Abstract
Minor alleles of polymorphisms in the fatty acid desaturase (FADS) gene cluster have been associated with reduced desaturation of the precursor polyunsaturated fatty acids (FAs) in small studies. The effects of these polymorphisms during progressive developmental stages have not previously been reported. Data from blood samples for 4342 pregnant women, 3343 umbilical cords reflecting the newborn's blood supply and 5240 children aged 7 years were analysed to investigate the associations of polyunsaturated FAs with rs1535 and rs174575—two polymorphisms in the FADS2 gene. Strong positive associations were observed between the minor G allele for these two markers, especially rs1535, and the substrates linoleic (18:2n-6) and α-linolenic (18:3n-3) acid. Negative associations were observed for the more highly unsaturated FAs such as arachidonic acid (20:4n-6), timnodonic acid (EPA, 20:5n-3) and cervonic acid (DHA, 22:6n-3). Bivariable genetic associations using the mother and child genotypes suggested that the newborn metabolism had a greater capacity to synthesize the more highly unsaturated omega-6 FAs than the more highly unsaturated omega-3 FAs. Nevertheless, despite the immaturity of the neonate, there was evidence that synthesis of DHA was occurring. However, by 7 years, no associations were observed with the maternal genotype. This suggested that the children's FA levels were related only to their own metabolism with no apparent lasting influences of the in utero environment.
INTRODUCTION
There are two main classes of polyunsaturated fatty acids (FAs), omega-3 and omega-6, characterized by a double bond at position 3 or 6 from the methyl end of the carbon chain. Omega-9 is a further class of polyunsaturated FAs, but appreciable amounts of these FAs occur only with omega-3 and omega-6 deficiency (1). The long-chain polyunsaturated FAs such as arachidonic acid (AA), timnodonic acid (EPA) and cervonic acid (DHA) are involved in key biological processes, including inflammatory responses, gene expression and cellular fluidity (2). Although both omega-3 and omega-6 FAs are involved in these processes, they infer different properties. For instance, the eicosanoids produced from AA tend to have a more inflammatory response than those from EPA.
Imbalance in the ratio of omega-6 to omega-3 FAs and/or deficiency in omega-3 FAs has been associated with adverse outcomes such as cardiovascular disease, type 2 diabetes and depression (3). Pregnant mothers may also be at particular risk of deficiency during the last trimester when the fetal brain undergoes a growth spurt and demands for DHA are at their highest. Supplementation and the consumption of fish may ease this burden with benefits for both the mother and child. These may include reductions in pregnancy-related depression (4) and improvements in child IQ and visual development (5–8).
A number of studies have investigated the association between variants in the fatty acid desaturas (FADS) gene cluster and blood FA levels (9–17). Within this cluster, FADS1 and FADS2 genes encode the Δ-5 and Δ-6 desaturases. FADS3 is also likely to be involved but as yet its function is unknown (18). These studies tended to report associations that were stronger for omega-6 FAs rather than omega-3 with AA having the most consistent results. Associations with the minor allele of FADS polymorphisms tended to be in a positive direction above AA/EPA in the cascade (see Fig. 1) whereas negative for the highly unsaturated FAs. These results suggest that the enzymes with the minor allele are less efficient at producing the derivatives with a complementary increase in pre-cursors left un-metabolized. However, with sample sizes ranging from 69 to 1231, many of these associations lacked statistically robustness. In particular, the association of FADS variants with DHA levels remains unclear.
Figure 1.
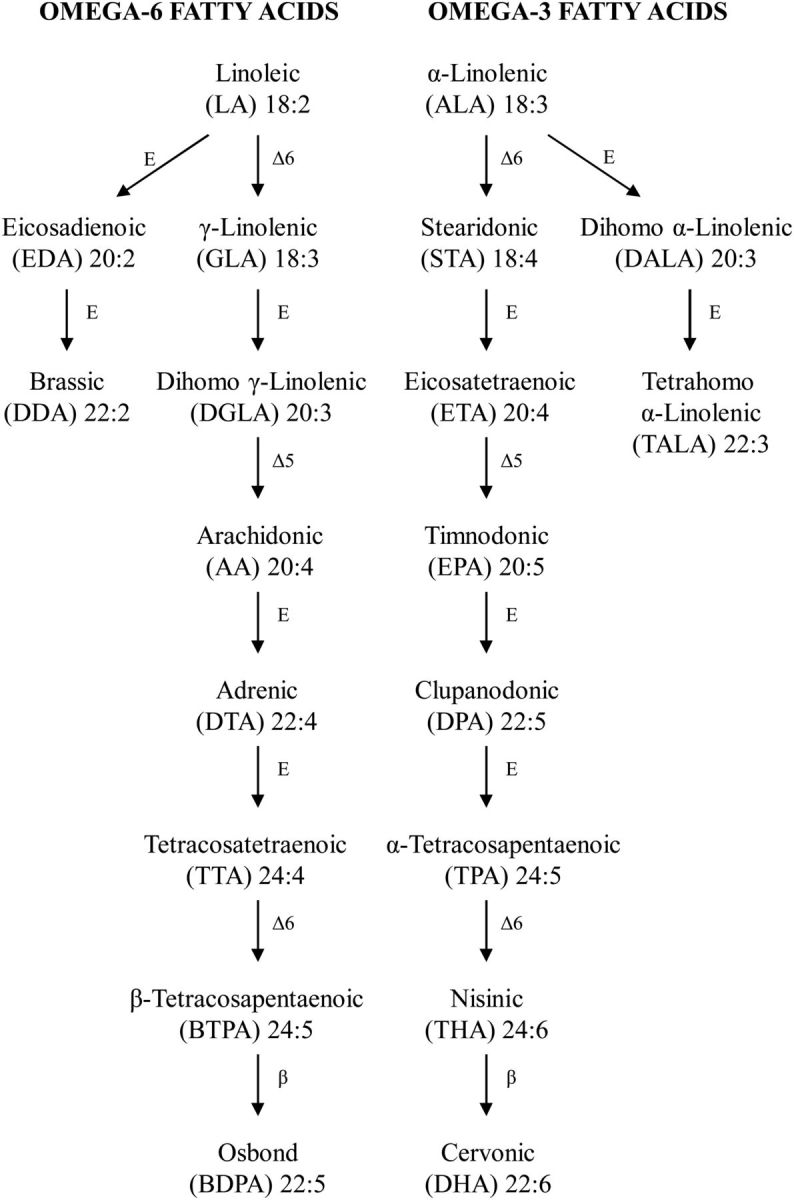
Metabolic pathways in the synthesis of omega-6 and omega-3 FAs. Omega-6 and omega-3 are shown with their common/scientific names, their abbreviations used in this study and their chemical structure (length of carbon chain: number of unsaturated bonds). Metabolic stages are labelled with E for elongation with two carbon atoms, β for beta oxidation or Δ-5 or Δ-6 indicating desaturation at positions 5 and 6 from the carboxyl terminus. The two isomers for docosapentaenoic and tetracosapentaenoic acids have been arbitrarily labelled as α or β for the omega-3 or omega-6 variants, respectively. There is an equivalent pathway for omega-9 FAs (not shown) starting with oleic acid (18:1) converting to mead acid (22:3) via Δ-6, elongation, Δ-5 and elongation stages.
