Abstract
Metastatic dissemination in prostate cancer is often early, however not all cancer cells form clinical metastases. Map kinase kinase 4 (MKK4) suppresses metastasis in a preclinical prostate cancer model. We hypothesize that MKK4 will specifically inhibit metastatic colonization through impaired proliferation.
Three highly metastatic rat prostate cancer cell lines (AT6.1, Mat-Lu, AT3.1) were employed. Stably over-expressing HA-MKK4 or vector control lines were injected into immunocomprimised mice. These experiments validated that HA-MKK4 specifically affects metastatic colonization and increases survival. Median survival (days) with HA-MKK4 vs. vector was 42 vs. 28 (p<0.0001) for AT6.1, 25 vs. 19 (p<0.0001) for Mat-Lu and 27 vs. 20 (p<0.0001) for AT3.1. HA-MKK4 suppresses colonization within 14 days post dissemination, after which, exponential proliferation resumes. Although overt metastases retain HA-MKK4, it is inactive within these lesions. Nonetheless, metastasis derived cell lines were shown to retain functional HA-MKK4 and like their parental HA-MKK4 line, are suppressed for experimental metastasis formation in vivo. Disseminated AT6.1-HA-MKK4 cells were analyzed and were found to have an alteration in cell cycle. Specifically, there was an accumulation of cells in G1-phase (p=0.024), and decrease in S-phase (p=0.037) compared to vector.
In multiple prostate cancer lines, HA-MKK4 suppresses an early step in metastatic colonization. These data support a model in which MKK4 activation at the metastatic site causes a cell-cycle arrest, which is eventually overcome despite presence of functional HA-MKK4. Further studies will specifically interrogate the regulation of MKK4 activation within the metastatic microenvironment and the down-stream molecular events critical for metastasis suppression.
Keywords: metastasis suppressor, metastatic colonization, prostate cancer, cell cycle
Introduction
This year, it is estimated that 569,490 Americans will die from cancer; most of them from metastatic disease1. There is growing recognition that cancer cells disseminate from the primary tumor early in the natural history of many cancers. For example, although more than 90% of prostate cancers are considered to be localized at the time of diagnosis, after definitive local therapy, 20–40% of patients will develop clinically detectable recurrent disease2. Accumulated evidence supports that prostate cancer cells disseminate to metastatic sites early in the course of disease and can remain undetected for extended periods of time3–5. Such findings have sparked intense interest in understanding metastatic dormancy in an effort to prevent or control late-arising metastases6–10. The factors that control the survival and subsequent growth of disseminated cells are largely unknown. Thus, there is a crucial need to discern cellular and molecular mechanisms that regulate metastatic colonization, which is the progressive growth of disseminated cells at metastatic sites.
There is increasing use of metastasis suppressor proteins as tools to query the clinically tractable process of metastatic colonization. Our efforts have focused on the metastasis-suppressive effect of the c-Jun NH2-terminal kinase activating kinase 1/mitogen-activated protein kinase (MAPK) kinase 4 (JNKK1/MKK4; hereafter referred to as MKK4), a key member of the stress-activated protein kinase (SAPK) signaling cascade. Multiple studies support a role for MKK4 in the suppression of metastatic growth in ovarian as well as prostate cancer11–14. Ectopic expression of MKK4 in AT6.1 Dunning prostate cancer cells, reduces spontaneous metastases formation by ~80% (p<0.0001), and increases survival by ~60% (p<0.0001) in immunocompromised mice and syngeneic rats15, 16. Preliminary studies showed that MKK4 is not active within the primary tumor, but becomes activated after cells lodge within the lung. These findings raise important questions: Can MKK4 directly impair the ability of highly metastatic cells to colonize target sites? If so what is the magnitude and duration of this suppression? Can MKK4-expressing cells become resistant to or adapt to the effects of MKK4? Activation of MKK4 and its down stream targets p38 and JNK can lead to various cellular sequelae including cell cycle arrest and apoptosis17. In the SKOV3.ip ovarian cancer experimental metastasis model, ectopic expression of MKK4 leads to inhibition of proliferation, possibly mediated by the cell cycle inhibitor p2113. The molecular mechanism of metastasis suppression in the prostate cancer model is not known.
Experiments detailed herein were designed to test the hypothesis that ectopic expression of MKK4 specifically suppresses metastatic colonization by highly metastatic variants of the Dunning model of rat prostatic cancers. The Dunning model has been used successfully for many years in basic and translational studies of prostate cancer. It originated in a spontaneous rat prostate adenocarcinoma and is comprised of multiple distinct well-characterized cell lines (Fig 1). This model is especially useful in studies of metastasis as many Dunning cell lines form reproducible numbers of spontaneous metastatic lesions18, 19. In particular, the Dunning model has proven to be a powerful tool in the identification and evaluation of metastasis suppressor genes15, 16, 20–31.
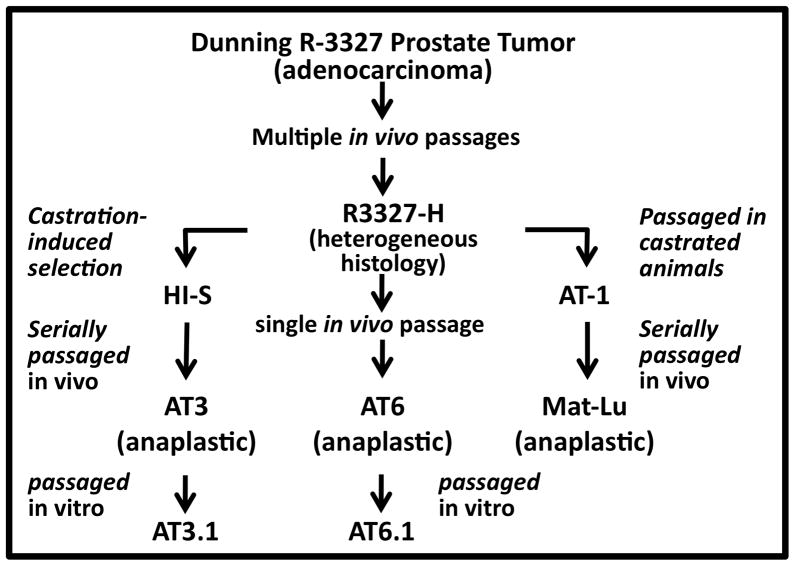
Figure 1. Summary of the derivation of the family of Dunning rat prostatic cancers used in this study.
The Dunning rat prostate cancer lines include multiple cell lines of various histologies, aggressiveness, and pattern of spread derived from a spontaneously originating prostate adenocarcinoma. Adapted from Ref. 18.
Using robust in vivo studies, we show that MKK4 significantly reduces the ability of highly metastatic, Dunning Mat-Lu, AT3.1, and AT6.1 cells to colonize target organs through a transient cell cycle arrest. Our data also show that contrary to conventional wisdom32, the eventual outgrowth of MKK4-expressing cells is not due to a discrete genetic selection event. Rather, our data support a model in which the population of MKK4-expressing cells adapts to the consequences of MKK4 activation.
