Abstract
Mitogen-activated protein kinases (MAPKs) are key regulators of cellular physiology and immune responses and abnormality in MAPKs is implicated in many diseases. MAPKs are activated by MAPK kinases through phosphorylation of the threonine and tyrosine residues in the conserved Thr-Xaa-Tyr domain, where Xaa represents amino acid residues characteristic of distinct MAPK subfamilies. Since MAPKs play a crucial role in a variety of cellular processes, a delicate regulatory network has evolved to control their activities. Over the past two decades, a group of dual specificity MAPK phosphatases (MKPs) have been identified that deactivate MAPKs. Since MAPKs can enhance MKP activities, MKPs are considered as an important feedback control mechanism that limits the MAPK cascades. This review outlines the role of MKP-1, a prototypical MKP family member, in physiology and disease. We will first discuss the basic biochemistry and regulation of MKP-1. Next, we will present the current consensus on the immunological and physiological functions of MKP-1 in infectious, inflammatory, metabolic, and nervous system diseases as revealed by studies using animal models. We will also discuss the emerging evidence implicating MKP-1 in human disorders. Finally, we will conclude with a discussion of the potential for pharmacomodulation of MKP-1 expression.
Keywords: MKP-1, MAPK, immunology, mouse, human, inflammation
Introduction
MAPKs are a group of highly conserved serine/threonine protein kinases in eukaryotes. Based on their signature Thr-Xaa-Tyr motifs, MAPKs can be categorized into three MAPK subfamilies: ERK, JNK, and p38 (Su and Karin 1996). The ERK subfamily contains a Thr-Glu-Tyr motif. The JNK subfamily contains a Thr-Pro-Tyr motif, and potently phosphorylates c-Jun, a component of AP-1 transcription factor. The p38 subfamily contains a Thr-Gly-Tyr motif. The MAPK subfamilies are differentially regulated (Johnson and Lapadat 2002). In general, mitogens, including growth factors, potently activate the ERK subfamily, whereas stress has little effect. In contrast, the JNK and p38 subfamilies are highly activated by stress and therefore are also referred to as Stress-Activated Protein Kinases (SAPKs) (Rincon and Davis 2009). MAPKs play a pivotal role in a variety of cellular processes, including cell proliferation, differentiation, stress responses, inflammation, apoptosis, and immune defense (Boutros et al. 2008; Duan and Wong 2006; Jeffrey et al. 2007).
MAPK pathways are activated through a cascade of sequential phosphorylation events, beginning with the activation of MAPK kinase kinase. MAPK kinase kinase activates MAPK kinase by phosphorylating two serine residues. MAPK kinase in turn phosphorylates MAPK at the adjacent threonine and tyrosine residues in the conserved Thr-Xaa-Tyr motif located in a regulatory loop between the kinase subdomains VII and VIII. Activated MAPK can phosphorylate a wide array of downstream targets, including other protein kinases and transcription factors that facilitate transcription of MAPK-regulated genes (Whitmarsh and Davis 1996).
MAPKs can regulate gene expression through several mechanisms. MAPKs can facilitate gene transcription through modulating chromatin structure (Cheung et al. 2000). MAPKs can also enhance the activities of numerous transcription factors, including AP-1 (Whitmarsh and Davis 1996), CREB (Arthur and Cohen 2000; Arthur et al. 2004; Eliopoulos et al. 2002; Wong et al. 2004), SRF (Posern and Treisman 2006), and C/EBPβ(Davis 1993). In addition to transcriptional regulation, MAPK can regulate gene expression by altering stability, transport, and translation of mRNA species that contain an AU-rich element (ARE), AUUUA, in their 3′ untranslated region (UTR) (Carballo et al. 1998; Mahtani et al. 2001; Stoecklin et al. 2004). Tristetraprolin (TTP) binds to the AREs of several cytokine mRNAs and promotes the deadenylation and degradation of these ARE-containing mRNAs (Carballo et al. 1998). However, when TTP is phosphorylated by MAPK-activated protein kinase (MK)-2 (Mahtani et al. 2001), a downstream target of p38, TTP-mediated degradation of ARE-containing transcripts is inhibited (Stoecklin et al. 2004). Many cytokine transcripts, including TNF-α, IL-1β, IL-6, granulocyte macrophage colony stimulating factor (GM-CSF), and IL-2, contain ARE(s) in their mRNAs and are targets of TTP (Lai et al. 2006). Recently, MAPKs have been shown to regulate the expression of several micro RNAs, including miR21 (Huang et al. 2009), miR125a (Monk et al. 2010), and miR155 (O’Connell et al. 2007; Yin et al. 2008). On the protein level, MAPK-mediated phosphorylation regulates the stability of numerous proteins. For example, ERK-mediated phosphorylation of GATA-1, a key hematopoietic transcription factor, accelerates its degradation, while the MEK inhibitor U0126 stabilizes GATA protein (Hernandez-Hernandez et al. 2006).
Since MAPKs are regulated by reversible phosphorylation, phosphatase-mediated dephosphorylation is likely the most efficient mode of MAPK deactivation. Indeed, a number of protein phosphatases deactivate MAPKs, including tyrosine, serine/threonine, and dual specificity phosphatases (Keyse 2000). In mammalian cells, the dual specificity protein phosphatases (DUSPs) are the primary phosphatases responsible for dephosphorylation/deactivation of MAPKs in vivo (Keyse 2000). These phosphatases are often referred to as MAPK phosphatases (MKPs). By dephosphorylating the adjacent threonine and tyrosine residues essential for the activation of the MAPKs, MKPs deactivate MAPKs to terminate kinase cascades. To date, at least 10 MKPs have been identified from mammalian cells (Keyse 2000), with MKP-1 being the archetype of this family. These MKPs exhibit distinct properties in substrate preferences, patterns of tissue- and cell type-specific expression, subcellular localization, and transcriptional induction. Several excellent general reviews on MKPs/DUSPs have been published recently, including those focusing on cell proliferation and cancer (Boutros et al. 2008; Keyse 2008) and the immune response (Jeffrey et al. 2007; Lang et al. 2006; Li et al. 2009; Liu et al. 2007; Salojin and Oravecz 2007; Wang and Liu 2007).
This review will focus on the function and regulation of MKP-1. Particular emphasis will be placed on the current understanding of MKP-1’s role in immunology, physiology, and disease. First, we will summarize findings on the biochemical function and regulation of MKP-1. We will review how MKP-1 regulates immunological and physiological responses in animal models of inflammatory, infectious, metabolic, and central nervous system diseases. Next, we will briefly discuss the emerging evidence supporting a role for MKP-1 in specific human diseases. Finally, we will conclude with a review of currently available pharmacological agents that modify MKP-1 expression, since these agents are potential therapies for diseases associated with abnormal MAPK activation.
MKP-1 – history and basic biochemistry
MKP-1 has also been referred to as DUSP1 (Keyse 2008), hVH1 (Zheng and Guan 1993), CL100 (Keyse and Emslie 1992), 3CH134 (Charles et al. 1992), and Erp (Dorfman et al. 1996). The mouse MKP-1 cDNA was initially identified over 20 years ago as an immediate-early gene induced by serum through differential hybridization screening of a BALB/c 3T3 cDNA library (Lau and Nathans 1985). Originally referred to as 3CH134, the cDNA clone encodes a protein of ~40 kDa. The DNA sequence of 3CH134 was first reported in 1992 (Charles et al. 1992), and a human homolog (CL100) was identified shortly thereafter as an oxidative stress-induced tyrosine phosphatase gene (Keyse and Emslie 1992). Structurally, both 3CH134 and CL100 contain a (I/V)HCXAGXXR(S/T)AG signature motif characteristic of the catalytic domain of the tyrosine phosphatases. They also share considerable homology with the dual specificity phosphatase of vaccinia virus, VH1, particularly at the catalytic site. The 3CH134 protein or its human homologue exhibited highly specific phosphatase activity towards the ERK MAPK, both in vitro and in cultured cells (Alessi et al. 1993; Charles et al. 1993; Sun et al. 1993; Zheng and Guan 1993). Since this was the first protein phosphatase specific for MAPKs, selectively targeting their phosphotyrosine and phosphothreonine residues, it was designated as MAPK phosphatase (MKP)-1 (Sun et al. 1993). While initially MKP-1 was considered to be ERK-specific, subsequent studies showed that MKP-1 effectively dephosphorylates both JNK and p38 (Liu et al. 1995; Raingeaud et al. 1995). Subsequently, by titrating MKP-1 expression levels, Franklin et al. have shown that p38 and JNK are the preferred substrates of MKP-1 (Franklin and Kraft 1997; Franklin et al. 1998). This is consistent with findings observed in studies using MKP-1 knockout cells (Dorfman et al. 1996), as knockout of MKP-1 in mouse embryonic fibroblasts does not affect the kinetics of ERK activation during serum stimulation (Dorfman et al. 1996). In primary macrophages stimulated by lipopolysaccharide (LPS), knockout of MKP-1 prolongs JNK and p38 activity but has little effect on ERK activity (Chi et al. 2006; Zhao et al. 2006). These results suggest that under most physiological conditions, p38 and JNK, but not ERK, are the targets of MKP-1. However, this does not preclude the possibility in conditions or tissues with high MKP-1 expression, MKP-1 may play an important role in ERK deactivation. In fact, when MKP-1 expression levels are high, all three MAPKs can be effectively dephosphorylated by MKP-1 (Chu et al. 1996). For example, lungs from LPS-challenged Mkp-1−/− mice exhibited higher activities of all three MAPK subfamilies (Wang et al. 2009).
Regulation of MKP-1
MKP-1 expression can be regulated at several levels, including gene transcription, protein stability, and phosphatase activity. This multi-level regulation allows for tight control of MAPK activities. Over the years, many extracellular stimuli, including growth factors, cytokines, and stress have been shown to induce MKP-1 expression (Keyse 1995; 1999). Moreover, MKP-1 protein stability can be modulated by phosphorylation. Interestingly, MKP-1 has been shown to undergo acetylation in LPS-stimulated macrophages, which enhances the affinity between MKP-1 and p38 and facilitates MKP-1-mediated p38 dephosphorylation (Cao et al. 2008). Further adding to the complexity is the finding that the interaction of MKP-1 with MAPKs can result in conformational changes that substantially enhanced MKP-1’s catalytic activity (Hutter et al. 2000; Slack et al. 2001).
Transcription
A wide variety of extracellular stimuli can activate MKP-1 transcription (Keyse 2000), including growth factors, bacterial toxins, stress, and chemical agents. However, the mechanisms mediating MKP-1 transcription remain elusive. For example, MKP-1 mRNA levels are rapidly increased by 10-100 fold within 15-60 minutes in response to extracellular stimulation, such as serum or LPS. In most systems, the stability of MKP-1 mRNA does not appear to change significantly (Laderoute et al. 1999), making it likely that MKP-1 induction is primarily due to enhanced gene transcription. Since many extracellular stimuli activate cell membrane-associated receptors, it is very likely that signaling events initiated at the cell membrane are pivotal for MKP-1 transcriptional induction in the nucleus. As MKP-1 functions to deactivate MAPKs, it was postulated that MAPKs might activate MKP-1 transcription, forming a feedback control loop (Liu et al. 1995; Sun et al. 1993). Subsequent studies supported the idea that MAPKs play an important role in MKP-1 transcription in numerous biological systems. MKP-1 induction in serum-treated CCL-39 cells is mediated by ERK (Brondello et al. 1997). While the signaling events mediating MKP-1 induction during stress are likely to be more complex than those initiated by receptor ligands, there is strong evidence that stress-mediated MKP-1 induction may be partially mediated by growth factor receptors. For example, UV and H2O2 activate the EGFR tyrosine kinase (Sachsenmaier et al. 1994; Wang et al. 2000). We have shown that arsenite, a potent inducer of MKP-1 transcription (Li et al. 2001), induces EGFR activation and tyrosine phosphorylation of Shc (Chen et al. 1998).
