Abstract
Introduction
Numerous epidemiological studies have linked consumption of cruciferous vegetables to a reduced risk of colorectal cancer (CRC) in individuals. It is currently well accepted that chronic inflammation is a contributing factor in 15-20% malignancies including CRC. Many chemopreventive compounds are effective in preclinical systems and many on-going clinical trials are showing promising findings. Many of these compounds could activate the antioxidant responsive element (ARE), a critical regulatory element for phase II protective/detoxification and anti-oxidative stress enzymes mediated by nuclear factor-erythroid 2-related factor 2 (Nrf2). Recently, Nrf2 has emerged as a novel target for the prevention of CRC.
Areas covered
A full literature search was performed using PubMed with the key words ‘ARE, Nrf2, colon, colorectal cancer, chemoprevention, cancer prevention’, and all relevant publications are included.
Expert opinion
The use of Nrf2 knockout mice has provided key insights into the toxicological and chemopreventive importance of this pathway. Mounting evidence has revealed that Nrf2 is a critical regulator of inflammation as well, a major driving force for CRC progression and formation. Targeting the Nrf2/ARE pathway may present a novel therapeutic approach for the treatment of not only colorectal inflammatory diseases but the frequent subsequent development of CRC as well.
Keywords: Antioxidant responsive element (ARE), colorectal cancer (CRC), inflammation, Keap 1, Nuclear factor-erythroid 2-related factor 2 (Nrf2), oxidative stress, phase II enzymes
1. Introduction
The focus of cancer research is often to find molecular targets for cancer chemotherapy, but molecules that can be potential targets for cancer chemoprevention would need equal consideration. Many studies have shown that various compounds have effective chemopreventive properties in vitro and in vivo, as well as in many on-going clinical trials. Many of these active compounds have been identified in various fruits, vegetables and other naturally occurring dietary phytochemicals. Numerous epidemiological studies have linked consumption of cruciferous vegetables to a reduced risk of colorectal cancer (CRC) which currently is the third highest cancer mortality in the US, after lung and genital cancers [1]. It thus becomes important to more clearly understand the mechanism leading to this cancer and how to prevent it. Many of these dietary cancer chemopreventive compounds could activate the antioxidant responsive element (ARE), a critical regulatory element in the promoter sequence of genes encoding cellular phase II detoxifying and antioxidant enzymes. Transcriptional activation of ARE is typically mediated by the transcription factor, nuclear factor-erythroid 2-related factor 2 (Nrf2) [2-4]. Recently, Nrf2 has emerged as a novel target for the prevention of CRC since it is currently well accepted that chronic inflammation is a contributing factor in 15-20% malignancies including CRC [5] and that this inflammation can be attributed to a number of factors including oxidative stress, reactive oxygen species (ROS) and reactive nitrogen species (RNS). The understanding and importance of targeting Nrf2 for the prevention of CRC is the focus of this review. Targeting the Nrf2/ARE signaling pathway may represent a novel therapeutic approach for the treatment of not only colorectal inflammatory diseases but also the frequently occurred subsequent development of CRC.
Carcinogenesis is a multi-steps process involving initiation, promotion and progression, transitioning of normal cells to mutant cells and ultimately invasive carcinoma. Therefore, this provides ample opportunities for cancer chemoprevention either using non-toxic approved drugs such as non-steroidal anti-inflammatory drugs (NSAID) or relatively non-toxic dietary phytochemicals [6, 7]. While cancer chemoprevention currently may appear to be costly, however, it would be the best approach towards cancer management(s). The American society would appear to be willing to pay for this potential costly cancer prevention programs since the disease inflicts great pains and the burden for the cost of treatment is tremendous. The good news is, the vast majority of cancers could be a preventable disease, and as only 5-10% of all cancers can be attributed to genetic defects, while the majority remaining 90-95% can be attributed to non-genetic, environmental, and lifestyle factors [8]. Current understanding of cancer prevention has been enhanced and increasing evidence has shown that chronic inflammations could be associated in driving malignancies. It is estimated that about 15-20 % of all deaths from cancer worldwide are linked to infections and inflammations [5]. With many factors triggering chronic inflammation, the risk of developing cancer(s) is increased. CRC is one of the more classic examples of cancer with a strong association with chronic inflammation [9]. Therefore, it would critical to prevent chronic inflammation and it typically precedes cancer development [10]. Recently many studies have shown that many chemopreventive compounds are effective not only in in vitro and in vivo systems, they are also shown to be promising in many completed and on-going clinical trials [11-19]. Critical targets for chemoprevention often encompass multiple molecular pathways involved in; inflammatory pathways; cell survival, proliferation, and invasion; angiogenesis of tumor cells [20-23]; enhancement of the cellular antioxidant response [2] and metabolism of carcinogenic species [3]. A key player that has been found to be involved in many of these pathways is the transcription factor; nuclear factor-erythroid 2-related factor 2 (Nrf2) [4]. Therefore as discussed above, targeting the Nrf2 pathway [24-27] as an approach to CRC prevention, will be the main focus of the present review. The colorectal, colon and rectal cancers are considered collectively as CRC herein, unless specified otherwise.
2. Epidemiology, diet and CRC
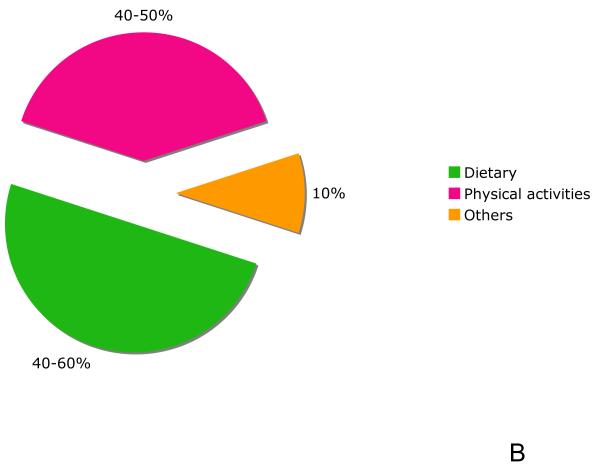
Cancer arises from an accumulation of mutations that promote clonal selection of cells with increasingly aggressive behavior. The vast majority of mutations in cancer are somatic and only 1% of patients would have hereditary cancer syndromes who carry a particular germline mutation [28]. The non-inherited genetic causes of cancers can be logically modified to reduce the cancer risk and these include cigarette smoking, diet, alcohol consumption, exposure to sunlight and environmental pollutants, infections, stress, obesity, and physical inactivity. An individual who carries a mutant allele of an inherited cancer gene would appear to have a variable and possible risk of cancer that can be influenced by other genetic and non-genetic factors, which would appear to occur in every one of us, due to random statistical considerations of our genome. As with most cancers, the contributing factors to CRC can be broken down into heritable genetic, sporadic genetic and non-genetic components [28], and furthermore these factors appear to be strongly interacting with one another [29]. Approximately 15% of CRC has genetic roots. Less than 1 % of this is represented by hereditary with familial adenomatous polyposis (FAP) [30], and 5-15% represented by hereditary non-polyposis colon cancer (HNPCC) [31]. Both FAP and HNPCC are classified as inherited cancer among others cancers [28]. However, remarkably 85% of CRC are considered sporadic (non-hereditary) cases [32] (Fig. 1A). The fact that most CRC cases are not caused by heritable genetic factors highlights the importance of changes in dietary, lifestyle and environmental factors in preventing CRC (Fig. 1B). In 1981 Doll and Peto reported a review, who first estimated that approximately 35% of the cancer deaths in the US were potentially avoidable by modification of diet [33]. Since then epidemiological data on diet and cancer have grown rapidly. Later, Willet et al. had also reviewed and presented a similar estimation that 20-42% of cancer could be avoided by dietary modification [34]. In referencing specifically to CRC, while Doll and Peto estimated that about 90% of CRC was avoidable by dietary change, whereas Willet et al. was more conservative, estimating that only 50% diet-related CRC could be avoided by dietary changes due to the increasing evidence suggesting that, instead of diet alone, the addition of physical activity could also account for a 40-50% reduction in CRC risk [35, 36]. This estimate was later corroborated by later findings showing that physical activity could indeed reduce CRC risk by 40-50% [37, 38]. Taken together, these epidemiological literature indicate that environmental and lifestyle factors, would in principal, be modified in this context to reduce the majority of CRC. Over the past decade, the impact of genetic factors and environmental/lifestyle factors including diet [39], lifestyle such as smoking [40, 41] and alcohol consumption [42, 43], as well as metabolism phenotypes on CRC have been extensively investigated [29]. A recent completed survey among woman revealed that both genetic and environmental factors are believed to be the predominant causes of breast and CRC cancers [44]. Therefore in this context, it is important to identify the modifiable risk factors, especially dietary factor in prevention of CRC [45].