Materials and Methods
Cell lines and culture conditions
AT6.1, AT3.1, and Mat-Lu Dunning rat prostate carcinoma cells were the generous gift of Dr. John Isaacs, The Johns Hopkins School of Medicine 18,19. All cell lines, which have low endogenous levels of MKK4 relative to rat brain (positive control), tested mycoplasma negative with the Mycoplasma PCR ELISA per manufacturer’s specifications (Roche Applied Science). Cells were maintained in standard media as described previously15. The construction of AT6.1-HA-MKK4 and AT6.1-pLNCX2 vector-only control cell lines has been reported previously15. The same methodology was used to derive AT3.1-HA-MKK4, Mat-Lu-HA-MKK4 and their corresponding pLNCX2 vector-only control cell lines. Clonal cell lines were established by limited dilution cloning and maintained in growth media containing G41815. Stable transfection of pmCherry into AT6.1 cell lines was similarly performed. First, the pmCherry expression cassette (Clontech) was subcloned into the pLHCX vector. Transfection of packaging cells with pLHCX-pmCherry constructs and retroviral infection of the target cancer cells were conducted as above. One representative AT6.1-HA-MKK4/Vector clone was engineered to also stably express pLHCX-pmCherry. Infected cells were selected and maintained in medium containing 400 μg/mL active Hygromycin B (Invitrogen) to select for stable pools. Stable pmCherrry expression was verified by fluorescence imaging and flow cytometry.
Establishment of metastasis-derived cell lines
At the experimental endpoint, lungs were harvested, PBS was injected through the trachea to inflate the lungs, and individual surface metastases were removed, minced, and placed in a 12 well plate with 1mL of standard media containing 500 μg/mL active G418. Viable cells were cultured in vitro and expanded for immunoblotting and kinase assays.
Quantitation of disseminated AT6.1 cells in the lungs of mice
Primers and a probe (Integrated DNA Technologies) specific to an intronic portion of the rat β-globin gene were used to detect rat cells in mouse lung. The sequences were as follows: F: 5′-GTTTCAACATGAAGTAGAACAACAATATCA-3′, R: 5 ′-TCAGTAGTCATTCTGCCTGTCTTTTAA-3′, reporter probe: 5 ′-FAM-CACTGCAGGCCCATTTCAAATGGAG-3BHQ-1–3′. To normalize against mouse DNA, mouse-specific primers specific to an intronic portion of β-globin were also designed: F: 5′-GGCTGCCTGCCTTTAATTCA-3′, R: 5′-GGTTAGCTTGGATAACCTGCTTTTT-3′, reporter probe: 5′-FAM-AGGGATTGTCCTGTCCTTCCACGCTT-BHQ-1–3′. All DNAs were prepared with the PUREGENE DNA Purification Kit (Gentra Systems). DNA from each lung was purified into 200-μL Tris-EDTA and subsequently diluted 1:10 before adding 1 μL of DNA to the quantitative reverse transcription-PCR (qRT-PCR) reaction. Quantitative RT-PCR reactions were conducted as described13. All reactions were done in triplicate, with appropriate negative controls (100% mouse DNA for rat primers, 100% rat DNA for mouse primers, and water). To calculate the number of rat AT6.1 cells disseminated within the lung tissues, a standard curve was generated by qRT-PCR amplification for the rat β-globin gene from DNA isolated from resected lungs spiked ex vivo with various known numbers (1×102–1×107) of AT6.1 cells. The experimental amplification cycles were then compared to the standard curve to determine the number of AT6.1 cells in a given sample.
Immunoblotting and kinase assays
Monolayer cell cultures at 70–80% confluence were washed in ice-cold PBS, and protein lysates were prepared as previously described13, 15. For protein isolated from metastases, individual metastases were rapidly washed in ice-cold PBS, and homogenized using a 1-mL tissue grinder (Fisher Scientific) with 300μL of the lysis buffer; after which, the preparation steps were the same as for cells in culture. SDS-PAGE and immunoblotting were conducted as previously described15. The antibodies and dilutions are as follows: HA.11 (Covance) at 1:1,000 with anti-mouse secondary (Sigma) at 1:5000; p38 (Cell Signaling) at 1:1,000 with anti-rabbbit (Cell Signaling) 1:5000; and JNK (Cell Signaling) at 1:1000 with anti-rabbit (Cell Signaling) 1:5000. As a loading control, membranes were probed for actin (Calbiochem) at 1:5000 followed by incubation with a goat anti-mouse secondary antibody (Calbiochem) at 1:20,000.
Kinase assays
Cell lines stimulated with 50ng/μl anisomycin (Sigma) or unstimulated, were scraped in 250μl Triton X lysis buffer with protease inhibitors15. Tissues were washed in cold PBS and homogenized in 1.5mL Triton X buffer with inhibitors. Tissue lysates were pre-cleared by rotating the lysates overnight at 4°C with 30μL of Protein A/G PLUS Agarose Beads (Santa Cruz Biotechnology) and 0.667μL per 100μL of lysate of Normal Mouse IgG (Santa Cruz Biotechnology). Five hundred micrograms of cell lysate or 1.0 mg of tissue lysate were brought to a final volume of 600μL with cold M2 immunoprecipitation buffer15. To this mixture, 4μg (1:150) of 1mg/ml of HA.11 antibody (Covance) and 30μL of Protein A/G PLUS Agarose Beads were added. The mixture was rotated at 4°C for 16 hours. The beads were centrifuged and washed three times with ice-cold M2 buffer and three times with ice-cold 50 mmol/L HEPES (Media Tech). The washed beads were resuspended in 19μL of cold double-distilled deionized water (d3H2O) and mixed with 0.1μg inactive recombinant p38α (Upstate); 5μL of ATP mix [0.03 μL of magnesium/ATP mix (Upstate), 0.05μL 10 mCi/mL γ-32P-ATP (Perkin Elmer), and 4.92μL d3H2O per sample], and 5μL of chilled 6X kinase reaction buffer15 and 10μM of p38 inhibitor SB03580 (Calbiochem). The kinase reactions were incubated at 30°C for 1 hour, with gentle mixing every 10 minutes. The reactions were spun down and 20μL of supernatant were withdrawn, and subjected to denaturing SDS-PAGE as described above. The gel was transferred to a nitrocellulose membrane and exposed to autoradiograph film which was visualized for radioactively labeled p38 substrate at ~36 hours. After detection of radiolabeled substrate, the membrane was immunoblotted for total p38 as a loading control.
Metastasis Assays
All animal experiments are part of an Institutional Animal Care and Use Committee (IACUC)-approved protocol at The University of Chicago. All cell lines were maintained below passage 10, cultured for a minimum of two passages, and not grown beyond 80% confluence. Male 6- to 8-week-old athymic nude mice (Harlan) were utilized for all animal experiments. Spontaneous metastasis assays were conducted as previously described15, 16. For experimental metastasis assays, 1 × 104 cancer cells in 100μL PBS were injected via lateral tail vein. Metastases were quantitated by visual inspection after the lungs were excised and inflated with 10% buffered formalin or cold PBS. Surface lung metastases (>1 mm2 in size) were counted. For endpoint experiments, animals were followed closely for signs of advanced stage metastatic disease including weight loss, lethargy, breathing difficulty and euthanized according to standard IACUC protocols.