In addition, these studies had left a number of questions unanswered. First, they had only considered the individual's genotype and its associations with FAs at one point in time in adolescence or adulthood. In this study, we examine the influence of the child genotype in combination with the maternal genotype at birth and at 7 years. This may elucidate any long-term influence of the in utero environment on FA metabolism in the offspring. Secondly, comparisons of the associations with maternal FAs in pregnancy and neonate FAs may identify the extent of the fetal dependence on the maternal supply. To date, there is only limited evidence that the supply of DHA and AA may be supplemented by the fetal synthesis (19). Finally, there is some evidence that oestrogen may enhance the metabolism of DHA in females (20). While this has been demonstrated in adults, it is less clear whether, in pre-pubertal children when differences in hormones levels are less, girls already exhibit a metabolic advantage.
This study will investigate two genetic variants of the FADS2 gene that have been implicated in an interaction with breastfeeding on child IQ (21,22). The importance of this gene may also be inferred from its role in two stages of the pathway (see Fig. 1) and from the Δ-6 desaturation generally being recognized as the rate-limiting step (23).
RESULTS
FA data
Of the 8012 maternal erythrocyte (RBC) samples taken at various times during pregnancy (median 28 weeks), the 4192 cord blood plasma samples and the 5632 plasma samples at 7 years, ∼1–3% of the data were excluded due to problems during the FA analysis (see Fig. 2). Descriptive statistics for these data are shown in Table 1. There was some evidence of bio-magnification, the preferential transfer across the placenta (19,24), with relative increases in AA of 46%, DHA of 17%, BDPA (22:5n-6) of 105% and DGLA (22:3n-6) of 83%, such that concentrations in cord blood were higher than in the maternal circulation (all P< 4.9 × 10−19, n = 1644 paired samples).
Figure 2.
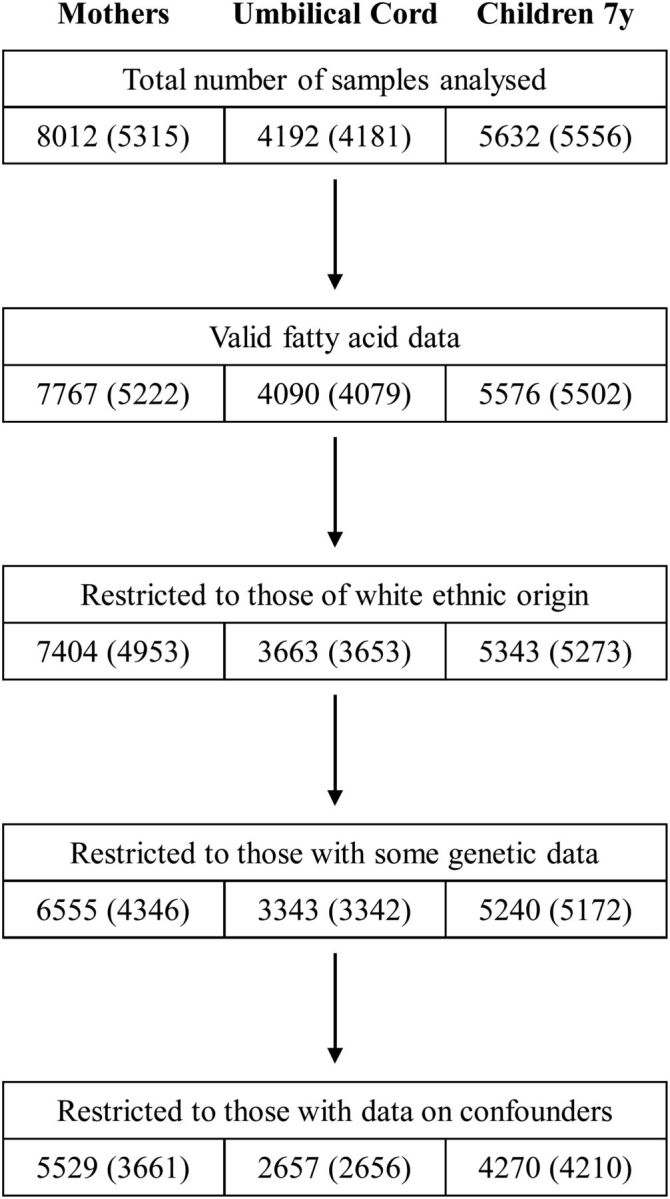
Availability of FA and genetic data used in these analyses. Sample sizes are shown at various stages with the number of corresponding mothers shown in parentheses. For maternal FA data, the number of mothers was less than the number of observations due to repeat measurements obtained at various times during pregnancy. For neonate and child data, this feature reflected multiple pregnancies. The availability of genetic data varied by FADS2 polymorphism and for mothers and offspring. The numbers reported for these data reflect those observations with at least one genotype available.
Table 1.
Median (IQR) of maternal and child FAs (as % of total FAs)
| Fatty acida |
Maternal RBC | Cord plasma | Child plasma 7 years | |
|---|---|---|---|---|
| Omega-9 | ||||
| Mead | 20:3 | 0.06 (0.04–0.09) | 0.14 (0.11–0.18) | |
| Omega-6 | ||||
| LA | 18:2 | 11.14 (9.61–12.58) | 9.75 (8.67–11.06)* | 30.63 (28.6–32.6) |
| GLA | 18:3 | 0.02 (0.005–0.03) | 0.35 (0.26–0.74)* | 0.36 (0.29–0.44) |
| EDA | 20:2 | 0.26 (0.21–0.30) | 0.29 (0.25–0.34)* | 0.20 (0.18–0.23) |
| DDA | 22:2 | 0.02 (0.005–0.03) | ||
| DGLA | 20:3 | 1.38 (1.05–1.69) | 2.64 (2.20–3.08)* | 1.69 (1.48–1.94) |
| AA | 20:4 | 6.09 (3.89–8.36) | 8.94 (7.16–11.53)* | 6.34 (5.49–7.27) |
| DTA | 22:4 | 0.93 (0.53–1.40) | 0.41 (0.33–0.52)* | 0.24 (0.21–0.28) |
| BDPA | 22:5 | 0.22 (0.14–0.32) | 0.47 (0.35–0.62)* | 0.18 (0.15–0.21) |
| Omega-3 | ||||
| ALA | 18:3 | 0.14 (0.10–0.18) | 0.14 (0.11–0.18) | 0.66 (0.53–0.84) |
| STA | 18:4 | 0.06 (0.04–0.08) | ||
| DALA | 20:3 | 0.005 (0.005–0.02) | ||
| TALA | 22:3 | 0.005 (0.005–0.005) | ||
| EPA | 20:5 | 0.24 (0.16–0.36) | 0.19 (0.14–0.25)* | 0.62 (0.51–0.74) |
| DPA | 22:5 | 0.66 (0.35–1.05) | 0.20 (0.15–0.27)* | 0.62 (0.53–0.71) |
| DHA | 22:6 | 2.01 (1.24–3.05) | 2.45 (1.85–3.44)* | 1.82 (1.52–2.17) |
| Gestation (range)b | 28 (4–44) | 40 (27–44) | 40 (25–44) | |
| nc | 6555d | 3394 | 5240 | |
aFor a description of the FA abbreviations, see Figure 1.