Figure 1.
(A) The contribution role of familial (hereditary) and sporadic factors in the development of human colorectal cancer. (B) Modifiable factors to reduce risk of CRC. Dietary change can reduce the risk in half; other means including increasing physical activity, reducing alcohol drinking and avoiding smoking can further reduce the risk. Please see text for discussion.
3. Inflammation and oxidative stress: carcinogenesis of CRC
It is well accepted that the developmental process of CRC in patients with sporadic cancer (as opposed to familial cancer) occurs over a period of decades. Patients with inflammatory bowel disease (IBD), which includes both ulcerative colitis (UC) and Crohn’s disease, are at increased risk of developing CRC [32]. Indeed IBD ranks among the top three high-risk conditions for CRC; the risk for developing CRC increases with duration of colitis. For reasons still unclear, CRC is rarely encountered before 7 years of colitis, however thereafter, the risk increases at a rate of ~0.5-1.0% per year and the risk is directly proportionate to the colonic surface that is involved with colitis as well [32]. Despite extensive research, at present there is no genetic basis found to be able to explain the predisposition of CRC in IBD patients in developing CRC. Regardless of the underlying condition, essentially all CRC develops from a dysplastic precursor lesion. For instance, as with sporadic CRC, the “adenoma-carcinoma” setting becomes "inflammation-dysplasia-carcinoma" sequence in IBD [32]. Recent case studies of UC patients have demonstrated that histological inflammation scores are significantly correlated (odds ratio, 4.7; p < 0.001) with the risk of developing colorectal neoplasia [46]. How chronic inflammation contributes to the carcinogenesis of CRC remains an important question. It appears to be logical to assume that factors associated with inflammation, such as oxidative stress, ROS and RNS are contributing to the development of CRC [17]. An increase release of ROS at the site of inflammation is accompanied with activation of the inflammatory cells. In chronic inflammation, excessive levels of ROS could react with DNA, forming oxidative DNA adducts and causing impaired cell cycle contributing to genotypic changes that drive the shift from mutation to carcinogenesis [47]. In fact, clinical studies involving IBD patients demonstrate that these patients have reduced plasma antioxidant(s) concentrations and increased oxidative DNA damage [48]. More specifically, oxidative DNA adduct, 8-hydroxydeoxyguanosine (8-OHdG) accumulation was correlated significantly with the duration of the disease [49], which may explain why the risk of developing CRC from colitis is increased by 0.5-1.0% every year after 7 years of the disease [32]. RNS are also thought to play the same role as ROS in CRC carcinogenesis [50]. Putting all these together, a simplified illustration of the carcinogenesis in human CRC is shown in Fig. 2. For a more detailed discussion of the pathogenesis of CRC, the readers would refer to the existing literature [32, 51, 52].
Figure 2.
A simplified illustration of the carcinogenesis in human CRC and the role of inflammation and oxidative stress. (A) 85% sporadic CRC and patients with FAP tend to proceed via genetic modifications that contributes to colon carcinogenesis as a result of chromosomal instability (CIN). Usually one or two of focal areas of dysplastic lesions are found. (B) Clinically, colitis-associated CRC and HNPCC demonstrate similar features to (A), and unlike sporadic CRC, usually they have multiple dysplastic lesions. The remaining 15% sporadic CRC originates from genetic modifications that contributes to colon carcinogenesis as a result of microsatellite instability (MSI). This is also a pathways preferred by majority of patients with HNPCC. Emerging evidence has shown that in colitis-associated CRC, the frequency of CIN and MIS are also about the same as sporadic CRC, 85% and 15% respectively. Inflammation and oxidative stress, together with the accumulation of genetic alterations over the patients lifetime, will result in the formation of CRC. It is important to take note that in reality, cancer can arise without proceeding through each of these steps. For a more detailed pathogenesis of CRC, please refer to existing literature [32, 51, 52].
Recently intense research has focused on discovering critical signaling pathways involved in mediating oxidative stress commonly associated with chronic inflammation leading to carcinogenesis such as CRC. The following sections will introduce key factors that mediate this anti-oxidative stress-anti-inflammatory signaling pathway and may provide useful molecular targets for cancer chemoprevention of CRC. Nrf2 is a novel transcriptional factor that is involved.
4. Nrf2 pathway
4.1 Nrf2-ARE mediated signal transduction
The first isolation of Nrf2 was reported in 1994 by Y.W. Kan’s group [53]. Since then its function has been widely investigated. The current understanding of the most important role of Nrf2 is activating the ARE-mediated anti-oxidative responses [54]. Initial testing with hydrogen peroxide, phenolic antioxidant compounds and metabolizable planar aromatic compounds such as β-naphthoflavone resulted in ARE activation therefore establishing its role in responding to oxidative stress [55]. Under physiological conditions, ROS and other endogenous reactive molecules are constantly produced during normal aerobic metabolism. The role of ARE in controlling the phase II detoxifying and anti-oxidative gene expression is critical in maintaining cellular redox homeostasis under both stressed and non-stressed conditions [54]. Moreover, many compounds, including the well-known dietary chemopreventive compounds, such as isothiocyanates (ITCs) [56] and curcumin [57], are also found to be potent ARE activators. Therefore, the study of whether such compounds could specifically target Nrf2 for CRC prevention could provide valuable information.
4.2 Keap 1-Nrf2-ARE mediated pathway
Recently our laboratory has found evidence suggesting that Nrf2 could be self-sufficient to sense and transduce oxidative stress signals into the nucleus and triggers the transcription of antioxidant genes [58]. Currently it is well established that the activity of Nrf2 is controlled in part by the cytosolic protein Kelch-like ECH-associated protein 1 (Keap 1). The mechanistic pathway of Keap 1 repression on Nrf2 has been discussed previously [54], however more research is needed to fully characterize the Keap 1-Nrf2 pathway especially in human based on our current understanding [2, 26]. Additionally, translational controlled of Nrf2 in response to oxidative stress was recently found [59]. The mechanism appears to involve an internal ribosomal entry site (IRES) found within the 5′ untranslated region of the human Nrf2 mRNA that mediates redox-sensitive translation of Nrf2 [59]. Figure 3A illustrates the latest consideration of the most relevant findings in our understand how the Keap 1-Nrf2-ARE signaling pathway could be targeted in CRC for cancer chemoprevention. This Nrf2-ARE pathway appears to be the major mechanism in the cellular defense against oxidative and electrophilic stress by inducing proteins that are involved in the anti-oxidative stress by induction of anti-oxidative enzymes, as well as detoxification and elimination of carcinogens and electrophiles through conjugation by induction of detoxifying enzymes. Increased intake of dietary chemopreventive compounds will further enhance the capacity of this cellular antioxidant mechanism from its basal levels, leading to their cancer preventive effects. Many other cellular signaling pathways can also coordinately regulate Nrf2 signaling, including a wide array of kinase signaling pathways such as the mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3-kinase (PI3K), protein kinase C (PKC) and PKR-like endoplasmic reticulum kinase (PERK) [60-63]. It appears that these kinase signaling pathways are also activated by chemopreventive compounds and that in turn would enhance Nrf2 transcriptional activity, thus the chemopreventive compounds may not only act directly on Keap 1.