Evaluation of the cell cycle distribution of AT6.1-mcherry cells in the lung microenvironment
1 ×104 AT6.1-Vector or AT6.1-HA-MKK4 cells, which stably express pmCherry, were injected via tail vein as above. Mice were euthanized and their lungs harvested after perfusion with 1mL cold PBS to wash out potentially autofluorescent erythrocytes33. The excised lungs were minced, placed in 5mL DMEM with 8000 units Collagenase Type III (Worthington Biochemical) and then incubated at 42°C for 45 minutes with rotational mixing. An additional 5mL DMEM was added to the lung mixture, which was then dissociated using a Seward Stomacher 80 Biomaster (Brinkmann) on low for 15 minutes. The mix was filtered through a 48μm nylon-mesh (Small Parts) and the filtrate centrifuged at 224 x g for 5 minutes. The cell pellet was resuspended in 5ml DMEM. To prevent efflux of Hoechst dye out of the cells34, 50μl of 50mM Verapamil hydrochloride (Sigma) was added to the lung mixture and the suspension incubated at 37°C for 30 minutes with rotational mixing. The cells were then stained with 50μl of 1mg/ml Hoechst 33342 (Invitrogen) by incubation at 37°C for 45 minutes with rotational mixing. After staining, the mix was centrifuged at 224 x g for 5 minutes, and the cell pellet was resuspended in 2mL PBS. Cell cycle analysis was performed via flow cytometry using a BD LSRII cytometer and the analysis was performed using FlowJo v.9.0.1. The entire volume of lung suspension was analyzed for each sample. Cellular aggregates and erythrocytes were excluded using a FSC vs. SCC plot. The pmCherry+ population was determined using a 561nm yellow-green laser with a 610/20 filter with fluorescence plotted vs. SSC. Cell-cycle data was collected using a 355nm UV laser with a 450/50 filter plotted as a linear histogram. Due to relatively small populations, the pmCherry+ cell-cycle distribution could not be directly determined. Therefore, the G1, S and G2/M gates were determined using the entire Hoechst stained population within each sample, transposed onto the mCherry+ cell-cycle histogram, and the percentage of pmCherry+ cells within each gate was determined. Analysis of lungs spiked with 1×106 AT6.1 pmCherry+ cells grown in vitro and processed as above served as the positive control and confirmed that the G1, S and G2/M gated populations for the entire lung corresponded to the respective populations of pmCherry+ cells. Healthy lung tissues processed as above served as the negative control.
Statistical Analyses
All statistical calculations were performed using SigmaPlot 11 (Systat Software, Inc) or STATA (Stata Corp, LP) in consultation with the biostatistics core at the University of Chicago. For surface lung metastasis comparison, the Mann-Whitney Rank Sum Test (t-test) was used to compare cohorts. For overall survival, log-rank Kaplan–Meier analyses were performed. A non-linear regression analysis was performed to determine the relationship between time and surface lung metastases. A modified, 2-parameter, exponential growth curve with the form f(t) = exp[b0 + b1*(t–t0)]) was used to model the data. In this equation, f(t) is equal to the number of measured surface metastases as a function of time, where t is the days post injection, t0 represents the delay in days, b0 is a calculated constant, and b1 is the exponential growth parameter. Finally, for cell cycle analyses, the percent of cells within a given phase of the cell cycle (G1, S, G2) was calculated as a percentage of the total cells within all three groups. The mean and standard error of the means for each animal cohort was calculated for each phase of the cell cycle, and a two-way analysis of variance (ANOVA) was performed to determine the potential interactions between days post injection and MKK4 status for each cell cycle phase.
Results
Ectopic MKK4 specifically suppresses the ability of highly metastatic cells to colonize target sites
Our laboratory previously identified MKK4 as a prostate cancer metastasis suppressor protein35, 36. Optimization of the Dunning AT6.1 model system has enabled us to discern the time-ordered pattern of metastasis formation by AT6.1-Vector and AT6.1-HA-MKK4 cells using the spontaneous metastasis assay (Fig 2A). Qualitative analyses of excised lung tissues (e.g. histology and clonogenic assays) show that both AT6.1-Vector and AT6.1-HA-MKK4 tumor-bearing mice have disseminated cells and microscopic disease by 21 days post injection (dpi) of cancer cells subcutaneously suggesting that ectopic MKK4 impairs the formation of overt metastases after cancer cells lodge at the secondary site15. This implies that ectopic MKK4 should not have a significant effect on the number of AT6.1 cells lodging in the lungs. To test this assertion, the number of AT6.1-Vector and AT6.1-HA-MKK4 cells present in the lungs of tumor bearing mice at 21 dpi was quantitated using qRT-PCR for rat β-globin DNA. This assay showed that in both AT6.1-Vector and AT6.1-HA-MKK4 tumor-bearing animals had approximately 1 × 104 cancer cells present in the lungs at 21 dpi (Fig 2B). This finding is consistent with the observation that histological evaluation of specimens for both groups revealed no difference in the size or relative abundance of microscopic lesions by a pathologist (TL) (Fig 2C).
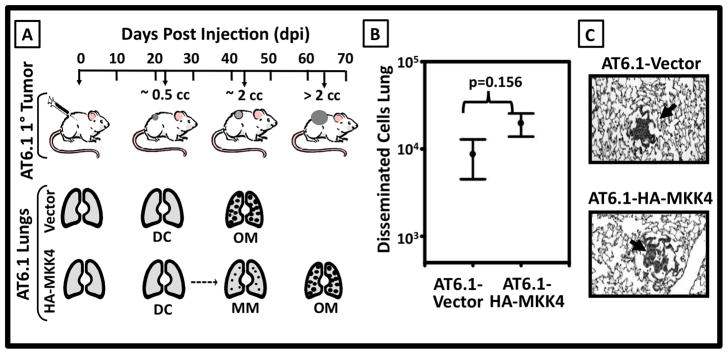
Figure 2. MKK4-mediated suppression of spontaneous metastasis formation is due to impaired metastatic colonization.
A. Schema for spontaneous metastasis assay. Upper Panel Injection of 2 ×105 AT6.1-Vector or AT6.1-HA-MKK4 cells subcutaneously into the flank of immunodeficient mice or syngeneic rats yields progressively growing tumors. Lower Panel The location and disposition of cancer cells at specific time points during spontaneous metastasis: disseminated cells (DC), microscopic metastases (MM), and overt metastases (OM) found in lungs at characteristic days post injection (dpi). B. Quantitation of the number of disseminated cells within the lungs at 21 dpi using q-RT PCR. Data is shown as the mean ± standard error [i.e. AT6.1-HA-MKK4 (8680±4174 cells) vs. AT6.1-Vector (19684±5842 cells) p=0.156]. C. Histologic appearance of microscopic metastases 21 dpi. Arrows denote foci of metastatic AT6.1-Vector and AT6.1-HA-MKK4 cells.
The spontaneous metastasis model has been well validated as a means of interrogating the entire metastatic cascade, from tumor cell invasion through overt metastatic outgrowth. However, as tumor cells can be shed continuously from the primary tumor, cancer cell dissemination to the lungs may occur continuously over time, leading to potential heterogeneity of metastatic tumor biology at a given specific time point post injection. To directly test the ability of MKK4 to suppress metastatic colonization and to temporally synchronize lodging at the metastatic site, an experimental metastasis assay using 1 × 104 cancer cells injected into the blood stream directly via tail vein was utilized for subsequent experiments (Fig 3A). Assays using three independent clonal AT6.1-Vector and AT6.1-HA-MKK4 cell lines showed that ectopic MKK4 caused an 87% reduction (p<0.001) in metastatic colonization at 28 dpi (Fig 3B, 3C). Specifically, the mean (± standard error of the mean) number of overt surface lung tumors formed with AT6.1-Vector was 54.9 ± 6.0 as compared to 6.8 ± 1.6 for animals injected with AT6.1-HA-MKK4 cells. This decrease translated into a significant increase in survival with animals injected with AT6.1-HA-MKK4 cells showing a median survival of 42 dpi compared to 28 dpi for those injected with AT6.1-Vector cells [p<0.0001; (Fig 3D)]. Using spontaneous and experimental metastasis assays, it is therefore clear that ectopic expression of HA-MKK4 in AT6.1 prostate cancer specifically and powerfully inhibits metastasis formation through disruption of metastatic colonization.
