Abstract
Background
We hypothesized that annuloplasty ring implantation alters mitral annular strains in a normal beating ovine heart preparation.
Methods and Results
Sheep had 16 radiopaque markers sewn equally spaced around the mitral annulus. Edwards Cosgrove partial flexible band (COS, n=12), St. Jude complete rigid saddle-shaped annuloplasty ring (RSA, n=10), Carpentier-Edwards Physio (PHY, n=11), IMR ETlogix (ETL, n=11), and GeoForm (GEO, n=12) annuloplasty rings were implanted in a releasable fashion. Four-dimensional marker coordinates were obtained using biplane videofluoroscopy with the ring inserted (Ring) and after ring release (Control). From marker coordinates, a functional spatio-temporal representation of each annulus was generated through a best fit using 16 piecewise cubic Hermitian splines. Absolute total mitral annular strains were calculated from the relative change in length of the tangent vector to the annular curve as strains occurring from Control to Ring state at end-systole. In addition, average Green-Lagrange strains occurring from Control to Ring state at end-systole along the annulus were calculated. Absolute total mitral annular ring strains were smallest for COS and greatest for ETL. Strains for RSA, PHY and GEO were similar. Except for COS in the septal mitral annular segment, all rings induced compressive strains along the entire annulus with greatest values occurring at the lateral mitral annular segment.
Conclusions
In healthy, beating ovine hearts, annuloplasty rings (COS; RSA, PHY, ETL and GEO) induce compressive strains that are: 1.) Predominate in the lateral annular region; 2.) Smallest for flexible partial bands (COS) and greatest for an asymmetric rigid ring type with intrinsic septal-lateral downsizing (ETL). However, the ring type with the most drastic intrinsic septal-lateral downsizing (GEO) introduced strains similar to physiologically shaped rings (RSA and PHY) indicating that rings effects on annular strain profiles cannot be estimated from the degree of septal-lateral downsizing.
Keywords: mitral valve annulus, strain, animal model, mitral regurgitation, surgery
INTRODUCTION
During surgical valve repair, annuloplasty devices are commonly sewn to the mitral annulus, a junctional zone between the left atrium and ventricle (LV) that consists of a fibrous and a muscular part along the septal and lateral mitral annulus, respectively. Although the mitral annulus is a dynamic structure that undergoes considerable changes of its dimensions and its three-dimensional, saddle-like shape during the cardiac cycle [1], the most commonly implanted annuloplasty ring type (Carpentier-Edwards Physio (PHY)) is semi-rigid and flat. As a consequence, rigid, saddle-shaped annuloplasty rings have been designed to mimic a more physiological mitral annular shape (e.g., St. Jude Medical RSAR (RSA), Medtronic Profile 3-D, or Carpentier-Edwards Physio II). Rings with shapes that are intentionally not physiological, however, also exist (e.g., Edwards GeoForm (GEO) and IMR ETLogix (ETL)). These rings address disease-specific alterations of the mitral annulus and the ventricle in patients with functional or ischemic mitral regurgitation, including a substantial disproportionate reduction of the septal-lateral diameter compared to the PHY ring [2].
Recently, mitral annular strain has been measured to assess alterations in the dynamic geometry of the native mitral annulus during the cardiac cycle [3, 4]. Rausch et al. demonstrated tensile strains along the septal part of the annulus, whereas compressive strains were observed along the lateral annular region during systole [4]. While it is reasonable to infer that the implantation of any of the above described rings would alter physiological mitral annular strain patterns, the effects of different ring types on mitral annular strains have never been quantified.
Our goal was to assess the effects of one flexible partial band (Edwards Cosgrove (COS)) and four complete annuloplasty rings (RSA, PHY, ETL and GEO) on mitral annular strains in healthy, beating ovine hearts.
METHODS
All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (DHEW [NIH] Publication 85 to 23, revised 1985). This study was approved by the Stanford University Medical Center Laboratory Research Animal Review Committee and conducted according to Stanford University policy.
Surgical Preparation
Fifty six adult, Dorsett-hybrid, male sheep (49±3kg) were premedicated with ketamine (25mg/kg intramuscularly), anesthetized with sodium thiopental (6.8mg/kg intravenously), intubated and mechanically ventilated with inhalational isoflurane (1.0–2.5%). The surgical preparation used for this dataset has been reported in detail earlier [5]. In brief, a left thoracotomy was performed and the heart was suspended in a pericardial cradle. Thirteen miniature radiopaque tantalum markers were surgically implanted into the sub-epicardium to silhouette the LV chamber at the intersections of two longitudinal and three crosswise meridians. Using cardiopulmonary bypass and cardioplegic arrest 16 radiopaque tantalum markers were sewn equally spaced around the mitral annulus (Figure 1 A). After marker placement, five different annuloplasty ring models, COS, RSA, PHY, ETL and GEO were implanted in a releasable fashion (see Ref. [6] for details). Ring and band sizes were determined by assessing the entire area of the anterior mitral leaflet using a sizer from Edwards Lifesciences. All annuloplasty devices were true-sized (as all animals had similarly sized leaflets, each received size 28 rings or bands). The animals were then transferred to the experimental catheterization laboratory for data acquisition under acute open-chest conditions.