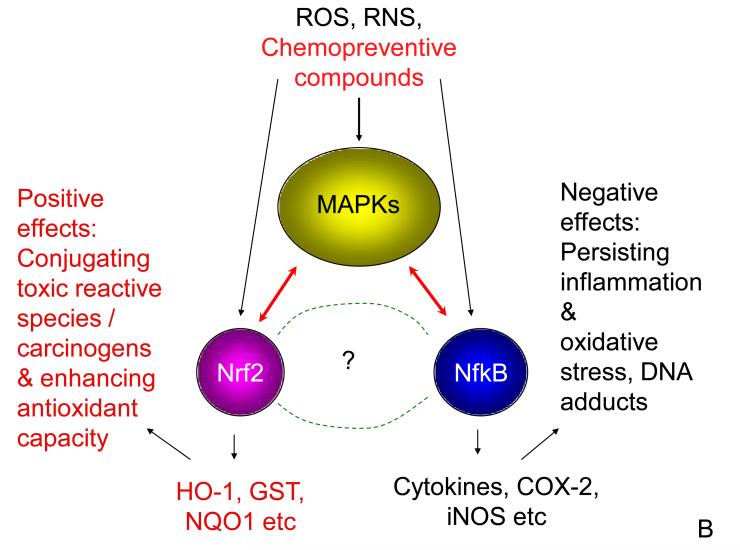
Figure 3.
(A) Summary of the regulation of phase II and anti-oxidative genes via the Keap 1-Nrf2-ARE pathway. In the cytoplasm, under basal level, newly synthesized Nrf2 is constitutively bound to Keap 1 forming a dimmer. Nrf2-Keap 1 which is then directed to go through ubiquitination (ubiquitin, Ub) and converted to Nrf2-Ub is degraded by proteasomal degradation (blue arrows). Oxidants such as ROS, RNS and dietary chemopreventive compounds react with redox reactive cysteines in Keap 1 (SH groups), disrupting the interaction between Nrf2 and Keap 1, hence allowing the transcriptional factor Nrf2 to translocate to the nucleus. In the nucleus, Nrf2 dimerizes with small MAF (sMAF)-family proteins and bind to antioxidant response element (ARE), which is located in the promoter of the phase II and anti-oxidative genes, resulting in increased transcriptional activity (red arrows). Numerous reports have found that chemopreventive agents also interact with other kinase signaling pathways, please see the text for more detailed discussion. (B) A simplified putative model for crosstalk between Nrf2 and NfkB in inflammation and carcinogenesis. Stimulus from ROS, RNS and / chemopreventive compounds could directly interact with members of MAPKs family, as well as interacting with Nrf2 and NfkB pathways concurrently, such multiple interactions allow chemopreventive compounds to exert their beneficial cancer preventive and therapeutic properties.
4.3 Cytoprotective role of Nrf2: lessons from Nrf2 knockout mice
Many cancer chemopreventive compounds have been reported to act at more than one of these three-phases; initiation, promotion and progression. Some can even suppress the final step when metastatic cells have formed, meaning they also possess cancer therapeutic properties such as anti-angiogenesis and inducing apoptosis in cancerous cells [20-23]. In addition, many chemopreventive agents can activate the Nrf2-ARE antioxidative stress pathway as described above that would confer their potential cancer preventive activities [2-4]. The ARE is a critical regulatory element present in the promoter sequence of many genes encoding cellular phase II protective/detoxification and anti-oxidative stress enzymes mediated by Nrf2. ARE containing genes have been shown to be activated by the Nrf2 transcription factor. We have recently summarized the Nrf2-regulated phase II detoxifying enzymes and their potential ARE sequences that are involved [64]. Electrophiles as well as ROS and RNS are generally detoxified by various phase II enzymes including NAD(P)H-quinone reductase-1 (NQO1), glutathione S-transferase (GST) and uridine-diphospho-glucuronosyltransferase (UGT) that could be mediated by the Nrf2-ARE pathway [64-67].
The use of Nrf2 knockout (Nrf2 KO) versus wild type (Nrf2 WT) mice has provided key insights into the carcinogenesis and chemopreventive importance of this pathway. Our laboratory [68], as well as Kensler’s laboratory [69] have shown that Nrf2 KO mice develop more severe colitis in response to treatment with the inflammatory agent dextran sulfate sodium (DSS) as compared to the Nrf2 WT mice. The work was followed up with induction of CRC using the carcinogen azoxymethane (AOM) followed by DSS treatment in the Nrf2 KO mice [70]. The Nrf2 KO mice were found to be more susceptible to the formation of colorectal cancers and dysplasia than WT mice when treated with AOM-DSS [70]. The AOM-DSS treated Nrf2 KO mice had significant increased incidence of colonic tumor and prolapsed rectum/anal bleeding as compared to the WT mice. Nitrotyrosine staining of inflamed mucosa in Nrf2 KO mice indicated higher cellular damage in response to inflammation. These findings show that Nrf2 is required in the protection against inflammation-associated CRC and thus corroborating with the finding that Nrf2 KO increases susceptibility to inflammation-associated aberrant crypt foci (ACF) [69]. The common theme among these studies is the decrease expression of phase II detoxifying and anti-oxidative stress genes with concurrent increased expression of inflammatory markers in Nrf2 KO mice as compared to their WT counterparts. Taken together, these results clearly illustrate the role of Nrf2 in regulating an adaptive response that protects against early-phase inflammation-mediated CRC carcinogenesis.
5. Nrf2 as a therapeutic target in CRC
Nrf2 acts as a major regulator for the cellular defense against oxidative and electrophilic stresses by inducing enzymes that are involved in the detoxification and elimination of ROS, RNS and electrophiles through conjugation and excretion. Because of the close relationship between these reactive intermediates and the development of disease states, Nrf2 is a highly likely candidate for human diseases prevention. In the following sections, we will discuss the available findings on dietary constituents as chemopreventive agents for treating and preventing CRC as they are related to the Nrf2/ARE pathway, which is a major target of dietary compounds, via the well-known mechanism: (a) maintenance of oxidative stress homeostasis and (b) enhancing the expression of detoxifying metabolizing enzymes.
5.1 Preclinical data
Table 1 summarizes the animal studies evaluating Nrf2-dependent gene expression with and without dietary chemopreventive compounds for CRC, only experiments that had examined colon, Nrf2 and/or the dependent genes are tabulated.
Table 1.