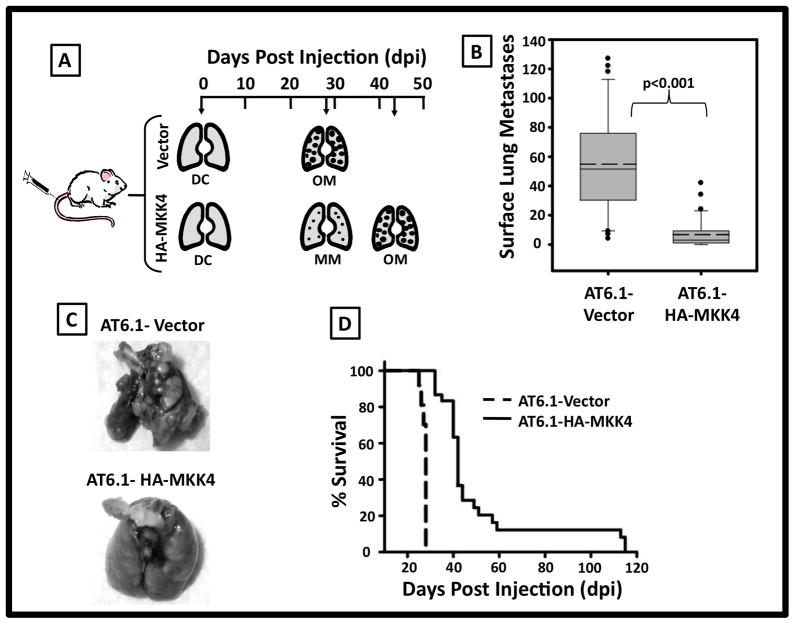
Figure 3. Ectopic expression of MKK4 suppresses metastatic colonization of AT6.1 prostate cancer cells in an experimental metastasis assay.
A. Schematic of the results from an experimental metastasis assay in which 1×104 cells are injected intravenously via tail vein venopuncture. B. Box plot of the number of overt surface lung lesions present in mice injected with either AT6.1-Vector or AT6.1-HA-MKK4 cells via experimental metastases assay. Boundaries of box are 25th and 75th percentiles, with solid line indicating median, dashed line indicating mean, whiskers indicating 10th and 90th percentiles. The mean (± standard error of the mean) number of overt metastases AT6.1-Vector was 54.9 ± 6.0 as compared to 6.8 ± 1.6 for AT6.1-HA-cells (p<0.001). C. Representative gross pathology of lungs harvested from mice 28 dpi of either AT6.1-Vector or AT6.1-HA-MKK4 cells. D. Kaplan-Meier survival analyses showed that the median survival for AT6.1 Vector animals was 28 days vs. 42 days for AT6.1-HA-MKK4 animals (p<0.0001).
Suppression of metastatic colonization by ectopic MKK4 expression is conserved across highly metastatic prostate cancer cell lines
The Dunning model is comprised of independent cell lines, which vary in histologies, hormonal dependence and metastatic abilities derived from a spontaneous rat prostate cancer (Fig 1)18, 19. In particular the Mat-Ly-Lu, Mat-Lu, AT3, and AT6 cell lines are independently derived highly metastatic variants19. To test the hypothesis that ectopic MKK4 expression suppresses metastatic colonization by more aggressive phenotypes than AT6.1, the Mat-Lu and AT3.1 cell lines were employed. Ectopic expression of HA-MKK4 was confirmed in independent, clonal Mat-Lu-HA-MKK4 and AT3.1-HA-MKK4 cell lines (Fig 4A left). Since MKK4 can signal through both the p38 and JNK MAPKs, and to confirm that these signaling networks were intact, their expression was also confirmed by immunoblotting of both Vector- and HA-MKK4-expressing cell lysates (Fig 4A left). To confirm that the ectopically expressed HA-MKK4 was functional, cells were activated with anisomycin, proteins were immunoprecipitated, and kinase activity was assayed (KA). In vitro, activated HA-MKK4 immunoprecipitated from cell lysates phosphorylated a purified p38 (GST-p38) substrate (Fig 4A right). Results from experimental metastasis assays showed that ectopic MKK4 caused an 84% reduction in Mat-Lu metastatic colonization (Fig 4B and C, p=0.045). Animals injected with Mat-Lu-MKK4 cells also had a significant extension of median survival when compared to animals injected with Vector-only cell lines (Fig 4D, p<0.0001). Ectopic MKK4 similarly caused a dramatic (94%) reduction in AT3.1 colonization of the lung (Fig 4E and F, p<0.001) and also extended lifespan (Fig 4G, P<0.0001). Thus, the phenotype of suppression on metastatic colonization is generalizable to other, even more aggressive, Dunning prostate cancer cell lines. Experiments were thus undertaken, using the AT6.1 cell line as a representative model, to elaborate the details of how the suppression is imparted and overcome.
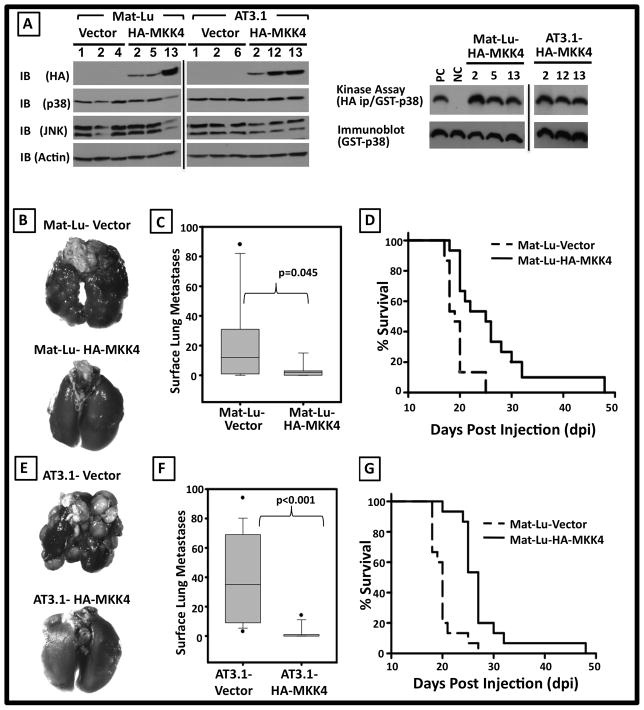
Figure 4. Ectopic HA-MKK4 specifically inhibits Mat-Lu and AT3.1 metastatic colonization.
A. Biochemical characterization of Mat-Vector, Mat-Lu-HA-MKK4, AT3.1-Vector and AT3.1-HA-MKK4 cell lines. Left: Lysates of three independent Vector and HA-MKK4 clones from each cell line were immunoblotted (IB) for the expression of a HA-tagged protein, p38, and JNK. Actin was used as a loading control. Right: Kinase assays (KA) showing functionality of ectopically expressed HA-MKK4. B. Representative gross pathology of lungs harvested at 21 dpi from mice injected via tail vein with Mat-Lu-Vector and Mat-Lu-HA-MKK4 cells. C. Box plot representation of the mean number ± standard error of the mean of overt surface experimental metastases formed by Mat-Lu-Vector was 25.3 ± 7.7 as compared to 4 ± 1.4 for Mat-Lu-HA-MKK4 cells (p=0.045). D. Kaplan-Meier survival analyses showed that the median survival for Mat-Lu-Vector animals was 19 days vs. 25 days for Mat-Lu-HA-MKK4 animals (p<0.0001). E. Representative gross pathology of lungs harvested at 21 dpi from mice injected with AT3.1-Vector and AT3.1-HA-MKK4 cells. F. Box plot representation of the mean number ± standard error of the mean of overt surface metastases formed by AT3.1-Vector was 38.3 ± 7.6 as compared to 2.3 ± 1.1 for AT3.1-HA-MKK4 cells (p<0.001). G. Kaplan-Meier survival analyses showed that the median survival for AT3.1-Vector animals was 20 days vs. 27 days for AT3.1-HA-MKK4 animals (p<0.0001).