Fig. 1.
Array of the 16 radiopaque markers delineating the mitral annulus. Total annular average strains were calculated from all 16 annular markers (A) while regional annular strains for the septal, lateral, anterior commissure (ACOM) and posterior commissure region (PCOM) were calculated from markers #1–5, #9–13, #14–16, and #6–8, respectively (B). Marker #3 represents the saddle horn.
Data Acquisition and Cardiac Cycle Timing
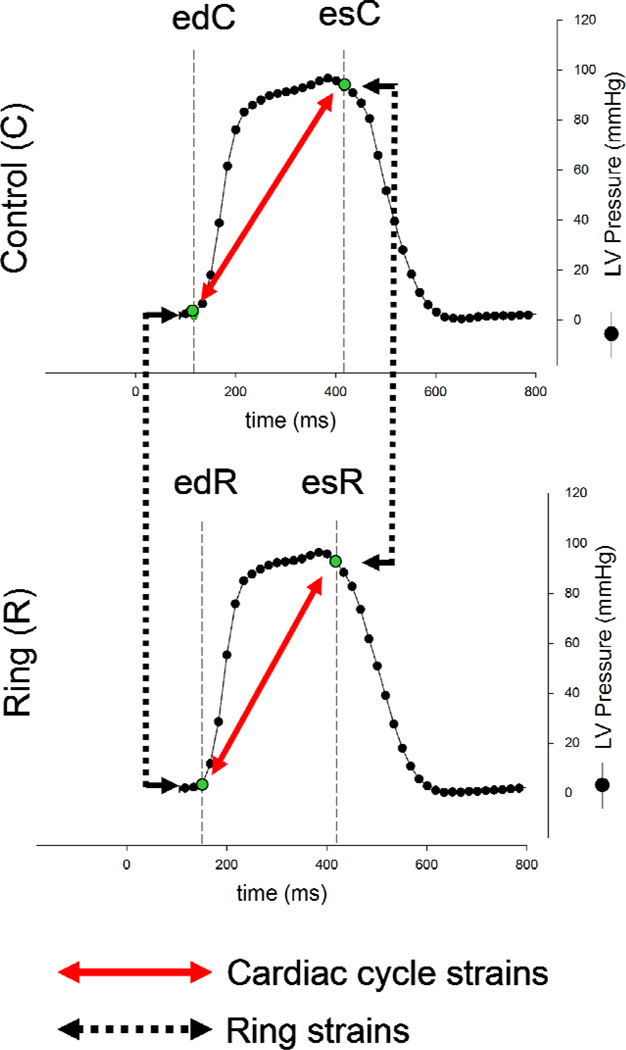
Videofluoroscopic images (60Hz) of all radiopaque markers were acquired using biplane videofluoroscopy (Philips Medical Systems, North America, Pleasanton, CA, USA). First, images were acquired under baseline conditions with the ring inserted (Ring, R). As reported earlier, a brief period of ischemia (90 seconds) was then induced and another dataset with ring and ischemia was acquired [7]. After hemodynamics returned to normal values, the ring was released and data were acquired under baseline conditions without ring (Control, C). Marker coordinates from two sinus rhythm heart beats from both biplane views were then digitized and merged to yield the 3-D coordinates of each marker centroid in each frame using semi-automated image processing and digitization software [8]. Simultaneously, analog left ventricular pressures (LVP) were recorded in real-time on the video images during data acquisition. For each beat, end-systole (es) and end-diastole (ed) were derived from LV pressure curves as shown in Figure 2.
Fig. 2.
Schematic illustrating cardiac cycle timing and the calculation of cardiac cycle and ring strains. The two curves represent left ventricular (LV) pressure curves from one representative animal with ring (RING) and without ring (CONTROL). End diastole (ed) and end systole (es) were derived from LV pressure curves as shown in the figure. Cardiac cycle strains were defined as strains from ed to es either with ring (RING, lower red solid arrow) or without ring (CONTROL, upper red solid arrow). Ring strains describe strains occurring upon ring implantation at ed (left black dotted arrow), or es (right black dotted arrow, see Methods for details).