In vivo studies showing cytoprotective role of Nrf2 in CRC models
| Testing model |
Study design/testing compounds |
Outcome effects | Reference |
|---|---|---|---|
| Nrf2 KO vs. C57BL/6 WT mice |
DSS-induced colitis Gp 1: WT + 1% DSS Gp 2: KO + 1% DSS Gp 3: WT + water Gp 4: KO + water |
Nrf2 KO mice had significant shorter colon as compared to Nrf2 WT mice (p<0.05). Nrf2 KO mice: ↑DSS-induced colitis ↓antioxidant/phase II detoxifying enzymes: HO-1, NQO1, UGT1A1, GSTM1 ↑proinflammatory mediators/cytokines: COX-2, iNOS, iL-1b, iL-6 |
[68] |
|
| |||
| Nrf2 KO vs. C57BL/6J WT mice |
AOM/DSS-induced CRC Gp 1: WT + water Gp 2: KO + water Gp 3: WT + AOM/DSS Gp 4: KO + AOM/DSS |
93% Nrf2 KO mice had colonic tumor vs. 53% Nrf2 WT (p<0.05), 80% were adenocarcinoma vs. 29% respectively. Nrf2 KO mice: ↑COX-2, 5-LOX, PGE2, LTB4, inflamed colonic mucosa (nitrotyrosine expression) Nrf2 WT mice: ↑NQO1, UGT1A1 |
[70] |
|
| |||
| Nrf2 KO vs. ICR/129S VJ WT mice |
AOM/DSS-induced CRC Gp 1: WT + AOM Gp 2: KO + AOM Gp 3: WT + AOM/DSS Gp 4: KO + AOM/DSS |
DSS treatment significantly increased ACF, inflammation and mucosal damage in Nrf2 KO mice but not WT (p<0.05). |
[69] |
|
| |||
| BALB/cA nude mice |
Gp 1: Basal diet Gp 2: IQ (50 mg/kg) Gp 3: IQ (50 mg/kg) + EGCG (5 mg/kg) Gp 4: IQ (50 mg/kg) + EGCG (10 mg/kg) Gp 5: IQ (50 mg/kg) + EGCG (20 mg/kg) |
IQ suppressed Nrf2 and UGT1A10 expression. As compared to IQ treated, EGCG groups have: ↓atypical hyperplasia, total ACF and total AC significantly ↑Nrf2, UGT1A10 (all p<0.01) |
[71] |
|
| |||
| Orthotopic HT-29 cancer cells implanted in the cecum of BALB/cA nude mice |
Gp 1: Basal diet Gp 2: EGCG (5 mg/kg) Gp 3: EGCG (10 mg/kg) Gp 4: EGCG (20 mg/kg) |
EGCG treated groups: ↓growth (all p<0.001, vs. control) and liver metastases (p<0.01 for Gp 3, p<0.05 for Gp 4 vs. control) ↑Nrf2, UGT1A, UGT1A8, UGT1A10 (all p<0.01) |
[72] |
|
| |||
| Fischer 344 rats |
Gp 1: Modified (high fat, fiber free) AIN76 diet Gp 2: IQ (100 mg/kg/day for 10 alternate days) Gp 3: IQ+Brussels sprouts juices 5 days before IQ and during IQ Gp 4: IQ+Red cabbage juices 5 days before IQ and during IQ |
Brussels sprouts: ↓ACF /rat 41-52% in the colon, 85-91% liver GST-P+ foci Red cabbage: ↓ACF/rat 17-20%, 41-83% liver GST-P+ foci ↑UGT-2 & P4501A2 increased by both vegetables |
[73] |
|
| |||
| F344 rats | 2% curcumin in diet by oral, 500 mg/kg by i.g. gavage vs. control treated with CCl4 |
Curcumin treated rats: ↑16% Hepatic GST ↓36% Colon M1G |
[74] |
Abbreviations used: Nrf2 knockout (Nrf2 KO); Nrf2 wild-type (Nrf2 WT); Dextran sulfate sodium (DSS); Group (Gp); heme-oxygenase-1 (HO-1); NAD(P)H-quinone reductase-1 (NQO1); Uridine-diphospho-glucuronosyltransferase (UGT), glutathione S-transferase (GST); cyclo-oxygenase 2 (COX-2); inducible nitric oxide synthase (iNOS); interleukin-1beta (iL-1b); interleukin 6 (iL-6); azoxymethane (AOM); aberrant crypt foci (ACF); aberrant crypts (AC); 2-amino-3-methylimidazo[4,5-f]quinoline (IQ); epigallocatechin gallate (EGCG); liver glutathione-S-transferase placental positive (GST-P+, an early preneoplastic lesion in liver); cytochrome P4501A2 (P450, an enzyme catalyses the activation of HAA); adducts of malondialdehyde with DNA (M1G)
As mentioned earlier, Nrf2 KO mice have confirmed the importance of Nrf2 in protecting against inflammation-associated CRC [68-70]. Other studies have also shown consistent evidence regarding the role of Nrf2 and its downstream proteins such as UGT in protecting against well-known genotoxic chemicals formed during food preparation, such as heterocyclic aromatic amines (HAA), and other HAA members such as 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), which is commonly found in the human diet [71]. Using a nude mouse system, it was demonstrated that mice treated with IQ have suppressed Nrf2 and UGT1A10 expression [71]. Mice treated with IQ also have significant increased number of total aberrant crypt foci (ACF) and total aberrant crypts (AC) as compared to epigallocatechin gallate (EGCG) treated groups [71]. EGCG, the major polyphenol in green tea, was found to inhibit colon carcinogenesis through various pathways, including enhancing the expression of Nrf2 and its downstream gene, UGT1A10 significantly [71, 72], consistent with our previous findings that EGCG activate the ARE transcription [75] as well as using Nrf2KO mice coupled with Affymetric chip assay [76]. The relevance of the role of Nrf2 is further confirmed by such observations that using EGCG was able to up-regulate the expression of Nrf2 and UGT1A10 in a dose-dependent manner, and the effect was most prominent in the high dose of EGCG (20 mg/kg) that could reverse the histopathological damage of colon tissue in mice treated with IQ back to normal anatomy [71]. It is postulated that increase in UGT1A10 expression in colon cancer could promote the conjugation of IQ with glucuronic acid, increasing the water solubility and elimination of IQ through urine and bile, thereby reducing or mitigating the carcinogenicity of IQ in colon tissue [71]. When mice were fed with EGCG only, it was demonstrated that there was an increase of Nrf2 and UGT1A in colon mucosa, further confirming the potential role of Nrf2 in attenuating carcinogenic toxicity of IQ, in part, by enhancing the expression of UGT1A [77].
The above logical argument could also explain in part, for the chemopreventive properties of cruciferous vegetables such as brussels sprouts and red cabbage when demonstrated by other scientists using similar approach by feeding the rats with IQ to induce colon lesions in the rats [73, 78]. Curciferous vegetables contain effective chemopreventive constituents similar to EGCG in the protection of the animals against IQ-induced colon lesions and at the same time the consumption of the cruciferous vegetables was found to up-regulate the expression of UGT (Table 1). There are several promising chemopreventive efficacy studies of sulforaphane (SFN) and phenethyl isothiocyanate (PEITC), the major ITCs in cruciferous vegetables, on various CRC animal models. Genetic model such as APC (adenomatous polyposis coli) Min/+ mice which mimics human pre-neoplastic FAP intestinal polyps spontaneously and that mutation in the APC gene is common in the majority of human colon cancers [79-81]. We had tested SFN in APCMin/+ mice, we found that compared to control, mice fed with SFN developed significantly lesser and smaller intestinal polyps [82]. Comparing Nrf2 KO and Nrf2 WT mice, it was also found that SFN and broccoli seeds produced about a 1.5 fold increase in NQO1 and GST in the small intestine and liver of Nrf2 WT mice but not Nrf2 KO [83, 84]. Using Nrf2 WT and Nrf2 KO mice, our group has also published the hepatic gene expression profiles of SFN [85] and PEITC [86]. We found that both SFN and PEITC enhanced expression of various genes including drug detoxifying enzymes, through the Nrf2 signaling pathway. These data show that SFN and PETIC are effective in inducing antioxidant and detoxifying proteins, both in in vivo and ex vivo, and that Nrf2 plays a critical role.
Another chemopreventive compound, curcumin, a major active compound found in turmeric and in ancient medicine, has also been reported to induce many antioxidant and phase II enzymes such as GST [74, 87-89] that taken together would also explain in part, the reduction of the incidence of ACF seen in a AOM-induced rat colon cancer model [90].