MKK4 specifically delays exponential growth of prostate cancer cells early after lodging within the lung
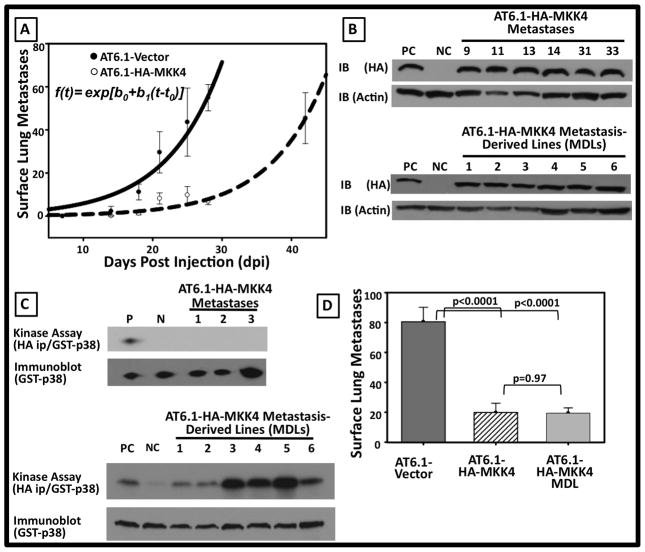
Ectopic MKK4 significantly reduces the number of overt experimental metastases and extends animal survival in both highly metastatic prostate and ovarian cancer xenograft models [Figs 3 and 4 and Ref. 9]. Eventually, however, mice injected with HA-MKK4–expressing cells develop macroscopic metastases and succumb to their disease burden. This raises the important question of how disseminated cells may ultimately escape or bypass metastasis suppression. A long-standing paradigm of metastasis biology is that the process of metastasis selects for clones that have undergone permanent molecular changes (such as DNA mutation or deletion) enabling them to complete all steps of the metastatic cascade32. Studies were conducted to address the possibility that the outgrowth of MKK4-expressing prostate cancer metastases is due to such a canonical selective process through deletion or inactivation of the MKK4 kinase, or alternatively, that the eventual metastatic colonization is the result of a population-wide adaptation of cells to SAPK signaling. As a first step to discerning between these possibilities, the accumulation of overt AT6.1-Vector and AT6.1-HA-MKK4 experimental metastases over time was quantitated (Fig 5A), with the hypothesis that similar to the ovarian cancer metastasis model13, AT6.1-HA-MKK4 and AT6.1-Vector lung colonization could be modeled with parallel exponential growth curves indicating a transient delay in the initiation of metastatic colonization. The nonlinear regression model used to estimate the exponential growth rate and delay in growth imparted by HA-MKK4 expression was number of metastases = exp[b0 + b1*(t–t0)], as described in the methods above. In this equation, the b0 parameter was calculated to be 0.5319, and b1, was calculated at 0.1246. The adjusted R2 of the model was 0.67. Tumor growth rates (b1) were not statistically significantly different between HA-MKK4 and Vector mice (p=0.99). Estimated tumor growth delay was 15.7 days (95% CI: 12.8–18.6) in the HA-MKK4 mice relative to vector. Thus, the HA-MKK4 growth curve was shifted in time, with metastatic colonization delayed by approximately 2 weeks (Fig 5A).
Figure 5. Transient suppression of metastatic colonization imparted by MKK4 not due to clonal loss or dysfunction of HA-MKK4 construct.
A. Non-linear regression analysis of overt surface lung lesions as a function of time. Each point represents the mean ± SEM surface metastases from a minimum of 5 animals per time point. The Adjusted R2=0.67. The calculated t0 delay for the AT6.1-HA-MKK4 curve is 15.7 days relative to the AT6.1-Vector curve. B. Representative immunoblots (top) from>30 AT6.1-MKK4 overt experimental metastases and (bottom) from multiple independent metastasis derived cell lines (MDLs) demonstrating presence of HA-MKK4 construct within overt metastases and MDL’s. C. Representative in vitro kinase assay (top) AT6.1-MKK4 overt metastases and (bottom) from multiple independent metastasis derived cell lines (MDLs) showing inactive HA-MKK4 kinase within overt metastases; however HA-MKK4 remains functionally intact and activatable in cell lines derived from overt metastases. D. Persistent suppression of metastatic colonization at 28 DPI with MDL’s. The mean and SEM for surface metastases were 80.5±9.7 for Vector, 19.8±6.3 for HA-MKK4 parental, and 19.4±3.5 for HA-MKK4 MDLs (p=0.97, MDL versus parental AT6.1-vector p<0.0001).
Based on our findings, we hypothesized that outgrowth of HA-MKK4-expressing cells is due to an adaptive response of the population of cells and not a selection of cells that have deleted or inactivated the HA-MKK4 transgene. This hypothesis predicts that overt AT6.1-HA-MKK4 surface lung lesions will retain and express functional HA-MKK4. However, the protein will not be activated in overt metastases. Consistent with this hypothesis, biochemical analyses showed that ectopic HA-MKK4 is expressed in multiple, independent overt experimental metastases and metastasis-derived lines (MDLs) (Fig 5B, Upper and Lower Panels respectively). However, MKK4 is not activated in these overt tumors as it does not phosphorylate GST-p38 in our ex vivo kinase assay (Fig 5C, Upper Panel (KA)). To rule out the possibility that ectopic HA-MKK4 in overt metastases has been rendered non-functional, AT6.1-HA-MKK4 MDLs were stimulated with anisomycin, and used for in vitro kinase assays (Fig 5C, Lower Panel (KA)). HA-MKK4 is functional in vitro and phosphorylates GST-p38 substrate in a manner similar to parental cell line controls (positive control, first lane). As a loading control, the blot was also probed for the GST-p38 substrate (bottom). Similar results were observed with protein lysates and MDL’s in the AT3.1 and Mat-Lu cell lines [data not shown].
Although AT6.1-HA-MKK4 MDLs express HA-MKK4, which can be artificially activated in vitro, it is possible that these cells have undergone other permanent molecular changes that will inactivate other aspects of the SAPK signaling pathway to compensate for ectopic HA-MKK4 expression. If a selection is indeed occurring, an experimental metastasis assay utilizing the AT.61-HA-MKK4 MDLs should result in increased experimental metastasis formation compared with the parental HA-MKK4-expressing AT6.1 clones. To this end, metastasis-derived AT6.1-HA-MKK4 cell lines were assayed in vivo for the ability to suppress metastatic colonization after tail vein injection. Compared with vector-only cells, MKK4 MDL’s displayed a significantly reduced ability to form overt experimental metastases (p<0.001 MDCL vs. Vector; Fig 5D). Taken together, these in vitro and in vivo data strongly suggest that the eventual outgrowth of HA-MKK4-expressing cells is not due to selection for clones of cells that have permanently altered their MKK4 signaling status, but is rather due to adaptation of the population to the biological consequences of SAPK signaling.