Absolute Total and Regional Mitral Annular Strains
From 4-D marker fiduciary coordinates obtained every 16.7 msec, a functional spatio-temporal representation of each annulus was generated through a best fit using sixteen piecewise cubic Hermitian splines as described earlier [4, 9]. From these smooth curves, annular strains were calculated from the local relative changes in length between two distinct time points for each animal and then averaged. In total, four sets of strains are calculated as shown in Figure 2: Strains from ed to es with ring (edR-esR) and without ring (edC-esC) are termed "cardiac cycle strains". Strains from the control state to the implanted state at ed (edR-edC) and at es (esR-esC) are termed "ring strains".
To quantify the largest cardiac cycle and ring strains in four different annular regions, absolute mitral annular strains were calculated individually in the septal (marker #1–5), lateral (marker #9–13), anterior commissure (ACOM(marker #14–16)) and posterior commissure (PCOM(marker #6–8)) regions as depicted in Figure 1 B.
Tensile and Compressive Mitral Annular Strains
Positive strains reflecting increases in lengths or dilation are termed "tensile"; negative strains reflecting decreases in length or contraction are termed "compressive". To characterize changes in tensile and compressive strain patterns across the entire mitral annulus, average Green-Lagrange strains along the annulus were calculated for each group and projected onto an average geometric representation of their representative annuli. Note that the absolute strains described above may be either tensile or compressive.
Annular Height
Because strains mainly describe alterations of the mitral annulus along the mitral annular circumference, annular height was determined to assess 3-D geometry of the mitral valve annulus with and without annuloplasty ring. Annular height was referenced to the best fit plane through the spatio-temporal representation of each annulus and defined as the orthogonal distance from each point along the annulus to this plane.
Statistical Analysis
Average values of all animals in the respective groups were reported as mean ± 1 standard deviation. Hemodynamic data with and without annuloplasty ring (or band) were compared using 1-way repeated-measures analysis of variance with a Holm–Sidak post hoc test (Sigmaplot 11.0, Systat Software Inc, CA, USA). Mitral annular strain data were statistically evaluated by comparing cardiac cycle strains with and without annuloplasty ring (edC-esC vs. edR-esR). Similarly, ring strains were compared at ed and es (edR-edC vs. esR-esC). Furthermore, differences between ring types (COS vs. RSA vs. PHY vs. ETL vs. GEO) were assessed. We applied linear mixed models including an interaction term between strains and ring types. We used unstructured covariance pattern to account for different variances and correlation between strains. A P-value of less than 0.05 was considered statistically significant. To adjust for multiplicity we applied Holm's sequential Bonferroni method for pairwise comparison. Statistical strain analyses were performed using SAS 9.3 (SAS Institute Inc., NC, USA).
RESULTS
Hemodynamics
Table 1 shows selected hemodynamic variables, including heart rate, LVEDV, and LV dP/dtmax. Except for COS, where dP/dtmax was slightly higher compared to Control, no relevant differences were found between Ring and Control states in all five groups.
TABLE 1.
Hemodynamics
| HR (min−1) |
P vs. CTRL |
LVEDV (ml) |
P vs. CTRL |
dP/dtmax (mmHg) |
P vs. CTRL |
|
|---|---|---|---|---|---|---|
| COS-CTRL | 98±14 | 120±15 | 1360±317 | |||
| COS | 98±13 | 0.91 | 121±16 | .392 | 1527±386 | 0.001 |
| RSA-CTRL | 85±16 | 120±24 | 1309±444 | |||
| RSA | 85±13 | 0.73 | 120±21 | .810 | 1232±324 | 0.28 |
| PHY-CTRL | 93±11 | 123±22 | 1350±313 | |||
| PHY | 93±10 | 0.76 | 123±24 | .854 | 1395±311 | 0.52 |
| ETL-CTRL | 82±6 | 125±20 | 1169±368 | |||
| ETL | 80±9 | 0.53 | 125±20 | .833 | 1190±363 | 0.26 |
| GEO-CTRL | 92±10 | 114±13 | 1313±315 | |||
| GEO | 93±10 | 0.49 | 113±13 | .223 | 1388±41 | 0.070 |
All values are mean±1SD, COS=Edwards Cosgrove band, RSAR=St Jude Medical rigid saddle-shaped annuloplasty ring, PHY=Carpentier-Edwards Physio, ETL= Edwards IMR ETlogix, GEO=Edwards GeoForm, SD=standard deviation.