5.2 Clinical data
Although some epidemiological studies may not have found a significant association between high intake of cruciferous vegetable with reduced CRC risk, there was a significant inverse correlation found among individuals null for both GST genotypes GSTM1 and T1, of which the GST phase II enzymes are important for the metabolism of ITCs [91]. Similar to conjugation of IQ with glucuronic acid by UGT enzymes for metabolism and excretion, conjugation with glutathione by GST is an important step in the metabolism and subsequent detoxification of carcinogens such as polycyclic aromatic hydrocarbons. GSTs are also known to metabolize ITCs, resulting in the formation of N-acetylcysteine conjugates, which are excreted in the urine [92, 93]. In individuals with both GSTM1 and T1 null genotypes, it was found that those consuming high dietary ITCs had a 57% reduction in risk as compared to low consumers of ITCs (odds ratio 0.43, 95% confidence interval 0.20–0.96), in particular for colon cancer (odds ratio 0.31, 95% confidence interval 0.12–0.84) [91]. These findings suggest that higher plasma levels of ITCs would be expected in individuals with null GST metabolizing enzymes and may be responsible for the chemoprotective effects of ITCs [91]. Similar findings were also found in another study [94], however, inconsistency exists within this study [95]. Such inconsistent evidence regarding the role of GST polymorphisms in CRC susceptibility might be associated with the ethnic differences in allele frequency for these polymorphisms [96] and or with the gender [95] or the presence of other genetic/environmental factors. For a more detailed review on the relationship between GST polymorphism, risk of CRC and chemoprevention in CRC, please refer to reviews available [96, 97]. Very recently, single nucleotide polymorphisms (SNP) in the UGT1A7 and UGT1A1 have been identified to be associated with higher risk for metastasis in human CRC [98]. Such findings could offer opportunity in identifying patients at risk of CRC and reducing their risk for severe CRC using individualized pharmacogenomic approaches. Thus, targeting Nrf2 is a novel approach since Nrf2 regulates numerous phase II detoxifying and antioxidant genes that protect against oxidative stress-induced CRC carcinogenesis.
6. Interplay between inflammatory and Nrf2 pathways
Many signaling pathways are involved in the induction of cytokines and the inflammatory responses, such as the nuclear factor-kappa-B (NfkB) [99, 100] and the MAPKs [101] signaling pathways. Interestingly, many chemopreventive compounds that work through Nrf2 pathways also have anti-inflammatory activities [24, 25, 102]. There is also increasing evidence showing that there is an interplay between the inflammatory NfkB pathway and the antioxidative stress Nrf2 pathway. For instance it has been reported that GSH/glutathione peroxidase 2 (Gpx2) is able to reduce COX-2 expression, and is believed to provide a physiological buffering system in preventing undue activation of COX-2 [103]. Thus GSH/Gpx2 aids in preventing the exacerbation of inflammation induced by COX-2 expression, and thereby attenuating inflammation-driven initiation of carcinogenesis [103]. It was also found that the protective effects of curcumin in the smooth muscle cellular response against toxic aldehydes and oxidative stress by inducing the expression of detoxifying enzyme aldose reductase, a member of the NADPH-dependent aldo-keto reductase family, were regulated by both Nrf2 and NfkB pathways [104]. Indeed oxidative stress and inflammation are inter-related, it was also found that activation of ARE-regulated genes via adenovirus-mediated expression of Nrf2 protected endothelial cells from oxidant injury induced by hydrogen peroxide and inhibited some inflammatory genes expression such as interleukin 1-beta (IL-1b) [105]. The activation of Nrf2/ARE pathway also causes an increase in interleukin (IL)-8 and there is a strong possibility that under certain circumstances IL-8 may serve an anti-inflammatory role, and thereby contributing to the resolution of tissue injury [106].
Since Nrf2 and NfkB are two important transcriptional regulators in the etiopathogenesis of many cancers as well as inflammation-associated diseases, our laboratory has recently attempted to explore the crosstalk between Nrf2 and NfkB using a bioinformatics approach [107]. Multiple alignments of Nrf2 and NfkB1 genes were performed for human, chimpanzee, dog, mouse and rat, and a canonical regulated network for concerted modulation of Nrf2 and NfkB1 in inflammation/carcinogenesis was constructed. It was found that out of 59 members in the canonical first-generation regulatory network members, representing the potential putative crosstalk between Nrf2 and NfkB1 in inflammation-associated carcinogenesis, 44 are MAPKs members. These findings demonstrate that MAPKs constitute 75% of the signaling molecules involved in crosstalk between Nrf2 and NfkB and that as described above, the MAPKs play an active role in the Nrf2-Keap1-ARE signaling pathway [62, 108-111]. Moreover, MAPKs are also known to modulate NfkB signaling pathways [112, 113]. Furthermore, most recently our laboratory as well as Paul Talalay’s laboratory found that the anti-inflammatory effects of the ITC sulforaphane are mediated by Nrf2 comparing the Nrf2 KO and WT mice [114, 115]. Similarly, we found that Nrf2 plays a role in suppressing LPS-Induced Inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids (PUFA) docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) [116]. Based on all these findings, we are presenting a simplified putative crosstalk model between Nrf2 and NfkB via upstream MAPKs pathway (Fig. 3B).
7. Expert opinion
The best evidence for the causal relationship between inflammation and CRC comes from epidemiological studies that patients taking NSAIDs appear to have lower risk in incidences of cancer, including CRC risk [117]. This is due to the fact that COX-2 is highly expressed in the CRC patients, COX-2 inhibitors were tested clinically for prevention of CRC [118]. Indeed celecoxib, a second generation COX-2 specific NSAID, was found to be an effective agent for the prevention of colorectal adenomas, as shown by celecoxib-treated patients having a lower total percentage of adenomas as compared to the placebo group (37.5-43.2% vs. 60.7%, p<0.001). However, celecoxib may not be routinely recommended as a therapeutic chemopreventive agent due to its potential risk of increasing cardiovascular events in patients (3 fold of increased risk as compared to the placebo group) [118]. Hence, one of the recommendations for better approaches in preventing inflammation-induced CRC is to use relatively non-toxic dietary cancer chemopreventive compounds [7, 18, 24].
Based on the promising chemopreventive properties of various dietary phytochemicals as discussed above, therefore it appears that the dietary approach for CRC prevention would be the most logical approach for cancer management. Phytochemicals do not carry the concern of the higher risks of adverse cardiovascular and thrombotic events and gastrointestinal bleeding frequently seen in non-steroidal anti-inflammatory drugs (NSAIDs) [18, 119]. The poor bioavailability of some dietary cancer chemopreventive agents (such as curcumin and EGCG), can be advantageous towards the colon since this can lead to higher levels of the chemopreventive agents being attained in the colon and thus may contribute to the enhanced effects on the Nrf2 and Nrf2-related signaling pathway in the intestines.
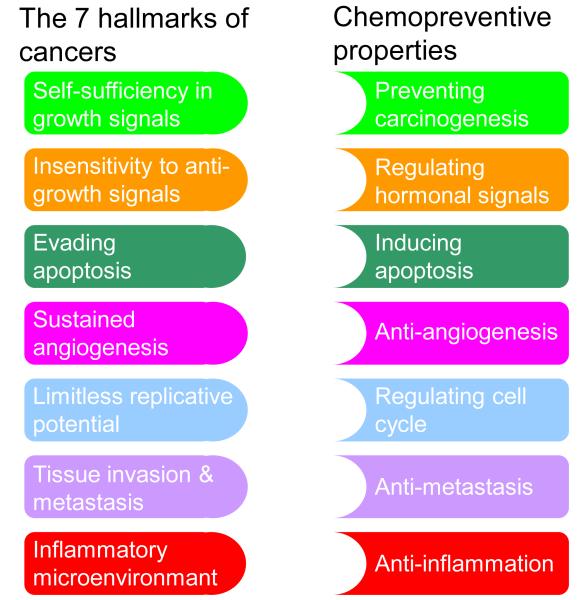
Recently cancer-related inflammation (inflammatory microenvironment) has been included as the seventh hallmark of cancer [51, 120], which is an additional hallmark to the identified six hallmarks of cancer summarized by Hanahan and Weinberg [121], namely a) self-sufficiency in growth signals, b) insensitivity to anti-growth signals, c) evading apoptosis, d) sustained angiogenesis, e) limitless replicative potential, f) tissue invasion and metastasis. Irrespective of the diverse molecular pathogenesis of CRC, most of CRC, if not all, have acquired the seven functional capabilities during their development, although through varied mechanisms. Thus, to prevent or treat CRC, targeting the Nrf2 pahtway would indeed be one of the logical and promising pathways, as many of the chemopreventive compounds targeting Nrf2 also possess other molecular targeting ability such as preventing the formation of cancer (as primary, secondary and tertiary chemopreventive agents that are able to block initiation, and suppressing promotion and progression of cancers respectively), inducing apoptosis in cancer cells, regulating cell cycle, regulating hormonal signaling, anti-angiogenesis, anti-inflammation, anti-metastasis, and most importantly as a whole, ultimately preventing carcinogenesis (Fig. 4).