bMaternal blood samples were taken throughout pregnancy. This is reflected in the low median gestation and wide range. Gestations at birth are reported for cord and child plasma data.
cNumber of observations relate to mothers/children of white ethnic origin for whom genotype data (for rs174575 and rs1535) are available.
dThis total n relates to 4346 mothers with 2648, 1251, 391, 49, 6 and 1 mothers having 1 to 6 samples, respectively.
*P< 4.9 × 10−19 (minimum <1.2 × 10−278) for difference between 1644 paired maternal-cord samples.
It was most noticeable that LA (18:2n-6) levels varied between the three data sets. Such results have been reported elsewhere and appear to be a facet of differences between RBC and plasma and variations in plasma levels with age. A fuller discussion of these points is given in Supplementary Material, Results S1.
All FA data were derived from non-fasting blood samples. To explore the impact of different lengths of fasting, we analysed the 7-year FA data in relation to the time of blood samples (see Supplementary Material, Table S1). Total FA concentrations increased throughout the day with the difference from the earliest to the latest time reflecting a relative change of 11.4%. While monounsaturated FAs (as a percentage of total FAs) followed this pattern, the increase was less at 4.8%. In contrast, polyunsaturated FAs decreased by 3.6% with saturated FAs changing the least with an increase of 1.1%. Despite the overall pattern for polyunsaturated FAs, specific FAs deviated to varying extents. LA, the FA with the highest level, showed a decrease of 2.4%; AA showed a decrease of 7.3% while DHA, one of the FAs with the lowest levels, showed a decrease of 9.6%. ALA (18:3n-3) showed the opposite trend, increasing by 19.8%. The largest change in total FAs, FA groups or specific FAs, occurred after lunch.
FADS2 genotypes
Both genotypes for mother and child were in Hardy–Weinberg equilibrium (all P> 0.39). The minor allele frequencies were 26% (G allele for rs174 575) and 33% (G allele rs1535). Major alleles were C and A, respectively. Genotype models did not materially improve the explanation of FA data compared with the additive model in most analyses. However, there was evidence of non-linearity for the child genotype and EPA. In cord blood, GG children had a greater percentage of EPA than expected from the additive model (rs1535: genotype effect P= 0.029, deviation from additive model P= 0.011). In contrast at 7 years, GG children had less EPA than expected from the additive model (genotype effect P= 2.5 × 10−72, deviation from additive model P= 0.000033). Similar results were observed for child rs174575 genotype but not for the maternal genotypes. Overall, these discrepancies from the general pattern did not change the conclusions and consequently only results for the additive model are reported in Tables 2, 3, S2, S5 and S6.
Table 2.
Univariable associations of FADS2 polymorphism rs1535 (for mother and offspring genotype)a on maternal and child FAs (as % of total FAs)
| FAb |
Maternal RBC | Cord plasma | Child plasma 7 years | |||
|---|---|---|---|---|---|---|
| Mother | Mother | Offspring | Mother | Offspring | ||
| Omega-9 | ||||||
| Mead | 20:3 | −0.067 (0.023)* | −0.245 (0.026)*** | −0.552 (0.020)*** | ||
| Omega-6 | ||||||
| LA | 18:2 | 0.140 (0.020)*** | 0.247 (0.029)*** | 0.163 (0.028)** | 0.082 (0.027) * | 0.196 (0.021)*** |
| GLA | 18:3 | −0.028 (0.020) | 0.022 (0.030) | −0.000 (0.028) | −0.202 (0.027)*** | −0.412 (0.020)*** |
| EDA | 20:2 | 0.151 (0.021)*** | 0.175 (0.030)** | 0.099 (0.026)* | 0.145 (0.027)** | 0.337 (0.021)*** |
| DDA | 22:2 | 0.054 (0.021)** | ||||
| DGLA | 20:3 | 0.378 (0.020)*** | 0.465 (0.029)*** | 0.525 (0.026)*** | 0.204 (0.026)*** | 0.381 (0.021)*** |
| AA | 20:4 | −0.168 (0.020)*** | −0.175 (0.030)** | −0.247 (0.028)*** | −0.329 (0.026)*** | −0.640 (0.019)*** |
| DTA | 22:4 | −0.146 (0.020)*** | −0.110 (0.030)* | −0.166 (0.028)** | −0.138 (0.026)** | −0.287 (0.021)*** |
| BDPA | 22:5 | −0.173 (0.021)*** | −0.078 (0.030) * | −0.134 (0.028)** | −0.097 (0.027)* | −0.183 (0.021)*** |
| Omega-3 | ||||||
| ALA | 18:3 | 0.102 (0.020)*** | 0.039 (0.032) | 0.115 (0.028)* | 0.067 (0.026)* | 0.118 (0.021)** |
| STA | 18:4 | 0.091 (0.019)*** | ||||
| DALA | 20:3 | −0.014 (0.028) | ||||
| TALA | 22:3 | 0.006 (0.028) | ||||
| EPA | 20:5 | −0.074 (0.020)*** | −0.052 (0.035) | −0.022 (0.029) | −0.191 (0.027)*** | −0.359 (0.020)*** |
| DPA | 22:5 | −0.037 (0.020) | −0.026 (0.034) | 0.004 (0.028) | −0.187 (0.026)*** | −0.319 (0.021)*** |
| DHA | 22:6 | −0.106 (0.020)*** | −0.122 (0.035)* | −0.109 (0.028)* | −0.125 (0.026)** | −0.197 (0.021)*** |
| n | 6432 | 2427 | 2862 | 3095 | 5029 | |
aAssociations are reported as regression coefficient per copy of the minor G allele (SE) and significance. All fatty acids were standardized to have a variance of one.
bFor a description of the FA abbreviations, see Figure 1.