While as a whole it is clear that MAPKs play an important role in MKP-1 induction, the specific MAPK subfamilies involved depend on stimulation and cellular context. This is not surprising, since MAPKs are differentially activated by distinct extracellular stimuli. In vascular smooth muscle cells, platelet-derived growth factor (PDGF), phorbol ester, and angiotensin II, which activate ERK, but not JNK and p38, induced a transient expression of MKP-1 (Bokemeyer et al. 1998). MKP-1 induction by PDGF is markedly inhibited by PD 098059, a pharmacological inhibitor of MEK1/2, thereby demonstrating the importance of ERK in MKP-1 induction. On the other hand, anisomycin, a potent stimulus for JNK and p38, also induced MKP-1 mRNA expression. This effect of anisomycin was significantly attenuated in the presence of the p38 inhibitor SB203580. We found that in C3H 10T1/2 murine fibroblasts, MKP-1 induction by heat shock and H2O2 is primarily dependent on ERK, whereas MKP-1 induction by arsenite and UVC is primarily mediated by p38 (Li et al. 2001). However, in NIH 3T3 fibroblasts, MKP-1 is highly induced by stress through a JNK-mediated process, while ERK has little effect on MKP-1 induction (Bokemeyer et al. 1996). In Hirc B cells, MKP-1 induction by insulin is mediated by both ERK and JNK (Byon et al. 2001). Likewise, MKP-1 induction in macrophages by LPS involves all three MAPK subfamilies (Ananieva et al. 2008; Chen et al. 2002b; Kim et al. 2008; Sanchez-Tillo et al. 2007). We found that MKP-1 induction in macrophages is primarily mediated by ERK, with p38 playing a minor role. This is consistent with the notion that knockout of both MSK1 and MSK2, downstream targets of both ERK and p38, abolished MKP-1 induction in LPS-treated primary macrophages (Ananieva et al. 2008; Kim et al. 2008). Additioanlly, JNK plays an important role in MKP-1 induction by both LPS and M-CSF (Sanchez-Tillo et al. 2007).
In addition to the MAPK pathways, other signaling pathways can regulate MKP-1 induction. For example, TRIF is essential for MKP-1 induction by poly(I-C) (Chi et al. 2006) and plays a significant role in MKP-1 induction by LPS. Members of the protein kinase C (PKC) family have also been shown to regulate MKP-1 induction in several systems. In cardiomyocytes treated with angiotensin II, PKC inhibitors or intracellular calcium chelation decrease MKP-1 expression while calcium ionophores increase MKP-1 mRNA levels (Hiroi et al. 2001). PKCε plays a critical role in MKP-1 induction in macrophages (Kar et al. 2010; Valledor et al. 2000), while PKCζ overexpression upregulates MKP-1 in vascular fibroblasts (Short et al. 2006). While these pathways may cross-talk with the MAPK cascades to regulate MKP-1 induction, MAPK-independent pathways could also be involved.
Since MKP-1 is an immediate-early gene whose transcription occurs in the absence of de novo protein synthesis (Lau and Nathans 1985), the transcriptional induction of MKP-1 is likely mediated by post-translational modification of pre-existing transcription factors or remodeling of MKP-1 chromatin. A variety of consensus transcription factor-binding sites have been found on the MKP-1 promoter, including AP-1, AP-2, cAMP response element (CRE), SP-1, CTF/NF-1, and E-Box (Kwak et al. 1994; Noguchi et al. 1993). Several transcription factors modulate MKP-1 transcription in response to particular stimuli. For example, MKP-1 induction by LPS or M-CSF in bone marrow-derived macrophages is mediated at least in part by binding of c-Jun and CREB to an AP-1/CRE box in the MKP-1 promoter (Casals-Casas et al. 2009). In murine macrophages, deletion of both MSK1 and MSK2 abolished phosphorylation of CREB and ATF1 and prevented the association of these transcription factors to the MKP-1 promoter (Ananieva et al. 2008). The transcription factor C/EBPβ has been identified as necessary for LPS-mediated MKP-1 induction in RAW264.7 macrophages (Cho et al. 2008). Recently, it has been shown that in enterocytes, NF-κB plays a significant role in MKP-1 induction by TLR ligands and MKP-1 induction is crucial for desensitization to TLR ligands (Wang et al. 2010). NF-κB also contributes to MKP-1 induction in response to γ-radiation, with recruitment of NF-κB RelA/p50 heterodimer to the MKP-1 gene promoter (Wang et al. 2008b). Deletion of the NF-κB-binding site or inactivation of NF-κB by small interfering RNA significantly decreased the radiation-induced MKP-1 promoter activity. Additionally, p53 has been implicated in MKP-1 induction, with p53 binding to a consensus p53 binding site in the second intron of the MKP-1 gene and stimulating MKP-1 transcription in reporter gene assays (Li et al. 2003).
Despite intensive efforts to identify transcription factors that regulate MKP-1 induction, few transcriptional factors are currently recognized with high certainty as mediators of MKP-1 induction. This is largely due to the discordance between the transcriptional pattern of the endogenous gene and the MKP-1 promoter in reporter assays. While endogenous MKP-1 expression can be enhanced 10-100-fold upon extracellular stimulation, extracellular treatment usually enhances reporter activity by a few fold. Unlike the very low basal expression of the endogenous gene, the MKP-1-promoter reporter exhibits a very high basal level, suggesting that transcriptional repressors in the distal promoter may suppress endogenous MKP-1 gene expression. Additionally, we have found that in response to stress, histone H3 was rapidly phosphorylated and acetylated at the MKP-1 locus, suggesting that chromatin remodeling is involved in MKP-1 induction (Li et al. 2001). Alternatively, basal MKP-1 expression may be inhibited by a yet unknown mechanism. Additionally, the 3′ UTR of MKP-1 mRNA contains several putative AREs, raising the possibility that MKP-1 expression could be regulated by a post-transcriptional mechanism, at least in some cellular contexts. For example, the RNA-binding proteins HuR and NF90 were recently shown to enhance MKP-1 mRNA stability in HeLa cells treated with H2O2 (Kuwano et al. 2008). Moreover, it was recently shown that miR-101 directly targets and suppresses MKP-1 through the 3′ UTR, thereby prolonging p38 and JNK activation (Zhu et al. 2010). Since miR-101 is also induced by TLR ligands, it is unlikely that the initial MKP-1 induction is due to the abrupt removal of miR-101-mediated MKP-1 inhibition. It is more likely that miR-101 mediated MKP-1 down-regulation following the initial induction, restoring homeostasis.
Protein
Over the years, a number of post-translational modifications have been identified that modulate MKP-1 activity. The degradation of MKP-1 protein is mediated by the ubiquitin-directed proteasome complex, which can be blocked by proteasome inhibitors (Brondello et al. 1999; Lin et al. 2003). MKP-1 is phosphorylated by ERK on ser-259 and 364 in serum-stimulated CCL-39 cells, protecting MKP-1 from proteasome-mediated degradation (Sohaskey and Ferrell 2002). This serves as a negative feedback mechanism to deactivate ERK. In LPS-stimulated macrophages, ERK also inhibits MKP-1 degradation, likely through catalyzing MKP-1 phosphorylation, since pretreatment of cells with the MEK inhibitor U0126 substantially decreased the half-life of MKP-1 (Chen et al. 2002b). Since JNK and p38, rather than ERK, are the physiological targets of MKP-1 in this system, ERK-mediated stabilization of MKP-1 constitute a cross-talk mechanism between distinct MAPK cascades. While in most cases phosphorylation of MKP-1 by ERK enhances MKP-1 stability, phosphorylation of MKP-1 by ERK can also accelerate MKP-1 degradation. Lin et al have shown that sustained activation of ERK results in phosphorylation of MKP-1 at Ser-296 and Ser-323. This phosphorylation facilitates the interaction of MKP-1 with the Skp-cullin-F-box (SCFSkp2) ubiquitin ligase, which targets MKP-1 for proteasomal degradation (Lin et al. 2003; Lin and Yang 2006). Thus, it appears that transient versus prolonged ERK activation exerts different effects on MKP-1 stability.
Another posttranslational modification of MKP-1 is acetylation. Cao et al. studied the mechanism underlying the anti-inflammatory effects of histone deacetylase (HDAC) inhibitors. They found that HDAC inhibitors attenuate the expression of iNOS, TNF-α, IL-6 and IL-1β in LPS-treated RAW264.7 macrophages by blocking activation of p38 and ERK, but not JNK. They further demonstrated that MKP-1 is acetylated upon LPS stimulation while MKP-1 is not acetylated in control cells. MKP-1 acetylation is increased by the HDAC inhibitor trichostatin A, which enhanced the interaction between MKP-1 and histone acetylase p300. MKP-1 acetylation occurred at Lys-57 in the basic motif required for interactions with p38, and findings from immunoprecipitation assays indicated that acetylation of MKP-1 enhances its interaction with p38. By using MKP-1-derived peptides containing acetylated Lys-57, they demonstrated that acetylation of Lys-57 increases the affinity between MKP-1 and p38. While acetylation of MKP-1 does not directly alter its phosphatase activity, activity is indirectly increased via increased affinity for substrate MAPKs.
Finally, MKP-1 can be inactivated by oxidation. TNF-α can trigger rapid oxidation of MKP-1 and several other MKPs at the critical cysteine residue in their catalytic sites in the absence of NF-κB. Oxidation of MKP-1 renders MKP-1 more susceptible to proteasome-mediated degradation, prolonging JNK activation and leading to apoptosis (Kamata et al. 2005). It appears that MKP-1 is maintained in different redox states depending on cell type. For example, MKP-1 exists primarily in a reduced state in alveolar macrophages but in an oxidized state in blood monocytes (Tephly and Carter 2007). The redox status of MKP-1 may contribute in part to cell responses to irritants, including asbestos. Asbestos-treated alveolar macrophages exhibit a minimal cytokine response, while blood monocytes rapidly degrade MKP-1 and produce a robust cytokine response to asbestos (Tephly and Carter 2007). Overall, the mechanisms regulating the redox states of MKP-1 are not completely understood.
Catalytic activity
The catalytic activity of MKP-1 protein can be regulated by interactions with its substrate MAPKs (Hutter et al. 2000; Slack et al. 2001). We and another group have independently shown that binding of recombinant MKP-1 protein to ERK, JNK, and p38 results in a 6-8 fold increase in MKP-1 activity (Chen et al. 2002a; Hutter et al. 2000; Hutter et al. 2002; Slack et al. 2001). Analysis of the crystal structure of a related phosphatase, MKP-3, has demonstrated that binding of MKP-3 to ERK may enable the MKP-3 catalytic site to adopt a more efficient conformational configuration (Stewart et al. 1999). Since MKP-1 and MKP-3 are highly similar, binding of MKP-1 to its substrate MAPKs may produce a more efficient catalytic site conformation (Stewart et al. 1999). Biochemical studies from several laboratories demonstrated that the interactions between MAPKs and MKPs depend on a basic kinase-interaction domain at the amino terminus of the phosphatases and an acidic domain at the carboxyl terminus of the kinases (Camps et al. 1998; Chu et al. 1996; Hutter et al. 2000; Nichols et al. 2000; Tanoue et al. 2000). The kinase-interaction domain in the MKP family contains the ψψXRRψXXG consensus sequence (where ψ represents a hydrophobic residue and × is any amino acid), flanked by two Cdc25-homology domains (Keyse 2000).