Figure 4.
“Lock and Key” model for chemopreventive compounds neutralizing the acquired capabilities of cancer. The seven hallmarks of cancer (simplified from [51, 120, 121]), and the multifaceted properties of chemopreventive compounds in preventing and treating cancers. Targeting at Nrf2, is one of the promising pathways as many of the chemopreventive compounds targeting Nrf2, also possess other molecular targeting ability such as a) blocking the initiation and suppressing the promotion and progression of cancers, b) regulating hormonal signaling, c) inducing apoptosis in cancer cells, d) anti-angiogenesis, e) regulating cell cycle, f) anti-metastasis, g) anti-inflammation, and most importantly prevent the carcinogenesis as a whole.
Increasing evidence has shown that chemopreventive compounds are also effective in suppressing various inflammatory models, and, clinically, COX-2 inhibitors have been proven to be effective for preventing colitis-associated CRC. Due to its cardiovascular toxicity risk it may not be prescribed and recommended as a routine practice as discussed early. However, more work needs to be done to further explore and define the relationship between Nrf2 and inflammation, so that a better understanding of its role in the pathogenesis of CRC, and thereby prevention of the disease. This will lead to better design of clinical trials using chemopreventive agents for the prevention of CRC.
Development of effective chemopreventive compounds against CRC is important and requires conclusive evidence from animal models that emulate human cancers. For colorectal carcinogenesis and intervention studies, APC (adenomatous polyposis coli) Min/+ mice are also used in many laboratories including ours. APC Min/+ spontaneously develop pre-neoplastic intestinal polyps and mutation in the APC gene which is common to the majority of human colon cancers, hence APC Min/+ appears to be a highly relevant model to study cancer chemoprevention on the intestines. Since Nrf2 is a crucial transcriptional factor in cytoprotection against oxidative and electrophilic stresses as well as inflammation, APC Min/+ knockout Nrf2 function mice would potentially be a very good model for further research on Nrf2 as a chemopreventive target for CRC. AOM-DSS-Nrf2 KO model is another ideal model to study both inflammation- and oxidative stress-induced carcinogenesis of colon in a single system, which will enable a better appreciation and understanding of the emerging roles of crosstalk between the Nrf2 and NfkB transcriptional factors.
While NfkB is a key player in the inflammatory response, aberrant activation of NfkB is frequently observed in many cancers. It was found that NfkB suppression could limit the proliferation of cancer cells. Hence methods of inhibiting NfkB have potential therapeutic applications in cancer and inflammatory diseases [122, 123]. Increasing evidences have shown that Nrf2 and NfkB are inter-related and our group have shown recently [24, 26, 114, 116], we believe that targeting Nrf2 by natural or synthetic chemopreventive agents that could enhance the Nrf2/ARE pathways while suppress the NfkB/inflammatory pathways in pre-cancerous tumors including CRC conditions, would greatly contribute to the prevention of CRC.
Nrf2 is ubiquitously distributed in many tissues in human and animals, and Nrf2 is shown to be the key transcriptional factor in maintaining the oxidative stress homeostasis via increased expression of anti-oxidative and detoxifying enzymes. There is a very close relationship between ROS/RNS and the development of cancers, we believe that chemopreventive agents targeting Nrf2 not only block the aberrant inflammation and decrease oxidative stress thus blocking the development of CRC, they can also work in preventing other cancers. To achieve these goals, several critical issues regarding Nrf2 as a chemopreventive target in CRC should be addressed in future investigations, and the answers should also be applicable more globally to other cancers. They are as follow:
On one hand chemopreventive agents such as ITCs can induce the expression of phase II detoxifying and antioxidant enzymes to increase the metabolism of carcinogens and reactive intermediates, on the other hand individuals with some null phase II enzymes known to metabolize ITCs appear to benefit from the lower metabolizing rate of ITCs and have lowered CRC risk. Controlled clinical studies including detailed amount of consumption of cruciferous vegetables or chemopreventive compounds and the pharmacokinetic profile of the compounds as well as the polymorphism of the genes involved should be carefully studied and defined, to better provide a pharmacogenomic profile in the use of Nrf2 as a potential target of chemopreventive compounds for CRC prevention in clinical settings.
Activation of the Nrf2/ARE pathway may suppress the expression of redox-sensitive inflammatory genes and protect against oxidant-mediated injury in cells and animals. Because Nrf2 can also be activated by some pro-inflammatory cytokines, further characterization of endogenous Nrf2/ARE signaling as well as exogenous activated Nrf2/ARE pathway will eventually lead to the development of Nrf2 targeting chemopreventive compounds that also has a role in suppressing the aberrant inflammation in CRC formation.
Combination approaches targeting multiple pathways in conjunction with the Nrf2 pathway, such as the NfkB, COX-2 and iNOS pathways (depending on the etiology) would enhance the overall success of cancer chemoprevention of CRC in the future.