*P< 0.05, **P< 0.00001, ***P< 10−9 (minima 7.3 × 10−80, 2.2 × 10−82 and 2.1 × 10−216 for maternal, cord and 7-year data sets, respectively).
Table 3.
Bivariable associations of the mother and offspring rs1535 genotypea with cord and child FA levels (% total FAs)
| FAb | Cord plasma (n = 1916) |
Child plasma 7 years (n = 2923) | |||
|---|---|---|---|---|---|
| Mother | Offspring | Mother | Offspring | ||
| Omega-9 | |||||
| Mead | 20:3 | 0.001 (0.029) | −0.516 (0.030)*** | ||
| Omega-6 | |||||
| LA | 18:2 | 0.214 (0.037)** | 0.058 (0.038) | −0.003 (0.031) | 0.172 (0.032)** |
| GLA | 18:3 | 0.070 (0.037) | −0.016 (0.039) | −0.017 (0.031) | −0.369 (0.031)*** |
| EDA | 20:2 | 0.195 (0.035)** | 0.007 (0.036) | −0.053 (0.031) | 0.397 (0.032)*** |
| DGLA | 20:3 | 0.284 (0.035)*** | 0.391 (0.036)*** | 0.009 (0.030) | 0.406 (0.031)*** |
| AA | 20:4 | −0.085 (0.037)* | −0.215 (0.039)** | −0.023 (0.028) | −0.622 (0.029)*** |
| DTA | 22:4 | −0.065 (0.038) | −0.141 (0.039)* | −0.023 (0.031) | −0.238 (0.032)*** |
| BDPA | 22:5 | −0.029 (0.037) | −0.129 (0.039)* | −0.022 (0.031) | −0.162 (0.032)** |
| Omega-3 | |||||
| ALA | 18:3 | 0.021 (0.035) | 0.088 (0.036)* | −0.008 (0.031) | 0.120 (0.032)* |
| EPA | 20:5 | −0.022 (0.037) | −0.029 (0.039) | −0.018 (0.030) | −0.335 (0.030)*** |
| DPA | 22:5 | −0.010 (0.037) | 0.004 (0.039) | −0.051 (0.030) | −0.277 (0.031)*** |
| DHA | 22:6 | −0.085 (0.038)* | −0.083 (0.039)* | −0.022 (0.031) | −0.189 (0.031)** |
aAssociations are reported as regression coefficient per copy of the minor G allele (SE) and significance. All FAs were standardized to have a variance of one.
bFor a description of the FA abbreviations, see Figure 1.
*P< 0.05, **P< 0.00001, ***P< 10−9 (minima 2.7 × 10−26 and 5.9 × 10−95 for cord and 7-year data sets, respectively).
For the maternal data, minor alleles of the FADS2 polymorphisms were generally positively associated with omega-6 FAs with two or three double bonds and with omega-3 FAs with three or four double bonds (see Table 2 and Supplementary Material, Table S2). The associations were negative for the more highly unsaturated FAs. DALA (20:3n-3) and TALA (22:3n-3), which do not require the Δ-6 desaturase, had no association with these genetic variants. However, the equivalent omega-6 FAs, EDA (20:2n-6) and DDA (22:2n-6), had positive associations, perhaps reflecting a greater quantity of LA left un-metabolized enhancing metabolism to these FAs via elongation. The variant rs1535 had consistently stronger associations than rs174575. This was most pronounced for the omega-6 FAs such as LA and EDA where the effect sizes were 60% larger on average.
A similar pattern emerged for omega-6 FAs measured in cord plasma. The maternal genotypes tended to have stronger associations than child genotypes where the minor allele was associated with higher levels. However, the reverse was apparent for the more highly unsaturated FAs where the minor allele was associated with lower levels. For omega-3 FAs, a somewhat different pattern emerged with weak or non-existent associations for ALA and EPA. In addition, there was some evidence that the relative strength of the associations with maternal or offspring genotypes was the opposite to that observed for omega-6 FAs, such that child genotypes exhibited stronger associations for the short-chain omega-3 FAs, but weaker associations for the long-chain FAs. However, the differences in univariable effect sizes were often small. The two polymorphisms had similar effects for these data although rs1535 had the larger effect sizes in 16 of the 22 analyses.
For the 7-year data, all FAs examined showed evidence of an association with both maternal and offspring genotypes. But in every case, a stronger association was observed with the offspring genotype. As for the maternal data, rs1535 tended to have the stronger association than rs174575 with larger effect sizes in 21 of the 24 analyses. However, for both maternal and offspring genotypes, rs174575 had the stronger association for DGLA.
Bivariable associations of maternal and offspring genotypes tended to confirm these univariable associations for cord plasma (see Table 3). However, it became apparent that the omega-6 associations were largely attributable to one source—either the maternal supply, as indexed by the maternal genotype (for the short-chain polyunsaturated FAs), or the newborn's own metabolism as indicated by the offspring genotype (for the long-chain polyunsaturated FAs). In contrast, the omega-3 associations were more equal. Both maternal and offspring genotypes contributed to the two important FAs such as AA and DHA, and also to DGLA. By 7 years, the pattern of associations had changed such that the influence of the maternal supply was minimal.
Despite the strong statistical evidence for these associations, many of the effect sizes were modest, in many cases accounting for <3% of the total variance in maternal and cord samples (see Supplementary Material, Table S3). DGLA was the exception, having the highest genetic explanations overall representing ∼7, 12 and 6% for maternal, cord and child levels, respectively, for rs1535. The equivalent R2 for AA were 1.4, 2.6 and 18%, and for DHA, 0.6, 0.9 and 1.7%, respectively. For 7-year plasma, 9 of the 12 FAs had explanations >3% for rs1535.
Multivariable adjustment for potential confounders
The putative confounders were generally highly associated with FAs but not with genetic variants (see Supplementary Material, Table S4). The associations observed in unadjusted analyses generally persisted after adjustment although some effect sizes attenuated in these analyses but usually by <5%. The largest attenuations were observed for AA (cord plasma) by 9.9%, DHA (cord plasma) by 7.7% and DHA (7-year plasma) by 7.2% (see Supplementary Material, Table S5). Some effect sizes increased after adjustment most notably for DGLA (cord plasma) and BDPA (7-year plasma). The limited extent of the changes in genetic effects after adjustment combined with the lack of any consistency in the direction of the changes is indicative of non-confounded associations. This is to be expected with genetic variants (25).