Role of MKP-1 in Physiology and Animal Models of Disease
While reproduction and growth in Mkp-1 deficient mice does not differ grossly from wildtype animals (Dorfman et al. 1996), Mkp-1−/− mice display numerous physiological alterations at baseline, and develop distinct disease phenotypes in numerous experimental models. In this section, we will briefly outline the role of MKP-1 in immunology, physiology, and disease, highlighting findings from rodent models of infectious, inflammatory, metabolic, and central nervous system diseases.
Immune regulation and infectious disease
Over the past 20 years, significant research effort has focused on understanding the role of MKP-1 in the immune system. Results from numerous cell culture and clinically relevant animal models implicate MKP-1 as a key factor in both innate and adaptive immunity.
Innate Immune Regulation
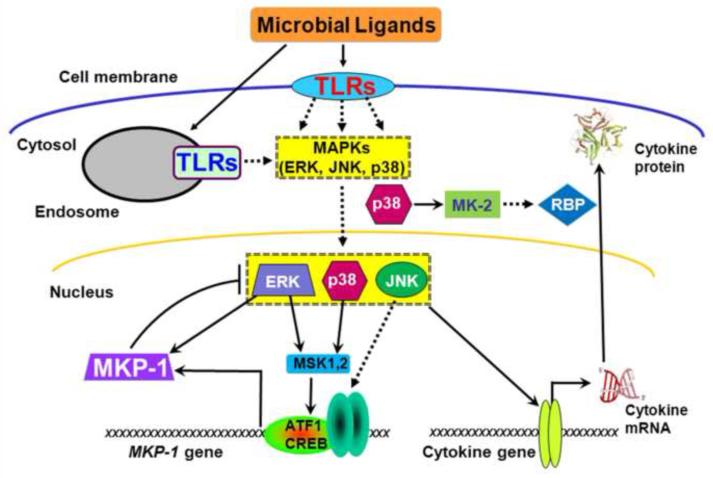
MAPKs are crucial regulators of the innate response, stimulation synthesis of numerous anti-microbial factors, including cytokines, chemokines, and other inflammatory mediators (Dong et al. 2002). Given the role of MKP-1 in inhibiting MAPK activation, it is not surprising that MKP-1 has emerged as a crucial feedback control regulator, limiting MAPK-mediated cytokine production and restoring immunologic homeostasis (Fig. 1).
Figure 1.
MKP-1 restrains pro-inflammatory cytokine biosynthesis. Microbial ligands stimulate toll-like receptors (TLRs), initiating the NF-κB and MAPK cascades and upregulating pro-inflammatory cytokines through both transcriptional and post-transcriptional mechanisms. p38 activates the downstream protein kinase MK-2. MK2 in turn enhances cytokine mRNA expression by two mechanisms: stabilizing the stabilities of cytokine mRNAs through modulating RNA-binding proteins (RBP) and augmenting the cytokine mRNA translation. Simultaneously, TLR-initiated signals also enhance MKP-1 transcription, which in turn dephosphorylates JNK and p38, destabilizes cytokine mRNA, and inhibits cytokine translation to terminate the inflammatory cascade. ERK phosphorylates MKP-1 to stabilize the protein. ERK and p38 signal through MSK-1 and MSK-2 to upregulate MKP-1 expression.
MKP-1 deficiency results in excessive production of numerous factors, including the pro-inflammatory cytokines TNF-α, IL-6, IL-1β (Chen et al. 2002b; Shepherd et al. 2004; Zhao et al. 2005), the anti-inflammatory cytokine IL-10 (Chi et al. 2006; Salojin et al. 2006; Zhao et al. 2006), the chemokines macrophage inflammatory protein (MIP)-1α (CCL3), MIP-1β (CCL4), MCP-1, and CXCL2 (Hammer et al. 2006; Salojin et al. 2006), iNOS (Calvert et al. 2008; Wang et al. 2009) and cyclooxygenase 2 (COX-2) (Lasa et al. 2002). MKP-1 mediated-inhibition of cytokine production was first documented in murine macrophage cell lines (Chen et al. 2002b; Shepherd et al. 2004; Zhao et al. 2005) using either over-expression or knockdown of MKP-1, and later confirmed in primary macrophages (Zhao et al. 2005). In LPS-stimulated RAW264.7 macrophages, ectopic expression of MKP-1, even at a modest level, substantially attenuated cytokine production, whereas MKP-1 knockdown enhanced cytokine release (Chen et al. 2002b; Shepherd et al. 2004; Zhao et al. 2005). Subsequent studies using macrophages or dendritic cells from Mkp-1−/− mice further confirmed the critical role of MKP-1 in the regulation of cytokine production (Chi et al. 2006; Hammer et al. 2006; Salojin et al. 2006; Zhao et al. 2005; Zhao et al. 2006). We and three other laboratories independently demonstrated that knockout of MKP-1 in TLR ligand-treated macrophages prolonged p38 and JNK activation, but had little effect on ERK activity. cDNA array analysis of spleens from LPS-challenged mice identified >480 genes with elevated expression levels in MKP-1-deficient compared to wildtype spleens, including numerous cytokines and chemokines (Hammer et al. 2006). Additionally, MKP-1 overexpression reduces the mRNA half-lives of several cytokines, including IL-6, IL-10, and TNF-α(Yu et al. 2011b). Ultimately, prolonged p38 and JNK activation in MKP-1-deficient cells provides a plausible mechanism for enhanced cytokine and chemokine production.
Localized and Systemic Bacterial Endotoxin Models
The Mkp-1−/− mouse is an effective tool for examining the role of MKP-1 in innate immunity in vivo, most notably in endotoxin-exposure models (Chi et al. 2006; Hammer et al. 2006; Salojin et al. 2006; Zhao et al. 2006). MKP-1 is crucial for the control of cytokine release locally and systemically. MKP-1 protects the oral cavity against inflammation triggered by bacterial ligands (Sartori et al. 2009; Yu et al. 2011a). In a rodent model of periodontitis (chronic inflammation of the oral cavity and surrounding bone), local delivery of LPS produced more severe maxillary bone loss and local inflammation in Mkp-1−/− mice (Sartori et al. 2009). This resorptive bone phenotype could be partially reversed through vector-transduced MKP-1 expression (Yu et al. 2011a). When LPS is administered systemically rather than locally, MKP-1 deficiency produces severe damage in vivo. Mkp-1−/− mice challenged systemically with LPS exhibit enhanced cytokine production (Chi et al. 2006; Hammer et al. 2006; Salojin et al. 2006; Zhao et al. 2006) and increased mortality compared to similarly treated wildtype mice. Additionally, Mkp-1−/− mice develop severe end-organ hypoperfusion, including profound iNOS up-regulation and hypotension (Calvert et al. 2008; Wang et al. 2009). It can be speculated that MKP-1 may support recovery from endotoxemia by attenuating iNOS expression and switching arginine metabolism from nitric oxide synthase to arginase (Nelin et al. 2007). Mkp-1−/− mice displayed similar responses after systemic challenge with either LPS or the Gram-positive cell ligand peptidoglycan, including increased mortality and cytokine release (Wang et al. 2007). Overall, these models clearly demonstrate that Mkp-1−/− mice undergo enhanced inflammation and organ damage in response to both localized and systemic bacterial ligands.
Localized and Systemic Bacterial Infections
Unlike the consistent results with Gram-positive and negative bacterial ligands, live-bacterial infections have sometimes produced unexpected results in Mkp-1−/− mice. Mkp-1−/− mice challenged systemically with either live Gram-negative Escherichia coli (Frazier et al. 2009) or LPS developed similar signs, including higher overall mortality and enhanced cytokine production compared to wildtype mice. E. coli-infected Mkp-1−/− animals also exhibited profound hemoconcentration and pulmonary edema (Frazier et al. 2009). Mortality was also higher in Mkp-1−/− mice during polymicrobial peritonitis (Frazier et al. 2009; Hammer et al. 2010).
Unlike purified ligands, live bacteria that are not quickly eliminated by the innate immune response can replicate and potentially overwhelm and kill the host. Loss of MKP-1 appears to hinder bacterial clearance of Gram-negative E. coli and Chlamydophila pneumoniae, despite hyper-inflammatory cytokine responses in Mkp-1−/− mice. Systemically-infected Mkp-1−/− mice had 10 fold higher blood and spleen E. coli loads 18 hours after infection than wildtype animals (Frazier et al. 2009). Additionally, Mkp-1−/− mice intranasally inoculated with C. pneumoniae maintained significantly higher pulmonary bacterial counts at three and six days post inoculation compared to wildtype mice (Rodriguez et al. 2010). Currently the mechanism underlying reduced bacterial clearance in Mkp-1−/− mice is unclear, although preliminary results implicate excessive production of IL-10 in E. coli infection (Frazier et al. 2009) and IL-6 in C. pneumoniae infection (Rodriguez et al. 2010). Intriguingly, reduced clearance alone does not account for increased mortality and inflammation in E. coli-infected Mkp-1−/− mice. Bactericidal antibiotics protected 100% of E. coli-infected wildtype mice without altering mortality rates or cytokine production in Mkp-1−/− animals (Frazier et al. 2009), highlighting the relative importance of controlling inflammation as opposed to infection.
In contrast to findings with Gram-negative agents, survival and bacterial tissue burdens were not different between Mkp-1−/− and wildtype mice infected intravenously with live Gram-positive Staphylococcus aureus (Wang et al. 2007). Since Gram-positive and Gram-negative pathogens signal through different pattern-recognition receptors and signal transduction pathways, the effects of MKP-1 deficiency may vary between bacteria. Counter to this argument are the earlier observations of near-identical phenotypes following administration of LPS (Chi et al. 2006; Hammer et al. 2006; Salojin et al. 2006; Zhao et al. 2006) and peptidoglycan (Wang et al. 2007). MKP-1 also appears to influence energy usage during E. coli infection. Intravenous infection with live E coli incites a broad catabolic response in wildtype mice, with massively increased serum triglyceride levels and depletion of hepatic glycogen stores (Frazier et al. 2009). In contrast, Mkp-1−/− mice failed to mount a significant catabolic response and maintained pre-infection glycogen and fat stores. This profound, and possibly lethal, energy mobilization defect among Mkp-1−/− mice is not fully characterized and remains an area for ongoing investigation.