Acknowledgments
Declaration of interest The authors would like to thank members of Ah-Ng Tony Kong’s lab, especially to Mr. Ka Lung Cheung for helpful discussions and critical reading of this manuscript. This work was supported in part by the National Institute of Health R01 CA-073674 (A.N.K.).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu S, Kong AN. Targeting carcinogen metabolism by dietary cancer preventive compounds. Curr Cancer Drug Targets. 2007;7:416–24. doi: 10.2174/156800907781386669. [DOI] [PubMed] [Google Scholar]
- 4.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52(Suppl 1):S128–38. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Kong AN. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic Biol Med. 2004;36:1505–16. doi: 10.1016/j.freeradbiomed.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Kong AN. Dietary cancer-chemopreventive compounds: from signaling and gene expression to pharmacological effects. Trends Pharmacol Sci. 2005;26:318–26. doi: 10.1016/j.tips.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McConnell BB, Yang VW. The role of inflammation in the pathogenesis of colorectal cancer. Curr Colorectal Cancer Rep. 2009;5:69–74. doi: 10.1007/s11888-009-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 11.Doherty GA, Murray FE. Cyclooxygenase as a target for chemoprevention in colorectal cancer: lost cause or a concept coming of age? Expert Opin Ther Targets. 2009;13:209–18. doi: 10.1517/14728220802653631. [DOI] [PubMed] [Google Scholar]
- 12.Gatof D, Ahnen D. Primary prevention of colorectal cancer: diet and drugs. Gastroenterol Clin North Am. 2002;31:587–623. xi. doi: 10.1016/s0889-8553(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 13.Turini ME, DuBois RN. Primary prevention: phytoprevention and chemoprevention of colorectal cancer. Hematol Oncol Clin North Am. 2002;16:811–40. doi: 10.1016/s0889-8588(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 14.Kumar N, Shibata D, Helm J, et al. Green tea polyphenols in the prevention of colon cancer. Front Biosci. 2007;12:2309–15. doi: 10.2741/2233. [DOI] [PubMed] [Google Scholar]
- 15.Reid ME, Duffield-Lillico AJ, Sunga A, et al. Selenium supplementation and colorectal adenomas: an analysis of the nutritional prevention of cancer trial. Int J Cancer. 2006;118:1777–81. doi: 10.1002/ijc.21529. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–80. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 17.Villegas I, Sanchez-Fidalgo S, Alarcon de la Lastra C. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Mol Nutr Food Res. 2008;52:1040–61. doi: 10.1002/mnfr.200700280. [DOI] [PubMed] [Google Scholar]
- 18.Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–9. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 19.Chu DZ, Chansky K, Alberts DS, et al. Adenoma recurrences after resection of colorectal carcinoma: results from the Southwest Oncology Group 9041 calcium chemoprevention pilot study. Ann Surg Oncol. 2003;10:870–5. doi: 10.1245/aso.2003.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–27. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatcher H, Planalp R, Cho J, et al. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Van Kuiken ME, Iyer LH, et al. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood) 2009;234:825–49. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khor TO, Yu S, Kong AN. Dietary cancer chemopreventive agents - targeting inflammation and Nrf2 signaling pathway. Planta Med. 2008;74:1540–7. doi: 10.1055/s-0028-1088303. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Cha YN, Surh YJ. A protective role of nuclear erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Nair S, Li W, Kong AN. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol Sin. 2007;28:459–72. doi: 10.1111/j.1745-7254.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 27.Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fearon ER. Human cancer syndromes: clues to the origin and nature of cancer. Science. 1997;278:1043–50. doi: 10.1126/science.278.5340.1043. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed FE. Gene-gene, gene-environment & multiple interactions in colorectal cancer. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24:1–101. doi: 10.1080/10590500600614295. [DOI] [PubMed] [Google Scholar]
- 30.Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. doi: 10.1186/1750-1172-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas HJ. Familial colorectal cancer. Bmj. 1993;307:277–8. doi: 10.1136/bmj.307.6899.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 33.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–308. [PubMed] [Google Scholar]
- 34.Willett WC. Diet, nutrition, and avoidable cancer. Environ Health Perspect. 1995;103(Suppl 8):165–70. doi: 10.1289/ehp.95103s8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garabrant DH, Peters JM, Mack TM, et al. Job activity and colon cancer risk. Am J Epidemiol. 1984;119:1005–14. doi: 10.1093/oxfordjournals.aje.a113805. [DOI] [PubMed] [Google Scholar]
- 36.Gerhardsson M, Norell SE, Kiviranta H, et al. Sedentary jobs and colon cancer. Am J Epidemiol. 1986;123:775–80. doi: 10.1093/oxfordjournals.aje.a114306. [DOI] [PubMed] [Google Scholar]
- 37.Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer. 1996;73:1134–40. doi: 10.1038/bjc.1996.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colditz GA, Cannuscio CC, Frazier AL. Physical activity and reduced risk of colon cancer: implications for prevention. Cancer Causes Control. 1997;8:649–67. doi: 10.1023/a:1018458700185. [DOI] [PubMed] [Google Scholar]
- 39.Satia-Abouta J, Galanko JA, Martin CF, et al. Associations of micronutrients with colon cancer risk in African Americans and whites: results from the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev. 2003;12:747–54. [PubMed] [Google Scholar]
- 40.Tsoi KK, Pau CY, Wu WK, et al. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2009;7:682–88. e1–5. doi: 10.1016/j.cgh.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124:2406–15. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- 42.Bongaerts BW, van den Brandt PA, Goldbohm RA, et al. Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. Int J Cancer. 2008;123:2411–7. doi: 10.1002/ijc.23774. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari P, Jenab M, Norat T, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC) Int J Cancer. 2007;121:2065–72. doi: 10.1002/ijc.22966. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Miller SM, Egleston BL, et al. Beliefs about the causes of breast and colorectal cancer among women in the general population. Cancer Causes Control. 2010;21:99–107. doi: 10.1007/s10552-009-9439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipkin M, Reddy B, Newmark H, et al. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–86. doi: 10.1146/annurev.nutr.19.1.545. [DOI] [PubMed] [Google Scholar]
- 46.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Guyton KZ, Kensler TW. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993;49:523–44. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- 48.D’Odorico A, Bortolan S, Cardin R, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289–94. doi: 10.1080/003655201317097146. [DOI] [PubMed] [Google Scholar]
- 49.D’Inca R, Cardin R, Benazzato L, et al. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis. 2004;10:23–7. doi: 10.1097/00054725-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Ohshima H, Sawa T, Akaike T. 8-nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid Redox Signal. 2006;8:1033–45. doi: 10.1089/ars.2006.8.1033. [DOI] [PubMed] [Google Scholar]
- 51.Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 52.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 53.Moi P, Chan K, Asunis I, et al. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–30. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–9. [PubMed] [Google Scholar]
- 56.Keum YS, Owuor ED, Kim BR, et al. Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC) Pharm Res. 2003;20:1351–6. doi: 10.1023/a:1025737622815. [DOI] [PubMed] [Google Scholar]
- 57.Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–95. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W, Yu SW, Kong AN. Nrf2 possesses a redox-sensitive nuclear exporting signal in the Neh5 transactivation domain. J Biol Chem. 2006;281:27251–63. doi: 10.1074/jbc.M602746200. [DOI] [PubMed] [Google Scholar]
- 59.Li W, Thakor N, Xu EY, et al. An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res. 2010;38:778–88. doi: 10.1093/nar/gkp1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–17. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 61.Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–74. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 62.Yu R, Chen C, Mo YY, et al. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J Biol Chem. 2000;275:39907–13. doi: 10.1074/jbc.M004037200. [DOI] [PubMed] [Google Scholar]
- 63.Lee JM, Hanson JM, Chu WA, et al. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem. 2001;276:20011–6. doi: 10.1074/jbc.M100734200. [DOI] [PubMed] [Google Scholar]
- 64.Shen G, Kong AN. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm Drug Dispos. 2009;30:345–55. doi: 10.1002/bdd.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–68. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 66.Rushmore TH, Kong AN. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 2002;3:481–90. doi: 10.2174/1389200023337171. [DOI] [PubMed] [Google Scholar]
- 67.Bock KW, Kohle C. UDP-glucuronosyltransferase 1A6: structural, functional, and regulatory aspects. Methods Enzymol. 2005;400:57–75. doi: 10.1016/S0076-6879(05)00004-2. [DOI] [PubMed] [Google Scholar]