Interactions with gender
We further explored whether the genetic associations were modified by gender. Given the more complex genetic associations with cord data, these analyses were confined to the 7-year data (see Supplementary Material, Table S6). Boys had a more pronounced negative genetic effects for AA with rs1535 and for mead acid with both polymorphisms. In contrast, the opposite effect was observed for DHA with rs174 575. These results reflected similar FA levels in boys and girls with GG genotype (homozygotes in the minor allele) but differences in levels for homozygotes in the major alleles (see Fig. 3). Adjustment for multiple comparisons (critical P= 0.0021) suggested that all these results could have occurred by chance. We also examined gender differences in FA levels after adjusting for confounders and exogenous or dietary sources of FAs. This is based on the assumption that any residual gender effect could be attributed to endogenous sources including interactions with genetic variants. Boys had higher levels for 8 of the 12 polyunsaturated FAs (EDA, P= 0.010; all other P< 3.4 × 10−5; n = 3951), with similar levels for LA, EPA and DHA (P = 0.75, 0.064 and 0.67) and lower levels for ALA (P = 0.016) (see Supplementary Material, Table S7). The critical P-value for these 12 comparisons was 0.038, using the false discovery rate.
Figure 3.
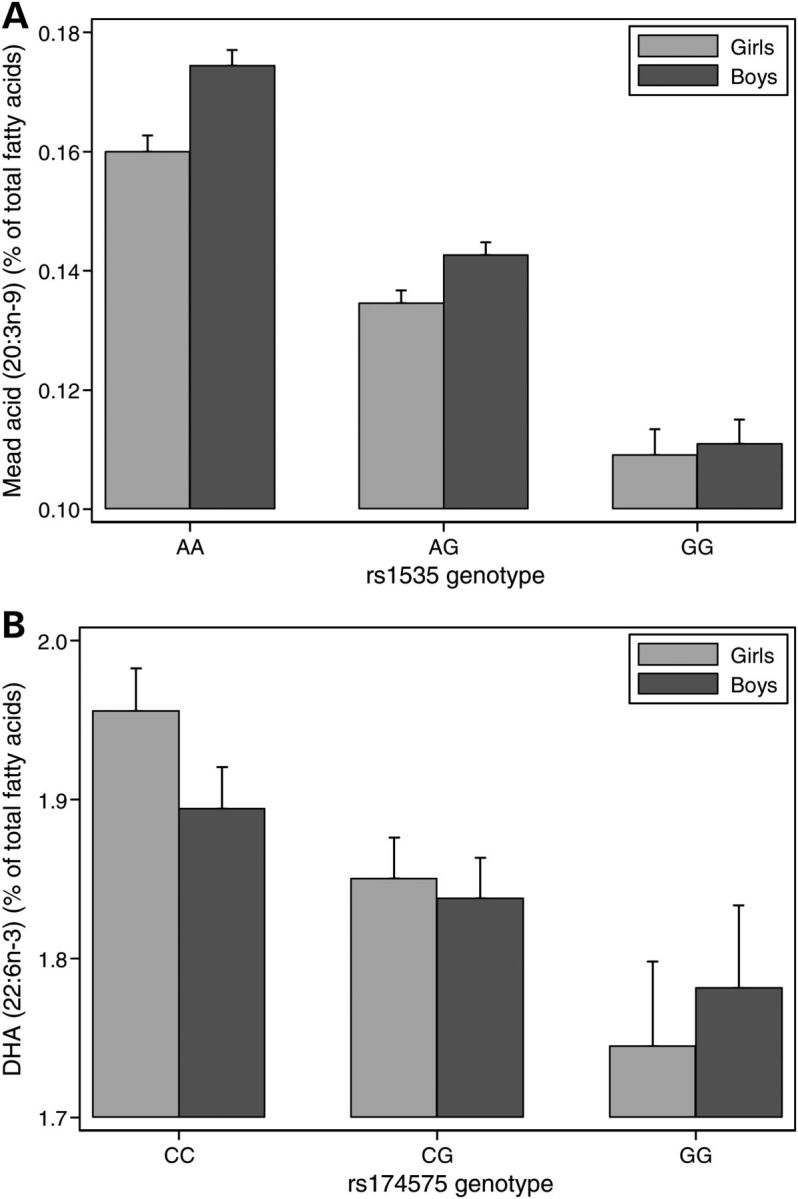
Gender interactions with FADS2 polymorphisms for DHA and mead acid levels at 7 years. Gender by genotype means are shown for two FAs with 95% CIs. (A) Boys showed a stronger genetic effect than girls for mead acid (P = 0.0021). In contrast for (B), girls showed a stronger effect for DHA (P = 0.039). Both these interactions tended to reflect similar levels for GG boys and girls with differences becoming most marked for homozygotes in the major allele.
DISCUSSION
This is the first large-scale study to compare the effect of two polymorphisms in the FADS2 gene on FA profiles at three time points: during pregnancy for the mother, at birth for the neonate and at 7 years old for the child. In all three data sets, the two genetic variants had strong associations with most of the polyunsaturated FAs reported in this study. These results were consistent with the minor allele being associated with lower activity leading to reduced amounts of the products but increased amounts of the un-metabolized precursors. These associations were not restricted to precursors or products of the specific Δ-6 metabolic stage, but included other associations possibly reflecting linkage disequilibrium (LD) with Δ-5 variants in the FADS1 gene.
The pattern of results was much clearer than that observed in other single studies probably due to the larger sample size. No previous study has reported robust associations for DHA (9,11–14) or for BDPA (14) for FADS2 single nucleotide polymorphisms (SNPs). The synthesis of DHA is particularly important for the development of the offspring as DHA accumulates in the brain and retina (26). The result from this study for DHA complements another study that showed an association with a deletion in the intergenic FADS1–FADS2 region considered the promoter region for FADS2 (17). In contrast, a recent meta-analysis of the results from five cohorts with European ancestry failed to observe robust associations between FADS2 and DHA after correction for multiple testing despite a combined n of 8866 (27). In our 7-year data, a negative association with the minor allele was observed for GLA (18:3n-6). This association would be expected since GLA is the product of a Δ-6 desaturase on LA. This association has previously been reported in one study (14) but not in another study (13). The lack of an association with DALA and TALA is consistent with the metabolic pathway for these FAs which do not require a Δ-6 desaturase for their synthesis. However, the positive associations for the equivalent omega-6 FAs, EDA and DDA, may also suggest that the lack of association for these omega-3 FAs arises due to extensive conversion of ALA into EPA leaving low levels of the substrate available for conversion to DALA and TALA (28).
