. Amino acids and peptides. XXIX. Synthesis and antimetastatic effects of peptides and peptide-poly(ethylene glycol) hybrids related to the core sequence of the type III connecting segment domain of fibronectin. Biol Pharm Bull. 1996;19:1574–1579. doi: 10.1248/bpb.19.1574. [DOI] [PubMed] [Google Scholar]
- Kratz F. DOXO-EMCH (INNO-206): the first albumin-binding prodrug of doxorubicin to enter clinical trials. Expert Opin Investig Drugs. 2007;16 doi: 10.1517/13543784.16.6.855. [DOI] [PubMed] [Google Scholar]
- Kratz F EG, Kauffmann HM, Unger C. Acute and repeat-dose toxicity studies of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH), an albumin-binding prodrug of the anticancer agent doxorubicin. Hum Exp Toxicol. 2007;26:19–35. doi: 10.1177/0960327107073825. [DOI] [PubMed] [Google Scholar]
- Kratz F, Warnecke A, Scheuermann K, Stockmar C, Schwab J, Lazar P, Druckes P, Esser N, Drevs J, Rognan D, Bissantz C, Hinderling C, Folkers G, Fichtner I, Unger C. Probing the cysteine-34 position of endogenous serum albumin with thiol-binding doxorubicin derivatives. Improved efficacy of an acid-sensitive doxorubicin derivative with specific albumin-binding properties compared to that of the parent compound. J Med Chem. 2002;45:5523–5533. doi: 10.1021/jm020276c. [DOI] [PubMed] [Google Scholar]
- Kratz F, Warnecke A, Schmid B, Chung DE, Gitzel M. Prodrugs of anthracyclines in cancer chemotherapy. Curr Med Chem. 2006;13:477–523. doi: 10.2174/092986706776055751. [DOI] [PubMed] [Google Scholar]
- Lecornet E, Ahmed HU, Moore CM, Emberton M. Conceptual basis for focal therapy in prostate cancer. J Endourol. 2010;24:811–818. doi: 10.1089/end.2009.0654. [DOI] [PubMed] [Google Scholar]
- MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat Mater. 2009;8:993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay JA, Chilkoti A. Temperature sensitive peptides: engineering hyperthermia-directed therapeutics. Int J Hyperthermia. 2008;24:483–495. doi: 10.1080/02656730802149570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Maluta S, Dall’Oglio S, Romano M, Marciai N, Pioli F, Giri MG, Benecchi PL, Comunale L, Porcaro AB. Conformal radiotherapy plus local hyperthermia in patients affected by locally advanced high risk prostate cancer: preliminary results of a prospective phase II study. Int J Hyperthermia. 2007;23:451–456. doi: 10.1080/02656730701553260. [DOI] [PubMed] [Google Scholar]
- Massodi I, Bidwell GL, 3rd, Davis A, Tausend A, Credit K, Flessner M, Raucher D. Inhibition of ovarian cancer cell metastasis by a fusion polypeptide Tat-ELP. Clin Exp Metastasis. 2009;26:251–260. doi: 10.1007/s10585-009-9237-z. [DOI] [PubMed] [Google Scholar]
- Massodi I, Bidwell GL, 3rd, Raucher D. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J Control Release. 2005;108:396–408. doi: 10.1016/j.jconrel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Massodi I, Moktan S, Rawat A, Bidwell GL, 3rd, Raucher D. Inhibition of ovarian cancer cell proliferation by a cell cycle inhibitory peptide fused to a thermally responsive polypeptide carrier. Int J Cancer. 2010;126:533–544. doi: 10.1002/ijc.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson DT, Xu J, Urry DW. Product purification by reversible phase transition following Escherichia coli expression of genes encoding up to 251 repeats of the elastomeric pentapeptide GVGVP. Protein Expr Purif. 1996;7:51–57. doi: 10.1006/prep.1996.0008. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Chilkoti A. Purification of Recombinant Proteins by Fusion with Thermally Responsive Polypeptides. Nat. Biotechnol. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Kong GA, Dewhirst MW, Zalutsky MR, Chilkoti A. Targeting a Genetically Engineered Elastin-like Polypeptide to Solid Tumors by Local Hyperthermia. Cancer Res. 2001;61:1548–1554. [PubMed] [Google Scholar]
- Moktan S, Ryppa C, Kratz F, Raucher D. A thermally responsive biopolymer conjugated to an acid-sensitive derivative of paclitaxel stabilizes microtubules, arrests cell cycle, and induces apoptosis. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9560-x. [DOI] [PubMed] [Google Scholar]
- Moktan S, Ryppa C, Kratz F, Raucher D. A thermally responsive biopolymer conjugated to an acid-sensitive derivative of paclitaxel stabilizes microtubules, arrests cell cycle, and induces apoptosis. Inv New Drugs. 2010 doi: 10.1007/s10637-010-9560-x. [DOI] [PubMed] [Google Scholar]
- Nelson WG LL, Coffey DS. Newly replicated DNA is associated with DNA topoisomerase II in cultured rat prostatic adenocarcinoma cells. Nature. 1986;322:187–189. doi: 10.1038/322187a0. [DOI] [PubMed] [Google Scholar]
- Raucher D, Chilkoti A. Enhanced uptake of a thermally responsive polypeptide by tumor cells in response to its hyperthermia-mediated phase transition. Cancer Res. 2001;61:7163–7170. [PubMed] [Google Scholar]
- Ryppa C, Mann-Steinberg H, Fichtner I, Weber H, Satchi-Fainaro R, Biniossek ML, Kratz F. In vitro and in vivo evaluation of doxorubicin conjugates with the divalent peptide E-[c(RGDfK)2] that targets integrin alphavbeta3. Bioconjug Chem. 2008;19:1414–1422. doi: 10.1021/bc800117r. [DOI] [PubMed] [Google Scholar]
- Sadler K, Eom KD, Yang JL, Dimitrova Y, Tam JP. Translocating proline-rich peptides from the antimicrobial peptide bactenecin 7. Biochemistry. 2002;41:14150–14157. doi: 10.1021/bi026661l. [DOI] [PubMed] [Google Scholar]
- Shinohara K. Thermal ablation of prostate diseases: advantages and limitations. Int J Hyperthermia. 2004;20:679–697. doi: 10.1080/02656730412331286876. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Emi Y, Hasuda S, Kakeji Y, Maehara Y, Sugimachi K. Clinical application of hyperthermia combined with anticancer drugs for the treatment of solid tumors. Surgery. 2002;131:S78–84. doi: 10.1067/msy.2002.119308. [DOI] [PubMed] [Google Scholar]
- Takakura Y, Fujita T, Hashida M, Sezaki H. Disposition characteristics of macromolecules in tumor-bearing mice. Pharm. Res. 1990;7:339–346. doi: 10.1023/a:1015807119753. [DOI] [PubMed] [Google Scholar]
- Tatham AS, Shewry PR. Elastomeric proteins: biological roles, structures and mechanisms. Trends Biochem Sci. 2000;25:567–571. doi: 10.1016/s0968-0004(00)01670-4. [DOI] [PubMed] [Google Scholar]
- Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J. Pharm. Sci. 1994;83:601–606. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]