Absolute Total Cardiac cycle and Ring Strains
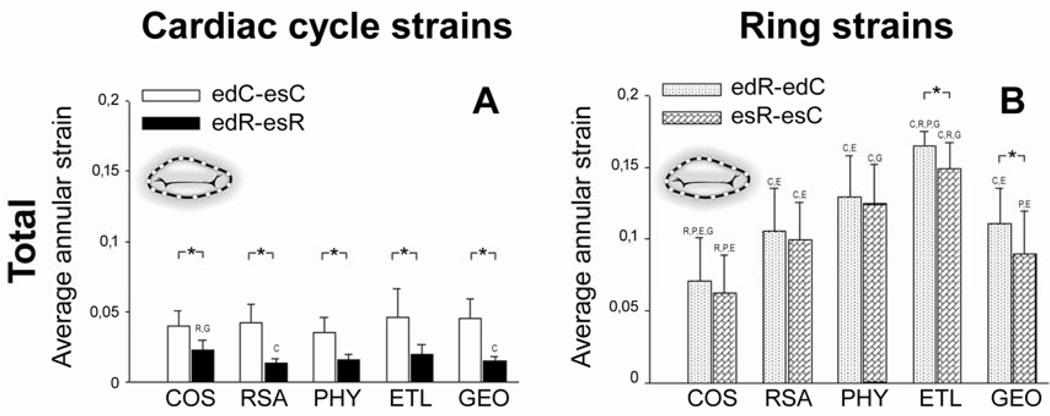
Figure 3 A and B depicts absolute total cardiac cycle and ring strains, respectively. Total cardiac cycle strains with ring were significantly smaller than without ring (edR-esR vs.edC-esC , Figure 3 A) for all rings. Total annular ring strains (edR-edC and esR-esC, Figure 3 B) were greater than cardiac cycle strains with no ring implanted (edC-esC, Figure 3 A); smallest for COS and greatest for ETL. Total annular ring strains for GEO were similar to RSA and PHY. There was a tendency for total mitral annular ring strains to be greater at ed than es (edR-edC vs. esR-esC, Figure 3 B) for all rings (but this was only statistically significant for ETL and GEO).
Fig. 3.
Absolute total average mitral annular cardiac cycle (A) and ring strains (B). Note that absolute strains are characterized by positive values only and, thus, do not reflect lengthening or shortening (see Methods for details). COS=Edwards Cosgrove band, RSAR=St Jude Medical rigid saddle-shaped annuloplasty ring, PHY=Carpentier-Edwards Physio, ETL= Edwards IMR ETlogix, GEO=Edwards GeoForm. C,R,P,E and G=p<0.05 vs. COS, RSA, PHY, ETL and GEO, respectively, *=p<0.05. ed=end diastole, es=end systole, C=Control, R=Ring.
Absolute Regional Cardiac cycle and Ring Strains
Figure 4 A-F shows absolute cardiac cycle and ring strains in the septal (Figure 4 A, B), lateral (Figure 4 C, D), ACOM (Figure 4 E, F) and PCOM (Figure 4 G, H) regions of the mitral annulus.
Fig. 4.
Absolute regional cardiac cycle (A, C, E, G) and ring strains (B, D, F, H) for the septal (A, B), lateral (C, D), anterior commissure (ACOM (E, F)) and posterior commissure region (PCOM (G, H)) of the annulus. Note that absolute strains are characterized by positive values only and, thus, do not reflect lengthening or shortening (see Methods for details). COS=Edwards Cosgrove band, RSAR=St Jude Medical rigid saddle-shaped annuloplasty ring, PHY=Carpentier-Edwards Physio, ETL= Edwards IMR ETlogix, GEO=Edwards GeoForm. C,R,P,E and G=p<0.05 vs. COS, RSA, PHY, ETL and GEO, respectively, *=p<0.05. ed=end diastole, es=end systole, C=Control, R=Ring.
In the septal annular region, representing the fibrous part of the annulus, ring strains (edR-edC and esR-esC) were greater with RSA, PHY, ETL and GEO compared to cardiac cycle strains in the Control state (edC-esC), while ring strains with COS were similar to cardiac cycle strains without ring in this annular region (Figure 4 A and B).
In the lateral region cardiac cycle strains in the Control state (edC-esC) were significantly greater than cardiac cycle strains with ring implanted (edR-esR) in all ring groups (COS; RSA, PHY, ETL and GEO, edR-esR, Figure 4 C). Ring strains (edR-edC and esR-esC) in the lateral annular region were greater with all rings (COS, RSA, PHY, ETL and GEO) compared to cardiac cycle strains in the Control state (edC-esC, Figure 4 C and D).