Results for the cord data showed contrasting results for the maternal and child genotypes. For some FAs, the maternal association was stronger while for others the child genotype showed the stronger association. This pattern of results suggests that two different mechanisms are operating. Since the fetus is dependent on the maternal supply, it was to be expected that the maternal signal as observed in the maternal blood would be reflected in the cord blood. The modification of this signal suggests that the placenta is compensating for variations in the maternal circulation particularly for the highly unsaturated omega-6 FAs. A recent paper has indicated that the FA-binding protein gene, FABP-3, may be involved in this process (Steer et al., submitted for publication). Secondly, these results may also reflect differential stages of maturity of the newborn's metabolic systems with some FAs being dominated by the maternal supply but others less so. If this explanation is valid, it suggests that the newborn has a greater capacity to synthesize the more highly unsaturated omega-6 as for the similar omega-3 FAs (19,24). For instance, the effect of the child genotype was twice that of the maternal genotype for AA although this difference was present only when the precursor LA was in abundance. In contrast, for the omega-3 DHA, the effect sizes were similar.
There was evidence of bio-magnification for DGLA, AA, BDPA and DHA in this study. Although some of these increases in cord FAs might have been due to differences in the FA profiles of RBC and total plasma, other studies have shown similar relative increases between maternal-cord RBC and plasma phospholipids of 66, 87, 93 and 59%, respectively, on average (29,30). While the variation between these studies and blood fraction is large, the increase in DHA being less than BDPA is consistent with this study. Given the results from our bivariable genetic associations, it seems probable that some of the increases may be a reflection of the newborn's own metabolism.
It has been suggested that the maternal diet during pregnancy can have long-lasting influences on child development, including behavioural and cognitive outcomes (6). From our results, there is no evidence that this is due to any ‘programmed’ impairment in the synthesis of DHA. By 7 years of age, we found no association between FA levels and the maternal genotype after adjustment for the child genotype. More plausible explanations for such long-term developmental effects may be subtle changes to the neuroanatomy or neurochemistry resulting from nutritional deficiencies at critical time points (31).
A companion study on the same cohort has examined nine other FADS2 polymorphisms in relation to maternal FA levels (32). As expected, our results supported the findings from that study. The strength of the associations varied between polymorphisms with perhaps rs174578, rs174574 and then rs1535 having the strongest associations overall across different FAs. From the present study, rs1535 had the strongest associations for DGLA and AA compared with the other nine SNPs and rs174575.
Although, in general, the FADS2 polymorphisms explained a modest proportion of the variability in FAs, by 7 years, this was not always the case. The explanation of AA in this study of 18% for rs1535 was equivalent to the reported explanation of 19% for rs174537, 28% by five SNPs in the FADS1/FADS2 region and 19% for rs174546 (12,14,33) but higher than 10% for rs174537 and 2.6% for rs174546 in other studies (34,35). While we might expect a functional SNP to have the strongest associations with particular FAs, which reflect the substrates or products of its associated enzyme, it is surprising that rs174537 and rs174546 (FADS1) had the strongest associations with AA as well as rs1535. The explanation appears to be that, despite being related to different genes, all three SNPs were in perfect LD (HapMap build 21, CEU database). This would suggest that other criteria may be needed to identify functional SNPs.
The FADS2 variants examined in this study were related to changes in many FAs, and, as a consequence, cannot be used, in a straightforward fashion, as an instrument for changes in the level of any particular FA within a Mendelian randomization framework (36). However, they may be useful in identifying the role of the highly unsaturated FAs if such a role is largely unrelated to the specific FAs in the omega-6 or omega-3 pathways. In this context, rs1535 may be a candidate for future research. Interrogating further genetic variants in these pathways may allow for identification of more specific influences on particular FAs. Alternatively, interactions between dietary intake and the genetic variants identified in the present study can strengthen evidence regarding the causal effects of particular components of dietary intake on health outcomes (37).
Gender has been proposed to modify FA metabolism in two ways (20). First, women may have a reduced requirement for ALA as a substrate in β-oxidation perhaps due to their lower muscle mass. This increases the availability of ALA as a precursor for the long-chain omega-3 FAs such as DHA. Secondly, oestrogen may increase the activity of the desaturase and elongase enzymes. In this study, we found little or no evidence of increased Δ-6 desaturase activity after adjustment for multiple comparisons. A weaker genetic effect for mead acid in girls of borderline significance and lower levels for many of the omega-6 FAs in girls might possibly support the increased activity hypothesis if these results reflect enhanced preferences to convert the omega-3 rather than the omega-6 or the omega-9 FAs (38). We observed higher levels of ALA in girls as expected by the β-oxidation hypothesis; this did not translate to higher levels of EPA or DHA after adjustment. In contrast, there was evidence of differences in DPA (22:5n-3) levels. However, boys had higher levels not lower levels as expected. Overall, our results are somewhat equivocal that may reflect the smaller difference in oestrogen levels between pre-pubertal boys and girls compared with adults. Further work is needed to explore whether the genetic signal differs between pre-pubertal boys and girls and, if so, whether oestrogen or higher levels of ALA are amplifying the genetic signal in girls.
In summary, we have shown strong associations of two FADS2 SNPs with levels of polyunsaturated FAs. We have explored the impact of the in utero environment as influenced by the maternal genotype on the child's FA levels. At birth, the influence was strong but not for the very highly unsaturated omega-6 FAs (DTA (22:4n-6) or lower in the omega-6 pathway). Both AA and DHA showed some association with the maternal supply. The child's metabolism seemed to be mainly responsible for the variation between individuals in the highly unsaturated omega-6 FAs but also contributed to AA and DHA. By 7 years, the influence of the maternal supply had dissipated.
MATERIALS AND METHODS
Study population
The Avon Longitudinal Study of Parents and Children (ALSPAC) was established to explore the environmental, social, psychological and genetic factors associated with child health and development. It recruited 14 541 pregnant women in the Bristol area who had an expected delivery date between April 1991 and December 1992. The study area comprises a mixture of rural areas, inner-city deprivation, suburbs and moderate-sized towns as well as the city of Bristol. The cohort is broadly similar to the UK in terms of a range of demographic variables. A total of 13 988 children from the study were alive at age 1 year (39). Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees.
Maternal erythrocyte FAs
At least one blood sample was taken for FA analysis from 5144 mothers during pregnancy, of whom 4136 had at least one sample taken after 20 weeks of pregnancy. Up to six samples were available for each mother. Blood samples were collected in heparin-containing tubes and centrifuged at 1500g (2500 rpm) for 15 min at 4°C to separate the red cells, which were then stored at −20°C until 1993 and subsequently at −70°C. Frozen red blood cell samples were shipped to the laboratories of Scotia Pharmaceuticals in Canada in 1996 for FA composition analysis as described previously (40). Briefly, lipids from thawed red blood cells were extracted with chloroform and methanol, extracted lipids were redissolved in chloroform (100 μl) and phospholipids isolated by thin-layer chromatography with a mixture of hexane/diethyl ether/acetic acid (80:20:1 vol:vol:vol). FA methyl esters were prepared by incubation with 120 g/l boron trifluoride in methanol at 90°C for 30 min. FA methyl esters were taken up in hexane. The amounts of 40 FAs were measured by gas-liquid chromatography. FA methyl esters were identified by comparison with authenticated standards. FA concentrations below the limit of detection of the assay (0.01% of standards) were recoded to half that value. Thirteen saturated (12:0 to 24:0), 11 monounsaturated (14:1 to 24:1) and 16 polyunsaturated (1 omega-9, 8 omega-6 and 7 omega-3) FAs were measured.