HA-MKK4-mediated suppression of metastatic colonization is concomitant with an accumulation of cells in G1 phase of the cell cycle
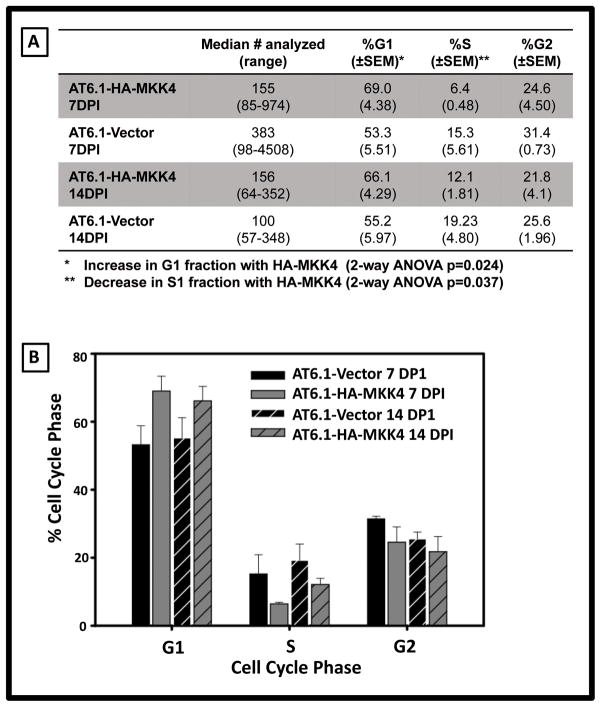
As shown in Fig 5A, metastasis suppression imparted by ectopic HA-MKK4 causes a delay ~14 day delay in metastatic colonization. Further, suppression is apparently homogeneous, with low variability in the number of overt metastases per lung at a given time point. Taken together, these data prompted the hypothesis that upon lodging, AT6.1-HA-MKK4 cells undergo a population-wide growth inhibition, perhaps via cell cycle arrest. To test this possibility, AT6.1-Vector and AT6.1 HA-MKK4 cells stably expressing mCherry protein were injected using the experimental metastasis assay, dissociated from the lung tissues at early time-points (7 and 14 days) post injection, and analyzed for DNA content using multi-parameter flow cytometry analysis. Five animals per cell line and per time point were analyzed and the number of cells analyzed and percentage of cells in each portion of the cell cycle is detailed (Fig 6A) and graphically illustrated (Fig 6b). There was no statistical difference in the number of cells analyzed in each cohort based on MKK4 status (p=0.80) or days post injection (p=0.60). A relative difference in the percentage of cells in the G1 and S phases of the cell cycle was noted in the HA-MKK4 expressing cells. There was a statistically significant increase in G1 cell cycle portion in AT6.1-HA-MKK4 cells compared to AT6.1-Vector controls (p=0.024) with a concordant decrease in S phase fraction (p=0.037). Taken together, these data depict a relative G1 arrest associated with ectopic MKK4 expression within the first 14 days upon metastatic dissemination, concordant with the delay in metastatic colonization.
Figure 6. Ectopic expression of HA-MKK4 leads to a shift in cell cycle from S to G1 within disseminated AT6.1 prostate cancer cells.
Disseminated AT6.1-Vector and AT6.1-HA-MKK4 cells were isolated from lungs at 7 and 14 days post injection and their cell cycle distribution was assessed via flow cytometry. A. Tabular details of the number of cells analyzed and percentage of cells in each of the cell cycle. B. Graphical representation of the cell cycle data. Bars represent the percentage of cells in each phase of the cell cycle with error bars representing standard error of the mean.
Discussion
Accumulated evidence shows that many cancers including prostate cancer disseminate to metastatic sites early in the natural history of disease and can remain undetected and quiescent for extended periods of time3, 4, 10. In one recent report, disseminated prostate cancer cells (DTC’s) can be detected in the bone marrow of 57% men who are without evidence of disease after prostatectomy. With a median follow-up of 42 months, 52% of those with DTC’s have not recurred, including patients who still have DTC’s 12 years after surgery5. Thus, in the context of prostate cancer recurrence, metastatic colonization, or the progressive outgrowth of disseminated cancer cells within a secondary site into clinically manifested metastases, is a particularly critical aspect to the multi-step metastatic process7, 37. The molecular factors that control the survival and eventual growth of these disseminated cells are largely unknown.
Experimental modulation of metastasis suppressor genes preclinically affords the unique opportunity to dissect the process of metastatic progression, specifically metastatic colonization, at the molecular level. This study shows that MKK4 has a powerful suppressive effect specifically on metastatic colonization in multiple highly metastatic prostate cancer cell lines, delaying the development of lethal metastases and significantly impacting survival. Prior work indicating that activation of the MKK4 kinase is critical for metastasis suppression15, along with data presented herein showing lack of MKK4 kinase activity within fully developed metastatic lesions, indicates that the alteration in biology within disseminated cells is critically tied to activation of the MKK4 kinase within the secondary microenvironment. Furthermore, the data on the kinetics of metastatic colonization are consistent with a populational dormancy associated with ectopic MKK4 expression in the immediate period after experimental dissemination. Adaptation and exponential metastatic colonization of the lungs by the MKK4 expressing cells following this period of suppression is eventual. Somewhat surprisingly, this adaptation was not due to a Darwinian permanent alteration in cancer cell biology promoting escape from suppression, but rather seemed to be due to alteration in SAPK signaling over time. The “stress” leading to time-limited MKK4 kinase activation is unknown. Our results support the notion that metastatic colonization can be a rate-limiting step to metastatic progression and that this process is dynamically regulated by complex interactions between disseminated cancer cells and the surrounding microenvironment. Current studies are pending using a novel ex vivo lung culture model38 to test the hypothesis that there are microenvironmental factors (potentially including macrophages within the lung or factors expressed by the lung endothelium) regulating this process.
It is well established that prostate cancer in humans is a disease that disseminates to and most frequently colonizes the bones. Unfortunately, there are very limited preclinical prostate cancer models that metastasize to the bones and quantifying the metastatic progression to the bone is imprecise39. The Dunning model is to date the only spontaneously derived prostate cancer model with multiple independent cell lines that reproducibly metastasize39. As such, it is well suited for this study of metastatic colonization. Nonetheless, the effect of MKK4 on metastatic colonization of the bone microenvironment will be studied in future studies utilizing human prostate cancer cell lines and genetically engineered mouse models that have an established pattern of bone metastasis39–41. Furthermore, interrogation of overtly metastastic tissue samples from rapid autopsy programs42 and disseminated prostate cancer cells isolated using cutting edge techniques5, 43, will be undertaken to explore the expression and activation of the SAPK pathway in metastatic patients samples.