There was a trend that ring strains (edR-edC and esR-esC) were greatest for ETL compared to the other ring types in all regions of the mitral annulus (septal, lateral, ACOM and PCOM, Figure 4 B, D, F and H). GEO, the ring that includes a non-physiological elevation of the lateral ring segment, had strain profiles in this annular portion that were similar to COS, RSA and PHY.
Tensile and Compressive Cardiac cycle and Ring Strains
Figure 5 shows color maps of average tensile and compressive cardiac cycle and ring strains. While cardiac cycle strains in the Control state (edC-esC) were tensile in all groups in the septal region, compressive cardiac cycle strains without ring were observed in the lateral region. Cardiac cycle strains with ring implanted (edR-esR) were minimal along the entire annulus with any of the rings implanted (COS, RSA, PHY, ETL and GEO) except for the septal region with COS, where tensile strain patterns similar to those during the Control state were observed. Except for the septal region of COS, ring strains (edR-edC and esR-esC) were compressive along the entire annulus with all rings (COS, RSA, PHY, ETL and GEO) and with highest values occurring in the lateral annular region.
Fig. 5.
Color maps of average tensile and compressive cardiac cycle and ring strains. COS=Edwards Cosgrove band, RSAR=St Jude Medical rigid saddle-shaped annuloplasty ring, PHY=Carpentier-Edwards Physio, ETL= Edwards IMR ETlogix, GEO=Edwards GeoForm. Markers #3, 7 and 15 represent saddle horn, posterior and anterior commissure, respectively (see Fig. 1). ed=end diastole, es=end systole, C=Control, R=Ring.
Annular Height
Figure 6 shows color maps of annular height. Compared to the Control state, COS and RSA have negligible effects on annular height while PHY and ETL reduce annular height especially in the septal (i.e. saddle horn (#3)) annular region. GEO reduces annular height in the saddle horn region, but an increase in annular height is observed in the lateral annular region.
Fig. 6.
Color maps of annular height represented by the distance of each annular segment to a best fit mitral annular plane. COS=Edwards Cosgrove band, RSAR=St Jude Medical rigid saddle-shaped annuloplasty ring, PHY=Carpentier-Edwards Physio, ETL= Edwards IMR ETlogix, GEO=Edwards GeoForm. Markers #3, 7 and 15 represent saddle horn, posterior and anterior commissure, respectively (see Fig. 1). ed=end diastole, es=end systole, C=Control, R=Ring.
DISCUSSION
The principal findings of this study were: 1.) Absolute total ring strains were greater with all rings than cardiac cycle strains in the Control state; 2.) Absolute total ring strains were smallest for COS and greatest for ETL while they were similar for GEO, RSA and PHY; 3.) Except for the septal region with COS, ring strains were compressive along the entire annulus with all rings with highest values occurring in the lateral annular region; and, 4.) PHY and ETL reduced annular height in the septal and the lateral annular segment, but COS and RSA did not. GEO reduced annular height in the septal region, but increased annular height in the lateral annular region.
Mitral annular ring strains, i.e. strains occuring upon the implantation of an annuloplasty ring, have never been described or quantified previously.
Expectedly, the COS, a flexible, partial band that spares the septal portion of the mitral annulus induced negligible ring strains to the septal mitral annular region. Furthermore, tensile systolic strain patterns similar to those during the Control state and no changes in annular height profiles were observed in this annular segment. The maintenance of physiological mitral annular dimensions, dynamics and shape in the septal annular portion could in part explain our recent finding that the COS, unlike complete, rigid rings, did not alter anterior mitral leaflet strains [5]. Furthermore, the observed preservation of annular height profiles could help to provide a maximum amount of leaflet coaptation as suggested by Vergnat et al. [10] and Jensen et al. [11]. To our surprise, the COS imposed significant ring strains to the lateral annular portion in an amount that was similar to RSA and PHY. Since mitral annular strain is a parameter that reflects alterations in the circumference of the mitral annulus, we speculate that the length of the COS is similar to the circumference of the lateral portion of RSA and PHY.
The finding that ring strain patterns observed with RSA and PHY were similar may be attributed to the fact that both rings have similar dimensions [2]; however, unlike RSA which preserved the physiological 3-D profile of the mitral annulus, the PHY ring reduced annular height. This reduction in annular height predominantly occured in the septal annular segment, a finding that may be attributed to the fact that the septal part includes the highest segment (in the natural mitral annulus) with the greatest distance to the annular plane. Consequently, it is expected that the most drastic effects occur in this mitral annular portion However, although the PHY ring had the greatest effects on the mitral annular 3-D profile in the septal annular region, the ring introduced the greatest strains to the lateral annular segment, indicating that changes in mitral annular strain patterns are not in direct proportion to alterations in the three-dimensional mitral annular profile. These findings reflect the complexity of the changes that are introduced to the mitral annulus upon the implantation of an annuloplasty ring and should be considered when a mitral repair is performed.