Adaptive immune regulation
MAPKs are integrally involved in adaptive immune responses, including modulating lymphocyte proliferation, cytokine production, and survival (Dong et al. 2002). As with innate immunity, MKP-1 also regulates the development of an appropriate adaptive immune response. MKP-1 is an abundantly transcribed MKP in the mouse thymus and its expression significantly increases at the transition from CD4/CD8 double positive to CD4 single positive T cells (Tanzola and Kersh 2006). MKP-1 deficiency in innate immune cells also favors CD4+ T cell differentiation toward the Th17 pathway. LPS-treated Mkp-1−/− dendritic cells produce less of the Th1-inducing cytokine IL-12 p40 and more of the Th17-inducing cytokine IL-6 (Huang et al. 2011); these cytokine changes were blocked by the p38 inhibitor SB203580, but not the JNK inhibitor SP600125 (Huang et al. 2011). T cells co-cultured with Mkp-1−/− dendritic cells produced less IFN-γ but more IL-17 and IL-17F, consistent with a Th17 phenotype (Huang et al. 2011). The Th17 phenotype could be reversed by the addition of exogenous IL-12 or an IL-6 blocking antibody (Huang et al. 2011).
To address whether this Th-17 phenotype was also present in vivo, Huang et al. produced mixed bone marrow chimera mice that had wildtype T cells with either 5:1 ratio of MKP-1−/−:MKP-1+/+ or 5:1 ratio of MKP-1+/+:MKP-1−/− innate immune cells. Chimera mice with more MKP-1−/− cells produced a Th17-like response, with much higher levels of IL-17-producing and lower levels of IFN-γproducing T lymphocytes compared to chimeras with predominantly MKP-1+/+ cells. Additionally, MKP-1 inhibits regulatory T cell induction by downregulating TGF-β2 production by dendritic cells (Huang et al. 2011). These results suggest that altered innate immune cytokine production play a key role in adaptive immune defects in MKP-1-deficient mice. MKP-1 also alters cytokine release and cell proliferation intrinsically in T cells. Mkp-1−/− T cells exhibit enhanced JNK activation and reduced nuclear translocation of NFATc, as well as reduced cytokine production and attenuated cellular proliferation after T cell receptor activation (Zhang et al. 2009). However, since T cell differentiation is influenced by MKP-1 in innate immune cells, it is unclear if T cell defects in Mkp-1−/− mice are caused by dysregulation in the innate, adaptive, or both immune systems.
MKP-1 also influences outcomes in auto-immune mediated diseases. MKP-1 plays a protective role in rheumatoid arthritis and may be a therapeutic target. Collagen-immunized Mkp-1−/− mice displayed increased joint swelling and elevated serum TNF-α and IL-6 levels than control animals (Salojin et al. 2006). The anti-rheumatoid arthritis drug aurothiomalate upregulates MKP-1 expression in chondrocytes and suppresses IL-1β-induced phosphorylation of p38 (Nieminen et al. 2010). MKP-1 siRNA blocked aurothiomalate-mediated inhibition of p38 phosphorylation and enhanced the expression of COX-2, IL-6, and MMP-3 in chondrocytes treated with IL-1β. Aurothiomalate attenuated IL-1β-induced COX-2, MMP-3, and IL-6 expression in articular cartilage explants from rheumatoid arthritis patients. Similar effects were seen with cartilage explants from wildtype, but not Mkp-1−/−, mice. The atteniuation of COX-2 expression by aurothiomalate and by the p38 inhibitor SB203580 were primarily due to destabilization of COX-2 mRNA, suggesting again that MKP-1 dampens inflammation, at least in part, by altering mRNA stability of pro-inflammatory factors. In contrast to the protective effects of MKP-1 in rheumatoid arthritis models, MKP-1 may promote disease progression in experimental autoimmune encephalomyelitis (EAE). Mkp-1−/− mice treated to induce EAE displayed a later onset of disease and lower overall disease scores than wildtype animals (Zhang et al. 2009). The reduced disease severity in Mkp-1−/− mice correlated with decreased infiltration of CD4+ T cells in the central nervous system and lower production of both INF-γ and IL-17 compared to wildtype animals (Zhang et al. 2009).
Energy Metabolism and Metabolic Disease
Obesity and related metabolic disorders are an important global concern, with an estimated 46 billion overweight adults worldwide in 2008 (Finucane et al. 2011). Given the magnitude of this problem, it is important to identify factors that control basal energy utilization. MKP-1 is known to modulate lipid metabolism in several tissues, promoting adipose tissue deposition at baseline (Sakaue et al. 2004) and altering lipid metabolism in multiple tissues when a high fat diet is consumed (Flach et al. 2011; Roth et al. 2009; Wu et al. 2006). MKP-1 promotes adipocyte maturation, as antisense inhibition of MKP-1 enhances ERK activation and blocks the differentiation of pre-adipocytes into the mature adipocytes (Sakaue et al. 2004). Similarly, mixed background Mkp-1−/− mice fed a chow diet maintained lower body weights, lipid stores, serum free fatty acid levels, and triglyceride levels compared to wildtype animals on the same diet, (Wu et al. 2006).
In mice fed high fat diets, MKP-1 suppresses lipid metabolism in adipocytes, hepatocytes, and skeletal muscle while Mkp-1−/− mice are protected from developing metabolic diseases including obesity, skeletal muscle atrophy, and hepatic steatosis. Mkp-1−/− mice fed high fat diets maintain lower body weights and lipid stores than similarly fed wild type animals (Roth et al. 2009; Wu et al. 2006). However, this lean phenotype did not protect Mkp-1−/− mice from developing signs of diabetes, as both wildtype and Mkp-1−/− animals displayed comparable glucose intolerance and insulin resistance on a high fat diet (Wu et al. 2006).
Excess dietary fat enhances MKP-1 expression in skeletal muscle and promotes loss of the oxidative myofibers by inhibiting p38 and indirectly suppressing peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α (PGC-1α), a factor which helps maintain oxidative myofibers. MKP-1 negatively regulates PGC-1α stability in C2C12 myoblasts, as inhibiting MKP-1 expression by MKP-1 siRNA increases PGC-1α half-life by 47% (Roth et al. 2009). Overexpression of MKP-1 reduces PGC-1α phosphorylation on both Ser265 and Thr298 by ~50% (Roth et al. 2009). This likely destabilizes PGC-1α, since p38 stabilizes PGC-1α by phosphorylating Ser265 and Thr298 of PGC-1α (Puigserver et al. 2001). In vivo, skeletal muscle from chow fed Mkp-1+/+ or Mkp-1−/− mice had similar levels of Ser265-phosphorylated PGC-1α, while muscle from wildtype mice fed a high fat diet had a significant decrease in Ser265-phosphorylated PGC-1α levels. In contrast, high fat fed Mkp-1−/− mice had significantly higher levels of phospho-Ser265 PGC-1α than high fat fed wildtype mice.
MKP-1 deficiency also protects mice in a model of hepatic steatosis, a condition which can predispose to diseases including cirrhosis and liver failure. Double knockout mice lacking the leptin receptor (db/db) and MKP-1 are resistant to hepatic steatosis compared to db/db-Mkp-1+/+ mice (Flach et al. 2011). MKP-1 deficiency resulted in PPAR-γ inhibition due to enhanced MAPK-dependent phosphorylation on PPARγ at its inhibitory residue of serine 112. Finally, PPAR-γ target genes that promote hepatic steatosis were downregulated in db/db-Mkp-1−/− mouse livers, including fat-specific protein 27 and cell death-inducing DNA fragmentation factor A-like effector C.
Central Nervous System Development and Disease
MKP-1 is increasingly recognized as an important regulator of the central nervous system, and is implicated in neuronal axonal development, inflammation, and depression. First, brain-derived neutrotrophic factor (BDNF) upregulates MKP-1 expression in both excitatory and inhibitory neurons, correlating with increased axonal branching (Jeanneteau et al. 2010). In order for axons to develop branches, axonal microtubule components must be destabilized and disassembled, an action opposed by JNK-mediated phosphorylation of tubule components such as stathmin. To determine if MKP-1 was involved in this process, Jeanneteau et al. produced a mutant MKP-1 that specifically targets JNK without an effect on either p38 or ERK. Expression of this MKP-1 mutant inactivated JNK, attenuated stathmin phosphorylation, and inhibited axon outgrowth, indicating that MKP-1 activity was sufficient to destabilize cortical microtubules. Conversely, expression of a JNK mutant resistant to MKP-1-mediated dephosphorylation reversed the negative effect of MKP-1 on axon outgrowth. This result was supported by finding that neurons from Mkp-1−/− mice are unable to form axon branches in response to BDNF.
Secondly, since MKP-1 suppresses systemic inflammation, the protein is a potential therapeutic target for inflammatory diseases of the central nervous system (CNS). MKP-1 is expressed in microglial cells, the endogenous brain macrophage. Eljaschewitsch et al. have shown that MKP-1 plays an important role in protecting neurons during CNS inflammation (Eljaschewitsch et al. 2006). The endocannabinoid system is highly activated during CNS inflammation and the endocannabinoid anandamide induces MKP-1 in microglial cells by activating the neuronal CB(1/2) cannabinoid receptor. Induction of MKP-1 is associated with histone H3 phoshorylation at the MKP-1 gene locus. As a result, in ligand-activated microglial cells, anandamide induces rapid increases in MKP-1 protein that switch off MAPK signal transduction and inhibit iNOS induction. The release of endocannabinoid anandamide during primary CNS injury tissue may represent a new mechanism of neuro-immune communication that controls and limits immune response.
Finally, MKP-1 expression is elevated in the brains of rodents in a model of major depressive disorder. Rats subjected to the chronic unpredictable stress (CUS) model, one of the most valid and relevant rodent models of depression, display depressive-like behaviors, including helplessness and anhedonia (inability to experience pleasure). At day 35, CUS-exposed rats displayed decreased sucrose preference and increased escape failures in an active avoidance test (Duric et al. 2010). Mkp-1 mRNA levels were significantly upregulated in the dentate gyrus and CA3 of CUS-stressed rats. Administration of the anti-depressant fluoxetine blocked CUS-induced anhedonia and helpless behavior and normalized the Mkp-1 mRNA levels in the dentate gyrus. Enhanced MKP-1 expression was specific to the hippocampus, as neither stress nor fluoxetine altered Mkp-1 gene expression in the cortex. When rats were administered a hippocampal-targeted adenoviral vector expressing MKP-1, unstressed rats exhibited anhedonic responses, mimicking behaviors in CUS-exposed rats. CUS-exposed Mkp-1−/− mice maintained normal sucrose consumption, while CUS-exposed wildtype mice developed a progressive and significant reduction in sucrose consumption, a sign associated with depression. These results indicate that induction of MKP-1 is not only a direct consequence of stress but also a key negative regulator of MAPK that contributes to the expression of depressive symptoms. The studies suggest that phosphatases such as MKP-1 represent promising new drug targets for treating depression and possibly other mood disorders.
MKP-1 in Human Disease
MAPKs may play key roles in the development of numerous human diseases, and there is increasing evidence of altered MKP-1 expression in conditions including asthma, sarcoidosis and cancer. While this section describes MKP-1’s role in these disorders, we emphasize that there is novel evidence of altered MKP-1 expression in other organs, including the brain. MKP-1 expression is elevated in patients with multiple sclerosis (Eljaschewitsch et al. 2006) and major depressive disorder (Duric et al. 2010), mirroring recent findings in rodent models.