Cord and child 7-year plasma FAs
Umbilical cord blood was collected at birth and stored at 4°C for 0–8 days until transported to the ALSPAC laboratory. Blood samples were also obtained from the children aged ∼7 years at a special ALSPAC clinic. From these samples, plasma were obtained after centrifugal separation and frozen immediately. All samples were stored at −70°C, thawed once to obtain a 100 μl aliquot, shipped airfreight on dry ice to Rockville, MD, and thawed a second time for analysis. Transmethylation of lipids with acetyl chloride and methanol was performed using a simplified method based on the Lepage and Roy procedure (41), using a high throughput automated method (42). Internal calibration was conducted by adding internal standards to each assay. A second standard was used to quantify the exact amount of internal standard in every batch for ongoing assay of experimental variability. Freedom Evo Instrument 200 (TECAN Trading AG, Switzerland) was utilized for the automatic transmethylation and extraction of FAs employing the customized control and automation software (EVOware v2.0, SP1, Patch3). Gas chromatography 6890 Plus LAN system (Agilent Technologies, Inc., Santa Clara, CA, USA) coupled with a fused-silica, narrow-bored DB-FFAP capillary column (Agilent 127–32H2, 15 m × 0.1 mm I.D. × 0.1 μm film thickness) which was used for chromatographic separation of the fatty acid methyl esters as reported previously (42). The assay was linear in the range of 1–600 μg/ml plasma. The within and between day imprecision was 3.26 ± 1.2 and 2.95 ± 1.6% for FA concentrations. Assays were undertaken in 2008–2010. In all, 22 FAs were measured including 11 polyunsaturates. For 7-year data, the omega-9 FA, mead acid, was additionally reported.
FADS2 genotyping
DNA was extracted from all available stored samples relating to 9656 children and 8678 mothers. Genotyping was undertaken by KBioscience Ltd using their own form of competitive allele-specific polymerase chain reaction system (KASPar) for SNP analysis. The failure rates for rs174575 and rs1535 were 6.5 and 5.2% for children and 4.9 and 4.2% for mothers. The error rate for genotyping of duplicate samples was <0.2%.
Statistical analysis
Linear regression was used to analyse the FA data. Although untransformed results are reported for ease of interpretation, log-transformed data were also analysed to investigate sensitivity to non-normality. Adjustments were made for repeat observations in maternal data using random effect models. The effects of genotypes were modelled using additive (1 df) or genotype (2 df) effects. Additive effects are reported per copy of the minor allele. Analyses were restricted to those mothers or children of white ethnic origin to avoid problems of Hardy–Weinberg disequilibria amongst the non-white sub-group. Ethnicity used in this selection was derived from questionnaire data as reported by the mother and school census data. Maternal FAs were analysed by the maternal genotype only. Due to the possible effects of the maternal supply and the infant's own metabolism on cord and child plasma FA levels, these data were analysed by the maternal and infant genotypes individually in univariable analyses and jointly in bivariable analyses to assess each genotype's independent effect. Analyses were also repeated adjusting for a number of potential confounders including gestation, multiple pregnancy, parity, maternal age, smoking during pregnancy, pre-pregnancy BMI, family adversity and gender (cord and child data only).
Due to the LD between rs1535 and rs174575 (r2 = 0.66, D′ = 0.97 for both maternal and child genotypes), the main results are presented for rs1535 with results for rs174575 reported in Supplementary Material.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the National Oceanic and Atmospheric Administration, USA and the Waterloo Foundation, UK.
Supplementary Material
ACKNOWLEDGEMENTS
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council (Grant ref: 74882), the Wellcome Trust (Grant ref: 076467) and the University of Bristol currently provide core support for ALSPAC. This publication is the work of the authors and C.D.S. will serve as guarantor for the contents of this paper.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press; 2005. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura M.T., Nara T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 3.Yashodhara B.M., Umakanth S., Pappachan J.M., Bhat S.K., Kamath R., Choo B.H. Omega-3 fatty acids: a comprehensive review of their role in health and disease. Postgrad. Med. J. 2009;85:84–90. doi: 10.1136/pgmj.2008.073338. [DOI] [PubMed] [Google Scholar]
- 4.Golding J., Steer C., Emmett P., Davis J.M., Hibbeln J.R. High levels of depressive symptoms in pregnancy with low omega-3 fatty acid intake from fish. Epidemiology. 2009;20:598–603. doi: 10.1097/EDE.0b013e31819d6a57. [DOI] [PubMed] [Google Scholar]
- 5.Helland I.B., Smith L., Saarem K., Saugstad O.D., Drevon C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 6.Hibbeln J.R., Davis J.M., Steer C., Emmett P., Rogers I., Williams C., Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 7.Williams C., Birch E.E., Emmett P.M., Northstone K. ALSPAC Study Team. Stereoacuity at age 3.5 y in children born full-term is associated with prenatal and postnatal dietary factors: a report from a population-based cohort study. Am. J. Clin. Nutr. 2001;73:316–322. doi: 10.1093/ajcn/73.2.316. [DOI] [PubMed] [Google Scholar]
- 8.Oken E., Radesky J.S., Wright R.O., Bellinger D.C., Amarasiriwardena C.J., Kleinman K.P., Hu H., Gillman M.W. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am. J. Epidemiol. 2008;167:1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gieger C., Geistlinger L., Altmaier E., de Angelis M.H., Kronenberg F., Meitinger T., Mewes H.W., Wichmann H.E., Weinberger K.M., Adamski J., et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet. 2008;4:e1000282. doi: 10.1371/journal.pgen.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinelli N., Girelli D., Malerba G., Guarini P., Illig T., Trabetti E., Sandri M., Friso S., Pizzolo F., Schaeffer L., et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am. J. Clin. Nutr. 2008;88:941–949. doi: 10.1093/ajcn/88.4.941. [DOI] [PubMed] [Google Scholar]
- 11.Malerba G., Schaeffer L., Xumerle L., Klopp N., Trabetti E., Biscuola M., Cavallari U., Galavotti R., Martinelli N., Guarini P., et al. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids. 2008;43:289–299. doi: 10.1007/s11745-008-3158-5. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T., Shen J., Abecasis G.R., Kisialiou A., Ordovas J.M., Guralnik J.M., Singleton A., Bandinelli S., Cherubini A., Arnett D., et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009;5:e1000338. doi: 10.1371/journal.pgen.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rzehak P., Heinrich J., Klopp N., Schaeffer L., Hoff S., Wolfram G., Illig T., Linseisen J. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br. J. Nutr. 2009;101:20–26. doi: 10.1017/S0007114508992564. [DOI] [PubMed] [Google Scholar]
