Abstract
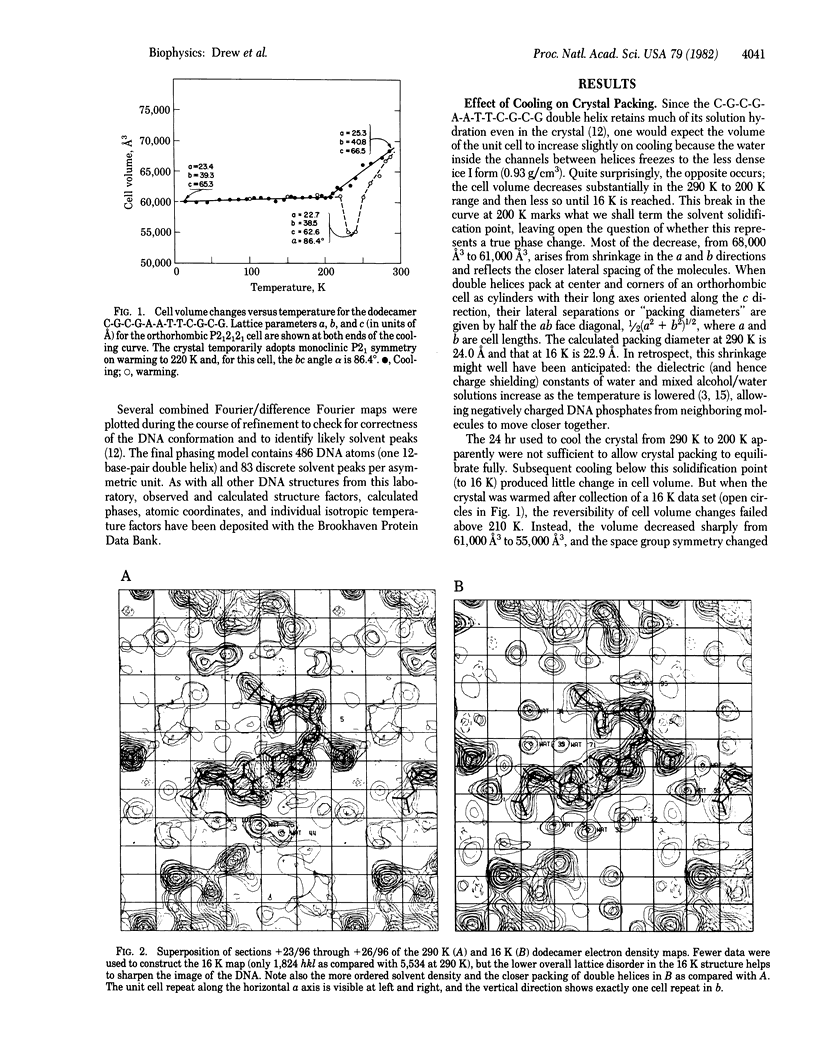
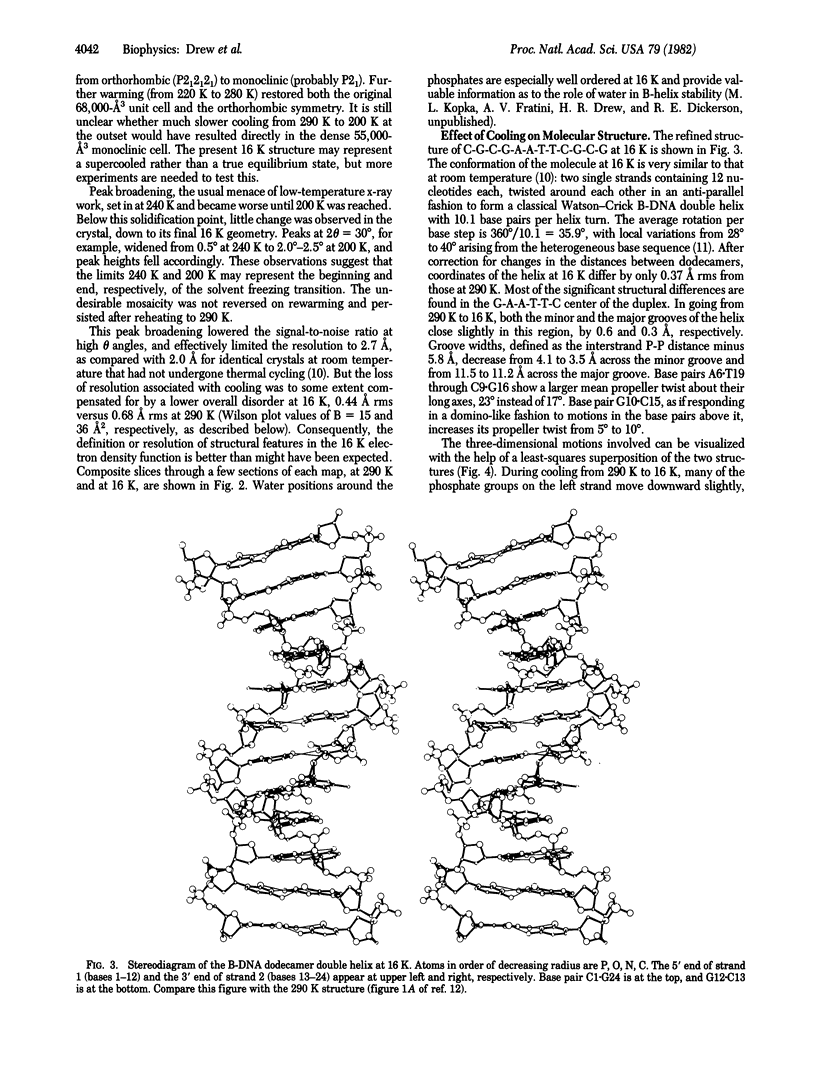
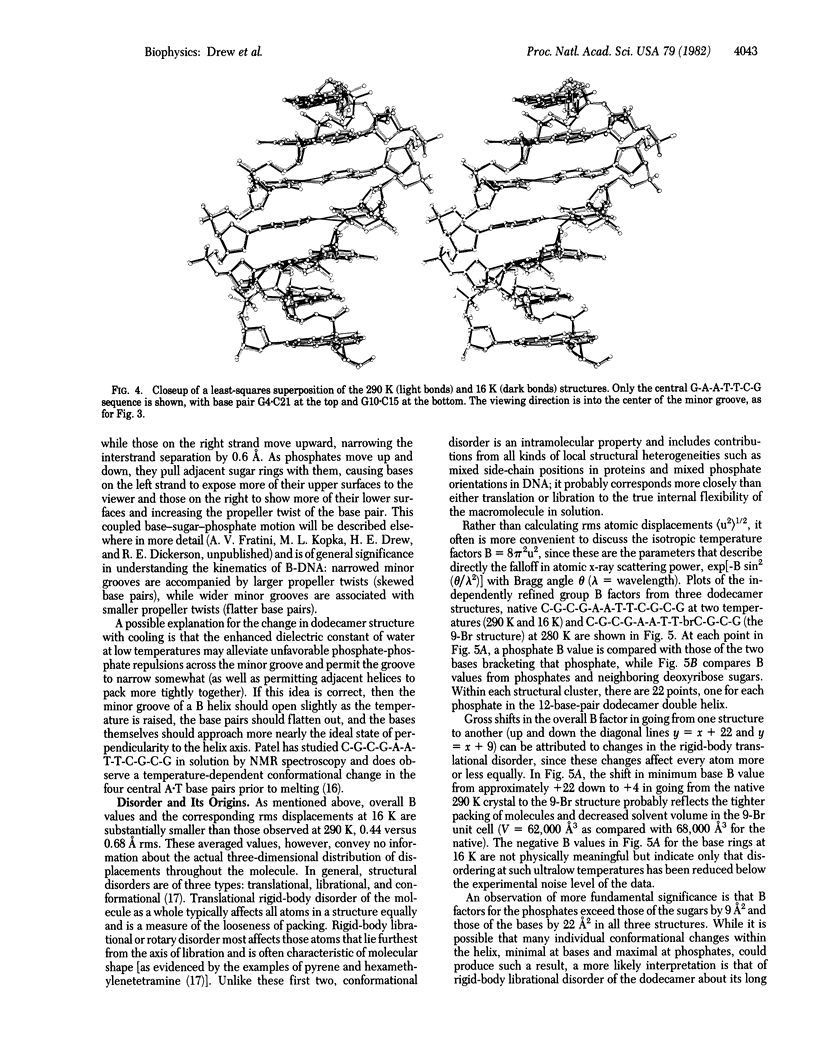
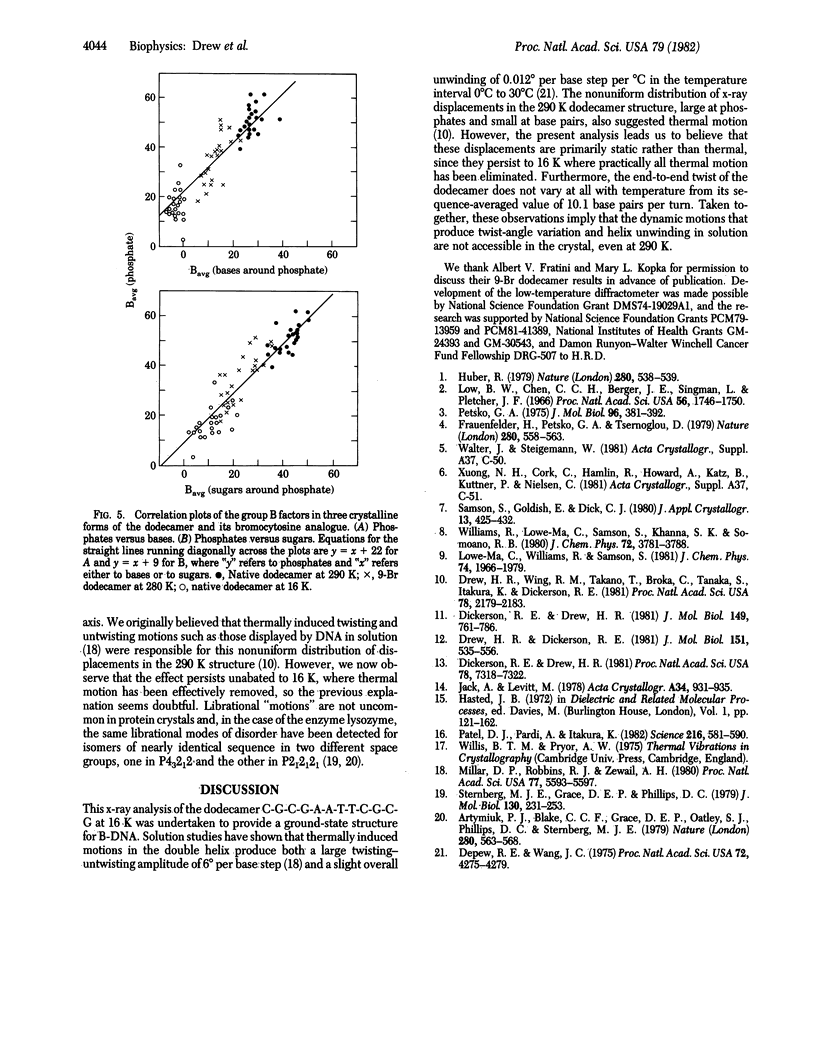
The crystal structure of the B-DNA dodecamer C-G-C-G-A-A-T-T-C-G-C-G, previously solved and refined at room temperature (290 K), has been analyzed at 16 K (-257 degrees C). The end-to-end winding of the helix does not vary with temperature but remains constant at 10.1 base pairs per turn. Negatively charged phosphate groups throughout the structure do move closer together on cooling, however, probably because of increase in the dielectric constant of water as the temperature is lowered. This has the two-fold effect of reducing the spacing between neighboring double helices from 24.0 to 22.9 A and of narrowing the helix grooves within any isolated molecule. Overall lattice displacements as deduced from crystallographic temperature factors are very much decreased in the 16 K structure, yet displacements at phosphates continue to exceed those of deoxyribose sugars by B = 9 A2 and those of base pairs by B = 22 A2, even at this very low temperature at which practically all thermal motion has been eliminated. These differences, formerly interpreted as evidence for thermal vibration, must now be attributed to static disorder.