Asthma and sarcoidosis
MAPKs are key mediators of airway inflammation and hyper-responsiveness in asthma (reviewed in (Duan and Wong 2006)), and MKP-1 expression is altered the immune cells of asthmatic patients. MKP-1 is readily induced by corticosteroids in human pulmonary tissues and immune cells, including bronchial biopsies (Kelly et al. 2011) and primary alveolar (Bhavsar et al. 2008) and sputum macrophages (Essilfie-Quaye et al. 2011). While LPS and dexamethasone can upregulate MKP-1 expression in alveolar macrophages isolated from both asthmatics and non-asthmatics, MKP-1 induction is muted in cells from severe asthmatics (Bhavsar et al. 2008). Similarly, peripheral blood mononuclear cells from obese asthmatics exhibit weaker MKP-1 induction in response to dexamethasone that did cells from non-asthmatics (Sutherland et al. 2008). Although MKP-1 responsiveness is suppressed in asthmatic immune cells, it remains to be seen if suppressed MKP-1 induction is an inciting event, secondary reaction to chronic inflammation, or a potential biomarker.
Sarcoidosis is a systemic inflammatory disorder characterized by the development and growth of tiny clusters of inflammatory cells in different organs, most commonly the lungs, lymph nodes, eyes and skin. While sarcoidosis is associated with up-regulation of Th1 cytokines, such as TNF-α and IL-12, the underlying mechanism remains unclear. Rastogi et al. tested the hypothesis that dysregulated MAPK signaling in response to microbial stimulation is important in mediating Th1 responses in sarcoidosis (Rastogi et al. 2011). Bronchoalveolar lavage (BAL) cells from sarcoidosis patients exhibited both higher basal p38 activity and a more robust increase in p38 phosphorylation after treatment with NOD1 or TLR4 ligand than cells from control subjects. Sarcoid BAL cells also produced more TNF-α and IL-12/IL-23p40. In contrast, control BAL cells exhibited greater basal ERK activity, and NOD1 and TLR4 agonists preferentially activated the ERK pathway. Inhibition of p38, but not ERK, attenuated IL12/IL23p40 and TNF-α production by sarcoid BAL cells. Interestingly, neither NOD1 nor TLR4 ligand could induce MKP-1 in sarcoid BAL cells. Adenovirus-mediated overexpression of MKP-1 attenuated p38 activation and decreased the production of IL12/IL23p40 and TNF-α in sarcoid BAL cells. These results suggest that defects in ERK activation prevent MKP-1 MKP-1 upregulation, contributing to enhanced p38 signaling and heightened inflammation in sarcoidosis.
Cancer
There is clear evidence that altered MKP-1 expression correlates with tumor progression in certain cancers (Wu 2007). Elevated MKP-1 expression is reported in melanoma (Kundu et al. 2010), advanced breast carcinoma (Loda et al. 1996; Rojo et al. 2009; Wang et al. 2003), pancreatic cancer (Liao et al. 2003), and non-small cell lung cancer (Chattopadhyay et al. 2006; Vicent et al. 2004). In contrast, MKP-1 expression decreases or is lost during cancer progression in prostate (Loda et al. 1996; Rauhala et al. 2005), colon (Loda et al. 1996), and bladder tumors (Loda et al. 1996). MKP-1 expression is more variable in ovarian cancer, with conflicting studies reporting decreased expression in higher stage tumors (Manzano et al. 2002) but enhanced protein expression in invasive ovarian cancer (Denkert et al. 2002). While the factors leading to altered MKP-1 expression in cancer are generally not known, oncogene mutations may be involved. MKP-1 expression is regulated by MAPKs, and mutations in the upstream regulators of the MAPK pathways, such as Ras and EGF receptor tyrosine kinase, are common in many human cancers (Harris and McCormick 2010; Vogelstein and Kinzler 1993). Since the tumor suppressor p53 regulates MKP-1 expression (Li et al. 2003; Yang and Wu 2004) and is often mutated or deleted in human cancers (Vogelstein and Kinzler 1993), loss of p53 activity may also alter MKP-1 expression. Alternatively, elevated MKP-1 could be caused by stress within tumor cells. Regardless of the mechanisms underlying the altered protein expression, MKP-1’s role in inhibiting the MAPKs likely has a significant effect on cell proliferation and apoptosis in tumors.
Modulation of MKP-1
Since MAPKs are implicated in the pathogenesis of many conditions (Chapman and Miner 2011; Dhillon et al. 2007), MAPK-modulating factors have potential therapeutic value. As an endogenous inhibitor of MAPKs, MKP-1 is a promising therapeutic target for diseases characterized by MAPK activation. Although currently there are no specific MKP-1 inhibitors, there are ongoing efforts to screen for compounds with enhanced specificity for MKP-1 (Lazo et al. 2007; Park et al. 2011; Vogt et al. 2008). Several reports have shown that MKP-1 expression is enhanced by nutritional compounds including L-glutamine (Ko et al. 2009; Singleton et al. 2005), fatty acid metabolites (Ichikawa et al. 2008; Metzler et al. 1998), and vitamin A derivatives (Choudhary et al. 2008; Palm-Leis et al. 2004). Immune factors commonly noted to alter MKP-1 expression are glucocorticoids and cytokines, including IFN-γ, MIF, and IL-10 (Fig. 2).
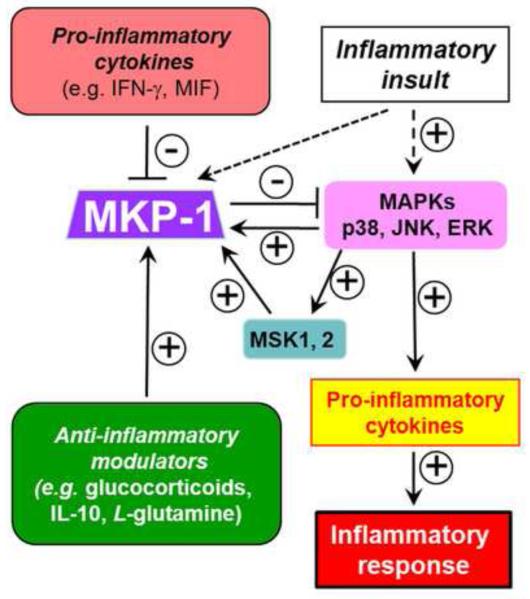
Figure 2.
Immunomodulatory agents regulate inflammation by modulating MKP-1 expression. Agents including glucocorticoids, IL-10, and L-glutamine induce MKP-1 expression, leading to dephosphorylation of p38 and JNK and attenuation of inflammation. In contrast, pro-inflammatory factors, including the cytokines IFN-γ and MIF, inhibit MKP-1 expression, prolonging p38 and JNK signaling pathways and enhancing inflammation. ERK and p38 upregulate MKP-1 expression through MSK-1 and MSK-2.
Glucocorticoids have long been recognized as a key inducer of MKP-1 gene transcription. The magnitude of glucocorticoid-mediated MKP-1 induction is influenced by factors including the cell type examined and the exogenous steroid tested. Glucocorticoids induce MKP-1 in numerous cell types and tissues, including macrophages (Bhavsar et al. 2008; Chen et al. 2002b; Usmani et al. 2005; Zhao et al. 2005), microglia (Huo et al. 2011), airway smooth muscle cells (Issa et al. 2007; Quante et al. 2008), rodent hearts (Fan et al. 2009), primary human neutrophils (Mortaz et al. 2008), primary human T cells (Maneechotesuwan et al. 2009), and primary human airway epithelial cells (Kaur et al. 2008; Wilson et al. 2009). Additionally, we have found that the magnitude of MKP-1 mRNA induction directly correlates with a corticosteroid’s anti-inflammatory potency, with higher induction by dexamethasone than prednisone (Zhao et al. 2005). This correlation suggests that part of the mechanism underlying the potent anti-inflammatory effects of certain corticosteroids is through upregulation of MKP-1.
Mechanistically, the glucocorticoid receptor (GR) is essential for steroid-mediated MKP-1 induction in macrophages, as glucocorticoid-treated peritoneal macrophages from GR knockout mice lacked significant MKP-1 induction (Bhattacharyya et al. 2007). Both human and mouse MKP-1 genes contain several putative consensus glucocorticoid response elements (GRE) binding sites in their promoter regions, located ~29, 28, 24, 4.6, and 1.3 kb upstream of the MKP-1 transcription start site (Tchen et al. 2010). Although these regions are conserved in mice and humans, glucocorticoid responsiveness was not always preserved in both species. For example, while the human and mouse glucocorticoid responsive regions at −1.3 and −4.6 were ≥ 65% identical in sequence and contain highly conserved glucocorticoid binding sequences, only the human elements were glucocorticoid-responsive when expressed in either mouse or human cells (Tchen et al. 2010). Other studies have identified p300 as being involved in glucocorticoid-mediated human MKP-1 transcription at the ~1.3 kb responsive site. p300 recruitment to the functional GRE in the MKP-1 promoter region between −1337 and −1323 was markedly enhanced by glucocorticoid treatment, and GR and p300 co-precipitated in the protein complex recruited to the DUSP1 GRE (Shipp et al. 2010).
Finally, it is important to emphasize that not all anti-inflammatory effects of corticosteroids are mediated through MKP-1. For example, while we found that the immediate inhibitory effects of glucocorticoids on p38 and JNK are mediated by MKP-1, glucocorticoids can inhibit cytokine production in LPS-challenged Mkp-1−/− mice (Wang et al. 2008a). In summary, glucocorticoids remain a widely researched family of potent MKP-1 inducers, and these drugs hold the potential to treat a wide variety of diseases in which MKP-1 upregulation would be beneficial.
In addition to glucocorticoids, cytokines are another class of immune mediators that modulate MKP-1 expression. While not all cytokines alter MKP-1 expression, there is clear evidence that the pro-inflammatory cytokines IFN-γ and macrophage inhibitory factor (MIF) inhibit MKP-1 expression, while the anti-inflammatory cytokine IL-10 stimulates MKP-1 expression. IFN-γ is a Th1 cytokine that primes macrophages to enhance release of other pro-inflammatory cytokines and NO upon LPS treatment (Collart et al. 1986; Gordon 1995). We demonstrated that priming resident peritoneal macrophages with IFN-γ prior to LPS treatment significantly attenuated MKP-1 induction while prolonging activation of p38 and JNK (Zhao et al. 2006). More recently, others have shown that IFN-γ-mediated inhibition of MKP-1 is directly responsible for both prolonged MAPK activation and growth inhibition in M-CSF-stimulated macrophages (Valledor et al. 2008a; Valledor et al. 2008b).
MIF is a pro-inflammatory cytokine that strongly induces other pro-inflammatory cytokines in macrophages. Elevated MIF expression is closely associated with lethality in models of bacterial sepsis, and antibody-mediated depletion of MIF protein can prevent mortality in septic mice (Calandra et al. 2000). Although the full details remain to be elucidated, MIF appears to act as a physiological counter-regulator to glucocorticoids (Calandra et al. 1995), and several independent investigators have discovered that MKP-1 is a critical mediator in the MIF-glucocorticoid crosstalk (Aeberli et al. 2006; Roger et al. 2005). Recombinant MIF antagonizes dexamethasone- and LPS-mediated induction of MKP-1 in wildtype macrophages (Roger et al. 2005). Treatment with recombinant MIF also restores TNF-α and IL-8 production in dexamethasone-treated macrophages (Roger et al. 2005). Similarly, peritoneal macrophages from MIF knockout mice produced elevated levels of MKP-1 mRNA, which can be suppressed by recombinant MIF (Aeberli et al. 2006).