- 68.Khor TO, Huang MT, Kwon KH, et al. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–4. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 69.Osburn WO, Karim B, Dolan PM, et al. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer. 2007;121:1883–91. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- 70.Khor TO, Huang MT, Prawan A, et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila Pa) 2008;1:187–91. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan JH, Li YQ, Yang XY. Protective effects of epigallocatechin gallate on colon preneoplastic lesions induced by 2-amino-3-methylimidazo[4,5-f ] quinoline in mice. Mol Med. 2008;14:590–8. doi: 10.2119/2007-00050.Yuan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan JH, Li YQ, Yang XY. Inhibition of epigallocatechin gallate on orthotopic colon cancer by upregulating the Nrf2-UGT1A signal pathway in nude mice. Pharmacology. 2007;80:269–78. doi: 10.1159/000106447. [DOI] [PubMed] [Google Scholar]
- 73.Kassie F, Uhl M, Rabot S, et al. Chemoprevention of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)-induced colonic and hepatic preneoplastic lesions in the F344 rat by cruciferous vegetables administered simultaneously with the carcinogen. Carcinogenesis. 2003;24:255–61. doi: 10.1093/carcin/24.2.255. [DOI] [PubMed] [Google Scholar]
- 74.Sharma RA, Ireson CR, Verschoyle RD, et al. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clin Cancer Res. 2001;7:1452–8. [PubMed] [Google Scholar]
- 75.Chen C, Yu R, Owuor ED, et al. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23:605–12. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- 76.Shen G, Xu C, Hu R, et al. Comparison of (-)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;22:1805–20. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- 77.Zhang ZM, Yang XY, Yuan JH, et al. Modulation of NRF2 and UGT1A expression by epigallocatechin-3-gallate in colon cancer cells and BALB/c mice. Chin Med J (Engl) 2009;122:1660–5. [PubMed] [Google Scholar]
- 78.Uhl M, Kassie F, Rabot S, et al. Effect of common Brassica vegetables (Brussels sprouts and red cabbage) on the development of preneoplastic lesions induced by 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) in liver and colon of Fischer 344 rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:225–30. doi: 10.1016/j.jchromb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 79.Chung FL, Conaway CC, Rao CV, et al. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–91. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 80.Shen G, Khor TO, Hu R, et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67:9937–44. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 81.Myzak MC, Dashwood WM, Orner GA, et al. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. Faseb J. 2006;20:506–8. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu R, Khor TO, Shen G, et al. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis. 2006;27:2038–46. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- 83.McMahon M, Itoh K, Yamamoto M, et al. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–307. [PubMed] [Google Scholar]
- 84.McWalter GK, Higgins LG, McLellan LI, et al. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 2004;134:3499S–506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- 85.Hu R, Xu C, Shen G, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–92. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 86.Hu R, Xu C, Shen G, et al. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006;79:1944–55. doi: 10.1016/j.lfs.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 87.Iqbal M, Sharma SD, Okazaki Y, et al. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol Toxicol. 2003;92:33–8. doi: 10.1034/j.1600-0773.2003.920106.x. [DOI] [PubMed] [Google Scholar]
- 88.Ye SF, Hou ZQ, Zhong LM, et al. Effect of curcumin on the induction of glutathione S-transferases and NADP(H):quinone oxidoreductase and its possible mechanism of action. Yao Xue Xue Bao. 2007;42:376–80. [PubMed] [Google Scholar]
- 89.Shen G, Xu C, Hu R, et al. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- 90.Volate SR, Davenport DM, Muga SJ, et al. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005;26:1450–6. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- 91.Seow A, Yuan JM, Sun CL, et al. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis. 2002;23:2055–61. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- 92.Brusewitz G, Cameron BD, Chasseaud LF, et al. The metabolism of benzyl isothiocyanate and its cysteine conjugate. Biochem J. 1977;162:99–107. doi: 10.1042/bj1620099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiao D, Ho CT, Foiles P, et al. Identification and quantification of the N-acetylcysteine conjugate of allyl isothiocyanate in human urine after ingestion of mustard. Cancer Epidemiol Biomarkers Prev. 1994;3:487–92. [PubMed] [Google Scholar]
- 94.Skjelbred CF, Saebo M, Hjartaker A, et al. Meat, vegetables and genetic polymorphisms and the risk of colorectal carcinomas and adenomas. BMC Cancer. 2007;7:228. doi: 10.1186/1471-2407-7-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeh CC, Hsieh LL, Tang R, et al. Vegetable/fruit, smoking, glutathione S-transferase polymorphisms and risk for colorectal cancer in Taiwan. World J Gastroenterol. 2005;11:1473–80. doi: 10.3748/wjg.v11.i10.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cotton SC, Sharp L, Little J, et al. Glutathione S-transferase polymorphisms and colorectal cancer: a HuGE review. Am J Epidemiol. 2000;151:7–32. doi: 10.1093/oxfordjournals.aje.a010124. [DOI] [PubMed] [Google Scholar]
- 97.Pool-Zobel B, Veeriah S, Bohmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens -- focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005;591:74–92. doi: 10.1016/j.mrfmmm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 98.Tang KS, Chiu HF, Chen HH, et al. Link between colorectal cancer and polymorphisms in the uridine-diphosphoglucuronosyltransferase 1A7 and 1A1 genes. World J Gastroenterol. 2005;11:3250–4. doi: 10.3748/wjg.v11.i21.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dixit V, Mak TW. NF-kappaB signaling. Many roads lead to madrid. Cell. 2002;111:615–9. doi: 10.1016/s0092-8674(02)01166-2. [DOI] [PubMed] [Google Scholar]
- 100.Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Mol Cell. 2002;10:693–5. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 101.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–49. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 102.Li W, Khor TO, Xu C, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–9. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Banning A, Florian S, Deubel S, et al. GPx2 counteracts PGE2 production by dampening COX-2 and mPGES-1 expression in human colon cancer cells. Antioxid Redox Signal. 2008;10:1491–500. doi: 10.1089/ars.2008.2047. [DOI] [PubMed] [Google Scholar]
- 104.Kang ES, Kim GH, Kim HJ, et al. Nrf2 regulates curcumin-induced aldose reductase expression indirectly via nuclear factor-kappaB. Pharmacol Res. 2008;58:15–21. doi: 10.1016/j.phrs.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 105.Chen XL, Dodd G, Thomas S, et al. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290:H1862–70. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 106.Zhang X, Chen X, Song H, et al. Activation of the Nrf2/antioxidant response pathway increases IL-8 expression. Eur J Immunol. 2005;35:3258–67. doi: 10.1002/eji.200526116. [DOI] [PubMed] [Google Scholar]
- 107.Nair S, Doh ST, Chan JY, et al. Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br J Cancer. 2008;99:2070–82. doi: 10.1038/sj.bjc.6604703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Svehlikova V, Wang S, Jakubikova J, et al. Interactions between sulforaphane and apigenin in the induction of UGT1A1 and GSTA1 in CaCo-2 cells. Carcinogenesis. 2004;25:1629–37. doi: 10.1093/carcin/bgh169. [DOI] [PubMed] [Google Scholar]
- 109.Jakubikova J, Sedlak J, Mithen R, et al. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem Pharmacol. 2005;69:1543–52. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 110.Yuan X, Xu C, Pan Z, et al. Butylated hydroxyanisole regulates ARE-mediated gene expression via Nrf2 coupled with ERK and JNK signaling pathway in HepG2 cells. Mol Carcinog. 2006;45:841–50. doi: 10.1002/mc.20234. [DOI] [PubMed] [Google Scholar]
- 111.Shen G, Hebbar V, Nair S, et al. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem. 2004;279:23052–60. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- 112.Murakami A, Song M, Ohigashi H. Phenethyl isothiocyanate suppresses receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis by blocking activation of ERK1/2 and p38 MAPK in RAW264.7 macrophages. Biofactors. 2007;30:1–11. doi: 10.1002/biof.5520300101. [DOI] [PubMed] [Google Scholar]
- 113.de Sousa RR, Queiroz KC, Souza AC, et al. Phosphoprotein levels, MAPK activities and NFkappaB expression are affected by fisetin. J Enzyme Inhib Med Chem. 2007;22:439–44. doi: 10.1080/14756360601162063. [DOI] [PubMed] [Google Scholar]
- 114.Lin W, Wu RT, Wu T, et al. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem Pharmacol. 2008;76:967–73. doi: 10.1016/j.bcp.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc Natl Acad Sci U S A. 2008;105:15926–31. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang H, Khor TO, Saw CL, et al. Role of nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated Fatty acids docosahexaenoic Acid and eicosapentaenoic Acid. Mol Pharm. 2010;7:2185–93. doi: 10.1021/mp100199m. [DOI] [PubMed] [Google Scholar]
- 117.Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: an updated quantitative review to 2005. Cancer Causes Control. 2006;17:871–88. doi: 10.1007/s10552-006-0033-7. [DOI] [PubMed] [Google Scholar]
- 118.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 119.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev Res (Phila Pa) 2009;2:310–21. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–7. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 121.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 122.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–68. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 123.Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med (Maywood) 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]