- 14.Schaeffer L., Gohlke H., Muller M., Heid I.M., Palmer L.J., Kompauer I., Demmelmair H., Illig T., Koletzko B., Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 15.Bokor S., Dumont J., Spinneker A., Gonzalez-Gross M., Nova E., Widhalm K., Moschonis G., Stehle P., Amouyel P., De Henauw S., et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J. Lipid Res. 2010;51:2325–2333. doi: 10.1194/jlr.M006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie L., Innis S.M. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J. Nutr. 2008;138:2222–2228. doi: 10.3945/jn.108.096156. [DOI] [PubMed] [Google Scholar]
- 17.Baylin A., Ruiz-Narvaez E., Kraft P., Campos H. Alpha-linolenic acid, delta(6)-desaturase gene polymorphism, and the risk of nonfatal myocardial infarction. Am. J. Clin. Nutr. 2007;85:554–560. doi: 10.1093/ajcn/85.2.554. [DOI] [PubMed] [Google Scholar]
- 18.Pédrono F., Blanchard H., Kloareg M., D'Andréa S., Daval S., Rioux V., Legrand P. The fatty acid desaturase 3 gene encodes for different FADS3 protein isoforms in mammalian tissues. J. Lipid Res. 2010;51:472–479. doi: 10.1194/jlr.M000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haggarty P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010;30:237–255. doi: 10.1146/annurev.nutr.012809.104742. [DOI] [PubMed] [Google Scholar]
- 20.Burdge G.C., Calder P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 21.Steer C.D., Davey Smith G., Emmett P.M., Hibbeln J.R., Golding J. FADS2 polymorphisms modify the effect of breastfeeding on child IQ. PLoS One. 2010;5:e11570. doi: 10.1371/journal.pone.0011570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caspi A., Williams B., Kim-Cohen J., Craig I.W., Milne B.J., Poulton R., Schalkwyk L.C., Taylor A., Werts H., Moffitt T.E. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc. Natl Acad. Sci. USA. 2007;104:18860–18865. doi: 10.1073/pnas.0704292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta. 2000;1486:219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 24.Cetin I., Alvino G., Cardellicchio M. Long chain fatty acids and dietary fats in fetal nutrition. J. Physiol. Lond. 2009;587:3441–3451. doi: 10.1113/jphysiol.2009.173062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davey Smith G., Lawlor D.A., Harbord R., Timpson N., Day I., Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4:1985–1992. doi: 10.1371/journal.pmed.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Innis S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 27.Lemaitre R.N., Tanaka T., Tang W.H., Manichaikul A., Foy M., Kabagambe E.K., Nettleton J.A., King I.B., Weng L.C., Bhattacharya S., et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011;7:e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyens P.L., Spilker M.E., Zock P.L., Katan M.B., Mensink R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006;84:44–53. doi: 10.1093/ajcn/84.1.44. [DOI] [PubMed] [Google Scholar]
- 29.Pankiewicz E., Cretti A., Ronin-Walknowska E., Czeszynska M.-B., Konefal H., Hnatyszyn G. Maternal adipose tissue, maternal and cord blood essential fatty acids and their long-chain polyunsaturated derivatives composition after elective caesarean section. Early Hum. Dev. 2007;83:459–464. doi: 10.1016/j.earlhumdev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Vlaardingerbroek H., Hornstra G. Essential fatty acids in erythrocyte phospholipids during pregnancy and at delivery in mothers and their neonates: comparison with plasma phospholipids. Prostaglandins Leukot. Essent. Fatty Acids. 2004;71:363–374. doi: 10.1016/j.plefa.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 31.McNamara R.K., Carlson S.E. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Koletzko B., Lattka E., Zeilinger S., Illig T., Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: findings from the Avon Longitudinal Study of Parents and Children. Am. J. Clin. Nutr. 2011;93:211–219. doi: 10.3945/ajcn.110.006189. [DOI] [PubMed] [Google Scholar]
- 33.Rzehak P., Thijs C., Standl M., Mommers M., Glaser C., Jansen E., Klopp N., Koppelman G.H., Singmann P., Postma D.S., et al. Variants of the FADS1 FADS2 gene cluster, blood levels of polyunsaturated fatty acids and eczema in children within the first 2 years of life. PLoS One. 2010;5:e13261. doi: 10.1371/journal.pone.0013261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathias R.A., Vergara C., Gao L., Rafaels N., Hand T., Campbell M., Bickel C., Ivester P., Sergeant S., Barnes K.C., et al. FADS genetic variants and omega-6 polyunsaturated fatty acid metabolism in a homogeneous island population. J. Lipid Res. 2010;51:2766–2774. doi: 10.1194/jlr.M008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zietemann V., Kroger J., Enzenbach C., Jansen E., Fritsche A., Weikert C., Boeing H., Schulze M.B. Genetic variation of the FADS1 FADS2 gene cluster and n-6 PUFA composition in erythrocyte membranes in the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Br. J. Nutr. 2010;104:1748–1759. doi: 10.1017/S0007114510002916. [DOI] [PubMed] [Google Scholar]
- 36.Davey Smith G., Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 37.Davey Smith G. Use of genetic markers and gene–diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr. 2011;6:27–43. doi: 10.1007/s12263-010-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer S. Dietary polyunsaturated fatty acids and eicosanoid formation in humans. Adv. Lipid Res. 1989;23:169–198. doi: 10.1016/b978-0-12-024923-7.50008-x. [DOI] [PubMed] [Google Scholar]
- 39.Golding J., Pembrey M., Jones R. the ALSPAC Study Team. ALSPAC—the Avon Longitudinal Study of Parents and Children: I. Study methodology. Paediatr. Perinat. Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 40.Newson R.B., Shaheen S.O., Henderson A.J., Emmett P.M., Sherriff A., Calder P.C. ALSPAC Study Team. Umbilical cord and maternal blood red cell fatty acids and early childhood wheezing and eczema. J. Allergy Clin. Immun. 2004;114:531–537. doi: 10.1016/j.jaci.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Lepage G., Roy C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 42.Masood M.A., Salem N. High-throughput analysis of plasma fatty acid methyl esters employing robotic transesterification and fast gas chromatography. Lipids. 2008;43:171–180. doi: 10.1007/s11745-007-3130-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