In contrast to IFN-γ and MIF, IL-10 enhances MKP-1 expression after LPS stimulation. IL-10 can synergize with dexamethasone to induce MKP-1 mRNA expression and inhibit production of IL-6 and IL-12 (Hammer et al. 2005). In LPS-stimulated macrophages, IL-10-mediated upregulation of MKP-1 correlated with more rapid p38 deactivation, suggesting that MKP-1 induction may play a key role in the anti-inflammatory mechanism of IL-10.
Concluding thoughts
Research in the past 20 years has demonstrated that MKP-1 regulates numerous physiological and immunological functions, and that altered MKP-1 expression is a key factor in numerous animal disease models and in several human conditions. For these reasons, MKP-1 remains an exciting potential therapeutic target for the diseases with altered MAPK activity. Despite the wealth of knowledge about MKP-1 biology described here, there remain key unanswered questions that will aid in moving forward with novel therapies. For example, what are the specific factors that regulate MKP-1 induction at the promoter level? Are certain inflammatory diseases such as rheumatoid arthritis, Crohn’s disease, and psoriasis associated with defects in MKP-1 induction, as has been shown for sarcoidosis? Do MKP-1 polymorphisms previously identified in diseases such as ovarian cancer (Suzuki et al. 2001) affect the cancer prognosis or sensitivity to chemotherapeutic drugs? Addressing these and related questions will help move MKP-1-based therapeutics from the lab into the clinic.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Disease at the National Institutes of Health (R01 AI57798 and AI68956 toY.L.) and the National Research for Research Resources at the National Institutes of Health (K01 RR032139 to L.M.W and T32 RR007073 for stipend support of L.M.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- Aeberli D, Yang Y, Mansell A, Santos L, Leech M, Morand EF. Endogenous macrophage migration inhibitory factor modulates glucocorticoid sensitivity in macrophages via effects on MAP kinase phosphatase-1 and p38 MAP kinase. FEBS Lett. 2006;580:974–81. doi: 10.1016/j.febslet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Smythe C, Keyse SM. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993;8:2015–20. [PubMed] [Google Scholar]
- Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028–36. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- Arthur JS, Cohen P. MSK1 is required for CREB phosphorylation in response to mitogens in mouse embryonic stem cells. FEBS Lett. 2000;482:44–8. doi: 10.1016/s0014-5793(00)02031-7. [DOI] [PubMed] [Google Scholar]
- Arthur JS, Fong AL, Dwyer JM, Davare M, Reese E, Obrietan K, et al. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci. 2004;24:4324–32. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood. 2007;109:4313–9. doi: 10.1182/blood-2006-10-048215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63:784–90. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- Bokemeyer D, Lindemann M, Kramer HJ. Regulation of mitogen-activated protein kinase phosphatase-1 in vascular smooth muscle cells. Hypertension. 1998;32:661–7. doi: 10.1161/01.hyp.32.4.661. [DOI] [PubMed] [Google Scholar]
- Bokemeyer D, Sorokin A, Yan M, Ahn NG, Templeton DJ, Dunn MJ. Induction of mitogen-activated protein kinase phosphatase 1 by the stress-activated protein kinase signaling pathway but not by extracellular signal-regulated kinase in fibroblasts. J Biol Chem. 1996;271:639–42. doi: 10.1074/jbc.271.2.639. [DOI] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- Brondello JM, Brunet A, Pouyssegur J, McKenzie FR. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J Biol Chem. 1997;272:1368–76. doi: 10.1074/jbc.272.2.1368. [DOI] [PubMed] [Google Scholar]
- Brondello JM, Pouyssegur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–7. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- Byon JC, Dadke SS, Rulli S, Kusari AB, Kusari J. Insulin regulates MAP kinase phosphatase-1 induction in Hirc B cells via activation of both extracellular signal-regulated kinase (ERK) and c-Jun-N-terminal kinase (JNK) Mol Cell Biochem. 2001;218:131–8. doi: 10.1023/a:1007204508882. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–70. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- Calvert TJ, Chicoine LG, Liu Y, Nelin LD. Deficiency of mitogen-activated protein kinase phosphatase-1 results in iNOS-mediated hypotension in response to low-dose endotoxin. Am J Physiol Heart Circ Physiol. 2008;294:H1621–9. doi: 10.1152/ajpheart.01008.2007. [DOI] [PubMed] [Google Scholar]
- Camps M, Nichols A, Gillieron C, Antonsson B, Muda M, Chabert C, et al. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science. 1998;280:1262–5. doi: 10.1126/science.280.5367.1262. [DOI] [PubMed] [Google Scholar]
- Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205:1491–503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–5. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- Casals-Casas C, Alvarez E, Serra M, de la Torre C, Farrera C, Sanchez-Tillo E, et al. CREB and AP-1 activation regulates MKP-1 induction by LPS or M-CSF and their kinetics correlate with macrophage activation versus proliferation. Eur J Immunol. 2009;39:1902–13. doi: 10.1002/eji.200839037. [DOI] [PubMed] [Google Scholar]
- Chapman MS, Miner JN. Novel mitogen-activated protein kinase kinase inhibitors. Expert Opin Investig Drugs. 2011;20:209–20. doi: 10.1517/13543784.2011.548803. [DOI] [PubMed] [Google Scholar]
- Charles CH, Abler AS, Lau LF. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992;7:187–90. [PubMed] [Google Scholar]
- Charles CH, Sun H, Lau LF, Tonks NK. The growth factor-inducible immediate-early gene 3CH134 encodes a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1993;90:5292–6. doi: 10.1073/pnas.90.11.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Machado-Pinilla R, Manguan-Garcia C, Belda-Iniesta C, Moratilla C, Cejas P, et al. MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small-cell lung cancer. Oncogene. 2006;25:3335–45. doi: 10.1038/sj.onc.1209364. [DOI] [PubMed] [Google Scholar]
- Chen P, Hutter D, Liu P, Liu Y. A mammalian expression system for rapid production and purification of active MAP kinase phosphatases. Protein Expr Purif. 2002a;24:481–8. doi: 10.1006/prep.2001.1599. [DOI] [PubMed] [Google Scholar]
- Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002b;169:6408–16. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- Chen W, Martindale JL, Holbrook NJ, Liu Y. Tumor promoter arsenite activates extracellular signal-regulated kinase through a signaling pathway mediated by epidermal growth factor receptor and Shc. Mol Cell Biol. 1998;18:5178–88. doi: 10.1128/mcb.18.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5:905–15. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–9. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho IJ, Woo NR, Kim SG. The identification of C/EBPbeta as a transcription factor necessary for the induction of MAPK phosphatase-1 by toll-like receptor-4 ligand. Arch Biochem Biophys. 2008;479:88–96. doi: 10.1016/j.abb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Choudhary R, Palm-Leis A, Scott RC, 3rd, Guleria RS, Rachut E, Baker KM, et al. All-trans retinoic acid prevents development of cardiac remodeling in aortic banded rats by inhibiting the renin-angiotensin system. Am J Physiol Heart Circ Physiol. 2008;294:H633–44. doi: 10.1152/ajpheart.01301.2007. [DOI] [PubMed] [Google Scholar]
- Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- Collart MA, Belin D, Vassalli JD, de Kossodo S, Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986;164:2113–8. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–6. [PubMed] [Google Scholar]
- Denkert C, Schmitt WD, Berger S, Reles A, Pest S, Siegert A, et al. Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. Int J Cancer. 2002;102:507–13. doi: 10.1002/ijc.10746. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- Dorfman K, Carrasco D, Gruda M, Ryan C, Lira SA, Bravo R. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13:925–31. [PubMed] [Google Scholar]
- Duan W, Wong WS. Targeting mitogen-activated protein kinases for asthma. Curr Drug Targets. 2006;7:691–8. doi: 10.2174/138945006777435353. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, et al. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–32. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21:4831–40. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Essilfie-Quaye S, Ito K, Ito M, Kharitonov SA, Barnes PJ. Comparison of Symbicort((R)) versus Pulmicort((R)) on steroid pharmacodynamic markers in asthma patients. Respir Med. 2011 doi: 10.1016/j.rmed.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Fan WJ, Genade S, Genis A, Huisamen B, Lochner A. Dexamethasone-induced cardioprotection: a role for the phosphatase MKP-1? Life Sci. 2009;84:838–46. doi: 10.1016/j.lfs.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach RJ, Qin H, Zhang L, Bennett AM. Loss of Mitogen-activated Protein Kinase Phosphatase-1 Protects from Hepatic Steatosis by Repression of Cell Death-inducing DNA Fragmentation Factor A (DFFA)-like Effector C (CIDEC)/Fat-specific Protein 27. J Biol Chem. 2011;286:22195–202. doi: 10.1074/jbc.M110.210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–23. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci U S A. 1998;95:3014–9. doi: 10.1073/pnas.95.6.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier WJ, Wang X, Wancket LM, Li XA, Meng X, Nelin LD, et al. Increased Inflammation, Impaired Bacterial Clearance, and Metabolic Disruption after Gram-Negative Sepsis in Mkp-1-Deficient Mice. J Immunol. 2009;183:7411–9. doi: 10.4049/jimmunol.0804343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. The macrophage. Bioessays. 1995;17:977–86. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- Hammer M, Echtenachter B, Weighardt H, Jozefowski K, Rose-John S, Mannel DN, et al. Increased inflammation and lethality of Dusp1(−/−) mice in polymicrobial peritonitis models. Immunology. 2010 doi: 10.1111/j.1365-2567.2010.03313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M, Mages J, Dietrich H, Schmitz F, Striebel F, Murray PJ, et al. Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur J Immunol. 2005;35:2991–3001. doi: 10.1002/eji.200526192. [DOI] [PubMed] [Google Scholar]
- Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, et al. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, McCormick F. The molecular pathology of cancer. Nat Rev Clin Oncol. 2010;7:251–65. doi: 10.1038/nrclinonc.2010.41. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hernandez A, Ray P, Litos G, Ciro M, Ottolenghi S, Beug H, et al. Acetylation and MAPK phosphorylation cooperate to regulate the degradation of active GATA-1. EMBO J. 2006;25:3264–74. doi: 10.1038/sj.emboj.7601228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi Y, Hiroi J, Kudoh S, Yazaki Y, Nagai R, Komuro I. Two distinct mechanisms of angiotensin II-induced negative regulation of the mitogen-activated protein kinases in cultured cardiac myocytes. Hypertens Res. 2001;24:385–94. doi: 10.1291/hypres.24.385. [DOI] [PubMed] [Google Scholar]