The ETL ring is specifically designed for the treatment of patients with ischemic mitral regurgitation and includes a disproportionate reduction of approximately 10% of the septal-lateral diameter compared to the PHY ring [2]. In addition, the design of the ring is slightly asymmetric with a pronounced reduction of the region subtending the posteromedial mitral leaflet scallop and posterior commissure [12]. The ETL imposed the greatest ring strains on all regions of all rings. As discussed earlier, strain is a parameter that is most sensitive to changes in annular circumference. It may therefore be assumed that the ETL has the smallest circumference of all rings, resulting in the greatest ring strains.
Surprisingly, the GEO, which includes the most radical disproportionate reduction of the septal-lateral diameter of all rings (approximately 25%), did not impose the greatest ring strains compared to the other ring types. Since it is has been demonstrated that the GEO has a greater diameter in the ACOM-PCOM diameter than ETL [2, 13], it is presumed that GEO has a greater circumference which, in turn, may explain why it induces less ring strains than the ETL. These findings indicate that rings effects on annular strain profiles cannot be estimated by the degree of septal-lateral downsizing. Another feature of the GEO is the elevation of the mid-lateral annular segment. This ring characteristic has been associated with ring dehiscences clinically [14]. Interestingly, average annular strains in this area were similar to those of COS, RSA and PHY. It is therefore reasonable to speculate that the observed ring dehiscences may be attributed more to the alterations in the 3-D annular profile (an increase in annular height in this region (Figure 6)) than to an increase in mitral annular strains.
Mitral annular strains – What Are We Measuring?
Recent engineering studies have focused on characterizing annular strains to provide insight into temporal and regional variations of annular contraction and dilation. Cardiac cycle strains without ring (edC-esC) are a measure of how much the native annulus contracts and dilates throughout the cardiac cycle. The cardiac cycle strains reported in this manuscript are in excellent qualitative and quantitative agreement with the natural annular strains reported by Eckert et al. [3] and Rausch et al. [9]. Cardiac cycle strains with ring (edR-esR) are a measure of how much the annulus with ring contracts and dilates throughout the cardiac cycle. The cardiac cycle strains with rings reported in this manuscript are in agreement with previously published data [4]. Figure 5, column 2, confirms the general intuition that stiffer rings (RSA, PHY, ETL and GEO)constrain annular dynamics more than flexible annuloplasty bands (COS). Ring strains at ed and es (i.e. edC-edR and esC-esR) are measures of how much annuloplasty devices constrain the circumference of the native mitral annulus. Ring strains have never been reported to date. Only the combination of permanent implantable markers and the releasable ring technique allows us to quantify ring induced strains. Here we restrict ourselves to report kinematic measurements, i.e., displacements and strain, which are directly extracted from our raw data. These data are not a direct measure of force. Reporting forces would require assumptions which might induce errors. Although no force measurements are provided, regional strain maps as shown in Figure 5 provide mechanistic insight into the functionality of different annuloplasty rings and may help the rational design of more physiological devices with improved long-term durability.
Clinical Inferences
Despite successful mitral repair with annuloplasty ring implantation, recurrent mitral regurgitation may occur in about 5–10% of patients with organic mitral valve disease [15] and upwards of 30% or more of patients with functional/ischemic mitral regurgitation [16, 17]. A potential, device-related cause for recurrent mitral valve regurgitation includes ring dehiscence [18–20]. It is logical to think that major perturbations of physiological annular strain patterns and/or 3-D annular profiles – as our findings demonstrated - increase the risk for ring dehiscence. It is, however, of note that an increased risk for a postoperative ring dehiscence may also be present if mitral annular strain profiles after ring implantation are normal. Such an increased risk for dehiscence may be due to a non-physiological 3-D ring profile as e.g. the lateral portion of the GEO (see above).
Another contributing factor to suboptimal outcomes after surgical mitral valve repair may be the fact that several steps during a repair such as e.g. ring type or size selection are currently more based on surgical expertise and intuition than on quantitative data. To date, no studies exist comparing the effects of different ring types on mitral annular strains. Furthermore, the dimensions of mitral annuloplasty rings are not fully provided by manufacturers. This lack of provision may be due to commercial reasons or simply due to practical difficulties in measurements. With the introduction of complexly shaped 3-D rings it has become difficult to provide a reasonable circumference for a given annuloplasty ring. Consequently, it is almost impossible for a surgeon to estimate the effects of an annuloplasty ring on mitral annular strain patterns The quantitative and qualitative annular strain data with and without annuloplasty rings as well as the 3-D mitral annular profiles reported in this study may not only provide mechanistic insight into the functionality of different annuloplasty, but may also help to guide a more rational planning and conduction of surgical mitral valve repair and to potentially improve the designs of current annuloplasty rings and, ultimately, patient outcomes.