Optimization of methods to analyze the cell cycle distribution of relatively rare AT6.1-Vector and AT6.1-HA-MKK4 cells lodged within the lung allowed the interrogation of these disseminated cancer cells with respect to their proliferative status. This study demonstrates that MKK4-expressing cells have a population shift in cell cycle from S phase to G1 phase within the first 7–14 dpi. In the parallel ovarian cancer metastasis mode, ectopic MKK4 expression seems to be associated with a decrease in cancer cell proliferation and an upregulation of the cell cycle regulator p2113. However, in that model, MKK4 signals through p38, whereas in the prostate model, it seems to signal through JNK11, 15. Nonetheless, as this study supports a G1 cell cycle arrest as the mechanism for suppression of metastatic colonization, regulators of the G1 to S transition including p21, p27, p16, and cyclins D/E, represent potential direct or indirect targets of MKK4 activation that may be implicated as the mediators of MKK4 dependent metastasis suppression. Studies are currently underway using this prostate cancer experimental metastasis model and these flow cytometry techniques to elaborate the mechanistic underpinnings of the metastasis suppressor phenotype. Array-based expression studies of disseminated MKK4 expressing cancer cells could also identify inverse correlates of MKK4 expression, potentially negative regulators of this process, which could be targeted in an attempt to prolong the metastasis suppression.
Prostate cancer is a disease that can disseminate early, be variably quiescent over time, and which eventually completes the steps of the metastatic cascade to form lethal metastases. It is therefore essential to understand the precise biology that governs the kinetics of metastatic colonization. MKK4 appears to be enable a shift from active to dormant cell cycling, thereby limiting proliferation of disseminated cells. As this process is transient and dependent on MKK4 activation, extension of kinase activation and/or MKK4 pathway activation may prolong suppression (eg. constitutively active kinase, pharmacologic activation of the kinase, inhibition of potential inverse correlates of MKK4 activation, specific activation of upstream effectors). As such, these and future studies have significant clinical implications for the design of therapies aimed at controlling disseminated tumor growth. Further work specifically testing this hypothesis that prolonged SAPK pathway activation can extend metastatic dormancy is necessary to enable future translational efforts targeting this process in micrometastatic prostate cancer patients.
NOVELTY STAEMENT.
Studies herein use complementary approaches to quantitate temporal and spatial events involved in the transient cell cycle arrest and growth suppression mediated by ectopic MKK4 expression. This is the first study to demonstrate that ectopic MKK4 specifically inhibits early steps in metastatic colonization via induction of a cell cycle arrest. It is also the first study to show that the inhibition of metastatic colonization is conserved in three distinct but genetically related, highly malignant, prostate cancer cell lines. Finally, our data show that bypass of this suppression is due to a population-wide adaptation to the effect(s) of MKK4 signaling and not selection of clones which have undergone permanent alterations which abrogate SAPK pathway activity.
IMPACT STATEMENT.
Increasing evidence suggests that metastatic colonization is a process potentially susceptible to therapeutic intervention. Despite its clinical importance, metastatic colonization has not been rigorously studied. We believe that metastasis suppressors can be used to glean new insights into this process. The finding that MKK4-mediated suppression of metastatic colonization is due to a transient alteration in the cell cycle progression shortly after lodging within the metastatic site provides insight into mechanisms that may play a role in human prostate cancer metastatic latency. Similarly the observation that this suppression is overcome without functional loss of the MKK4 construct but rather loss of MKK4 activation challenges long-held views that metastasis formation is solely due to serial mutation selection that results in deranged biology. Taken together, these findings prompt translational work aimed at inducing a durable cell cycle arrest in disseminated cancer cells through sustained activation of the MKK4 pathway.
Acknowledgments
Walter M. Stadler and Donald Vander Griend for conceptual support and guidance, Masha Kocherginski for biostatistical support, the National Institutes of Health and Department of Defense for research funding support.
ABBREVIATIONS USED
- MKK4
c-Jun NH2-terminal kinase activating kinase 1/mitogen-activated protein kinase kinase 4
- SAPK
Stress activated protein kinase
- dpi
Days post injection
- Fig
Figure
- qRT-PCR
Quantitative real time polymerase chain reaction
- MDL
Metastasis derived cell line
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ward JF, Moul JW. Rising prostate-specific antigen after primary prostate cancer therapy. Nat Clin Pract Urol. 2005;2:174–82. doi: 10.1038/ncpuro0145. [DOI] [PubMed] [Google Scholar]
- 3.Melchior SW, Corey E, Ellis WJ, Ross AA, Layton TJ, Oswin MM, Lange PH, Vessella RL. Early tumor cell dissemination in patients with clinically localized carcinoma of the prostate. Clin Cancer Res. 1997;3:249–56. [PubMed] [Google Scholar]
- 4.Cher ML, de Oliveira JG, Beaman AA, Nemeth JA, Hussain M, Wood DP., Jr Cellular proliferation and prevalence of micrometastatic cells in the bone marrow of patients with clinically localized prostate cancer. Clin Cancer Res. 1999;5:2421–5. [PubMed] [Google Scholar]
- 5.Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15:677–83. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barkan D, Green JE, Chambers AF. Extracellular matrix: A gatekeeper in the transition from dormancy to metastatic growth. European Journal of Cancer. 2010;46:1181–88. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers A, Groom A, MacDonald I. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 8.Naumov G, MacDonald I, Weinmeister P, Kerkvliet N, Nadkarni K, Wilson S, Morris V, Groom A, Chambers A. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–8. [PubMed] [Google Scholar]
- 9.WIKMAN H, VESSELLA R, PANTEL K. Cancer micrometastasis and tumour dormancy. APMIS. 2008;116:754–70. doi: 10.1111/j.1600-0463.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 10.Stoecklein NH, Klein CA. Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int J Cancer. 126:589–98. doi: 10.1002/ijc.24916. [DOI] [PubMed] [Google Scholar]
- 11.Hickson J, Huo D, Vander Griend D, Lin A, Rinker-Schaeffer C, Yamada S. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 2006;66:2264–70. doi: 10.1158/0008-5472.CAN-05-3676. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Vander Griend D, Yang X, Benson D, Dubauskas Z, Yoshida B, Chekmareva M, Ichikawa Y, Sokoloff M, Zhan P, Karrison T, Lin A, et al. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res. 2001;61:2833–7. [PubMed] [Google Scholar]