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artymiuk P. J., Blake C. C., Grace D. E., Oatley S. J., Phillips D. C., Sternberg M. J. Crystallographic studies of the dynamic properties of lysozyme. Nature. 1979 Aug 16;280(5723):563–568. doi: 10.1038/280563a0. [DOI] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Kinematic model for B-DNA. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7318–7322. doi: 10.1073/pnas.78.12.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Wing R. M., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Structure of a B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2179–2183. doi: 10.1073/pnas.78.4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Huber R. Conformational flexibility in protein molecules. Nature. 1979 Aug 16;280(5723):538–539. doi: 10.1038/280538a0. [DOI] [PubMed] [Google Scholar]
- Low B. W., Chen C. C., Berger J. E., Singman L., Pletcher J. F. Studies of insulin crystals at low temperatures: effects on mosaic character and radiation sensitivity. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1746–1750. doi: 10.1073/pnas.56.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar D. P., Robbins R. J., Zewail A. H. Direct observation of the torsional dynamics of DNA and RNA by picosecond spectroscopy. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5593–5597. doi: 10.1073/pnas.77.10.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Pardi A., Itakura K. DNA conformation, dynamics, and interactions in solution. Science. 1982 May 7;216(4546):581–590. doi: 10.1126/science.6280281. [DOI] [PubMed] [Google Scholar]
- Petsko G. A. Protein crystallography at sub-zero temperatures: cryo-protective mother liquors for protein crystals. J Mol Biol. 1975 Aug 15;96(3):381–392. doi: 10.1016/0022-2836(75)90167-9. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Grace D. E., Phillips D. C. Dynamic information from protein crystallography. An analysis of temperature factors from refinement of the hen egg-white lysozyme structure. J Mol Biol. 1979 May 25;130(3):231–252. doi: 10.1016/0022-2836(79)90539-4. [DOI] [PubMed] [Google Scholar]