Study Limitations
Limitations inherent to this experimental model using normal sheep hearts have been addressed in detail previously [5]. Annuloplasty rings are implanted in patients with mitral regurgitation undergoing surgical valve repair in order to restore mitral annular dimensions that allow a sufficient amount of leaflet coaptation and to stabilize the repair. Typically, in both patients with structural or ischemic/functional mitral regurgitation, a large degree of mitral annular dilatation is present, which is not seen in this healthy heart preparation. It is reasonable to speculate, however, that the changes in strain patterns observed after ring implantation in healthy hearts might be even more pronounced when mitral annular dilatation is present. This notion is supported by the finding that there was a tendency that ring strains were greatest at ed, a time point in the cardiac cycle where the mitral annulus has its largest dimensions.
Conclusions
In healthy, beating ovine hearts, annuloplasty rings (COS; RSA, PHY, ETL and GEO) induce compressive ring strains that are: 1.) Greater than cardiac cycle strains without ring; 2.) Predominate in the lateral annular region; and, 3.) Smallest for flexible partial bands (COS) and greatest for an asymmetric rigid ring type with intrinsic septal-lateral downsizing (ETL). However, the ring type with the most drastic intrinsic septal-lateral downsizing (GEO) introduced strains that were similar to those induced by physiologically shaped rings (RSA and PHY) indicating that rings effects on annular strain profiles cannot be estimated from the degree of septal-lateral downsizing. Clinical investigation is necessary to whether annuloplasty rings affect annular strain patterns in human subjects and whether any such potential perturbation adversely influences clinical outcomes.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the expert technical assistance of Carolin Berg, Maggie Brophy, Sigurd Hartnett, Saideh Fahramandnia and George T. Daughters II.
FUNDING SOURCES
D. Craig Miller, M.D.: R01 research grant, NHLBI, National Institutes of Health HL29589 (1982–2008), R01 research grant, NHLBI, National Institutes of Health HL67025 (2001–2010)
Ellen Kuhl, Ph.D.: NSF CAREER Award CMMI-0952021
John-Peder Escobar Kvitting, M.D., Ph.D.: U.S.-Norway Fulbright Foundation, the Swedish Heart-Lung Foundation and the Swedish Society for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
D. Craig Miller, M.D.: Consultant, Medtronic CardioVascular Division.
REFERENCES
- 1.Timek TA, Miller DC. Experimental and clinical assessment of mitral annular area and dynamics: what are we actually measuring? Ann Thorac Surg. 2001;72:966–974. doi: 10.1016/s0003-4975(01)02702-3. [DOI] [PubMed] [Google Scholar]
- 2.Bothe W, Swanson JC, Ingels NB, Miller DC. How much septal-lateral mitral annular reduction do you get with new ischemic/functional mitral regurgitation annuloplasty rings? J Thorac Cardiovasc Surg. 2010;140:117–121. doi: 10.1016/j.jtcvs.2009.10.033. 121 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert CE, Zubiate B, Vergnat M, Gorman JH, 3rd, Gorman RC, Sacks MS. In vivo dynamic deformation of the mitral valve annulus. Ann Biomed Eng. 2009;37:1757–1771. doi: 10.1007/s10439-009-9749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rausch MK, Bothe W, Kvitting JP, Swanson JC, Miller DC, Kuhl E. Mitral valve annuloplasty: a quantitative clinical and mechanical comparison of different annuloplasty devices. Ann Biomed Eng. 2012;40:750–761. doi: 10.1007/s10439-011-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bothe W, Kuhl E, Kvitting JP, Rausch MK, Goktepe S, Swanson JC, Farahmandnia S, Ingels NB, Jr, Miller DC. Rigid, complete annuloplasty rings increase anterior mitral leaflet strains in the normal beating ovine heart. Circulation. 2011;124:S81–S96. doi: 10.1161/CIRCULATIONAHA.110.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bothe W, Chang PA, Swanson JC, Itoh A, Arata K, Ingels NB, Miller DC. Releasable annuloplasty ring insertion--a novel experimental implantation model. Eur J Cardiothorac Surg. 2009;36:830–832. doi: 10.1016/j.ejcts.