- 13.Lotan T, Hickson J, Souris J, Huo D, Taylor J, Li T, Otto K, Yamada S, Macleod K, Rinker-Schaeffer C. c-Jun NH2-terminal kinase activating kinase 1/mitogen-activated protein kinase kinase 4-mediated inhibition of SKOV3ip. 1 ovarian cancer metastasis involves growth arrest and p21 up-regulation. Cancer Res. 2008;68:2166–75. doi: 10.1158/0008-5472.CAN-07-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotan T, Lyon M, Huo D, Taxy J, Brendler C, Foster B, Stadler W, Rinker-Schaeffer C. Up-regulation of MKK4, MKK6 and MKK7 during prostate cancer progression: an important role for SAPK signalling in prostatic neoplasia. J Pathol. 2007;212:386–94. doi: 10.1002/path.2194. [DOI] [PubMed] [Google Scholar]
- 15.Vander Griend D, Kocherginsky M, Hickson J, Stadler W, Lin A, Rinker-Schaeffer C. Suppression of metastatic colonization by the context-dependent activation of the c-Jun NH2-terminal kinase kinases JNKK1/MKK4 and MKK7. Cancer Res. 2005;65:10984–91. doi: 10.1158/0008-5472.CAN-05-2382. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida B, Dubauskas Z, Chekmareva M, Christiano T, Stadler W, Rinker-Schaeffer C. Mitogen-activated protein kinase kinase 4/stress-activated protein/Erk kinase 1 (MKK4/SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res. 1999;59:5483–7. [PubMed] [Google Scholar]
- 17.Taylor JL, Szmulewitz RZ, Lotan T, Hickson J, Griend DV, Yamada SD, Macleod K, Rinker-Schaeffer CW. New paradigms for the function of JNKK1/MKK4 in controlling growth of disseminated cancer cells. Cancer Lett. 2008;272:12–22. doi: 10.1016/j.canlet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs JT, Isaacs WB, Feitz WFJ, Scheres J. Establishment and characterization of seven dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. The Prostate. 1986;9:261–81. doi: 10.1002/pros.2990090306. [DOI] [PubMed] [Google Scholar]
- 19.Tennant TR, Kim H, Sokoloff M, Rinker-Schaeffer CW. The Dunning model. Prostate. 2000;43:295–302. doi: 10.1002/1097-0045(20000601)43:4<295::aid-pros9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, Saito K, Commes T, Hayashi S, Watabe M, Watabe K. The Drg-1 Gene Suppresses Tumor Metastasis in Prostate Cancer. Cancer Research. 2003;63:1731–36. [PubMed] [Google Scholar]
- 21.Chekmareva MA, Kadkhodaian MM, Hollowell CM, Kim H, Yoshida BA, Luu HH, Stadler WM, Rinker-Schaeffer CW. Chromosome 17-mediated dormancy of AT6. 1 prostate cancer micrometastases. Cancer Res. 1998;58:4963–9. [PubMed] [Google Scholar]
- 22.Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11. 2. Science. 1995;268:884–6. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- 23.Gao AC, Lou W, Dong JT, Isaacs JT. CD44 is a metastasis suppressor gene for prostatic cancer located on human chromosome 11p13. Cancer Res. 1997;57:846–9. [PubMed] [Google Scholar]
- 24.Hamano M, Kuramochi H, Nihei N, Kamiya N, Suzuki H, Igarashi T, Barrett JC, Ichikawa T, Ito H. Mechanisms of metastasis suppression by introduction of human chromosome 10 into rat prostate cancer. Asian J Androl. 2002;4:123–9. [PubMed] [Google Scholar]
- 25.Hosoki S, Ota S, Ichikawa Y, Suzuki H, Ueda T, Naya Y, Akakura K, Igarashi T, Oshimura M, Nihei N, Barrett JC, Ichikawa T, et al. Suppression of metastasis of rat prostate cancer by introduction of human chromosome 13. Asian J Androl. 2002;4:131–6. [PubMed] [Google Scholar]
- 26.Ichikawa T, Ichikawa Y, Dong J, Hawkins AL, Griffin CA, Isaacs WB, Oshimura M, Barrett JC, Isaacs JT. Localization of metastasis suppressor gene(s) for prostatic cancer to the short arm of human chromosome 11. Cancer Res. 1992;52:3486–90. [PubMed] [Google Scholar]
- 27.Mashimo T, Bandyopadhyay S, Goodarzi G, Watabe M, Pai SK, Gross SC, Watabe K. Activation of the tumor metastasis suppressor gene, KAI1, by etoposide is mediated by p53 and c-Jun genes. Biochem Biophys Res Commun. 2000;274:370–6. doi: 10.1006/bbrc.2000.3139. [DOI] [PubMed] [Google Scholar]
- 28.Mashimo T, Goodarzi G, Watabe M, Cuthbert AP, Newbold RF, Pai SK, Hirota S, Hosobe S, Miura K, Bandyopadhyay S, Gross SC, Watabe K. Localization of a novel tumor metastasis suppressor region on the short arm of human chromosome 2. Genes Chromosomes Cancer. 2000;28:285–93. doi: 10.1002/1098-2264(200007)28:3<285::aid-gcc6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Nihei N, Kouprina N, Larionov V, Oshima J, Martin GM, Ichikawa T, Barrett JC. Functional evidence for a metastasis suppressor gene for rat prostate cancer within a 60-kilobase region on human chromosome 8p21-p12. Cancer Res. 2002;62:367–70. [PubMed] [Google Scholar]
- 30.Nihei N, Ohta S, Kuramochi H, Kugoh H, Oshimura M, Barrett JC, Isaacs JT, Igarashi T, Ito H, Masai M, Ichikawa Y, Ichikawa T. Metastasis suppressor gene(s) for rat prostate cancer on the long arm of human chromosome 7. Genes Chromosomes Cancer. 1999;24:1–8. doi: 10.1002/(sici)1098-2264(199901)24:1<1::aid-gcc1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Xia W, Unger P, Miller L, Nelson J, Gelman IH. The Src-suppressed C kinase substrate, SSeCKS, is a potential metastasis inhibitor in prostate cancer. Cancer Res. 2001;61:5644–51. [PubMed] [Google Scholar]
- 32.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 33.el-Rahman A, Hammouda MA, Fakeir A. Flow cytometric evaluation of erythrocyte response to oxidant stress. Cytometry. 1995;20:19–22. doi: 10.1002/cyto.990200105. [DOI] [PubMed] [Google Scholar]
- 34.Krishan A. Effect of drug efflux blockers on vital staining of cellular DNA with Hoechst 33342. Cytometry. 1987;8:642–5. doi: 10.1002/cyto.990080618. [DOI] [PubMed] [Google Scholar]
- 35.Vander Griend DJ, Kocherginsky M, Hickson JA, Stadler WM, Lin A, Rinker-Schaeffer CW. Suppression of metastatic colonization by the context-dependent activation of the c-Jun NH2-terminal kinase kinases JNKK1/MKK4 and MKK7. Cancer Res. 2005;65:10984–91. doi: 10.1158/0008-5472.CAN-05-2382. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida BA, Dubauskas Z, Chekmareva MA, Christiano TR, Stadler WM, Rinker-Schaeffer CW. Mitogen-activated protein kinase kinase 4/stress-activated protein/Erk kinase 1 (MKK4/SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res. 1999;59:5483–7. [PubMed] [Google Scholar]
- 37.Szmulewitz RZ, Rinker-Schaeffer CW. Metastatic Colonization. In: Schwab M, editor. Encyclopedia of Cancer. 2. New York: Springer; 2009. [Google Scholar]
- 38.Mendoza A, Hong SH, Osborne T, Khan MA, Campbell K, Briggs J, Eleswarapu A, Buquo L, Ren L, Hewitt SM, Dakir el H, Garfield S, et al. Modeling metastasis biology and therapy in real time in the mouse lung. J Clin Invest. 2010;120:2979–88. doi: 10.1172/JCI40252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pienta KJ, Abate-Shen C, Agus DB, Attar RM, Chung LW, Greenberg NM, Hahn WC, Isaacs JT, Navone NM, Peehl DM, Simons JW, Solit DB, et al. The current state of preclinical prostate cancer animal models. Prostate. 2008;68:629–39. doi: 10.1002/pros.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: a compendium of old and new lines--part 2. J Urol. 2005;173:360–72. doi: 10.1097/01.ju.0000149989.01263.dc. [DOI] [PubMed] [Google Scholar]
- 41.Sobel RE, Sadar MD. Cell lines used in prostate cancerresearch: a compendium of old and new lines--part 1. J Urol. 2005;173:342–59. doi: 10.1097/01.ju.0000141580.30910.57. [DOI] [PubMed] [Google Scholar]
- 42.Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, Vessella RL. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–53. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 43.Holcomb IN, Grove DI, Kinnunen M, Friedman CL, Gallaher IS, Morgan TM, Sather CL, Delrow JJ, Nelson PS, Lange PH, Ellis WJ, True LD, et al. Genomic alterations indicate tumor origin and varied metastatic potential of disseminated cells from prostate cancer patients. Cancer Res. 2008;68:5599–608. doi: 10.1158/0008-5472.CAN-08-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]