- Huang G, Wang Y, Shi LZ, Kanneganti TD, Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity. 2011;35:45–58. doi: 10.1016/j.immuni.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TH, Wu F, Loeb GB, Hsu R, Heidersbach A, Brincat A, et al. Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion. J Biol Chem. 2009;284:18515–24. doi: 10.1074/jbc.M109.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Rangarajan P, Ling EA, Dheen ST. Dexamethasone inhibits the Nox-dependent ROS production via suppression of MKP-1-dependent MAPK pathways in activated microglia. BMC Neurosci. 2011;12:49. doi: 10.1186/1471-2202-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter D, Chen P, Barnes J, Liu Y. Catalytic activation of mitogen-activated protein (MAP) kinase phosphatase-1 by binding to p38 MAP kinase: critical role of the p38 C-terminal domain in its negative regulation. Biochem J. 2000;352(Pt 1):155–63. [PMC free article] [PubMed] [Google Scholar]
- Hutter D, Chen P, Li J, Barnes J, Liu Y. The carboxyl-terminal domains of MKP-1 and MKP-2 have inhibitory effects on their phosphatase activity. Mol Cell Biochem. 2002;233:107–17. doi: 10.1023/a:1015502226940. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Zhang J, Chen K, Liu Y, Schopfer FJ, Baker PR, et al. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: a critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology. 2008;149:4086–94. doi: 10.1210/en.2007-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa R, Xie S, Khorasani N, Sukkar M, Adcock IM, Lee KY, et al. Corticosteroid inhibition of growth-related oncogene protein-alpha via mitogen-activated kinase phosphatase-1 in airway smooth muscle cells. J Immunol. 2007;178:7366–75. doi: 10.4049/jimmunol.178.11.7366. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci. 2010;13:1373–9. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kar S, Ukil A, Sharma G, Das PK. MAPK-directed phosphatases preferentially regulate pro- and anti-inflammatory cytokines in experimental visceral leishmaniasis: involvement of distinct protein kinase C isoforms. J Leukoc Biol. 2010;88:9–20. doi: 10.1189/jlb.0909644. [DOI] [PubMed] [Google Scholar]
- Kaur M, Chivers JE, Giembycz MA, Newton R. Long-acting beta2-adrenoceptor agonists synergistically enhance glucocorticoid-dependent transcription in human airway epithelial and smooth muscle cells. Mol Pharmacol. 2008;73:203–14. doi: 10.1124/mol.107.040121. [DOI] [PubMed] [Google Scholar]
- Kelly M, King E, Rider C, Gwozd C, Holden N, Eddleston J, et al. Corticosteroid-induced gene expression in allergen-challenged asthmatic subjects taking inhaled budesonide. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse SM. An emerging family of dual specificity MAP kinase phosphatases. Biochim Biophys Acta. 1995;1265:152–60. doi: 10.1016/0167-4889(94)00211-v. [DOI] [PubMed] [Google Scholar]
- Keyse SM. The role of protein phosphatases in the regulation of mitogen and stress-activated protein kinases. Free Radic Res. 1999;31:341–9. doi: 10.1080/10715769900300911. [DOI] [PubMed] [Google Scholar]
- Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–92. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–61. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature. 1992;359:644–7. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–27. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HM, Oh SH, Bang HS, Kang NI, Cho BH, Im SY, et al. Glutamine protects mice from lethal endotoxic shock via a rapid induction of MAPK phosphatase-1. J Immunol. 2009;182:7957–62. doi: 10.4049/jimmunol.0900043. [DOI] [PubMed] [Google Scholar]
- Kundu S, Fan K, Cao M, Lindner DJ, Tuthill R, Liu L, et al. Tyrosine phosphatase inhibitor-3 sensitizes melanoma and colon cancer to biotherapeutics and chemotherapeutics. Mol Cancer Ther. 2010;9:2287–96. doi: 10.1158/1535-7163.MCT-10-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano Y, Kim HH, Abdelmohsen K, Pullmann R, Jr., Martindale JL, Yang X, et al. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28:4562–75. doi: 10.1128/MCB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SP, Hakes DJ, Martell KJ, Dixon JE. Isolation and characterization of a human dual specificity protein-tyrosine phosphatase gene. J Biol Chem. 1994;269:3596–604. [PubMed] [Google Scholar]
- Laderoute KR, Mendonca HL, Calaoagan JM, Knapp AM, Giaccia AJ, Stork PJ. Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. J Biol Chem. 1999;274:12890–7. doi: 10.1074/jbc.274.18.12890. [DOI] [PubMed] [Google Scholar]
- Lai WS, Parker JS, Grissom SF, Stumpo DJ, Blackshear PJ. Novel mRNA targets for tristetraprolin (TTP) identified by global analysis of stabilized transcripts in TTP-deficient fibroblasts. Mol Cell Biol. 2006;26:9196–208. doi: 10.1128/MCB.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Hammer M, Mages J. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol. 2006;177:7497–504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol. 2002;22:7802–11. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985;4:3145–51. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo JS, Skoko JJ, Werner S, Mitasev B, Bakan A, Koizumi F, et al. Structurally unique inhibitors of human mitogen-activated protein kinase phosphatase-1 identified in a pyrrole carboxamide library. J Pharmacol Exp Ther. 2007;322:940–7. doi: 10.1124/jpet.107.122242. [DOI] [PubMed] [Google Scholar]
- Li J, Gorospe M, Hutter D, Barnes J, Keyse SM, Liu Y. Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Mol Cell Biol. 2001;21:8213–24. doi: 10.1128/MCB.21.23.8213-8224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen SF, Liu Y. MAP kinase phosphatase-1, a critical negative regulator of the innate immune response. Int J Clin Exp Med. 2009;2:48–67. [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhou JY, Ge Y, Matherly LH, Wu GS. The phosphatase MKP1 is a transcriptional target of p53 involved in cell cycle regulation. J Biol Chem. 2003;278:41059–68. doi: 10.1074/jbc.M307149200. [DOI] [PubMed] [Google Scholar]
- Liao Q, Guo J, Kleeff J, Zimmermann A, Buchler MW, Korc M, et al. Down-regulation of the dual-specificity phosphatase MKP-1 suppresses tumorigenicity of pancreatic cancer cells. Gastroenterology. 2003;124:1830–45. doi: 10.1016/s0016-5085(03)00398-6. [DOI] [PubMed] [Google Scholar]
- Lin YW, Chuang SM, Yang JL. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J Biol Chem. 2003;278:21534–41. doi: 10.1074/jbc.M301854200. [DOI] [PubMed] [Google Scholar]
- Lin YW, Yang JL. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J Biol Chem. 2006;281:915–26. doi: 10.1074/jbc.M508720200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J Biol Chem. 1995;270:8377–80. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases--regulating the immune response. Nat Rev Immunol. 2007;7:202–12. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- Loda M, Capodieci P, Mishra R, Yao H, Corless C, Grigioni W, et al. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am J Pathol. 1996;149:1553–64. [PMC free article] [PubMed] [Google Scholar]
- Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol. 2001;21:6461–9. doi: 10.1128/MCB.21.9.6461-6469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneechotesuwan K, Yao X, Ito K, Jazrawi E, Usmani OS, Adcock IM, et al. Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS Med. 2009;6:e1000076. doi: 10.1371/journal.pmed.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano RG, Montuenga LM, Dayton M, Dent P, Kinoshita I, Vicent S, et al. CL100 expression is down-regulated in advanced epithelial ovarian cancer and its re-expression decreases its malignant potential. Oncogene. 2002;21:4435–47. doi: 10.1038/sj.onc.1205542. [DOI] [PubMed] [Google Scholar]
- Metzler B, Hu Y, Sturm G, Wick G, Xu Q. Induction of mitogen-activated protein kinase phosphatase-1 by arachidonic acid in vascular smooth muscle cells. J Biol Chem. 1998;273:33320–6. doi: 10.1074/jbc.273.50.33320. [DOI] [PubMed] [Google Scholar]
- Monk CE, Hutvagner G, Arthur JS. Regulation of miRNA transcription in macrophages in response to Candida albicans. PLoS One. 2010;5:e13669. doi: 10.1371/journal.pone.0013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E, Rad MV, Johnson M, Raats D, Nijkamp FP, Folkerts G. Salmeterol with fluticasone enhances the suppression of IL-8 release and increases the translocation of glucocorticoid receptor by human neutrophils stimulated with cigarette smoke. J Mol Med. 2008;86:1045–56. doi: 10.1007/s00109-008-0360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelin LD, Wang X, Zhao Q, Chicoine LG, Young TL, Hatch DM, et al. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol. 2007;293:C632–40. doi: 10.1152/ajpcell.00137.2006. [DOI] [PubMed] [Google Scholar]
- Nichols A, Camps M, Gillieron C, Chabert C, Brunet A, Wilsbacher J, et al. Substrate recognition domains within extracellular signal-regulated kinase mediate binding and catalytic activation of mitogen-activated protein kinase phosphatase-3. J Biol Chem. 2000;275:24613–21. doi: 10.1074/jbc.M001515200. [DOI] [PubMed] [Google Scholar]
- Nieminen R, Korhonen R, Moilanen T, Clark AR, Moilanen E. DMARD aurothiomalate inhibits COX-2, MMP-3 and IL-6 expression in chondrocytes by increasing MKP-1 expression and decreasing p38 phosphorylation. Arthritis Rheum. 2010 doi: 10.1002/art.27409. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Metz R, Chen L, Mattei MG, Carrasco D, Bravo R. Structure, mapping, and expression of erp, a growth factor-inducible gene encoding a nontransmembrane protein tyrosine phosphatase, and effect of ERP on cell growth. Mol Cell Biol. 1993;13:5195–205. doi: 10.1128/mcb.13.9.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–9. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm-Leis A, Singh US, Herbelin BS, Olsovsky GD, Baker KM, Pan J. Mitogen-activated protein kinases and mitogen-activated protein kinase phosphatases mediate the inhibitory effects of all-trans retinoic acid on the hypertrophic growth of cardiomyocytes. J Biol Chem. 2004;279:54905–17. doi: 10.1074/jbc.M407383200. [DOI] [PubMed] [Google Scholar]
- Park H, Jeon JY, Kim SY, Jeong DG, Ryu SE. Identification of novel inhibitors of mitogen-activated protein kinase phosphatase-1 with structure-based virtual screening. J Comput Aided Mol Des. 2011;25:469–75. doi: 10.1007/s10822-011-9432-2. [DOI] [PubMed] [Google Scholar]
- Posern G, Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–96. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–82. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Quante T, Ng YC, Ramsay EE, Henness S, Allen JC, Parmentier J, et al. Corticosteroids reduce IL-6 in ASM cells via up-regulation of MKP-1. Am J Respir Cell Mol Biol. 2008;39:208–17. doi: 10.1165/rcmb.2007-0014OC. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–6. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Rastogi R, Du W, Ju D, Pirockinaite G, Liu Y, Nunez G, et al. Dysregulation of p38 and MKP-1 in response to NOD1/TLR4 stimulation in sarcoid bronchoalveolar cells. Am J Respir Crit Care Med. 2011;183:500–10. doi: 10.1164/rccm.201005-0792OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhala HE, Porkka KP, Tolonen TT, Martikainen PM, Tammela TL, Visakorpi T. Dual-specificity phosphatase 1 and serum/glucocorticoid-regulated kinase are downregulated in prostate cancer. Int J Cancer. 2005;117:738–45. doi: 10.1002/ijc.21270. [DOI] [PubMed] [Google Scholar]
- Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–24. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez N, Dietrich H, Mossbrugger I, Weintz G, Scheller J, Hammer M, et al. Increased inflammation and impaired resistance to Chlamydophila pneumoniae infection in Dusp1(−/−) mice: critical role of IL-6. J Leukoc Biol. 2010;88:579–87. doi: 10.1189/jlb.0210083. [DOI] [PubMed] [Google Scholar]
- Roger T, Chanson AL, Knaup-Reymond M, Calandra T. Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur J Immunol. 2005;35:3405–13. doi: 10.1002/eji.200535413. [DOI] [PubMed] [Google Scholar]
- Rojo F, Gonzalez-Navarrete I, Bragado R, Dalmases A, Menendez S, Cortes-Sempere M, et al. Mitogen-activated protein kinase phosphatase-1 in human breast cancer independently predicts prognosis and is repressed by doxorubicin. Clin Cancer Res. 2009;15:3530–9. doi: 10.1158/1078-0432.CCR-08-2070. [DOI] [PubMed] [Google Scholar]
- Roth RJ, Le AM, Zhang L, Kahn M, Samuel VT, Shulman GI, et al. MAPK phosphatase-1 facilitates the loss of oxidative myofibers associated with obesity in mice. J Clin Invest. 2009;119:3817–29. doi: 10.1172/JCI39054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf HJ. Involvement of growth factor receptors in the mammalian UVC response. Cell. 1994;78:963–72. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Sakaue H, Ogawa W, Nakamura T, Mori T, Nakamura K, Kasuga M. Role of MAPK phosphatase-1 (MKP-1) in adipocyte differentiation. J Biol Chem. 2004;279:39951–7. doi: 10.1074/jbc.M407353200. [DOI] [PubMed] [Google Scholar]
- Salojin K, Oravecz T. Regulation of innate immunity by MAPK dual-specificity phosphatases: knockout models reveal new tricks of old genes. J Leukoc Biol. 2007;81:860–9. doi: 10.1189/jlb.1006639. [DOI] [PubMed] [Google Scholar]
- Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- Sanchez-Tillo E, Comalada M, Xaus J, Farrera C, Valledor AF, Caelles C, et al. JNK1 Is required for the induction of Mkp1 expression in macrophages during proliferation and lipopolysaccharide-dependent activation. J Biol Chem. 2007;282:12566–73. doi: 10.1074/jbc.M609662200. [DOI] [PubMed] [Google Scholar]
- Sartori R, Li F, Kirkwood KL. MAP kinase phosphatase-1 protects against inflammatory bone loss. J Dent Res. 2009;88:1125–30. doi: 10.1177/0022034509349306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd EG, Zhao Q, Welty SE, Hansen TN, Smith CV, Liu Y. The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. J Biol Chem. 2004;279:54023–31. doi: 10.1074/jbc.M408444200. [DOI] [PubMed] [Google Scholar]
- Shipp LE, Lee JV, Yu CY, Pufall M, Zhang P, Scott DK, et al. Transcriptional Regulation of Human Dual Specificity Protein Phosphatase 1 (DUSP1) Gene by Glucocorticoids. PLoS One. 2010;5:e13754. doi: 10.1371/journal.pone.0013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MD, Fox SM, Lam CF, Stenmark KR, Das M. Protein kinase Czeta attenuates hypoxia-induced proliferation of fibroblasts by regulating MAP kinase phosphatase-1 expression. Mol Biol Cell. 2006;17:1995–2008. doi: 10.1091/mbc.E05-09-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KD, Beckey VE, Wischmeyer PE. Glutamine Prevents Activation of NF-kappaB and Stress Kinase Pathways, Attenuates Inflammatory Cytokine Release, and Prevents Acute Respiratory Distress Syndrome (ARDS) Following Sepsis. Shock. 2005;24:583–9. doi: 10.1097/01.shk.0000185795.96964.71. [DOI] [PubMed] [Google Scholar]
- Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J Biol Chem. 2001;276:16491–500. doi: 10.1074/jbc.M010966200. [DOI] [PubMed] [Google Scholar]
- Sohaskey ML, Ferrell JE., Jr Activation of p42 mitogen-activated protein kinase (MAPK), but not c-Jun NH(2)-terminal kinase, induces phosphorylation and stabilization of MAPK phosphatase XCL100 in Xenopus oocytes. Mol Biol Cell. 2002;13:454–68. doi: 10.1091/mbc.01-11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AE, Dowd S, Keyse SM, McDonald NQ. Crystal structure of the MAPK phosphatase Pyst1 catalytic domain and implications for regulated activation. Nat Struct Biol. 1999;6:174–81. doi: 10.1038/5861. [DOI] [PubMed] [Google Scholar]
- Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, et al. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313–24. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–11. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–93. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–7. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki C, Unoki M, Nakamura Y. Identification and allelic frequencies of novel single-nucleotide polymorphisms in the DUSP1 and BTG1 genes. J Hum Genet. 2001;46:155–7. doi: 10.1007/s100380170105. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–6. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- Tanzola MB, Kersh GJ. The dual specificity phosphatase transcriptome of the murine thymus. Mol Immunol. 2006;43:754–62. doi: 10.1016/j.molimm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Tchen CR, Martins JR, Paktiawal N, Perelli R, Saklatvala J, Clark AR. Glucocorticoid regulation of mouse and human dual specificity phosphatase 1 (DUSP1) genes: unusual cis-acting elements and unexpected evolutionary divergence. J Biol Chem. 2010;285:2642–52. doi: 10.1074/jbc.M109.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly LA, Carter AB. Differential expression and oxidation of MKP-1 modulates TNF-alpha gene expression. Am J Respir Cell Mol Biol. 2007;37:366–74. doi: 10.1165/rcmb.2006-0268OC. [DOI] [PubMed] [Google Scholar]
- Usmani OS, Ito K, Maneechotesuwan K, Ito M, Johnson M, Barnes PJ, et al. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am J Respir Crit Care Med. 2005;172:704–12. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]
- Valledor AF, Arpa L, Sanchez-Tillo E, Comalada M, Casals C, Xaus J, et al. IFN-{gamma}-mediated inhibition of MAPK phosphatase expression results in prolonged MAPK activity in response to M-CSF and inhibition of proliferation. Blood. 2008a;112:3274–82. doi: 10.1182/blood-2007-11-123604. [DOI] [PubMed] [Google Scholar]
- Valledor AF, Sanchez-Tillo E, Arpa L, Park JM, Caelles C, Lloberas J, et al. Selective roles of MAPKs during the macrophage response to IFN-gamma. J Immunol. 2008b;180:4523–9. doi: 10.4049/jimmunol.180.7.4523. [DOI] [PubMed] [Google Scholar]
- Valledor AF, Xaus J, Comalada M, Soler C, Celada A. Protein kinase C epsilon is required for the induction of mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2000;164:29–37. doi: 10.4049/jimmunol.164.1.29. [DOI] [PubMed] [Google Scholar]
- Vicent S, Garayoa M, Lopez-Picazo JM, Lozano MD, Toledo G, Thunnissen FB, et al. Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients. Clin Cancer Res. 2004;10:3639–49. doi: 10.1158/1078-0432.CCR-03-0771. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–41. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Vogt A, McDonald PR, Tamewitz A, Sikorski RP, Wipf P, Skoko JJ, 3rd, et al. A cell-active inhibitor of mitogen-activated protein kinase phosphatases restores paclitaxel-induced apoptosis in dexamethasone-protected cancer cells. Mol Cancer Ther. 2008;7:330–40. doi: 10.1158/1535-7163.MCT-07-2165. [DOI] [PubMed] [Google Scholar]
- Wang HY, Cheng Z, Malbon CC. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 2003;191:229–37. doi: 10.1016/s0304-3835(02)00612-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Ford HR, Grishin AV. NF-kappaB-mediated expression of MAPK phosphatase-1 is an early step in desensitization to TLR ligands in enterocytes. Mucosal Immunol. 2010;3:523–34. doi: 10.1038/mi.2010.35. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu Y. Regulation of innate immune response by MAP kinase phosphatase-1. Cell Signal. 2007;19:1372–82. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–31. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- Wang X, Meng X, Kuhlman JR, Nelin LD, Nicol KK, English BK, et al. Knockout of Mkp-1 enhances the host inflammatory responses to gram-positive bacteria. J Immunol. 2007;178:5312–20. doi: 10.4049/jimmunol.178.8.5312. [DOI] [PubMed] [Google Scholar]
- Wang X, Nelin LD, Kuhlman JR, Meng X, Welty SE, Liu Y. The role of MAP kinase phosphatase-1 in the protective mechanism of dexamethasone against endotoxemia. Life Sci. 2008a;83:671–80. doi: 10.1016/j.lfs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao Q, Matta R, Meng X, Liu X, Liu CG, et al. Inducible nitric-oxide synthase expression is regulated by mitogen-activated protein kinase phosphatase-1. J Biol Chem. 2009;284:27123–34. doi: 10.1074/jbc.M109.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cao N, Nantajit D, Fan M, Liu Y, Li JJ. Mitogen-activated protein kinase phosphatase-1 represses c-Jun NH2-terminal kinase-mediated apoptosis via NF-kappaB regulation. J Biol Chem. 2008b;283:21011–23. doi: 10.1074/jbc.M802229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Shen P, Rider CF, Traves SL, Proud D, Newton R, et al. Selective prostacyclin receptor agonism augments glucocorticoid-induced gene expression in human bronchial epithelial cells. J Immunol. 2009;183:6788–99. doi: 10.4049/jimmunol.0902738. [DOI] [PubMed] [Google Scholar]
- Wong K, Zhang J, Awasthi S, Sharma A, Rogers L, Matlock EF, et al. Nerve growth factor receptor signaling induces histone acetyltransferase domain-dependent nuclear translocation of p300/CREB-binding protein-associated factor and hGCN5 acetyltransferases. J Biol Chem. 2004;279:55667–74. doi: 10.1074/jbc.M408174200. [DOI] [PubMed] [Google Scholar]
- Wu GS. Role of mitogen-activated protein kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev. 2007;26:579–85. doi: 10.1007/s10555-007-9079-6. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Roth RJ, Anderson EJ, Hong EG, Lee MK, Choi CS, et al. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006;4:61–73. doi: 10.1016/j.cmet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Yang H, Wu GS. p53 Transactivates the phosphatase MKP1 through both intronic and exonic p53 responsive elements. Cancer Biol Ther. 2004;3:1277–82. doi: 10.4161/cbt.3.12.1370. [DOI] [PubMed] [Google Scholar]
- Yin Q, Wang X, McBride J, Fewell C, Flemington E. B-cell receptor activation induces BIC/miR-155 expression through a conserved AP-1 element. J Biol Chem. 2008;283:2654–62. doi: 10.1074/jbc.M708218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Li Q, Herbert B, Zinna R, Martin K, Junior CR, et al. Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene Ther. 2011a;18:344–53. doi: 10.1038/gt.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Sun Y, Haycraft C, Palanisamy V, Kirkwood KL. MKP-1 regulates cytokine mRNA stability through selectively modulation subcellular translocation of AUF1. Cytokine. 2011b doi: 10.1016/j.cyto.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Reynolds J, Chang SH, Martin-Orozco N, Chung Y, Nurieva RI, et al. MAP kinase phosphatase 1 is necessary for T cell activation and function. J Biol Chem. 2009 doi: 10.1074/jbc.M109.052472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem. 2005;280:8101–8. doi: 10.1074/jbc.M411760200. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–40. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CF, Guan KL. Dephosphorylation and inactivation of the mitogen-activated protein kinase by a mitogen-induced Thr/Tyr protein phosphatase. J Biol Chem. 1993;268:16116–9. [PubMed] [Google Scholar]
- Zhu QY, Liu Q, Chen JX, Lan K, Ge BX. MicroRNA-101 targets MAPK phosphatase-1 to regulate the activation of MAPKs in macrophages. J Immunol. 2010;185:7435–42. doi: 10.4049/jimmunol.1000798. [DOI] [PubMed] [Google Scholar]