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bothe W, Kvitting JP, Stephens EH, Swanson JC, Liang DH, Ingels NB, Jr, Miller DC. Effects of different annuloplasty ring types on mitral leaflet tenting area during acute myocardial ischemia. J Thorac Cardiovasc Surg. 2011;141:345–353. doi: 10.1016/j.jtcvs.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niczyporuk MA, Miller DC. Automatic tracking and digitization of multiple radiopaque myocardial markers. Comput Biomed Res. 1991;24:129–142. doi: 10.1016/0010-4809(91)90025-r. [DOI] [PubMed] [Google Scholar]
- 9.Rausch MK, Bothe W, Kvitting JP, Swanson JC, Ingels NB, Jr, Miller DC, Kuhl E. Characterization of mitral valve annular dynamics in the beating heart. Ann Biomed Eng. 2011;39:1690–1702. doi: 10.1007/s10439-011-0272-y. [DOI] [PubMed] [Google Scholar]
- 10.Vergnat M, Jackson BM, Cheung AT, Weiss SJ, Ratcliffe SJ, Gillespie MJ, Woo YJ, Bavaria JE, Acker MA, Gorman RC, Gorman JH., 3rd Saddle-shape annuloplasty increases mitral leaflet coaptation after repair for flail posterior leaflet. Ann Thorac Surg. 2011;92:797–803. doi: 10.1016/j.athoracsur.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen MO, Jensen H, Levine RA, Yoganathan AP, Andersen NT, Nygaard H, Hasenkam JM, Nielsen SL. Saddle-shaped mitral valve annuloplasty rings improve leaflet coaptation geometry. J Thorac Cardiovasc Surg. 2011;142:697–703. doi: 10.1016/j.jtcvs.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daimon M, Fukuda S, Adams DH, McCarthy PM, Gillinov AM, Carpentier A, Filsoufi F, Abascal VM, Rigolin VH, Salzberg S, Huskin A, Langenfeld M, Shiota T. Mitral valve repair with Carpentier-McCarthy-Adams IMR ETlogix annuloplasty ring for ischemic mitral regurgitation: early echocardiographic results from a multi-center study. Circulation. 2006;114:I588–I593. doi: 10.1161/CIRCULATIONAHA.105.001347. [DOI] [PubMed] [Google Scholar]
- 13.Bothe W, Kvitting JP, Swanson JC, Hartnett S, Ingels NB, Jr, Miller DC. Effects of different annuloplasty rings on anterior mitral leaflet dimensions. J Thorac Cardiovasc Surg. 2010;139:1114–1122. doi: 10.1016/j.jtcvs.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bonis M, Taramasso M, Grimaldi A, Maisano F, Calabrese MC, Verzini A, Ferrara D, Alfieri O. The GeoForm annuloplasty ring for the surgical treatment of functional mitral regurgitation in advanced dilated cardiomyopathy. Eur J Cardiothorac Surg. 2011;40:488–495. doi: 10.1016/j.ejcts.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 15.Mohty D, Orszulak TA, Schaff HV, Avierinos JF, Tajik JA, Enriquez-Sarano M. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation. 2001;104:I1–I7. doi: 10.1161/hc37t1.094903. [DOI] [PubMed] [Google Scholar]
- 16.Shiota M, Gillinov AM, Takasaki K, Fukuda S, Shiota T. Recurrent mitral regurgitation late after annuloplasty for ischemic mitral regurgitation. Echocardiography. 2011;28:161–166. doi: 10.1111/j.1540-8175.2010.01284.x. [DOI] [PubMed] [Google Scholar]
- 17.Tahta SA, Oury JH, Maxwell JM, Hiro SP, Duran CM. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Dis. 2002;11:11–18. discussion 18-9. [PubMed] [Google Scholar]
- 18.Shimokawa T, Kasegawa H, Katayama Y, Matsuyama S, Manabe S, Tabata M, Fukui T, Takanashi S. Mechanisms of recurrent regurgitation after valve repair for prolapsed mitral valve disease. Ann Thorac Surg. 2011;91:1433–1438. doi: 10.1016/j.athoracsur.2011.01.015. discussion 1438-9. [DOI] [PubMed] [Google Scholar]
- 19.Tsang W, Wu G, Rozenberg D, Mosko J, Leong-Poi H. Early mitral annuloplasty ring dehiscence with migration to the descending aorta. J Am Coll Cardiol. 2009;54:1629. doi: 10.1016/j.jacc.2009.03.090. [DOI] [PubMed] [Google Scholar]
- 20.Kronzon I, Sugeng L, Perk G, Hirsh D, Weinert L, Garcia Fernandez MA, Lang RM. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J Am Coll Cardiol. 2009;53:1543–1547. doi: 10.1016/j.jacc.2008.12.059. [DOI] [PubMed] [Google Scholar]